Chemical Composition, Antibacterial Activity, and Food Application of Sprouts from Fabaceae and Brassicaceae Species
Abstract
1. Introduction
2. Materials and Method
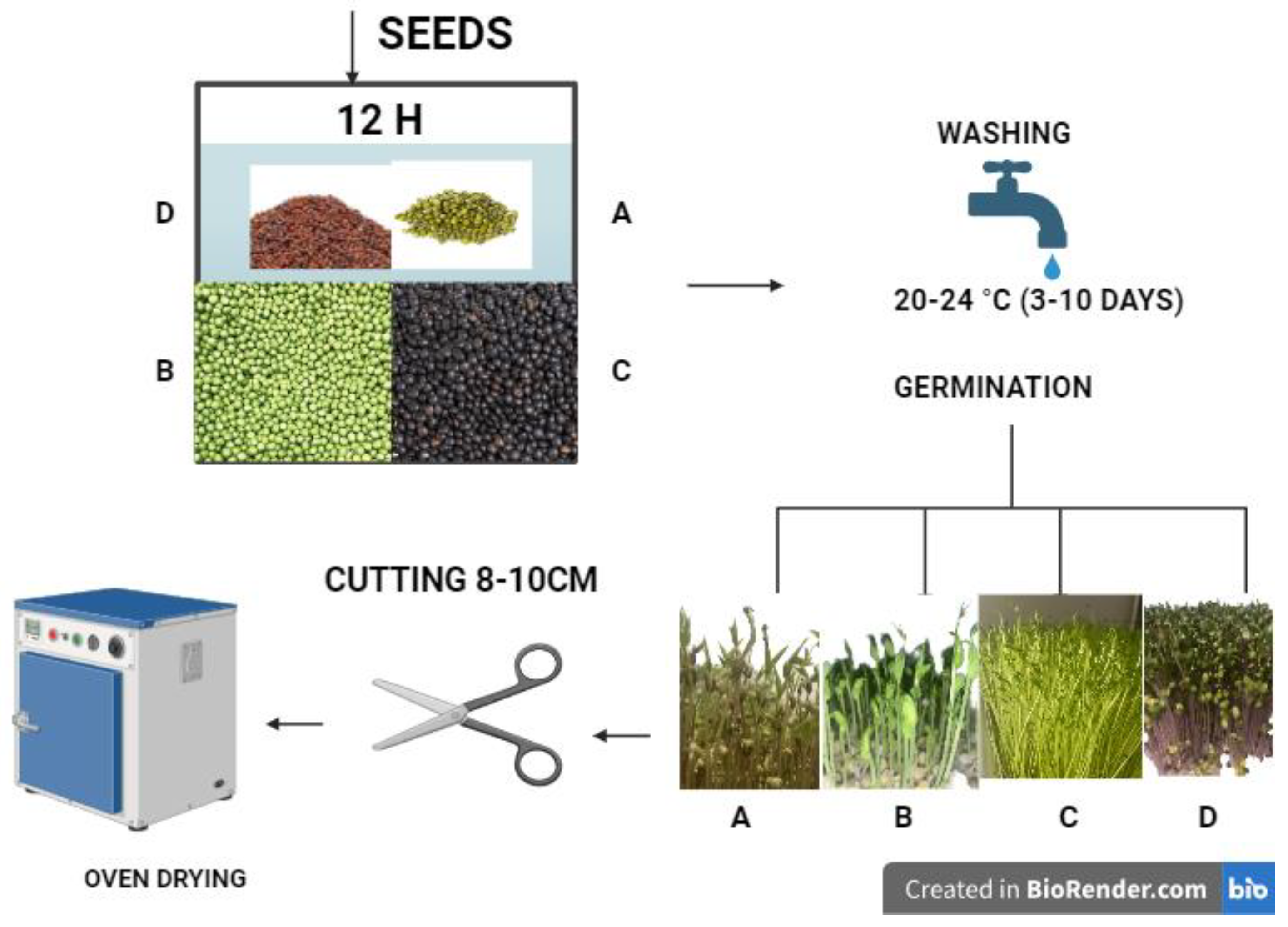
2.1. Sample Preparation and Seed Germination
2.2. Determination of Chemical Composition of Sprout Extracts
2.2.1. Total and Individual Polyphenol Content
- TPCsprouts—the content of TPC (mg GAE/100 g) of analyzed sprouts;
- TPCWF—the content of TPC (mg GAE/100 g) of wheat flour sample.
2.2.2. Macro- and Microelements Composition
2.3. Determination of Antibacterial Activity
2.3.1. Strains and Materials
2.3.2. The Disk Diffusion Method
- DV—vertical diameter (mm);
- DC—disc diameter (mm);
- DH—horizontal diameter (mm) [24].
- DS—Diameter of inhibition zone for sample (Ø mm);
- DPC—Diameter of inhibition zone for positive control (Ø mm).
