Polyphenol Composition of Traditional Decoctions from Polygoni Cuspidati Rhizoma et Radix of Different Origin and Their Impact on Human Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
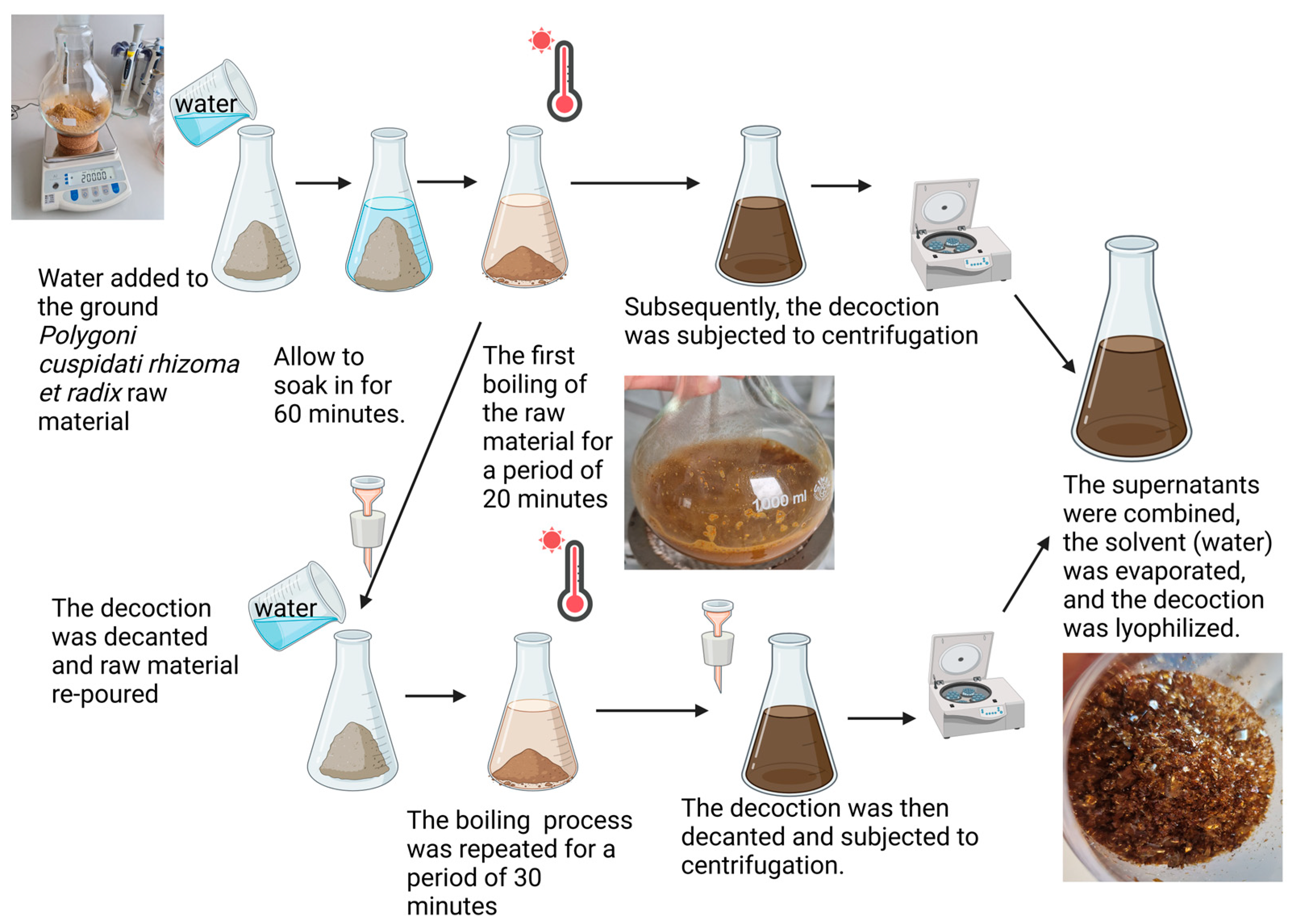
2.1. Plant Material and Decoction Preparation
2.2. UHPLC-DAD-qTOF-MS Qualitative and Quantitative Analysis
2.3. UPLC/QqQ-MS/MS Quantitative Analysis
2.4. Cell Viability
2.4.1. Cell Line and Conditions
2.4.2. Cytotoxic Activity, MTT Assay
2.5. Statistical Analysis
3. Results
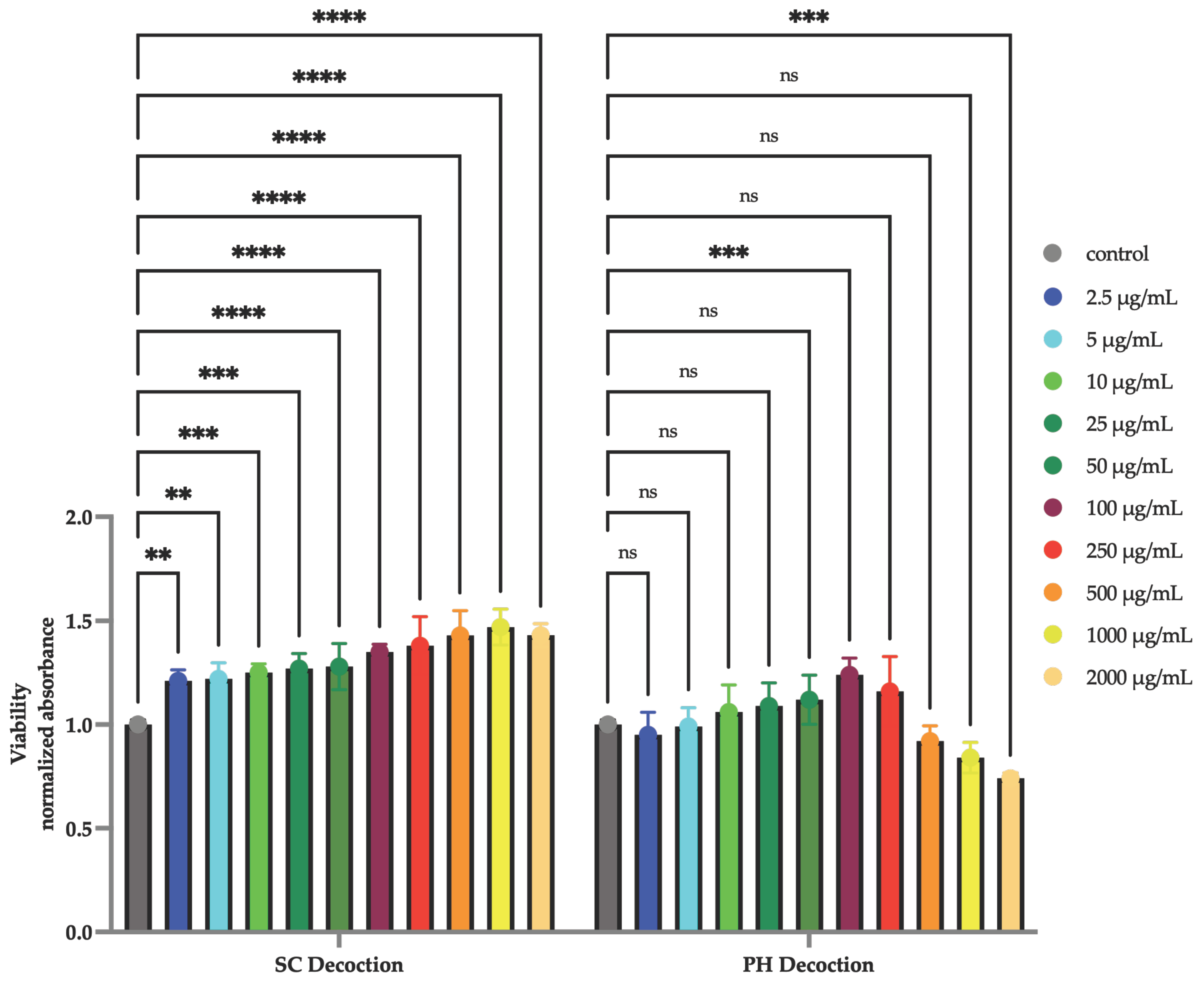
3.1. Cell Viability—MTT Assay
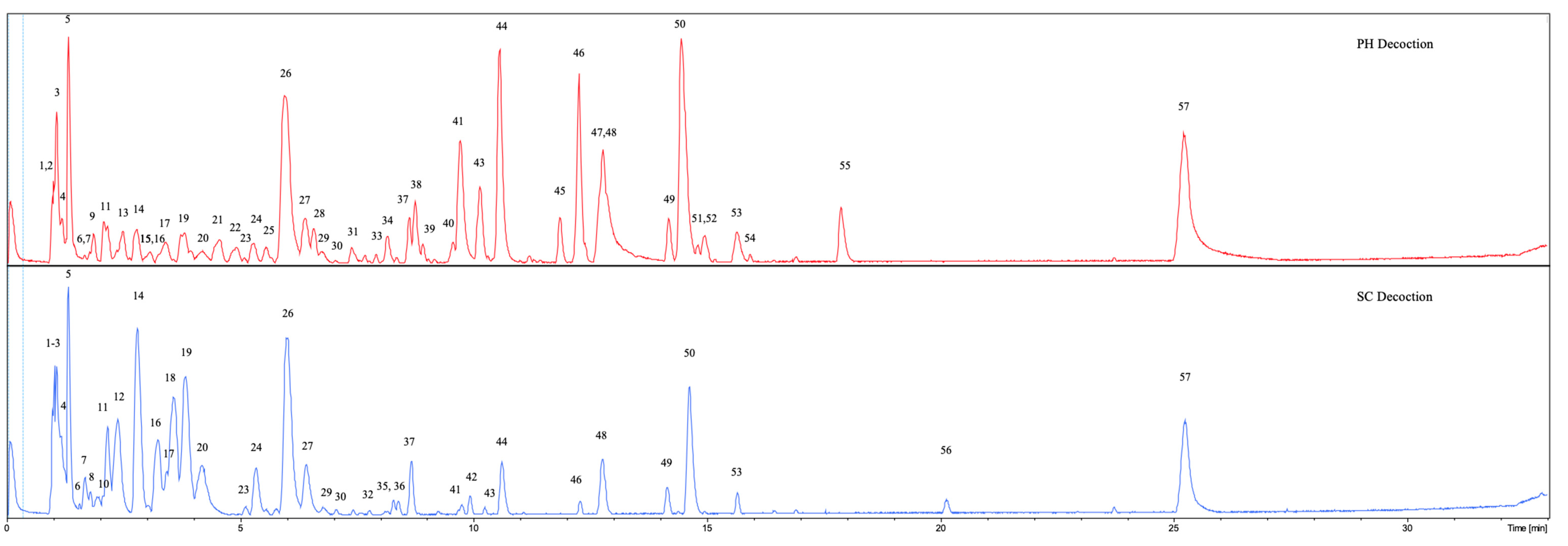
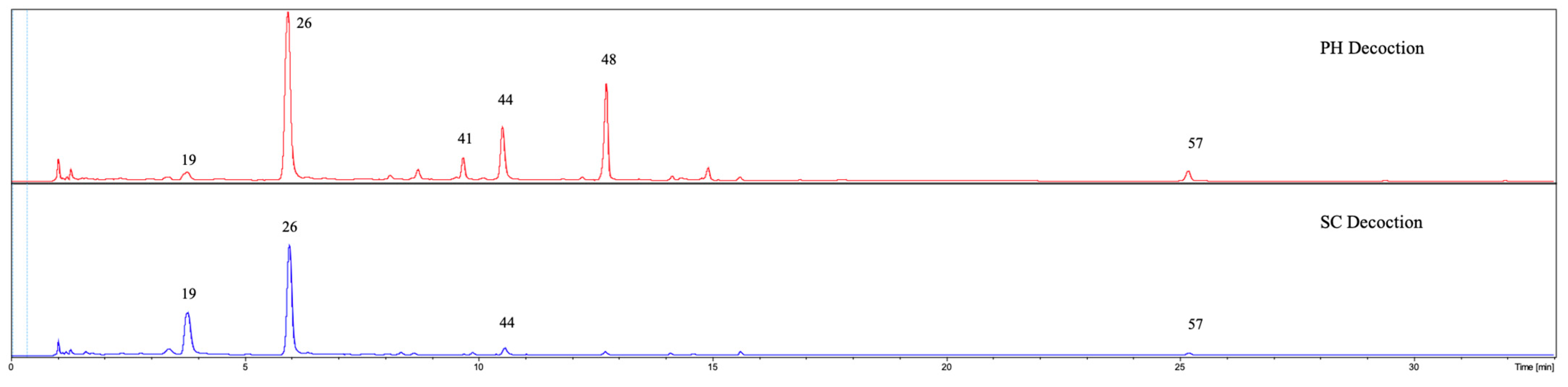
3.2. UHPLC-DAD-qTOF-MS Qualitative Analysis
3.3. UHPLC-DAD-qTOF-MS Quantitative Analysis
3.4. UPLC/QqQ-MS/MS Quantitative Analysis of Flavan-3-Ols and Procyanidins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Invasive Species Database. Species Profile: Polygonum Cuspidatum. Available online: https://www.iucngisd.org/gisd/speciesname/Polygonum+cuspidatum (accessed on 23 November 2024).
- Nawrot-Hadzik, I.; Hadzik, J.; Fleischer, M.; Choromańska, A.; Sterczała, B.; Kubasiewicz-Ross, P.; Saczko, J.; Gałczyńska-Rusin, M.; Gedrange, T.; Matkowski, A. Chemical composition of east Asian invasive knotweeds, their cytotoxicity and antimicrobial efficacy against cariogenic pathogens: An in-vitro study. Med. Sci. Monit. 2019, 25, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia, 2010th ed.; Medical Science and Technology Press: Beijing, China, 2010; pp. 194–195. [Google Scholar]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Kwok, S.T.; Zhang, Q.W.; Chan, S.W. A review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid.-Based Complement. Altern. Med. 2013, 2013, 208349. [Google Scholar] [CrossRef]
- Hempen, C.-H.; Fischer, T. A Materia Medica for Chinese Medicine: Plants, Minerals, and Animal Products; Churchill Livingstone: London, UK, 2009; ISBN 9780443100949. [Google Scholar]
- Song, J.H.; Kim, S.K.; Chang, K.W.; Han, S.K.; Yi, H.K.; Jeon, J.G. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch. Oral Biol. 2006, 51, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Luo, X.Q.; Gu, S.Y.; Xu, J.H. The effects of Polygonum cuspidatum extract on wound healing in rats. J. Ethnopharmacol. 2012, 141, 934–937. [Google Scholar] [CrossRef]
- Hadzik, J.; Choromańska, A.; Karolewicz, B.; Matkowski, A.; Dominiak, M.; Złocińska, A.; Nawrot-Hadzik, I. Oral Wound Healing Potential of Polygoni Cuspidati Rhizoma et Radix Decoction-In Vitro Study. Pharmaceuticals 2023, 16, 267. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Pitułaj, A.; Sterczała, B.; Olchowy, C.; Szewczyk, A.; Choromańska, A. In Vitro Gingival Wound Healing Activity of Extracts from Reynoutria japonica Houtt Rhizomes. Pharmaceutics 2021, 13, 1764. [Google Scholar] [CrossRef]
