Abstract
The aim of this paper is to evaluate, from a chemical and antibacterial point of view, sprouts from species of Fabaceae and Brassicaceae families, to establish a correlation between analyzed parameters and to test the possibility of using these functional compounds in the flour food industry. The material used was lentil sprouts (LS), pea sprouts (PS), bean sprouts (BS), and radish sprouts (RS), which were chemically analyzed by determining the content of total and individual polyphenols and macro- and microelements. The antimicrobial potential of the sprout extracts obtained was tested using the disk diffusion method on five bacterial strains: Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterococcus faecalis, and Staphylococcus aureus. In order to capitalize on the flour food industry, the sprouts were introduced in the recipes for the manufacture of some cookies, and sensory analysis was performed. The results obtained indicated that LS are the sprouts with the highest content of TPC (524.130 mg/100 g) and highest content for Ni, Cu, Zn, Fe, Mn, and Mg, while the highest values for Ca were recorded in the case of PS (256.575 ± 2.23 mg/kg), and for K in the case of the BS sample (4819.450 ± 1.1 mg/kg). The RS extract has been shown to be effective against E. faecalis (MIC-70 µL/mL), S. aureus (MIC 50 µL/mL), and PS against S. aureus (MIC 70 µL/mL). Regarding the sensory analysis of cookies, the panelists participating in the study preferred products based on BS and PS in a percentage of 2.5%.
1. Introduction
Legumes are an important group of agricultural crops that play a significant role in ensuring food security and promoting sustainable agriculture. Among them, lentils (Lens culinaris), peas (Pisum sativum), and beans (Phaseolus vulgaris) stand out for their agronomic versatility and nutritional value, serving as essential sources of plant-based proteins, dietary fiber, vitamins, and minerals [1,2]. Radish (Raphanus sativus L.) is an annual vegetable belonging to the Brassicaceae family [3], having multiple properties beneficial to health, especially due to the sulphuric compounds with antioxidant and anticancer properties, which protect cells against oxidative damage [4].
Recently, germination has been analyzed as an innovative approach in green food engineering, having the potential to increase the nutrient supply of plant matrices with promising uses in the fields of functional foods [5]. Germinated seeds can contain a 2- to 10-fold increase in phytochemicals [6]. Brassicaceae species are cultivated to produce microgreens, which are functional foods derived from plant seedlings harvested at the stage when they develop two cotyledonary leaves or, in some cases, upon the emergence of their first rudimentary true leaves [7]. These are considered novel plant-based foods due to their rich composition of bioactive compounds compared to mature plants. Sprouted beans, peas, or lentils contain natural antioxidants and are rich in vitamins (especially vitamins E and C) and polyphenols that reduce oxidative stress and protect cells from free radical damage [8], while sprouted radishes contain sulforaphane and other compounds that have antioxidant and anticancer effects [9,10,11].
The antimicrobial properties of germinated seeds from the Fabaceae and Brassicaceae families result from the presence of bioactive compounds produced during the germination process [11]. These compounds, such as polyphenols, flavonoids, and glucosinolates, demonstrate the ability to inhibit the growth of pathogenic bacteria, offering a natural alternative to synthetic antibiotics [11]. The antimicrobial activity of legumes against both Gram-positive and Gram-negative bacteria, including pathogens such as Staphylococcus aureus, Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae, was reported [12]. Pina-Pérez et al., 2018 explained that the antimicrobial potential of these extracts can be attributed to the protease inhibitors, which inhibit the protease secreted by microorganisms [12]. Among cruciferous vegetables, sprouted radishes are effective against bacteria and fungi due to sulfur compounds [13]. The study of the antibacterial effect of vegetable sprouts against human pathogens showed that sprouted radish, kohlrabi, and red cabbage exhibited inhibitory zones against Escherichia coli, Salmonella Typhimurium, Shigella flexneri, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus [14].
The impact of incorporating legume flour into biscuit formulations at replacement rates of 5–25% was previously studied [15]. The focus is not only on improving the nutritional profile but also on maintaining the rheological properties of the biscuit dough, which are crucial for preserving the system’s internal structure and overall quality [15]. The farinograph analysis revealed that the highest quality index was observed in the sample containing 5% sprouted legume flour. In the sample where 25% of the wheat flour was replaced with bean flour, the dough formation time extended to 5 min and 18 s, more than twice that of the control sample [15].
The use of legume flour in composite flours as an alternative to wheat flour is frequently practiced in the bakery and pastry industry in order to increase protein content, and this offers a better amino acid balance [16]. Lupin, soybean, or pea improves the quality of biscuits or bakery products [17,18,19], while sprouts are less frequently used in this regard, especially the sprouts of seeds from the Brassicaceae species, in particular radish seeds, which have not been reported in the literature.
In this study, sprouts were obtained from three species of legumes belonging to the Fabaceae family: lentil sprouts (LS), pea sprouts (PS), and bean sprouts (BS) and one species from Brassicaceae family: radish sprouts (RS). The elemental and phytochemical profiles were analyzed, and the antimicrobial potential of the sprout extracts obtained was tested against five bacterial strains. The novelty elements of this paper refer to the study of the phytonutrient content of some sprouts in correlation with their antibacterial properties against some strains and the impact of using these germinated seeds in the technology of obtaining biscuits, emphasizing the sensory properties of the products obtained and the acceptability of consumers.
2. Materials and Method
2.1. Sample Preparation and Seed Germination
Four samples were analyzed: black lentils (Lens culinaris), peas (Pisum sativum), beans (Phaseolus vulgaris), and radish (Raphanus sativus L.). Lentil and peas seeds were sourced from Pronat Romania, while radish and beans were obtained from BioSnacky Rapunzel Romania. The seeds were thoroughly washed and soaked in water for 12 h to facilitate and accelerate germination, then placed on a tray near a natural light source, where germination occurred over 2–3 days at a temperature of 20–28 °C [20].
The germination process continued, and the sprouts were harvested after 6–7 days. Their length was measured with a ruler, and they were collected once they reached 8–10 cm. After harvesting, the sprouts were dried in an oven (FloiLabo AC 60, Air Concept, Zülpich, Germany) at 50 °C for 5–6 days to ensure complete drying. To produce the powder, the dried sprouts were ground using a kitchen blender (Bosch TSM6013B, BSH Hausgeräte, Munich, Germany), ensuring uniform particle size and preserving nutrient integrity until a fine powder was obtained. Figure 1 shows the technological flow of the seed germination process.
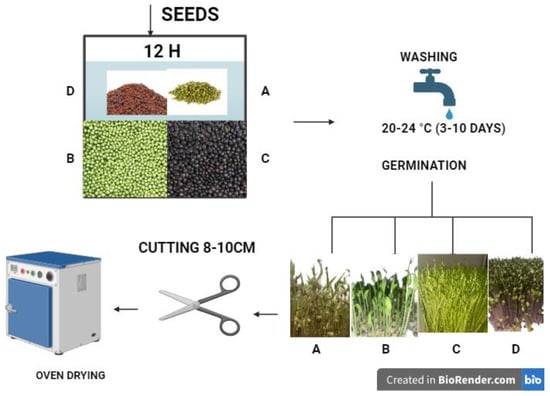
Figure 1.