2.3.3. Determination of the Minimum Inhibitory Concentration (MIC)
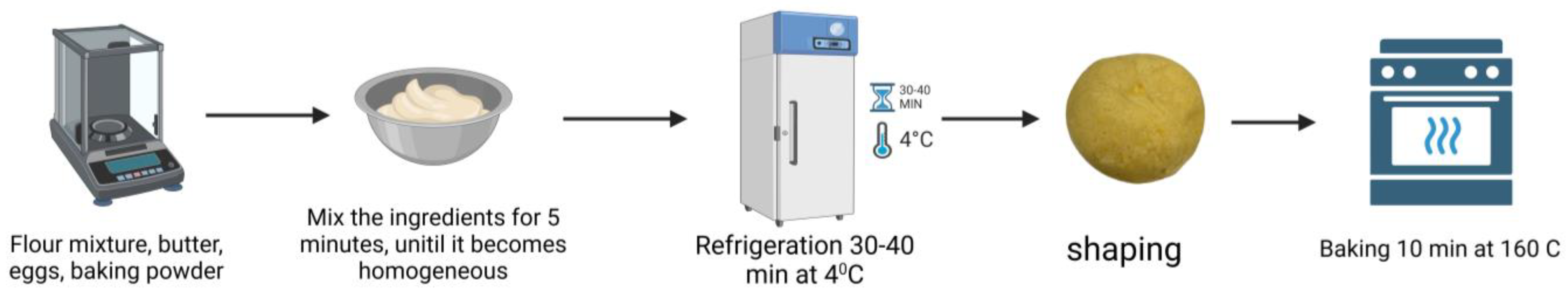
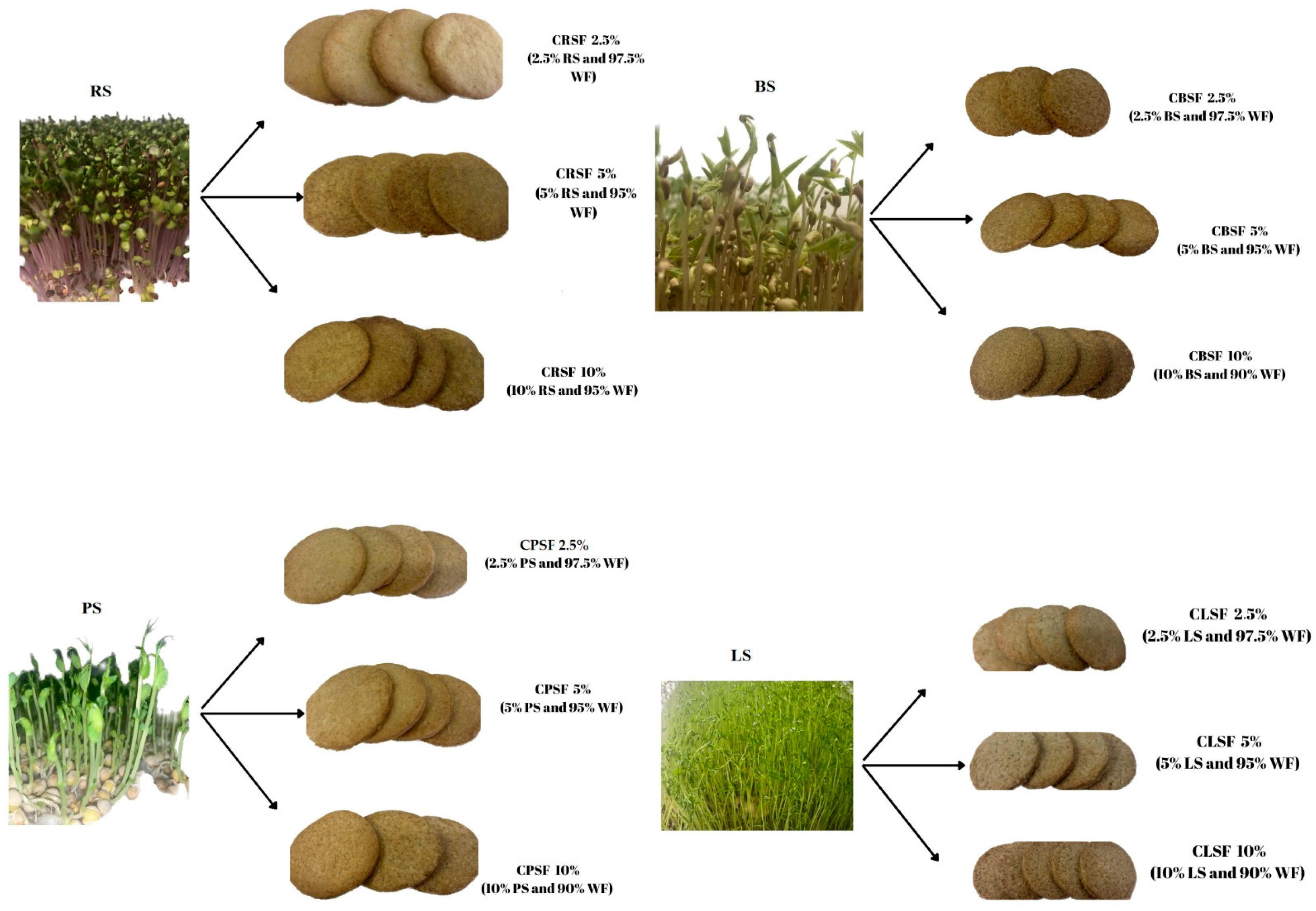
2.4. The Acquisition of Cookies with Sprouts
2.5. Sensory Analysis of Cookies Fortified with Sprouts
2.6. Statistical Analysis
3. Results and Discussion
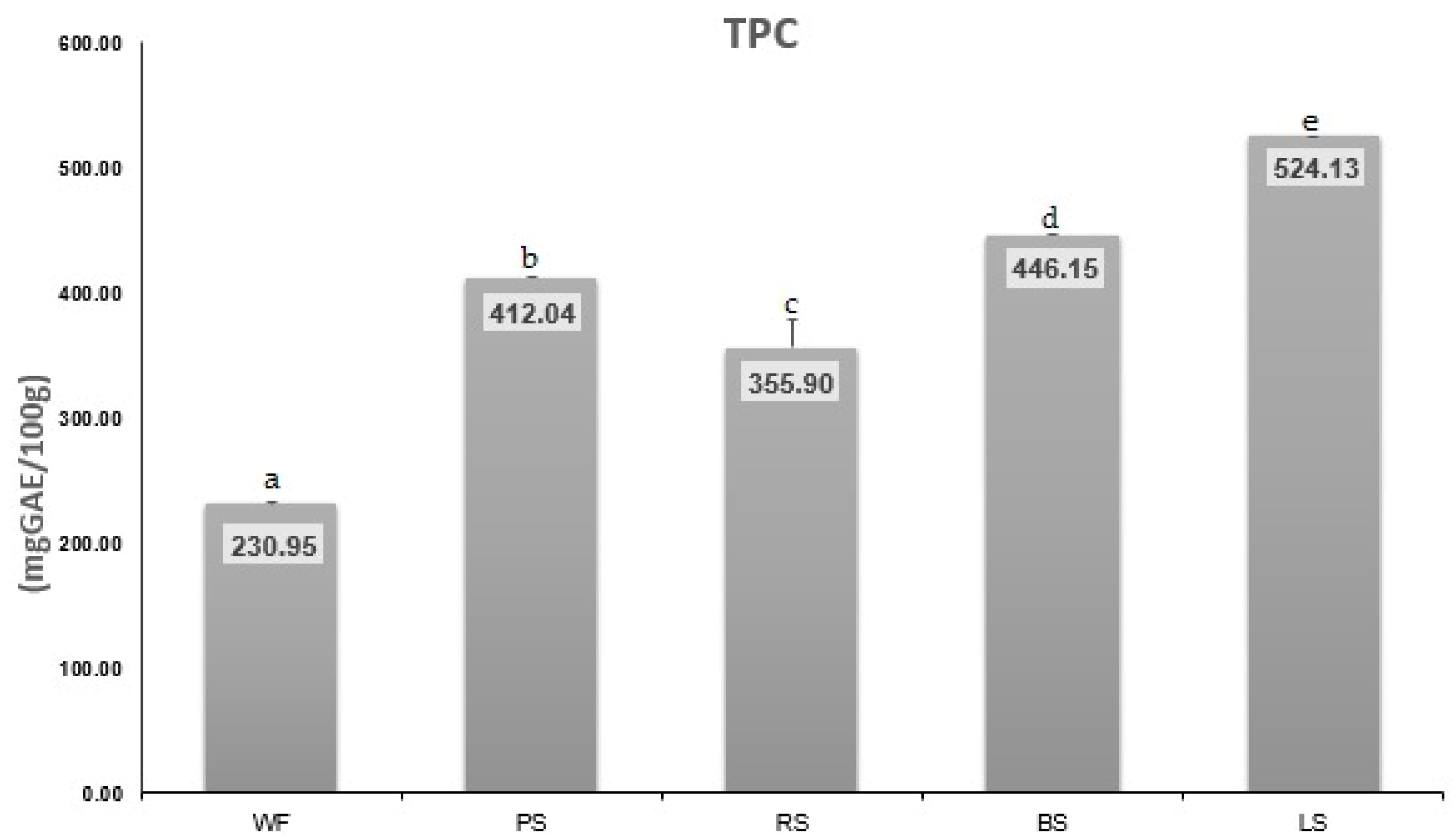
3.1. Chemical Composition
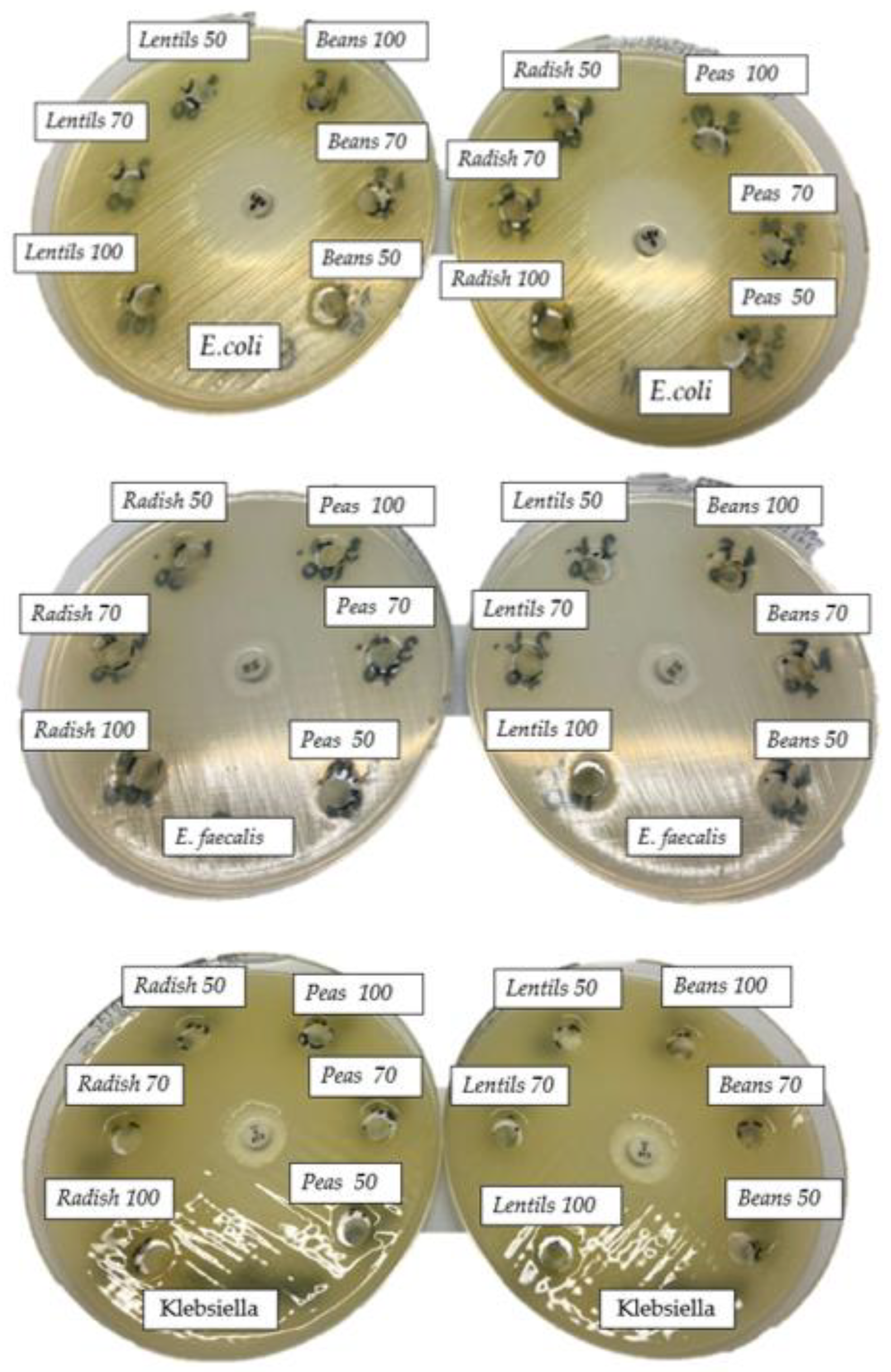
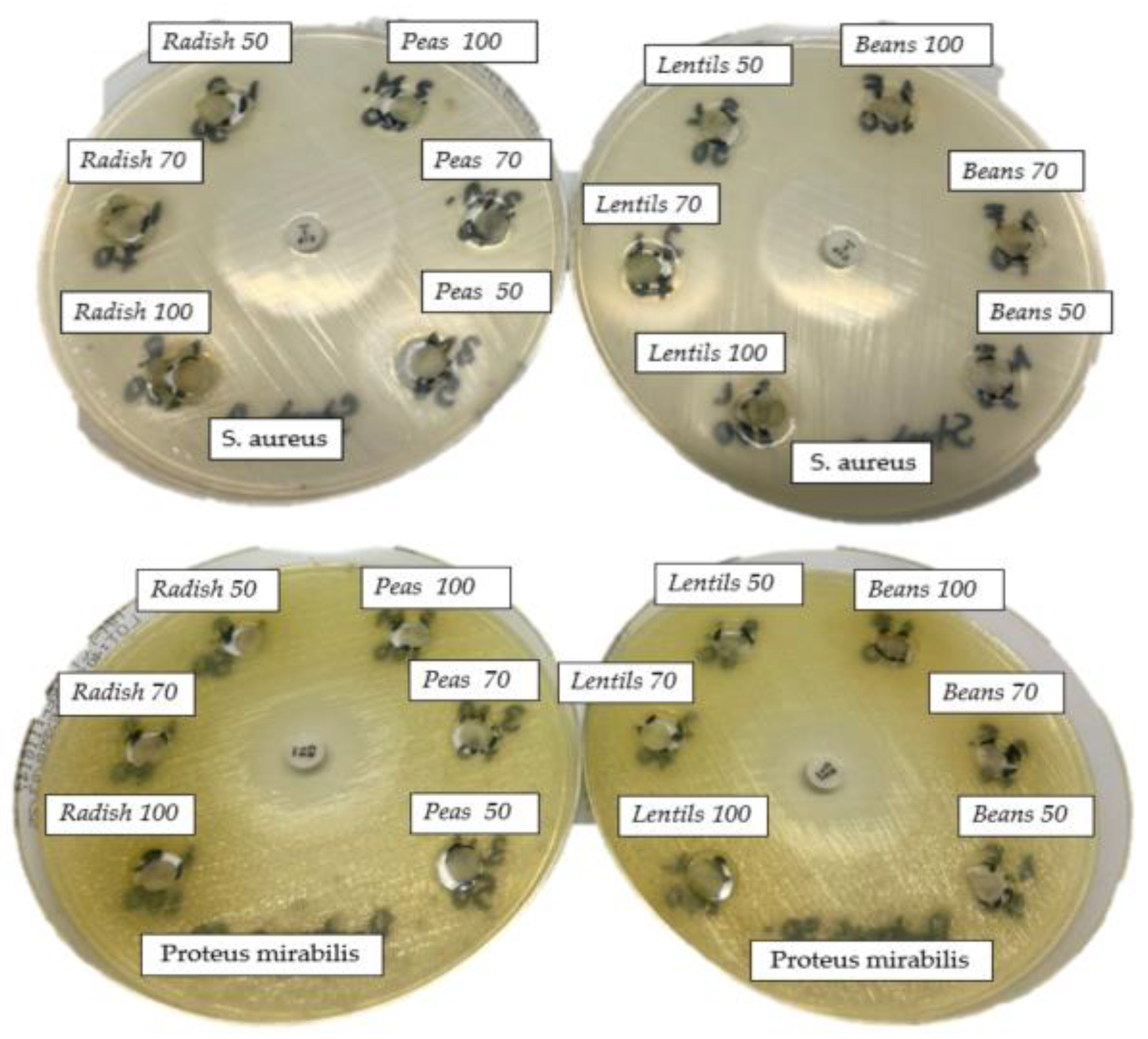
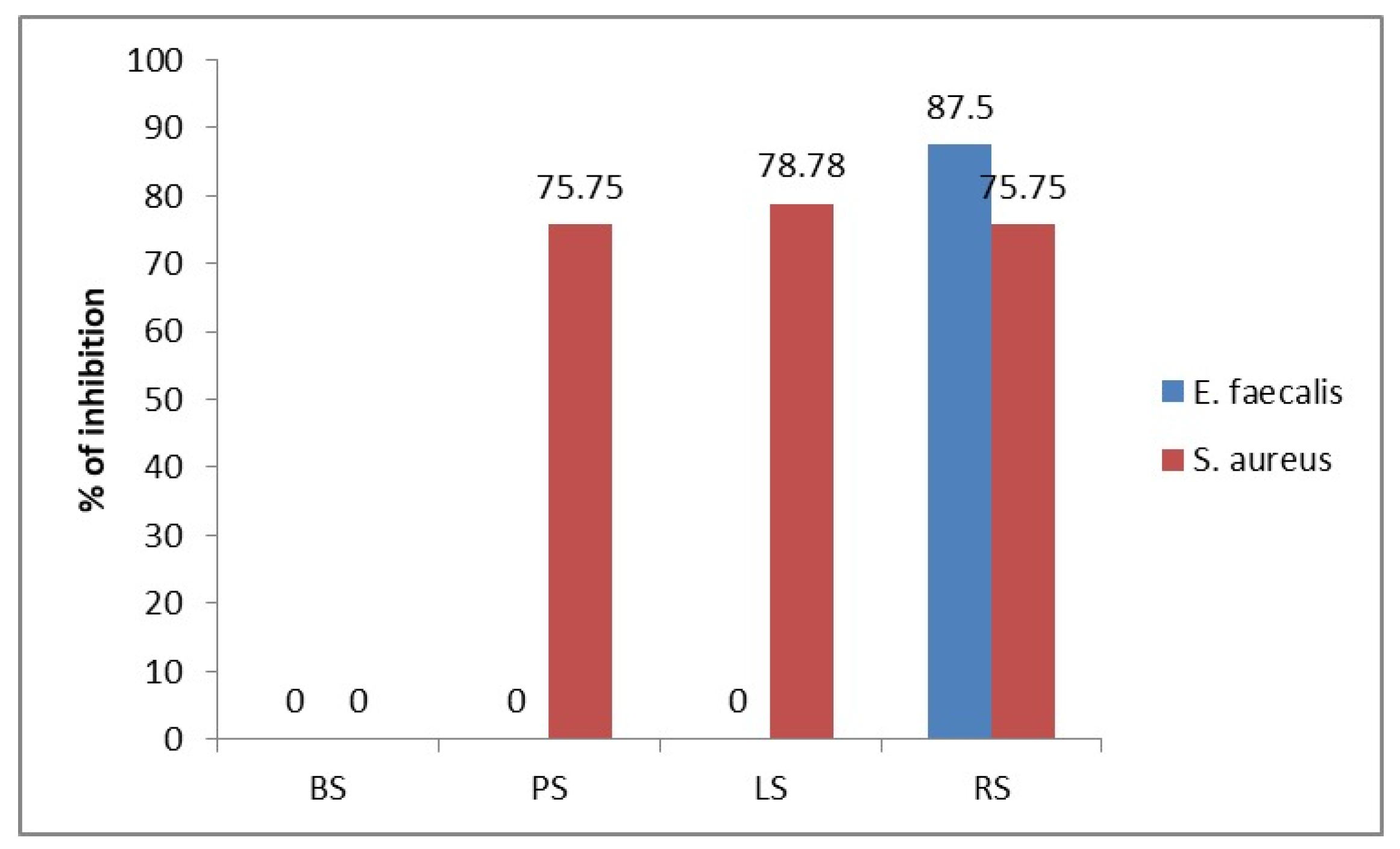
3.2. Antibacterial Activity
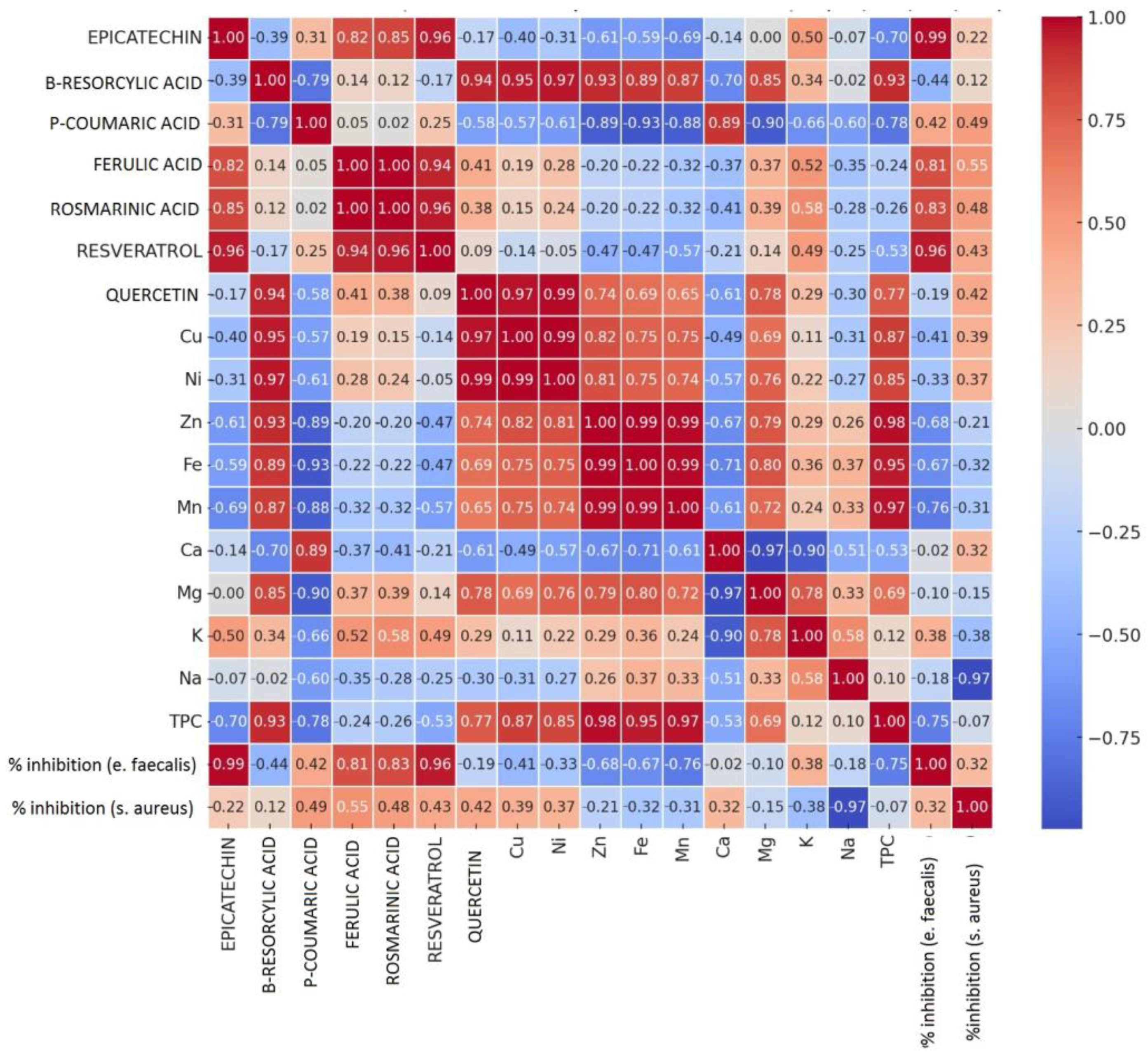
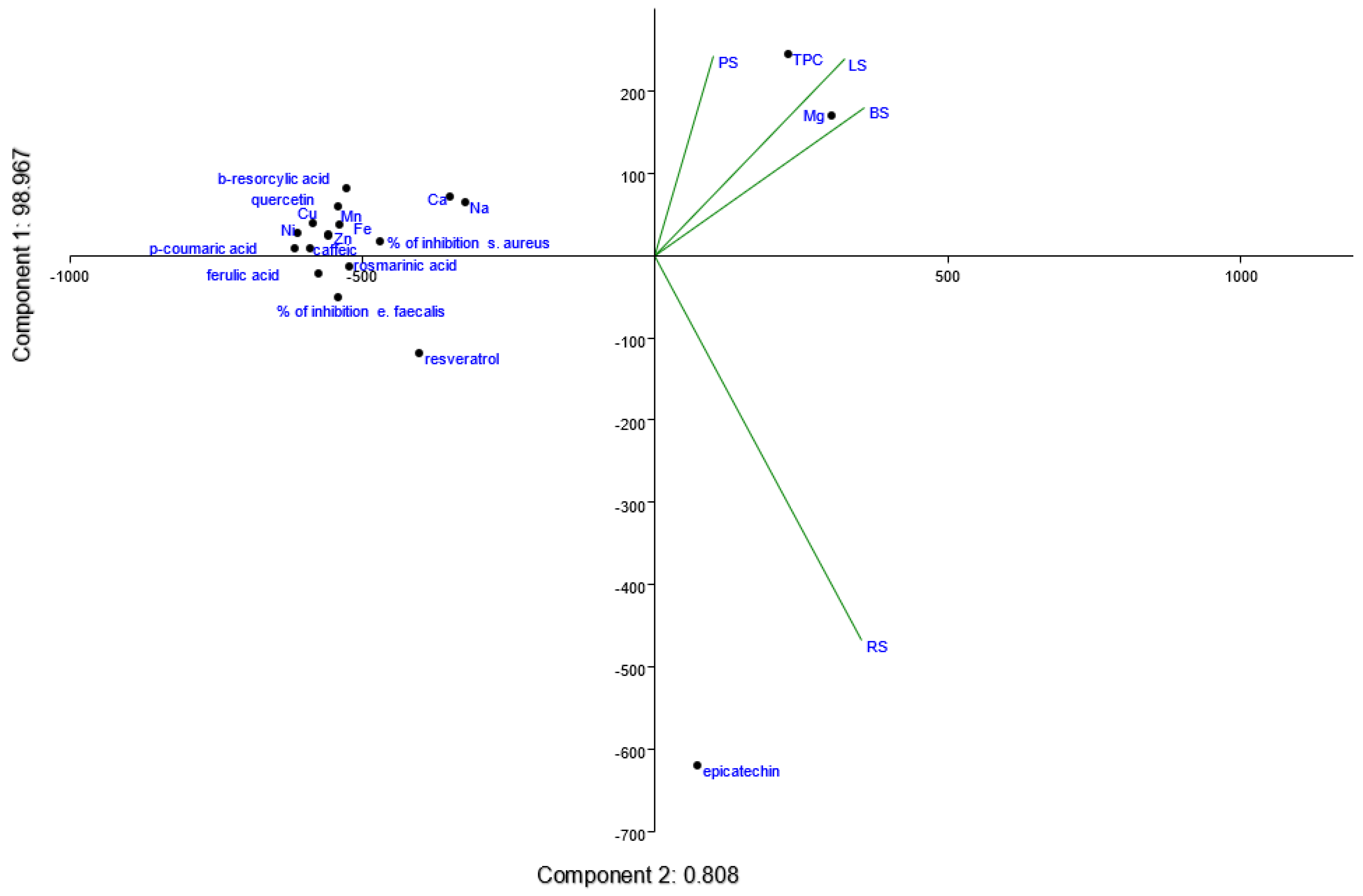
3.3. Correlation Between Chemical Composition and Antibacterial Activity
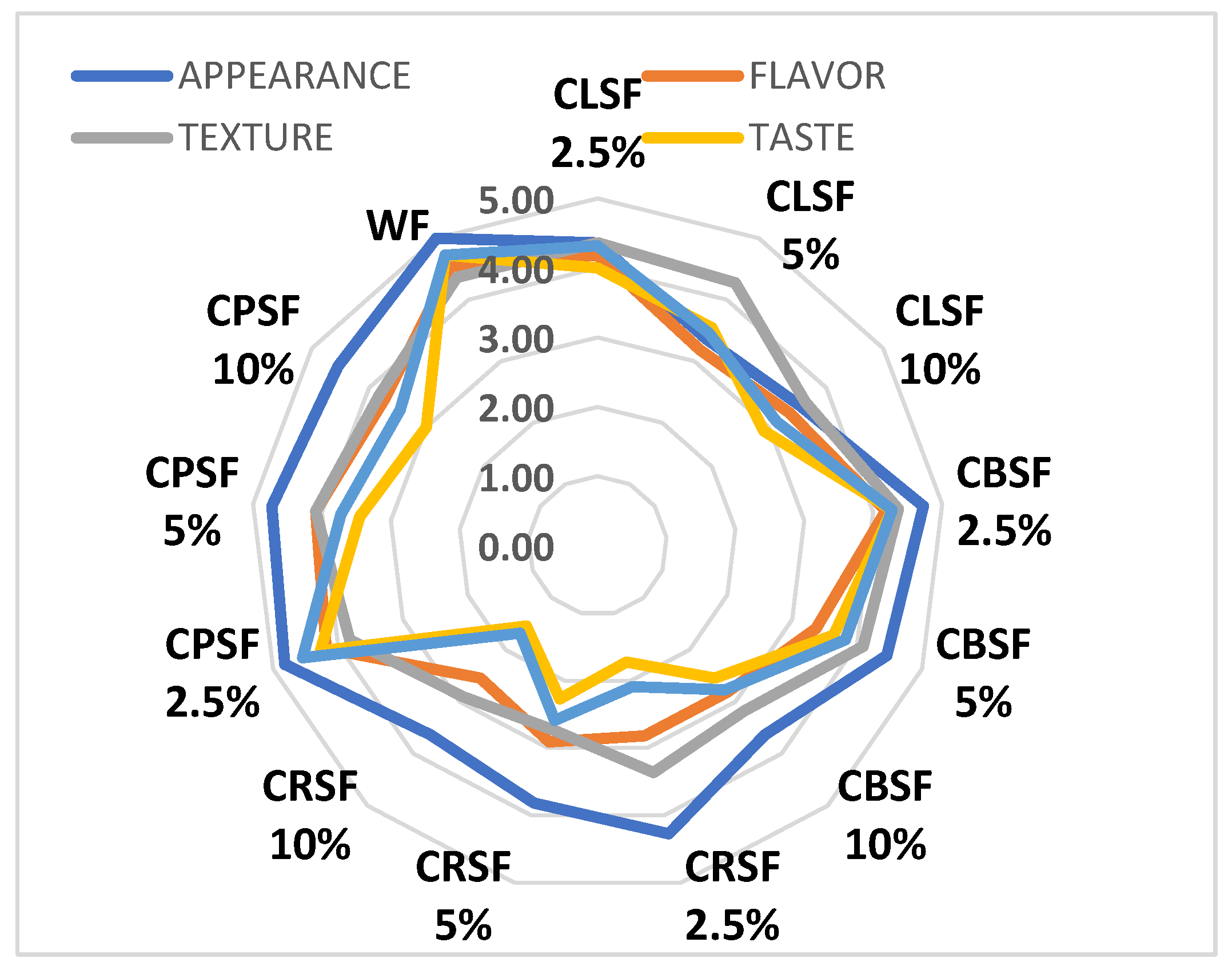
3.4. Sensory Analysis of Cookies Fortified with Germinated
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celac, V. Plantele leguminoase–Actualitate şi viitor. Rev. Ştiinţă Inovare Cult. Artă Akad. 2009, 13, 77–79. [Google Scholar]