- Alperth, F.; Melinz, L.; Fladerer, J.P.; Bucar, F. Uhplc analysis of reynoutria japonica houtt. Rhizome preparations regarding stilbene and anthranoid composition and their antimycobacterial activity evaluation. Plants 2021, 10, 1809. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Slusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical Diversity in Rhizomes of Three Reynoutria Species and their Antioxidant Activity Correlations Elucidated by LC-ESI-MS/MS Analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef]
- Magacz, M.; Oszajca, M.; Nawrot-Hadzik, I.; Drożdż, R.; Jurczak, A.; Hadzik, J.; Smakosz, A.; Krzyściak, W. Phenolic compounds of Reynoutria sp. As modulators of oral cavity lactoperoxidase system. Antioxidants 2021, 10, 676. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Yu, S.; Wang, H.; Tian, Z.; Zhu, S. Procyanidins and Their Therapeutic Potential against Oral Diseases. Molecules 2022, 27, 2932. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis—Antibacterial Effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Matkowski, A.; Kubasiewicz-Ross, P.; Hadzik, J. Proanthocyanidins and Flavan-3-ols in the Prevention and Treatment of Periodontitis-Immunomodulatory Effects, Animal and Clinical Studies. Nutrients 2021, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Granica, S.; Domaradzki, K.; Pecio, Ł.; Matkowski, A. Isolation and Determination of Phenolic Glycosides and Anthraquinones from Rhizomes of Various Reynoutria Species. Planta Med. 2018, 84, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A Versatile Targeted Metabolomics Method for the Rapid. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Organization for Standardization: Geneva, Switzerland, 2009.
- Xiao, K.; Xuan, L.; Xu, Y.; Bai, D.; Zhong, D. Constituents from Polygonum cuspidatum. Chem. Pharm. Bull. 2002, 50, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Xuan, L.; Xu, Y.; Bai, D. Stilbene Glycoside Sulfates from Polygonum cuspidatum. J. Nat. Prod. 2000, 63, 1373–1376. [Google Scholar] [CrossRef]