Technological flow for obtaining sprouts. Figure created with BioRender.com, accessed on 29 December 2024. (A) beans (Phaseolus vulgaris); (B) peas (Pisum sativum); (C) black lentils (Lens culinaris), and (D) radish (Raphanus sativus L.).
2.2. Determination of Chemical Composition of Sprout Extracts
2.2.1. Total and Individual Polyphenol Content
To determine the total polyphenol content (TPC), the Folin–Ciocalteu method [21] was used. To obtain the extract, 1 g sprouts were mixed with 10 mL 70% aqueous ethanol and shaken for 30 min on a mechanical shaker (IDL, Freising, Germany). Subsequently, 0.5 mL of the extract was mixed with the Folin–Ciocalteu reagent (diluted 1:10) (Sigma-Aldrich Chemie GmbH, Munich, Germany) and 1 mL of sodium carbonate solution (60 g/L) (Geyer GmbH, Renningen, Germany). The mixture was incubated at 50 °C for 30 min using an INB500 incubator (Memmert GmbH, Schwabach, Germany). Absorbance was measured at 750 nm using a UV-Vis spectrophotometer, specifically the Specord 205 model (Analytik Jena AG, Jena, Germany). The results were expressed as mg GAE/100 g sample. Each sample was determined in triplicate. The increase in TPC compared to the values obtained for wheat flour was calculated with the relation (1).
where:
[(TPCsprouts − TPCWF)/TPCWF] × 100
- TPCsprouts—the content of TPC (mg GAE/100 g) of analyzed sprouts;
- TPCWF—the content of TPC (mg GAE/100 g) of wheat flour sample.
The chromatographic analysis of individual polyphenols was performed by HPLC using a Shimadzu chromatograph equipped with SPD-10A UV and LC-MS 2010 detectors (Kyoto, Japan). Separation was conducted on an EC 150/2 NUCLEODUR C18 Gravity SB column (150 × 2 mm, 5 μm) under the following conditions: the mobile phase consisted of (A) water acidified with formic acid to pH 3 and (B) acetonitrile acidified with formic acid to pH 3. The gradient elution program was as follows: 0.01–20 min, 5% B; 20.01–50 min, 5–40% B; 50–55 min, 40–95% B; and 55–60 min, 95% B. The flow rate was maintained at 0.2 mL/min, and the column temperature was set to 200 °C [21]. The detection of the compounds was performed at wavelengths 280 and 320 nm (Supplementary Materials). The results reported in Table 1 represent the values obtained for the wavelength of 280 nm. Calibration curves were prepared for a concentration range of 20–50 μg/mL. The results were expressed as mg/kg. Each sample was analyzed in triplicate.

Table 1.
The individual polyphenol content expressed as mg/kg, detected as 280 nm.
2.2.2. Macro- and Microelements Composition
For the analysis, 3 g of the product was incinerated at 650 °C in a calcination oven (Nabertherm GmbH, Lilienthal/Bremen, Germany) until the organic matter was completely combusted. The resulting ash was dissolved in 20% HCl and filtered to prepare it for the analysis of macro- and microelements using the atomic absorption spectroscopy (AAS) method [17].
Calibration curves were generated using a certified mixed standard solution (ICP Multi-Element Standard Solution IV, CertiPUR). The results were reported in ppm, with all measurements performed in triplicate to ensure precision and reliability.
2.3. Determination of Antibacterial Activity
2.3.1. Strains and Materials
Five nosocomial bacterial strains were tested: three Gram strain negative, which belong to the Order (Ord.) of Enterobacterales Escherichia coli (E. coli), Proteus mirabilis (P. mirabilis), Klebsiella pneumoniae (K. pneumoniae), and two Gram stain positive: one belonging to Ord. LactobacillalesEnterococcus faecalis (E. faecalis) and one belonging to Ord. Bacillales—Staphylococcus aureus (S. aureus).
Isolation and confirmation of coliforms were carried out on different selective culture media—MacConkey agar (Mikrobiologie-Labor-Technik RO, Arad, Romania), Chromatic Detection Agar (Mikrobiologie-Labor-Technik RO) and Eosine Methylene Blue (EMB—Levine) as well as on confirmation media—Triple Sugar Iron (TSI) Agar, Simmons Citrate Agar and Mobility Indole Urease (MIU) Agar (Mikrobiologie-Labor-Technik RO), specifically for E. coli, P. mirabilis, and K. pneumoniae.
The isolation and confirmation of the E. faecalis germ were carried out on the selective culture media Chromatic Detection Agar (Mikrobiologie-Labor-Technik RO) and Bile Aesculin Azide Agar (Mikrobiologie-Labor-Technik RO), while for the isolation and confirmation of the S. aureus germ culture media, Chapman agar (Mannitol Salt agar) and Columbia agar with 5% ram red blood cells (Mikrobiologie-Labor-Technik RO) were used, respectively.
The cultivation was carried out in Petri plates with a diameter of 100 mm by streaking with the help of sterile microbiological loops in aseptic conditions, and the resulting plates were incubated for 24 h between 40 and 42 °C for coliforms and between 35 and 37 °C, for faecalis enterococci and for Staphylococcus [22].
2.3.2. The Disk Diffusion Method
The sprout extracts were prepared as presented in Section 2.2.1. In order to verify the antibacterial potential of sprouts on the five microbial strains, the Kirby–Bauer diffusometric method was used, taking as a standard (positive control) different antibiotics depending on bacterial strain characteristics. The microbial suspension at an optical density (OD) of 0.5 McFarland was read at a wavelength of 565 nm, using standard saline for each tested strain and then seeding it on Mueller–Hinton agar (Sigma-Aldrich, Dortmund, Germany). After drying the inoculum on the surface of the culture medium, the microtablet containing the antibiotic of choice (Liofilchem, Roseto degli Abruzzi, Italy) was placed centrally for the positive control, and the microtablets using sterile Whatman filter paper no. 1 soaked with different µL of the extracts to be tested were placed lateral to it. It should be noted that the diameter of the microtablets is 6 mm. The positive control was different depending on microorganism as follows: E. coli, Poteus mirabilis, and Klebsiella pneumoniae—positive control for levofloxacin; E. faecalis—positive control for vancomycin; and S. aureus—had a positive control for linezolid.
After placing the microtablets on the surface of the culture medium, the plates are incubated for 18–24 h at 35–37 °C, after which the size of the inhibition zones is checked by measuring the halo around the microtablets and expressing the results in millimeters [23].