- McKevith, B. Nutritional aspects of cereals. Br. Nutr. Found. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kimizuka-Takagi, C.; Bang, S.W.; Matsuzawa, Y. Radish. In Vegetables; Springer: Berlin/Heidelberg, Germany, 2007; pp. 141–160. [Google Scholar]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Park, Y.R.; Kwon, S.-J.; Kim, J.H.; Duan, S.; Eom, S.H. Light-Induced Antioxidant Phenolic Changes among the Sprouts of Lentil Cultivar. Antioxidants 2024, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Servan, A.; Nigam, D. Comparative study on antioxidant activity, phytochemical analysis and mineral composition of the Mung Bean (Vigna Radiata) and its sprouts. J. Pharmacogn. Phytochem. 2017, 6, 336–340. [Google Scholar]
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small functional foods: Comparative phytochemical and nutritional analyses of five microgreens of the Brassicaceae family. Foods 2021, 10, 427. [Google Scholar] [CrossRef]
- López-Amorós, M.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Khalid, M.; Ayayda, R.; Gheith, N.; Salah, Z.; Abu-Lafi, S.; Jaber, A.; Al-Rimawi, F.; Al-Mazaideh, G. Assessment of Antimicrobial and Anticancer Activity of Radish Sprouts Extracts. Jordan J. Biol. Sci. 2020, 13, 567. [Google Scholar]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial potential of legume extracts against foodborne pathogens: A review. Trends Food Sci. Technol. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Lin, J.C.; Bagal-Kestwal, D.; Chiang, B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Szabó, S.; Németh, Z.; Polyák, É.; Bátai, I.; Kerényi, M.; Figler, M. Antibacterial effect of sprouts against human pathogens in vitro. Acta Aliment. 2014, 43, 501–508. [Google Scholar] [CrossRef]