- Krenn, L.; Presser, A.; Pradhan, R.; Bahr, B.; Paper, D.H.; Mayer, K.K.; Kopp, B. Sulfemodin 8-O-β-d-Glucoside, a New Sulfated Anthraquinone Glycoside, and Antioxidant Phenolic Compounds from Rheum emodi. J. Nat. Prod. 2003, 66, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Abd Hamid, R.; Xiao, K.; Liu, C.; Wang, Y.; Han, L.; Wang, P.; Zhao, Q.; Zheng, F.; Dou, Z.; Yang, W.; et al. Rapid Discovery of the Potential Toxic Compounds in Polygonum multiflorum by UHPLC/Q-Orbitrap-MS-Based Metabolomics and Correlation Analysis. Front. Pharmacol. 2019, 10, 329. [Google Scholar] [CrossRef]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Chaniad Id, P.; Tewtrakul, S.; Sudsai, T.; Langyanai, S.; Kaewdana, K. Anti-inflammatory, wound healing and antioxidant potential of compounds from Dioscorea bulbifera L. bulbils. PLoS ONE 2020, 15, e0243632. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lechtenberg, M.; Sendker, J.; Petereit, F.; Deters, A.; Hensel, A. Wound-healing plants from TCM: In vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia 2013, 84, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef] [PubMed]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Rajeshkumar, S.; Thomas, S.; Kalarikkal, N. Nano formulated proanthocyanidins as an effective wound healing component. Mater. Sci. Eng. C 2020, 106, 110056. [Google Scholar] [CrossRef]
- Babich, H.; Krupka, M.E.; Nissim, H.A.; Zuckerbraun, H.L. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol. In Vitro 2005, 19, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Hsieh, M.T.; Lin, C.Y.; Kuo, P.J.; Yang, Y.C.S.H.; Shih, Y.J.; Lai, H.Y.; Cheng, G.Y.; Tang, H.Y.; Lee, C.C.; et al. 2,3,5,4′-tetrahydroxystilbene-2-O-β-glucoside isolated from Polygoni Multiflori ameliorates the development of periodontitis. Mediat. Inflamm. 2016, 2016, 6953459. [Google Scholar] [CrossRef]
- D’Amico, E.; Pierfelice, T.V.; Amoroso, R.; Cacciatore, I.; D’Arcangelo, C.; Lepore, S.; D’Ercole, S.; Di Pietro, N.; Di Rienzo, A.; Petrini, M.; et al. Emerging Effects of Resveratrol Derivatives in Cells Involved in Oral Wound Healing: A Preliminary Study. Int. J. Mol. Sci. 2023, 24, 3276. [Google Scholar] [CrossRef] [PubMed]
- Birar, V.C.; Sheerin, A.N.; Ostler, E.L.; Faragher, R.G.A. Novel resveratrol derivatives have diverse effects on the survival, proliferation and senescence of primary human fibroblasts. Biogerontology 2020, 21, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Chen, H.; Wan, Q.; Dai, J.; Sun, Y.; Wang, J.; Li, X. Emodin promotes fibroblast apoptosis and prevents epidural fibrosis through PERK pathway in rats. J. Orthop. Surg. Res. 2019, 14, 319. [Google Scholar] [CrossRef]
- Baczewska, I.; Hawrylak-Nowak, B.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Borowski, G.; Dresler, S. Towards the Use of Lichens as a Source of Bioactive Substances for Topical Applications. Molecules 2024, 29, 4352. [Google Scholar] [CrossRef]