The size of the zone of inhibition indicates the antibacterial efficacy of the analyzed extract and was determined using the formula:
where:
Diameter of inhibition zone (Ø mm) = [(DV − DC) + (DH − DC)]/2
- DV—vertical diameter (mm);
- DC—disc diameter (mm);
- DH—horizontal diameter (mm) [24].
The percentage of strain inhibition for each extract was calculated by relating the zone of inhibition generated by the sample to the zone of inhibition of the antibiotic; positive control was calculated using the following formula:
where:
Inhibition (%) = [DS/DPC] × 100
- DS—Diameter of inhibition zone for sample (Ø mm);
- DPC—Diameter of inhibition zone for positive control (Ø mm).
2.3.3. Determination of the Minimum Inhibitory Concentration (MIC)
Using the serial dilution method, the microbial suspension was adjusted to an OD of 5 × 105 CFU/mL (colony forming units) in Mueller–Hinton broth at a wavelength of 565 nm read on a McFarland densitometer (Biosan, Riga, Latvia). Thus, with each of the tested strains, the inoculum was made, at a concentration of 5 × 105 CFU/mL; afterwards, 100 µL were taken and distributed in 5 sterile standard test cups, over which 2, 50, 70, and 100 µL, respectively, of each of the four extracts were tested. In the fifth cup, only the inoculum was introduced, considered the negative control. Experiments were performed in triplicate for each microorganism tested. After 24 h of incubation at 37 °C, the development of microorganisms in each of the cups was checked and compared to the control test. It was considered positive the result in the wells which presented a cloudy suspension or sediment. The minimum inhibitory concentration (MIC) was considered to be the lowest concentration of antimicrobial agent, which completely inhibits the development of microorganisms, in the microdilution cups used. The working technique used for MIC determination is the one standardized by EUCAST to which minimal adjustments have been performed inour previous studies [22].
2.4. The Acquisition of Cookies with Sprouts
The cookies with added sprouts were prepared according to the technology presented in Figure 2. The sprouts were added in 3 different concentrations (2.5, 5, 10%) to wheat flour. For each sample (BS, LS, PS, RS), three experimental samples were obtained, as is presented in Figure 3. The recipe to make cookies was 100 g flour, 50 g butter with 82% fat, 1.4 g baking powder, 1 piece egg, and 34 g sugar [21]. The control cookies were produced from wheat flour (WF).
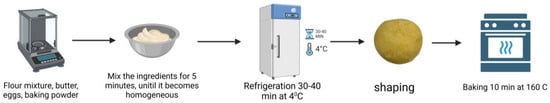
Figure 2.
The technological flowchart for obtaining cookies with sprouts. Figure created with BioRender.com, accessed on 1 December 2024.
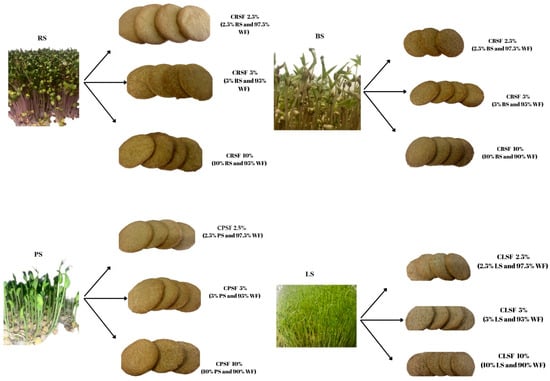
Figure 3.
Cookies made with wheat flour (WF) enriched with different proportions of BS, LS, PS, RS.
2.5. Sensory Analysis of Cookies Fortified with Sprouts
The sensory analysis was conducted following the methodologies described by Iannario et al. (2012) [25]. Written consent was obtained from each evaluator in accordance with the European Union’s ethical standards for food-related research [26]. The sensory evaluation involved panelists comprising semi-trained employees and students from the University of Life Sciences in Timișoara, Romania. The panel included 15 evaluators (7 men and 8 women), all non-smokers aged between 19 and 46 years, with no reported allergies or intolerances, particularly to gluten. Prior to the evaluation, the panelists underwent training to familiarize themselves with the identification of sensory attributes. Samples were presented individually in cardboard containers labeled with three-digit codes. Panelists were instructed to rinse their mouths with plain water after tasting each sample to ensure accurate assessment. Sensory characteristics, including appearance, flavor, texture, taste, and overall acceptability, were evaluated using a five-point hedonic scale [25]. Taste refers to the five basic sensations detected by the taste buds: sweet, salty, sour, or bitter. It is a physiological response to chemical compounds in food. Flavor is a broader experience that includes taste, smell, and other sensory factors like texture and temperature. Aroma plays a major role in flavor.
The scoring criteria for each attribute were as follows: Appearance: 1—unattractive, 2—slightly unattractive, 3—moderate, 4—attractive, 5—very attractive.
Flavor and overall acceptability: 1—dislike, 2—neither like nor dislike, 3—like slightly, 4—like moderately, 5—like very much.
Texture and taste: 1—very poor, 2—poor, 3—fair, 4—good, 5—very good [27].
2.6. Statistical Analysis
The results are presented as mean values ± standard deviation (SD). Differences between means were evaluated using one-way ANOVA, followed by multiple comparison analyses performed with a t-test (two samples, assuming equal variances) in Microsoft Excel 365. Statistical significance was set at p < 0.05. All determinations were performed in triplicate. Additionally, Principal Component Analysis (PCA) was conducted using the Past3 software (version 4.03). The correlation matrix was utilized as the basis for PCA, and data standardization was applied prior to analysis to normalize differences in scale. A biplot was generated to visualize the relationships between phenolic compounds, mineral content, and antibacterial inhibition. Factor loadings were analyzed to identify the key contributors to the variability within the dataset.
3. Results and Discussion
3.1. Chemical Composition
In order to highlight the application potential of sprouts in obtaining fortified flours intended for obtaining biscuits, the content of TPC and individual polyphenols in the BS, LS, PS, and RS extracts was determined, and wheat flour (WF) was used as a control.
Figure 4 presents the TPC composition, while Figure 5 illustrates the increase in TPC compared to the control (WF). Table 1 provides the content of individual polyphenols.
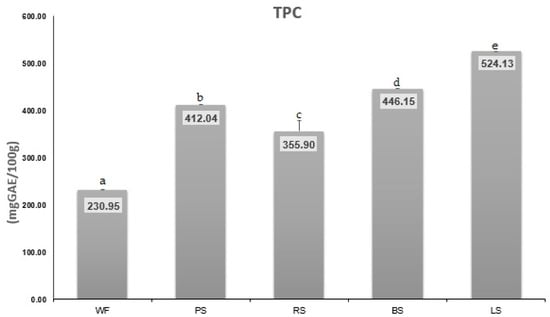
Figure 4.
TPC of pea sprouts (PS), radish sprouts (RS), bean sprouts (BS), lentil sprouts (LS) expressed as mg GAE/100 g. The values are expressed as mean values ± standard deviations of all measurements; data sharing different letters are significantly different (p < 0.05).