- Biletska, Y.; Gontar, A. Research of rheological indicators of the using the flour of germinated legumes. Technol. Audit Prod. Reserves 2020, 5, 55. [Google Scholar] [CrossRef]
- Duodu, K.G.; Minnaar, A. Chapter 18-Legume Composite Flours and Baked Goods: Nutritional, Functional, Sensory, and Phytochemical Qualitie. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridgem, MA, USA, 2011; pp. 193–203. ISBN 9780123808868. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G.; et al. Lupin (Lupinus spp.)-Fortified Bread: A Sustainable, Nutritionally, Functionally, and Technologically Valuable Solution for Bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef]
- Codină, G.G.; Marineac, A.R.; Todosi-Sănduleac, E. The influence of lupin flour addition on bread quality. Food Environ. Saf. 2016, 15, 216–226. [Google Scholar]
- Bashir, A.; Ashraf, S.A.; Khan, M.; Azad Azaz Ahmad, Z.R. Development and Compositional Analysis of Protein Enriched Soybean-Pea-Wheat Flour Blended Cookies. Asian J. Clin. Nutr. 2015, 7, 76–83. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Nutritional Quality and Antioxidant Properties of Brown and Black Lentil Sprouts. Horticulturae 2023, 9, 668. [Google Scholar] [CrossRef]
- Plustea, L.; Dossa, S.; Dragomir, C.; Cocan, I.; Negrea, M.; Obistioiu, D.; Poiana, M.-A.; Voica, D.; Berbecea, A.; Alexa, E. Comparative Study of the Nutritional, Phytochemical, Sensory Characteristics and Glycemic Response of Cookies Enriched with Lupin Sprout Flour and Lupin Green Sprout. Foods 2024, 13, 656. [Google Scholar] [CrossRef]
- Jianu, C.; Mişcă, C.; Muntean, S.G.; Gruia, A.T. Composition, antioxidant and antimicrobial activity of the essential oil of Achillea collina Becker growing wild in western Romania. Hem. Ind. 2015, 69, 381–386. [Google Scholar] [CrossRef]
- Available online: https://microchemlab.com/test/zone-inhibition-test-antimicrobial-activity/ (accessed on 29 December 2024).