- Yang, J.B.; Li, W.F.; Liu, Y.; Wang, Q.; Cheng, X.L.; Wei, F.; Wang, A.G.; Jin, H.T.; Ma, S.C. Acute toxicity screening of different extractions, components and constituents of Polygonum multiflorum Thunb. on zebrafish (Danio rerio) embryos in vivo. Biomed. Pharmacother. 2018, 99, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Franz, G. The role of the European Pharmacopoeia (Ph Eur) in quality control of traditional Chinese herbal medicine in European member states. World J. Tradit. Chin. Med. 2015, 1, 5–15. [Google Scholar] [CrossRef]
- Sheridan, H.; Krenn, L.; Jiang, R.; Sutherland, I.; Ignatova, S.; Marmann, A.; Liang, X.; Sendker, J. The potential of metabolic fingerprinting as a tool for the modernisation of TCM preparations. J. Ethnopharmacol. 2012, 140, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Hanlin, R.L. Comparison of Ethanol and Acetone Mixtures for Extraction of Condensed Tannin from Grape Skin. S. Afr. J. Enol. Vitic. 2016, 31, 154–159. [Google Scholar] [CrossRef]
- Stafiniak, M.; Bielecka, M.; Kujawa, K.; Jezierska-Domaradzaka, A.; Pencakowski, B.; Basiak, A.; Matkowski, A.; Nawrot-Hadzik, I. Integrative morphological, phytochemical, and molecular identification of three invasive and medicinal Reynoutria species. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Loschi, F.; Faggian, M.; Sut, S.; Ferrarese, I.; Maccari, E.; Peron, G.; Dall’acqua, S. Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials. Molecules 2022, 27, 1932. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Scientific Opinion on additional scientific data related to the safety of preparations of Rheum palmatum L., Rheum officinale Baill. and their hybrids, Rhamnus purshiana DC., Rhamnus frangula L. and Cassia senna L., submitted pursuant to Article 8(4) of R. EFSA J. 2024, 22, e8766. [Google Scholar] [CrossRef] [PubMed]


| Nr. | Compound | Rt. [min] | UV Max [nm] | m/z [M-H]− | Error (ppm) | Ion Formula | MS2 Main-Ion (Relative Intensity %) | MS2 Fragments (Relative Intensity %) | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown carbohydrate | 1.0 | ND | 341.1091 | −0.6 | C12H21O11 | 113.0258 (100) | 101 (66), 119 (63), 179 (18), 173 (16) | HMDB0000258 |
| 2 | Unknown carbohydrate | 1.05 | ND | 719.2017 | 3.2 | C30H39O20 | 377.0868 (100) | 379 (29), 341 (13), 215 (2.0), 179 (0.4) | - |
| 3 | Organic acid, e.g., citric acid | 1.1 | ND | 191.0195 | 1.2 | C6H7O7 | 111.0083 (100) | HMDB0000094 | |
| 4 | Organic acid, e.g., malic acid | 1.15 | ND | 133.0145 | −1.6 | C4H5O5 | 115.0014 (100) | HMDB0000156 | |
| 5 | Organic acid, e.g., citric acid | 1.3 | ND | 191.0199 | −0.7 | C6H7O7 | 111.0083 (100) | HMDB0000094 | |