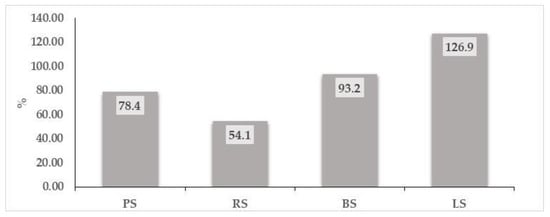
Figure 5.
The increase in TPC content (%) in sprouts compared to WF.
Polyphenols are vital secondary metabolites present in all plant tissues, including flowers and fruits. These compounds exhibit a wide range of structural variations, all characterized by at least one aromatic ring and one or more hydroxyl groups, along with additional chemical components. As essential antioxidants in the human diet, they play a crucial role in supporting and maintaining overall health [2]. The germination process can notably impact polyphenol concentration due to the metabolic changes that take place during this stage. The values obtained in our study (Figure 4) show that TPC varied in order: WF (230.959 mg GAE/100 g) < RS (355.900 mg GAE/100 g) < PS (412.040 mg GAE/100 g) < BS (446.147 mg GAE/100 g) < LS (524.130 mg GAE/100 g). It is noted that lentil sprouts contain the highest level of TPC compared to the other analyzed sprouts.
Previous studies have shown that lentil sprouts exhibit TPC values ranging from 450 to 600 mg GAE/100 g, while their antioxidant activity, measured by DPPH, ranges between 40% and 50% inhibition [4,28]. The recorded TPC values for mung beans and peas fall within 400–500 mg GAE/100 g and 350–450 mg GAE/100 g, respectively [28]. Khang et al. 2016 reported that seed germination enhances TPC and antioxidant activity in mung beans, with observed values of 14.97 mg GAE/g and 26.45% DPPH antioxidant activity [28]. Additionally, the study of González-Montemayor et al., 2021, assessed flours from whole pods of green bean, pea, and mesquite, identifying mesquite flour as the matrix with the highest TPC content at 474.77 mg GAE/g [29].
Francis H et al. 2022 studied radish sprouts obtained after different days of germination and observed that the TPC increased from 2.56 to 9.75 mg GAE/g dry matter after six days of germination [30]. Other studies conducted on five microgreens belonging to the Brassicaceae family have revealed a TPC content between 102 and 363 mg/100 g, depending on the analyzed species [7].
Figure 5 shows the increase in TPC content in analyzed sprouts compared to whole wheat flour. An increase between 54.1% and 126.9% is observed, depending on the type of sprouts analyzed, which suggests the possibility of using sprouts as functional ingredients in composite flours. The TPC content in germinated plants is influenced by the germination conditions: time, humidity, brightness, or sprout length at which the harvest was carried out, respectively [5].
The individual polyphenols determined in sprouts samples analyzed in our study (Table 1) include epicatechin, rosmarinic acid, quercetin, resveratrol, gallic acid, coumaric acid, ferulic acid, and β-resorcylic acid. The strong antioxidant properties and ability to scavenge free radicals contribute to their potential role in protecting against oxidative stress [31,32]. Gallic and caffeic acids were not quantified and detected in concentrations higher than the minimum detection limit for any of the samples analyzed (indicated as nd * in Table 1). In sample RS, the concentrations recorded for the individual polyphenols were epicatichin (919.94 mg/kg), ferulic acid (62.76 mg/kg), rosmarinic acid (62.11 mg/kg), resveratrol (228.95 mg/kg), values that are found in higher concentrations compared to other analyzed sprouts. The highest concentration of β-resorcylic acid was observed in LS, with a value of 92.23 mg/kg. In contrast, the concentrations of this compound in RS and BS are significantly lower, recording values of 13.014 mg/kg and 8.068 mg/kg, respectively. The β-resorcylic acid content in dry lentil seeds typically falls below 1 mg/100 g dry weight, making it a minor component of the phenolic profile [33]. The highest values for coumaric acid were found in RS (62.767 mg/kg), highlighting that radish is an excellent source of this compound. Also, in RS, the highest concentration of ferulic acid was recorded, followed by FS (37.209 mg/kg) and BS (7.119 mg/kg).
Germination enhances the accumulation of rosmarinic acid, renowned for its antioxidant properties. The highest concentration is observed in RS (228.954 mg/kg), with notable levels also detected in FS (77.358 mg/kg) and BS (19.832 mg/kg). Quercetin, a flavonoid known for its potent antioxidant, anti-inflammatory, and anti-carcinogenic effects, plays a crucial role in protecting sprouts from environmental oxidative stress factors such as light, temperature, and oxygen during germination. The highest concentration is observed in FS (74.919 mg/kg), highlighting the significant accumulation of quercetin throughout the germination process [31]. Guo X. et al., 2012 identified quercetin-3-O-glucoside in both raw and germinated mung bean extracts [34], and Kapravelou G. et al., 2020, highlighted that germination improves the polyphenolic profile and functional value of mung bean [35]. Li Z. et al. 2018, analyzed 12 cruciferous vegetables, detecting 74 phenolic compounds, primarily hydroxycinnamic acids and flavonoids [36]. Notably, caffeic acid, p-coumaric acid, ferulic acid, and quercetin were prevalent across different species [35]. Table 2 presents the macro- and microelements composition of sprouts and wheat flour.

Table 2.
The macro- and microelements composition of sprouts and wheat flour.
From Table 2, it can be seen that in terms of microelement content, their distribution increases in the order of Fe > Zn > Mn > Cu, while Ni was undetectable in most samples except LS. The highest level of microelements was found in the LS sample, while the lowest level was found in the RS sample. LS showed the highest Zn content (21.058 mg/kg), suggesting a beneficial use in supporting immune functions. Our results revealed that LS had the highest levels of Cu (5.654 mg/kg) and Mn (20.469 mg/kg), minerals essential for metabolic health and balance [36].
Dobrowolska-Iwanek et al., 2022 investigated the content of essential macro- and micronutrients such as Cu, Zn, Mn, Fe, Mg, and Ca in Brassicaceae family sprouts. The results obtained show significant variations among the species analyzed, with broccoli, cabbage, and radish sprouts being distinguished by high levels of minerals [37].
Regarding the macroelement composition, PS recorded the highest content of Ca (256.575 mg/kg) but the smaller content of Mg (405.819 mg/kg), K (1346.158 mg/kg), and Na (114.871 mg/kg). The LS was distinguished by a low Ca content (86.400 mg/kg) but higher Mg value (518.971 mg/kg) compared to the other analyzed sprouts. The maximum value for K was recorded in the BS sample (4819.450 mg/kg), while Na varied between 114.871 mg/kg in PS and 178.512 mg/kg in BS.