- The European Committee on Antimicrobial Susceptibility Testing. EUCAST Reading Guide for Broth Microdilution. Version 5.0. Available online: https://www.eucast.org/ (accessed on 29 December 2024).
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/research/participants/data/ref/fp7/89847/research-food_en.pdf (accessed on 29 December 2024).
- Poșta, D.S.; Radulov, I.; Cocan, I.; Berbecea, A.A.; Alexa, E.; Hotea, I.; Iordănescu, O.A.; Băla, M.; Cântar, I.C.; Rózsa, S.; et al. Hazelnuts (Corylus avellana L.) from Spontaneous Flora of the West Part of Romania: A Source of Nutrients for Locals. Agronomy 2022, 12, 214. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- González-Montemayor, A.M.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; López-Badillo, C.M.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Green Bean, Pea and Mesquite Whole Pod Flours Nutritional and Functional Properties and Their Effect on Sourdough Bread. Foods 2021, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. Optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Lemus-Conejo, A.; Rivero-Pino, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Nutritional composition and biological activity of narrow-leafed lupins (Lupinus angustifolius L.) hydrolysates and seeds. Food Chem. 2023, 420, 136104. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Phenolic Substance Characterization and Chemical and Cell-Based Antioxidant Activities of 11 Lentils Grown in the Northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Barakat, H.; Alshimali, S.I.; Almutairi, A.S.; Alkhurayji, R.I.; Almutiri, S.M.; Aljutaily, T.; Algheshairy, R.M.; Alhomaid, R.M.; Aljalis, R.A.; Alkhidhr, M.F. Antioxidative potential and ameliorative effects of green lentil (Lens culinaris M.) sprouts against CCl4-induced oxidative stress in rats. Front. Nutr. 2022, 9, 1029793. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Perazzoli, G.; Sánchez González, C.; Llopis, J.; Cantarero, S.; Goua, M.; Bermano, G.; Prados, J.; Melguizo, C.; et al. Germination Improves the Polyphenolic Profile and Functional Value of Mung Bean (Vigna radiata L.). Antioxidants 2020, 9, 746. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; Saraiva de Carvalho, I.; Paśko, P. Determination of essential minerals and trace elements in edible sprouts from different botanical families—Application of Chemometric analysis. Foods 2022, 11, 371. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://aurosan.de/images/mediathek/servicematerial/EUCAST_RefStaemme_Sollwerte.pdf (accessed on 29 December 2024).
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Oliveira, M.B.P.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control. 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Valli, S.A.; Gowrie, S.U. Synergistic effect of phytoconstituents in mixed sprouts-an approach towards therapeutic applications. J. Chem. Pharm. Res. 2017, 9, 95–112. [Google Scholar]
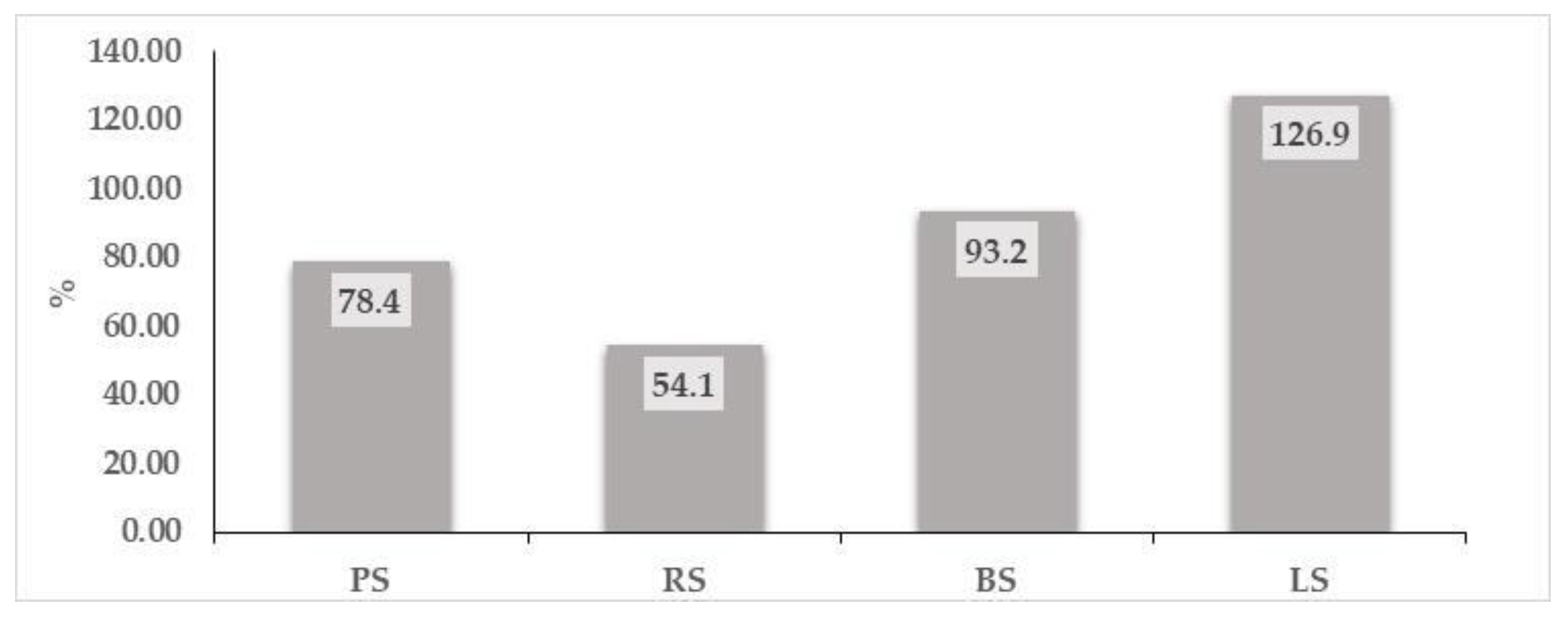
| Sample | Gallic Acid | Epicatechin | Caffeic Acid | Β-Resorcylic Acid | p-Coumaric Acid | Ferulic Acid | Rosmarinic Acid | Resveratrol | Quercetin |
|---|---|---|---|---|---|---|---|---|---|
| WF | nd * | 6.50 ± 0.05 a | nd * | 1.20 ± 0.01 a | nd * | nd * | 3.50 ± 0.02 a | 3.10 ± 0.01 a | 0.95 ± 0.02 a |
| PS | nd * | nd * | nd * | nd * | 14.50 ± 0.85 a | nd * | 2.16 ± 0.01 b | 14.26 ± 0.8 b | 4.07 ± 2.1 b |