| 6 | Unknown | 1.57 | 225, 280 | 443.1928 | −1.2 | C21H31O10 | 443.1941 (100) | 189 (17), 101 (9), 113 (8), 119 (8) | |
| 7 | Procyanidin dimer, Type B | 1.6–1.7 | 225, 280 | 577.1347 | 0.7 | C30H25O12 | 289.0722 (100) | 407 (60), 125 (33), 425 (18) | [12] |
| 8 | Procyanidin trimer, Type B | 1.8 | 225, 280 | 865.2002 | −2.0 | C45H37O18 | 575.1211 (100) | 577 (81), 287 (69), 695 (48), 713 (46) | [12] |
| 9 | Unknown/dihydroxyphenylvaleric acid sulfate | 1.9 | 280 | 289.0389 | −0.5 | C11H13O7S | 96.9585 (100) | 149 (5), 209 (2) | HMDB0240447 |
| 10 | Procyanidin dimer, Type B | 1.9–2.0 | 225, 280 | 577.1363 | −2.0 | C30H25O12 | 289.0719 (100) | 407 (68), 125 (42), 425 (20) | [12] |
| 11 | Catechin | 2.15 | 225, 280 | 289.0722 | −1.7 | C15H13O6 | 109.0280 (100) | 123 (89), 203 (71), 221 (64),151 (63) | [12] |
| 12 | Procyanidin dimer, Type B | 2.4 | 225, 280 | 577.1350 | 0.3 | C30H25O12 | 289.0744 (100) | 407 (60), 125 (34), 425 (19) | [12] |
| 13 | Unknown sulfate derivative | 2.5 | 280 | 313.0027 | −0.9 | C12H9O8S | 189.0561 (100) | 233 (4) | |
| 14 | Epicatechin | 2.8 | 225, 280 | 289.0723 | −0.8 | C15H13O6 | 109.0292 (100) | 123 (93), 221 (80), 125 (76) | [12] |
| 15 | Piceid sulfate | 3.08 | 280, 320 | 469.0825 | −3.3 | C20H21O11S | 227.0717 (100) | 307 (4), 243 (1), 269 (1), 389 (0.5) | HMDB0240553 |
| 16 | Lynioresinol 2a-sulfate | 3.25 | 280 | 499.1286 | −1.3 | C22H27O11S | 499.1292 (100) | 96.9585 (12), 110.9755 (6), 453.0840 (2) | HMDB0039926 [20] |
| 17 | Piceatannol glucoside | 3.4 | 220, 290, 318 | 405.1202 | −2.8 | C20H21O9 | 243.0672 (100) | 201 (1), 225 (0.5) | [17] |
| 18 | Isolariciresinol-2a-sulfate | 3.6 | 280 | 439.1081 | −2.8 | C20H23O9S | 439.1085 (100) | 96.9585 (24), 359.1514 (2), 110.9749 (1) | HMDB0240703 [20] |
| 19 | Resveratrolside | 3.8 | 220, 304, 315 | 389.1237, 435.1291 [M+COO]− | 1.2 | C20H21O8 | 227.0713 (100) | 228 (16), 225 (9), 185 (2) | [17] |
| 20 | Procyanidin dimer monogallate | 4.2 | 225, 280 | 729.1458 | 0.4 | C37H29O16 | 407.0789 (100) | 289 (62), 577 (45), 441 (28), 451 (28) | [12] |
| 21 | Resveratrol sulfoglucoside | 4.5 | 290, 320 | 469.0827 | −3.2 | C20H21O11S | 469.0827 (100) | 241 (45), 96.96 (12), 227 (2) | HMDB0037075 [21] |
| 22 | Resveratrol sulfoglucoside isomer | 4.9 | 290, 320 | 469.0824 | −3.1 | C20H21O11S | 241.0027 (100) | 227 (13), 96.95 (7), 138.96 (2) | HMDB0037075 [21] |
| 23 | Unknown | 5.1 | 270 | 233.0453 | 0.9 | C12H9O5 | 109.0280 (100) | 123 (89), 203 (71) | - |
| 24 | Unknown sulfate derivative | 5.35 | 280 | 269.0123 | 0.7 | C11H9O6S | 189.0556 (100) | 147 (0.5) | |