According to a study conducted by Wojdyło et al. in 2020, radish sprouts have a calcium content of approximately 211 mg/kg, while lentil sprouts contain about 71 mg/kg [38]. Broccoli sprouts exhibited the highest concentrations of Mg (518.971 mg/kg) and Fe (48.664 mg/kg), highlighting their potential role in preventing magnesium and iron deficiencies. Kapravelou et al. 2020 reported that mung bean sprouts contain high levels of essential minerals such as Mg, Mn, Fe, Cu, and Zn, which contribute to optimal antioxidant status [35]. Similarly, Li et al. 2018 found that broccoli and radish sprouts had elevated concentrations of Fe, Zn, and Mg, while Chinese cabbage displayed a well-balanced content of Ca and K—minerals crucial for bone health and electrolyte balance [36]. In comparison, wheat flour was found to be lower in all analyzed macro- and microelements. These findings emphasize that incorporating LS, BS, PS, and RS sprouts into wheat flour can enhance its nutritional value, potentially offering health benefits.
3.2. Antibacterial Activity
The in vitro antibacterial activity of the four sprout extracts analyzed on the five bacterial strains tested had been evaluated qualitatively and quantitatively by the presence or absence of inhibition zones, percentages of bacterial inhibition, and the resulting MIC values. Figure 6 shows images of Petri dishes in which different doses of extracts were applied to the four bacterial strains analyzed, and Table 3 shows the diameters (in mm) of the inhibition zones under the action of the various volumes of extracts that were analyzed.
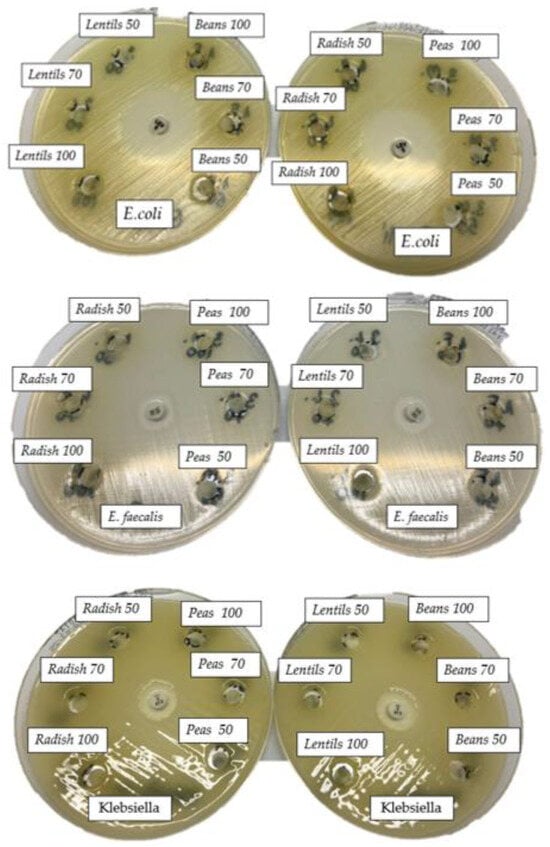
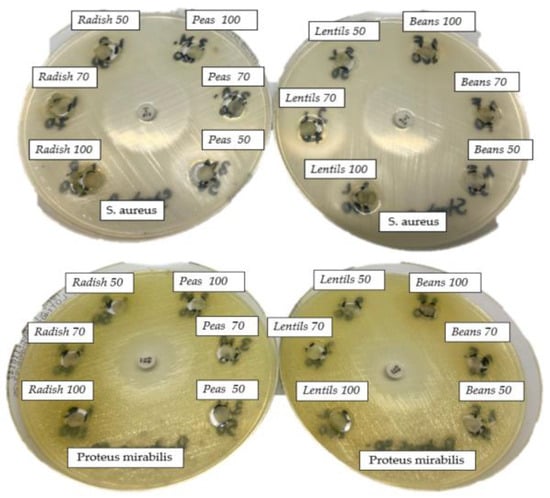
Figure 6.
The antimicrobial effect of BS, LS, PS, RS against E. coli, P. mirabilis, K. pneumoniae, and E. faecalis.

Table 3.
The diameter of inhibition zone (mm) for the analyzed sprouts at different extract concentrations.
It should be emphasized that the diameters of the inhibition zone for each positive control and, respectively, for each tested extract, included the 6 mm diameter of the micro-tablet. For the Escherichia coli strain, isolated from an infected wound, the effectiveness of the antibiotic used (microtablet containing 5 µg of levofloxacin) generated an inhibition zone (halo) of 33 mm. The tested extracts had no effect against E. coli, the colonies developing up to the proximity of the microtablets soaked with 2, 50, 70, and 100 µL of the extract.
For Proteus mirabilis, it was proven to be sensitive to gentamicin, which generated an inhibition zone of 25 mm. On the other hand, the inefficiency of the four analyzed extracts was proven, regardless of the concentration applied.
Regarding the K. pneumoniae strain, the microtablet containing 5 µg of ciprofloxacin, which was considered a positive control, generated a halo of 17 mm. The tested extracts were without effect for this strain at a volume of 2 µL of extract. It was also checked for antibacterial activity of the extracts at larger volumes of 50 μL, 70 μL, and 100 μL were without any bactericidal action.
Compared to the other strains studied, E. fecalis has been shown to be sensitive to radish sprout extract in concentrations of 50 µL, 70 µL, and 100 µL, with an inhibition diameter between 12 and 14 mm; also, the inhibition zone was observed lowering RS concentration (2 µL). The microtablet containing 5 µg of vancomycin, considered a positive control, generated a halo of 16 mm; the rest of the analyzed extracts were without any inhibitory effect for this strain.
For S. aureus, the microtablet with 10 µg of linezolid, considered a positive control, generated a halo of 33 mm. Two of the tested extracts, BS and LS, had no effect on the tested strain at volumes of 2 µL of extract, but the PS generated an 11 mm halo, while the microtablet, softened with 2 µL extract from RS, generated a 13 mm halo. For increased volumes of extracts microtablets, an antibacterial effect against S. aureus was observed when LS, PS, and RS were applied (Table 3); BS recorded no inhibition effect, regardless of the concentration used.
The MIC values for the analyzed extracts, in relation to the tested microorganisms, are as follows. PS—70 µL/mL and RS—50 µL/mL for Staphylococcus aureus and, for Enterococcus faecalis, RS—70 µL/mL. In the case of the other extracts, they are without effect for the analyzed microbial strains at the volume of the analyzed extract.
Regarding the size of the halos that indicate the sensitivity to antibiotics of various microorganisms, the antibiotic substances that generate halos (including the diameter of the micro-tablet) larger than 14 mm are considered effective, intermediately sensitive are those between 12 and 14 mm, and resistant are those that generate halos smaller than 12 mm (for Enterobacteriaceae family). In the case of the Staphylococcus aureus species, if we take linezolid as a standard, for a halo size greater than 24 mm, the strain is sensitive (S); between 21 and 24 mm, it is considered intermediate sensitive (IS); and below 21 mm is resistant (R), according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) [39].