| RS | nd * | 919.94 ± 2.56 b | nd * | nd * | 13.01 ± 0.2 b | 62.76 ± 2 a | 62.11 ± 0.06 c | 228.95 ± 1.10 c | 14.98 ± 1.10 c |
| BS | nd * | 135.63 ± 1.54 c | nd * | 25.43 ± 1.32 b | 8.06 ± 0.3 c | nd * | 7.11 ± 0.02 d | 19.83 ± 1.12 d | 5.01 ± 1.2 d |
| LS | nd * | 86.50 ± 3.20 d | nd * | 92.23 ± 2.22 c | 6.83 ± 0.60 d | 30.76 ± 4.1 b | 35.20 ± 1.8 e | 76.35 ± 4.20 e | 75.22 ± 0.5 e |
| Samples | Macro- and Microelements Content (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Ni | Zn | Fe | Mn | Ca | Mg | K | Na | |
| Composite flours | |||||||||
| WF | 3.530 ± 0.27 a | nd * | 14.37 ± 0.20 a | 11.781 ± 0.11 a | 2.050 ±0.04 a | 141.510 ± 0.64 a | 401.160 ± 0.09 a | 499.320 ± 3.4 a | 191.320 ± 0.80 a |
| PS | 5.235 ± 0.5 b | nd * | 16.912 ± 0.30 b | 27.458 ± 0.40 b | 16.023 ± 1.19 b | 256.575 ± 2.23 b | 405.819 ± 0.46 b | 1346.158 ± 0.7 b | 114.871 ± 0.75 b |
| RS | 5.175 ± 2.2 c | nd * | 15.848 ± 1.50 a | 23.239 ± 0.29 c | 14.144 ± 0.38 c | 148.147 ± 0.54 c | 458.727 ± 0.99 c | 4795.259 ± 0.8 c | 129.435 ± 1.34 c |
| BS | 5.202 ± 0.6 d | nd * | 19.102 ± 0.98 c | 41.478 ± 0.32 d | 18.796 ± 0.37 d | 111.161 ± 0.56 d | 478.203 ± 1.18 d | 4819.450 ± 1.1 d | 178.512 ± 1.55 d |
| LS | 5.654 ± 0.4 e | 0.367 ± 0.1 a | 21.058 ± 0.35 cd | 48.664 ± 0.08 e | 20.469 ± 0.47 e | 86.400 ± 0.36 e | 518.971 ± 2.93 e | 4378.643 ± 1.0 e | 125.878 ± 1.30 e |
| Concentration (µL) | E. coli | P. mirabilis | K. pneumoniae | E. faecalis | S. aureus | |
|---|---|---|---|---|---|---|
| BS | 2 | - | - | - | - | - |
| 50 | - | - | - | - | - | |
| 70 | - | - | - | - | - | |
| 100 | - | - | - | - | - | |
| PS | 2 | - | - | - | - | 11 |
| 50 | - | - | - | - | 24 | |
| 70 | - | - | - | - | 25 | |
| 100 | - | - | - | - | 25 | |
| LS | 2 | - | - | - | - | - |
| 50 | - | - | - | - | - | |
| 70 | - | - | - | - | 25 | |
| 100 | - | - | - | - | 26 | |
| RS | 2 | - | - | - | 12 | 13 |
| 50 | - | - | - | 12 | 15 | |
| 70 | - | - | - | 12 | 25 | |
| 100 | - | - | - | 14 | 25 | |
| Negative control | - | - | - | - | - | |
| Positive control | 30 for levofloxacin | 25 for gentamicin | 17 for levofloxacin 17 for ciprofloxacin | 16 for vancomycin | 33 for linezolid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomir, C.; Misca, C.D.; Dossa, S.; Jianu, C.; Radulov, I.; Negrea, M.; Paven, L.; Alexa, E. Chemical Composition, Antibacterial Activity, and Food Application of Sprouts from Fabaceae and Brassicaceae Species. Appl. Sci. 2025, 15, 1896. https://doi.org/10.3390/app15041896
Dragomir C, Misca CD, Dossa S, Jianu C, Radulov I, Negrea M, Paven L, Alexa E. Chemical Composition, Antibacterial Activity, and Food Application of Sprouts from Fabaceae and Brassicaceae Species. Applied Sciences. 2025; 15(4):1896. https://doi.org/10.3390/app15041896
Chicago/Turabian Style(Neagu) Dragomir, Christine, Corina Dana Misca, Sylvestre Dossa, Călin Jianu, Isidora Radulov, Monica Negrea, Loredana Paven, and Ersilia Alexa. 2025. "Chemical Composition, Antibacterial Activity, and Food Application of Sprouts from Fabaceae and Brassicaceae Species" Applied Sciences 15, no. 4: 1896. https://doi.org/10.3390/app15041896
APA StyleDragomir, C., Misca, C. D., Dossa, S., Jianu, C., Radulov, I., Negrea, M., Paven, L., & Alexa, E. (2025). Chemical Composition, Antibacterial Activity, and Food Application of Sprouts from Fabaceae and Brassicaceae Species. Applied Sciences, 15(4), 1896. https://doi.org/10.3390/app15041896