| 25 | Luteolin-7-O-glucoside | 5.6 | 220, 282 | 447.0940 | −1.6 | C21H19O11 | 285.0411 (100) | 447 (75), 327 (3) | HMDB0035588 |
| 26 | Piceid | 6.0 | 220, 304, 315 | 389.1245 | −0.8 | C20H21O8 | 227.0716 (100) | 228 (13), 185 (2), 225 (0.5) | [17] |
| 27 | Epicatechin-3-O-gallate | 6.4 | 220, 279 | 441.0828 | −0.2 | C22H17O10 | 169.0141 (100) | 289 (51), 125 (22), 245 (14) | [17] |
| 28 | Unknown sulfate derivative | 6.6 | 280 | 285.0076 | −0.4 | C11H9O7S | 205.0514 (100) | 190 (46) | |
| 29 | Epicatechin-O-gallate isomer | 6.78 | 220, 279 | 441.0842 | −3.3 | C22H17O10 | 169.0153 (100) | 289 (53), 125 (25), 245 (13) | [17] |
| 30 | Procyanidin trimer monogallate | 7.0 | 225, 280 | 1017.2127 | −3.1 | C52H41O22 | 729.1431 (100) | 577 (31), 865 (30), 287 (28), 441 (19) | [12] |
| 31 | Unknown/Citrusin A | 7.4 | 280 | 537.1987 | −1.8 | C26H33O12 | 329.1397 (100) | 341 (56), 385 (27) | HMDB0039230 |
| 32 | Resveratrol hexoside | 7.8 | 220, 304, 315 | 389.1244 | −0.5 | C20H21O8 | 227.0712 (100) | 228 (15), 185 (2) | [17] |
| 33 | Emodin-hexose-sulfate | 7.9 | 220, 280 | 511.0559 | −1.3 | C21H19O13S | 241.0027 (100) | 269 (25), 96 (10), 431 (5) | [22,23] |
| 34 | Resveratrol-(galloylglucoside) | 8.2 | 220, 280, 320 | 541.1362 | −1.9 | C27H25O12 | 541.1377 (100) | 313 (25), 169 (7), 227 (7) | HMDB0039341 |
| 35 | Unknown, Azelaic acid? | 8.3 | - | 187.0978 | −1.0 | C9H15O4 | 125.0960 (100) | 97 (14), 169 (9) | |
| 36 | Resveratrol derivative | 8.4 | 220, 282, 325 | 431.1355 | −1.8 | C22H23O9 | 227.0722 (100) | 228 (14), 185 (1) | [17] |
| 37 | Resveratrol hexoside | 8.6 | 220, 304, 315 | 389.1246 | −0.9 | C20H21O8 | 227.0721 (100) | 228 (16), 185 (1) | [17] |
| 38 | Resveratrol-(galloylglucoside) isomer | 8.7 | 220, 280, 320 | 541.1367 | −2.9 | C27H25O12 | 313.0577 (100) | 169 (10), 227 (9) | HMDB0039341 |
| 39 | Aloesone hexoside | 8.9 | 220, 270, 420 | 393.1196 | −1.4 | C19H21O9 | 231.0666 (100) | 232 (13), 187 (0,3) | HMDB0035734 |
| 40 | Resveratrol-(galloylglucoside) isomer | 9.6 | 220, 284, 320 | 541.1364 | −2.4 | C27H25O12 | 541.1376 (100) | 313 (33), 169 (28), 227 (11) | HMDB0039341 |
| 41 | Emodin-glucoside | 9.7 | 217, 252, 284, 423 | 431.0995 | −2.7 | C21H19O10 | 431.0994 (100) | 269 (81), 240 (8) | [17] |
| 42 | Lapathoside D | 9.9 | 213, 285, 315 | 633.1830 | −0.7 | C30H33O15 | 145.0298 (100) | 487 (44), 633 (34), 469 (7) | [10] |
| 43 | Emodin-hexose-sulfate | 10.1 | 220, 280 | 511.0562 | −2.0 | C21H19O13S | 269.0463 (100) | 431 (18), 241 (3) | [22,23] |
| 44 | Resveratrol | 10.6 | 220, 279, 307 | 227.0718 | −2.1 | C14H11O3 | 143.0506 (100) | 185 (85), 227 (47), 144 (11) | [17] |