In the case of the species Enterococcus faecalis (vancomycin as standard), for a halo size greater than 13 mm, the strain is sensitive (S); between 10 and 13 mm, it is considered intermediate sensitive (IS); and below 10 mm, it is resistant (R), according to EUCAST [39]. Based on these findings, we can say that the S. aureus strain is sensitive to the application of a concentration of 70 and 100 µL of PS, RS, and LS extract, with a halo of 25 mm and intermediate sensitivity (IS) for a concentration of 50 µL PS extract. Against Enterococcus faecalis, when 100 µL RS extract was applied and a halo of 14 mm was observed, it meant the strain was sensitive (S) at this concentration of RS extract. For lower concentrations, the strain is of intermediate sensitivity (IS) upon the application of RS extract
Figure 7 shows the percentage of inhibition of the analyzed extracts at the maximum concentration applied (100 µL/mL, against the two sensitive strains (S. aureus and E. faecalis) and shows the relation to the effect produced by the positive control antibiotic for which the maximum inhibition is considered. A high percentage of S. aureus inhibition of over 75% is observed with the application of three of the total four analyzed sprouts (PS, LS, RS) and an 87.5% inhibition efficacy against E. faecalis for the RS extract, respectively.
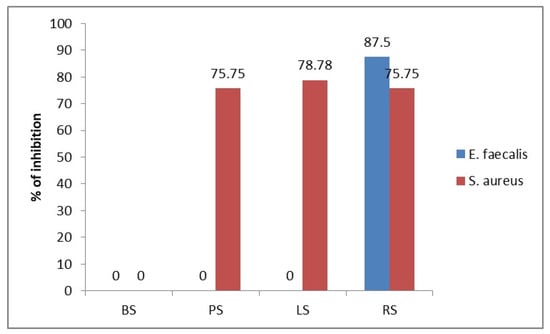
Figure 7.
The percentage of inhibition of the analyzed extracts, at the maximum concentration applied (100 µL/mL), against the 2 sensitive strains (S. aureus and E. faecalis). PS—pea sprouts, RS—radish sprouts, BS—bean sprouts, LS—lentil sprouts.
This fact indicates the antibacterial potential of sprouted legumes and radish extracts, with very promising results supporting their use as natural antibacterial agents in the food industry.
The study of the antibacterial activity of some extracts obtained from sprouts has been approached to a small extent in the literature, especially regarding certain legumes or radishes. Studies have demonstrated the antimicrobial properties of various sprouted vegetables against human pathogenic bacteria. Szabo et al. 2014 reported that sprouted radish, kohlrabi, and red cabbage exhibited inhibitory zones against Escherichia coli, Salmonella Typhimurium, Shigella flexneri, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus [14]. Research on different Brassica oleracea sprouts, including red cabbage, broccoli, Galega kale, and Penca cabbage, revealed that red cabbage and broccoli extracts exhibited the strongest antibacterial activity [40]. Vale et al. 2015 also found a strong correlation between this activity and the organic acid composition of the sprouts [40]. Additionally, the study on methanolic extracts of mixed leguminous sprouts (Chickpea, Green gram, and Horse gram) showed the highest inhibition zone (30 ± 0.6 mm) against Shigella flexneri and the lowest (3 ± 0.3 mm) against Escherichia coli [41].
3.3. Correlation Between Chemical Composition and Antibacterial Activity
In order to highlight the interdependencies between the chemical parameters and the antibacterial activity exerted by the four extracts against two of the five strains analyzed, namely Staphylococcus aureus and Enterococcus faecalis, the Pearson correlation between variables was performed (Figure 8). It is observed that there are strong positive correlations between the inhibition of E. faecalis and the individual polyphenols, namely between the pairs E. faecalis/epicatechin (r = 0.99), E. faecalis/resveratrol (r = 0.96), E. faecalis/rosmarinic acid (r = 0.83), E. faecalis/ferulic acid (r = 0.81). The existence of these strong positive correlations between the degree of inhibition of E. faecalis and the individual polyphenols analyzed suggests the possibility of using natural preparations based on these compounds, either as such or in combination with inhibitory chemicals, as antibacterial agents with applications in the food industry, hygiene, pharmacy or sanitation.
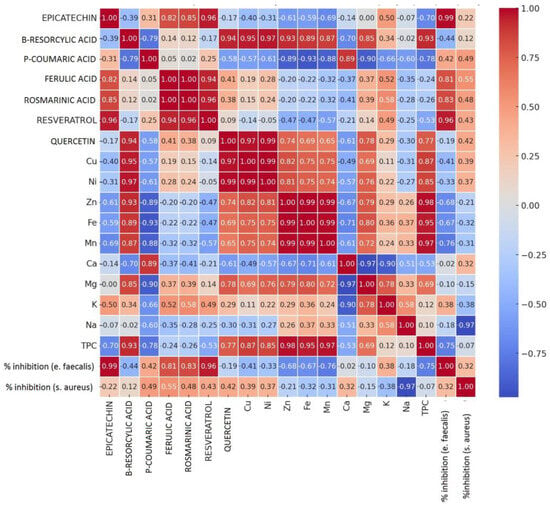
Figure 8.
Pearson correlation between chemical and microbiological parameters.
There are no positive, strong correlations between the inhibition of the E. faecalis strain and the content of macro- and microelements determined in the four types of sprouts analyzed. With the exception of K, which registers a moderate correlation (r = 0.38) in relation to E. faecalis, all other analyzed macro- and microelements are negatively correlated with the ability to inhibit the bacterial strain.
Regarding the inhibitory capacity of individual polyphenols against S. aureus, it is observed that there are no strong positive correlations between the bacteria strains and the determined chemical components, either individual polyphenols or elementar composition. A moderate positive correlation is nevertheless observed between the pairs S. aureus/ferulic acid (r = 0.55), S. aureus/coumaric acid (r = 0.49), S. aureus/rosmarinic acid (r = 0.48). It is worth noting a very strong negative correlation (r = −0.97) recorded between S. aureus/Na pairs, which suggests a negative influence of excess Na from plant matrices on the inhibitory capacity of bacterial strains. No strong or moderately positive correlations were identified between the inhibition capacity of S. aureus and the content of macro- or microelements. A weak positive correlation was recorded between the pairs S. aureus/Cu and S. aureus/Ca; the other analyzed elements have only negative correlations to the inhibition of the S. aureus strain.
The PCA analysis of the projection of the parameter by the first and second principal components (Figure 9) shows that component 1, the primary axis of variability, explains 98.967% of the variance. This component is dominated by variables strongly associated with TPC and the inhibition percentages of bacterial strains, particularly for S. aureus. Component 2 (0.808%), which represents a secondary source of variation, driven mainly by specific compounds such as epicatechin and the sample RS. Together, these components explain nearly all the variability in the data, indicating that the selected variables effectively capture the essential patterns in the dataset.
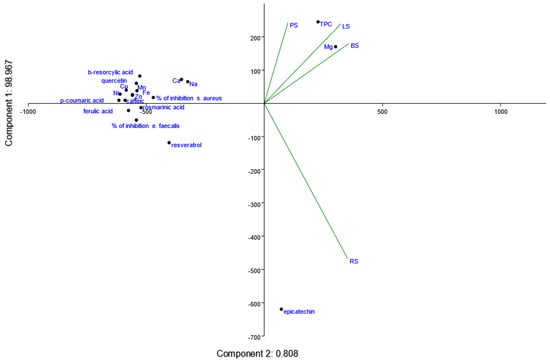
Figure 9.
Projection of the parameter by the first and second principal components.
TPC aligns strongly with Mg and LS/BS samples, highlighting a robust association between total polyphenols and magnesium concentrations in these samples. Resveratrol and quercitin are closely positioned near TPC, indicating their significant contribution to the total polyphenol content. These compounds are widely recognized for their potent antioxidant and antimicrobial activities.
The PCA plot demonstrates that the inhibition of E. faecalis and S. aureus is driven by different polyphenols, reflecting the specificity of antimicrobial mechanisms. It can be observed that inhibition of E. faecalis correlates strongly with resveratrol and ferulic acid. This suggests that these polyphenols are primary contributors to the antimicrobial efficacy against E. faecalis. These findings correspond with the Pearson correlation presented in Figure 8.
Inhibition of S. aureus shows a stronger association with rosmarinic acid, in line with Pearson correlation, suggesting distinct mechanisms of action for inhibiting this bacterium.
Macro- and microelement correlation with polyphenol content shows that Mg aligns closely with TPC, indicating its role in stabilizing or enhancing polyphenolic content in the samples. Zn and Cu are grouped with quercetin and beta-rezorciclic acid, suggesting a potential synergistic effect of these elements in promoting antioxidant or antimicrobial activity. Sodium is positioned separately, with minimal influence on TPC or microbial inhibition, reinforcing its potential inhibitory effect on polyphenol bioactivity.
From PCA analysis, this can be concluded with some sample-specific observations. A PS sample is positioned closer to Ca and Na, suggesting its lower polyphenolic content and minimal antimicrobial activity. An RS sample is distinctly associated with epicatechin and resveratrol, confirming its dominance in polyphenol-rich composition and antimicrobial efficacy. BS and LS samples are closely aligned with TPC, Mg, and microbial inhibition percentages, reflecting their balanced bioactive profiles.
3.4. Sensory Analysis of Cookies Fortified with Germinated
In order to evaluate certain applications in the food industry of the analyzed sprouts, biscuits were obtained with composite flour (wheat flour/sprouts) in three different compositional variants (2.5, 5, 10% sprouts in wheat flour), according to Figure 3. Also, a control sample from wheat flour was analyzed for comparison.
In order to evaluate the consumer acceptability of the cookies fortified with sprouts, a sensory evaluation was carried out by a panel of tasters who analyzed the following attributes: appearance, aroma, texture, taste, and general acceptability. The results of this evaluation are presented in Figure 10.
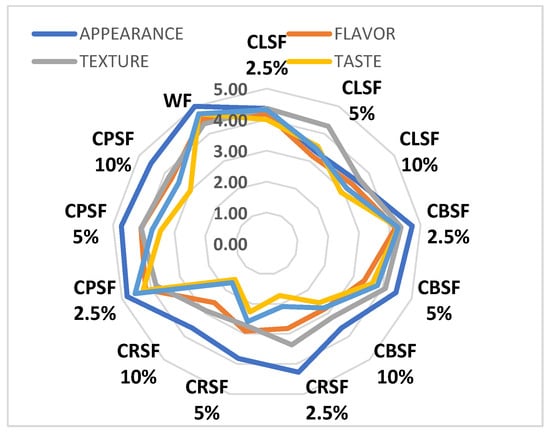
Figure 10.
Sensory analysis of cookies using a 5-point hedonic scale.
The analysis of data presented in Figure 10 shows that the control sample obtained only from wheat flour is the most appreciated product, achieving the highest scores: 5.00 ± 0; 4.55 ± 0.52; 4.36 ± 0.67; 4.73 ± 0.65; 4.73 ± 0.65 for appearance, aroma, texture, taste, and overall acceptability, respectively. This product was the most preferred by consumers. Among the analyzed germinated samples, it was observed that CPSF 2.5% (4.82 ± 0.40; 4.18 ± 0.60; 3.82 ± 0.75; 4.27 ± 0.47; 4.55 ± 0.52) and CBSF 2.5% (4.73 ± 0.47; 4.18 ± 0.60; 4.36 ± 0.67; 4.27 ± 0.65; 4.27 ± 0.47) received scores close to control, indicating they were also well-liked by consumers.
The least appreciated products were CRSF 2.5%, 5%, and 10%, which received low scores. For CRSF 2.5%, the scores were 4.27 ± 1.01; 2.28 ± 0.98; 3.36 ± 1.03; 1.73 ± 0.90; 2.09 ± 0.54, while for CRSF 5%, they were 3.82 ± 0.98; 2.91 ± 1.14; 2.73 ± 1.10; 2.27 ± 1.27; 2.59 ± 1.28, and for CRSF 10%, they were 3.64 ± 1.21; 2.55 ± 1.37; 2.91 ± 1.04; 1.55 ± 0.82; 1.68 ± 0.64. These samples were not well-received by consumers, likely due to their slightly spicy taste.
The obtained results confirm what other authors have reported regarding the possibility of using some legumes, either as such or in the form of sprouts, as functional ingredients in baking, namely that only in small percentages, up to 10%, flour products based on sprouts are acceptable from a sensory point of view [21,40]. The study on the sensory properties of biscuits with sprouted lupin added in the form of flour or in raw form highlighted that fortification with flour sprouts was more appreciated compared to the addition of green sprouts, and a percentage of 5% proved to be optimal for most respondents to the study.
4. Conclusions
The study of chemical and antibacterial activity of three species of legumes belonging to the Fabaceae family and from Brassicaceae family indicated that LS are the sprouts with the highest content of TPC, while RS recorded the highest amounts of individual polyphenols. LS has the highest content for Ni, Cu, Zn, Fe, Mn, and Mg, while the highest values for Ca were recorded in the case of PS and for K in the case of the BS sample.
In terms of antimicrobial potential, we can conclude that against strains E. coli, P. mirabilis, and K. pneumoniae, the sprouts have no efficiency, but RS at a concentration of 70 µL/mL, they exhibited antibacterial activity against E. faecalis and at 50 µL/mL against S. aureus. Also, PS extract inhibited S. aureus when a concentration of 70 µL/mL was applied.