| 45 | Emodin-hexose-sulfate | 11.8 | 220, 280 | 511.0563 | −2.3 | C21H19O13S | 241.0034 (100) | 431 (47), 269 (30) | [22,23] |
| 46 | Torachrysone-hexoside | 12.3 | 226, 266, 325 | 407.1359 | −2.7 | C20H23O9 | 245.0825 (100) | 246 (14), 230 (11) | [17] |
| 47 | Emodin-hexose-sulfate | 11.8 | 220, 280 | 511.0559 | −1.4 | C21H19O13S | 241.0034 (100) | 269 (32), 431 (10) | [22,23] |
| 48 | Emodin-glucoside | 12.8 | 221, 269, 281, 423 | 431.0989 | −1.2 | C21H19O10 | 269.0454 (100) | 431 (50), 311 (5) | [17] |
| 49 | Emodin-8-O-(6′-O-malonyl)-glucoside | 14.2 | 220, 281, 423 | 517.0998 | 0.0 | C24H21O13 | 473.1102 (100) | 269 (66), 311 (4) | [17] |
| 50 | Sulfonyl torachrysone/isomer | 14.6 | 220, 312 | 325.0392 | −1.5 | C14H13O7S | 245.0823 (100) | 230 (32), 215 (1) | [11] |
| 51 | Emodin-glucoside | 14.8 | 221, 269, 281, 423 | 431.0984 | −0.1 | C21H19O10 | 269.0461 (100) | 282 (12), 431 (3), 311 (2) | [17] |
| 52 | Physcionin/Rheochrysin | 15.0 | 221, 272, 423 | 445.1147 | −1.6 | C22H21O10 | 283.0616 (100) | 307 (3), 240 (3) | HMDB0040511/HMDB35931 |
| 53 | Hydropiperoside | 15.7 | 222, 290, 313 | 779.2179 | 1.7 | C39H39O17 | 779.2200 (100) | 145 (92), 633 (62), 453 (7), 615 (5) | [17] |
| 54 | Aloe-emodin 8-O-(6-O-acetyl)-glucoside | 15.9 | 220, 281, 423 | 473.1094 | −1.0 | C23H21O11 | 269.0466 (100) | 473 (78), 311 (5), 293 (1) | [24] |
| 55 | Sulfemodin | 17.9 | 220, 265, 286, 316 | 349.0037 | −3.9 | C15H9O8S | 269.0467 (100) | 225 (0.5) | PubChem SID 274505204 |
| 56 | Questin | 20.1 | 222, 286, 313, 430 | 283.0614 | −0.6 | C16H11O5 | 240.0431 (100) | 269 (0.6) | [17] |
| 57 | Emodin | 25.2 | 221, 248, 267, 288, 430 | 269.0458 | −0.9 | C15H9O5 | 269.0461 (100) | 225 (28), 241 (10), 197 (2), 181 (1) | [17] |
| Analyte | (μg/mL of Liquid Decotion) SD | (mg/g of Dry Decoction) SD | ||
|---|---|---|---|---|
| SC Decoction | PH Decoction | SC Decoction | PH Decoction | |
| Piceid | 104.26 ±0.42 | 173.86 ±11.39 | 20.85 ±0.08 | 34.77 * ±2.27 |
| Resveratrol | 3.11 ±0.017 | 23.08 ±1.54 | 0.62 ±0.003 | 4.62 * ±0.31 |
| Emodin | 3.95 ±0.03 | 14.85 ±0.96 | 0.78 ±0.006 | 2.97 * ±0.19 |
| Physcion | 1.24 a ±0.01 | 2.09 a ±0.14 | 0.25 ±0.003 | 0.42 ns ±0.02 |
| Sample Name | mg/g | LOQ | PH Decoction | SC Decoction | 25% EtOH | 40% EtOH | 60% Acetone |
|---|---|---|---|---|---|---|---|
| catechin gallate | mg/g | 0.001 | 0.351 | 0.212 | <LOQ | <LOQ | <LOQ |
| SD | 0.050 | 0.044 | - | - | - | ||
| catechin | mg/g | 0.02 | 1.467 | 4.449 | 2.339 | 2.595 | 3.030 |
| SD | 0.255 | 0.329 | 0.295 | 0.640 | 0.149 | ||
| epicatechin gallate | mg/g | 0.001 | 2.096 | 2.334 | 2.987 | 6.998 | 9.213 |
| SD | 0.083 | 0.050 | 0.061 | 0.109 | 0.019 | ||
| epicatechin | mg/g | 0.001 | 1.268 | 11.355 | 17.103 | 20.098 | 24.611 |
| SD | 0.040 | 0.309 | 0.250 | 0.383 | 0.145 | ||
| epigallocatechin gallate | mg/g | 0.001 | 0.011 | 0.004 | <LOQ | <LOQ | <LOQ |
| SD | 0.002 | 0.000 | - | - | - | ||
| epigallocatechin | mg/g | 0.001 | 0.004 | 0.008 | <LOQ | <LOQ | <LOQ |
| SD | 0.001 | 0.001 | - | - | - | ||
| gallocatechin gallate | mg/g | 0.001 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| SD | - | - | - | - | - | ||
| gallocatechin | mg/g | 0.001 | 0.004 | 0.010 | <LOQ | <LOQ | <LOQ |
| SD | 0.000 | 0.000 | - | - | - | ||
| procyanidin A2 | mg/g | 0.001 | 0.004 | 0.024 | 0.100 | 0.097 | 0.131 |
| SD | 0.002 | 0.009 | 0.006 | 0.008 | 0.035 | ||
| procyanidin B1 | mg/g | 0.001 | 1.436 | 6.421 | 7.466 | 7.803 | 8.129 |
| SD | 0.024 | 0.008 | 0.176 | 0.080 | 0.361 | ||
| procyanidin B2+B4 | mg/g | 0.001 | 1.499 | 13.780 | 18.699 | 19.422 | 22.817 |
| SD | 0.100 | 0.374 | 0.417 | 1.175 | 1.167 | ||
| procyanidin B3 | mg/g | 0.001 | 0.615 | 2.315 | 1.497 | 1.765 | 2.438 |
| SD | 0.033 | 0.201 | 0.115 | 0.121 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot-Hadzik, I.; Fast, M.; Gębarowski, T.; Zanoni, G.; Martens, S.; Matkowski, A.; Seweryn, P.; Hadzik, J. Polyphenol Composition of Traditional Decoctions from Polygoni Cuspidati Rhizoma et Radix of Different Origin and Their Impact on Human Gingival Fibroblasts. Appl. Sci. 2025, 15, 1914. https://doi.org/10.3390/app15041914
Nawrot-Hadzik I, Fast M, Gębarowski T, Zanoni G, Martens S, Matkowski A, Seweryn P, Hadzik J. Polyphenol Composition of Traditional Decoctions from Polygoni Cuspidati Rhizoma et Radix of Different Origin and Their Impact on Human Gingival Fibroblasts. Applied Sciences. 2025; 15(4):1914. https://doi.org/10.3390/app15041914
Chicago/Turabian StyleNawrot-Hadzik, Izabela, Magdalena Fast, Tomasz Gębarowski, Giorgio Zanoni, Stefan Martens, Adam Matkowski, Piotr Seweryn, and Jakub Hadzik. 2025. "Polyphenol Composition of Traditional Decoctions from Polygoni Cuspidati Rhizoma et Radix of Different Origin and Their Impact on Human Gingival Fibroblasts" Applied Sciences 15, no. 4: 1914. https://doi.org/10.3390/app15041914
APA StyleNawrot-Hadzik, I., Fast, M., Gębarowski, T., Zanoni, G., Martens, S., Matkowski, A., Seweryn, P., & Hadzik, J. (2025). Polyphenol Composition of Traditional Decoctions from Polygoni Cuspidati Rhizoma et Radix of Different Origin and Their Impact on Human Gingival Fibroblasts. Applied Sciences, 15(4), 1914. https://doi.org/10.3390/app15041914