The PCA highlights the strong influence of polyphenols on the bioactive potential of the samples. These insights are critical for optimizing the selection and formulation of bioactive ingredients for nutraceutical, pharmaceutical, and food applications, with a specific focus on enhancing antimicrobial properties through targeted enrichment of key polyphenols and supportive minerals.
As regards consumer preferences for floury foods such as cookies with added sprouts, the panelists participating in the study preferred products based on BS and PS with a percentage of 2.5%. The least appreciated products were biscuits with RS addition with lower scores for all analyzed parameters, while biscuits with 2.5% lentils, even though they showed good scores for texture and appearance, were poorly appreciated in terms of taste.
Future research prospects in this field refer to the study of the rheological properties of dough fortified with sprouts, as well as the effects that the technological process has on the inhibition of microbial growth and on the content of active principles in finished products enriched with sprouts from the analyzed plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15041896/s1. Figure S1. The individual polyphenols RS (radish sprouts), Ch1-280 nm, Ch2-320 nm. Figure S2. The individual polyphenols PS (pea sprouts), Ch1-280 nm, Ch2-320 nm. Figure S3. The individual polyphenols BS (bean sprouts) Ch1-280 nm, Ch2-320 nm. Figure S4. The individual polyphenols LS (lentil sprouts) Ch1-280 nm, Ch2-320 nm.
Author Contributions
All authors contributed to the study’s conception and design. Conceptualization and original draft preparation: C.D., C.D.M., S.D. and E.A.; methodology: C.D., C.D.M., S.D., M.N., L.P. and I.R.; formal analysis: C.D., C.D.M., S.D., M.N., C.J., I.R., L.P. and E.A.; review and editing and validation: C.D., C.D.M., S.D., M.N., C.J., I.R., L.P. and E.A.; supervising: E.A. and C.J. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of the present paper was supported by the University of Life Sciences “King Mihai I” from Timisoara, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Life Sciences “King Mihai I”, Timisoara (no. 202/10/03.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in the study are openly available at the University of Life Sciences “King Mihai I” from Timișoara and Misca Medical Center Timisoara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Celac, V. Plantele leguminoase–Actualitate şi viitor. Rev. Ştiinţă Inovare Cult. Artă Akad. 2009, 13, 77–79. [Google Scholar]
- McKevith, B. Nutritional aspects of cereals. Br. Nutr. Found. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kimizuka-Takagi, C.; Bang, S.W.; Matsuzawa, Y. Radish. In Vegetables; Springer: Berlin/Heidelberg, Germany, 2007; pp. 141–160. [Google Scholar]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Park, Y.R.; Kwon, S.-J.; Kim, J.H.; Duan, S.; Eom, S.H. Light-Induced Antioxidant Phenolic Changes among the Sprouts of Lentil Cultivar. Antioxidants 2024, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Servan, A.; Nigam, D. Comparative study on antioxidant activity, phytochemical analysis and mineral composition of the Mung Bean (Vigna Radiata) and its sprouts. J. Pharmacogn. Phytochem. 2017, 6, 336–340. [Google Scholar]
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small functional foods: Comparative phytochemical and nutritional analyses of five microgreens of the Brassicaceae family. Foods 2021, 10, 427. [Google Scholar] [CrossRef]
- López-Amorós, M.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Khalid, M.; Ayayda, R.; Gheith, N.; Salah, Z.; Abu-Lafi, S.; Jaber, A.; Al-Rimawi, F.; Al-Mazaideh, G. Assessment of Antimicrobial and Anticancer Activity of Radish Sprouts Extracts. Jordan J. Biol. Sci. 2020, 13, 567. [Google Scholar]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial potential of legume extracts against foodborne pathogens: A review. Trends Food Sci. Technol. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Lin, J.C.; Bagal-Kestwal, D.; Chiang, B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Szabó, S.; Németh, Z.; Polyák, É.; Bátai, I.; Kerényi, M.; Figler, M. Antibacterial effect of sprouts against human pathogens in vitro. Acta Aliment. 2014, 43, 501–508. [Google Scholar] [CrossRef]
- Biletska, Y.; Gontar, A. Research of rheological indicators of the using the flour of germinated legumes. Technol. Audit Prod. Reserves 2020, 5, 55. [Google Scholar] [CrossRef]
- Duodu, K.G.; Minnaar, A. Chapter 18-Legume Composite Flours and Baked Goods: Nutritional, Functional, Sensory, and Phytochemical Qualitie. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridgem, MA, USA, 2011; pp. 193–203. ISBN 9780123808868. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G.; et al. Lupin (Lupinus spp.)-Fortified Bread: A Sustainable, Nutritionally, Functionally, and Technologically Valuable Solution for Bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef]
- Codină, G.G.; Marineac, A.R.; Todosi-Sănduleac, E. The influence of lupin flour addition on bread quality. Food Environ. Saf. 2016, 15, 216–226. [Google Scholar]
- Bashir, A.; Ashraf, S.A.; Khan, M.; Azad Azaz Ahmad, Z.R. Development and Compositional Analysis of Protein Enriched Soybean-Pea-Wheat Flour Blended Cookies. Asian J. Clin. Nutr. 2015, 7, 76–83. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Nutritional Quality and Antioxidant Properties of Brown and Black Lentil Sprouts. Horticulturae 2023, 9, 668. [Google Scholar] [CrossRef]
- Plustea, L.; Dossa, S.; Dragomir, C.; Cocan, I.; Negrea, M.; Obistioiu, D.; Poiana, M.-A.; Voica, D.; Berbecea, A.; Alexa, E. Comparative Study of the Nutritional, Phytochemical, Sensory Characteristics and Glycemic Response of Cookies Enriched with Lupin Sprout Flour and Lupin Green Sprout. Foods 2024, 13, 656. [Google Scholar] [CrossRef]
- Jianu, C.; Mişcă, C.; Muntean, S.G.; Gruia, A.T. Composition, antioxidant and antimicrobial activity of the essential oil of Achillea collina Becker growing wild in western Romania. Hem. Ind. 2015, 69, 381–386. [Google Scholar] [CrossRef]
- Available online: https://microchemlab.com/test/zone-inhibition-test-antimicrobial-activity/ (accessed on 29 December 2024).
- The European Committee on Antimicrobial Susceptibility Testing. EUCAST Reading Guide for Broth Microdilution. Version 5.0. Available online: https://www.eucast.org/ (accessed on 29 December 2024).
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/research/participants/data/ref/fp7/89847/research-food_en.pdf (accessed on 29 December 2024).
- Poșta, D.S.; Radulov, I.; Cocan, I.; Berbecea, A.A.; Alexa, E.; Hotea, I.; Iordănescu, O.A.; Băla, M.; Cântar, I.C.; Rózsa, S.; et al. Hazelnuts (Corylus avellana L.) from Spontaneous Flora of the West Part of Romania: A Source of Nutrients for Locals. Agronomy 2022, 12, 214. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- González-Montemayor, A.M.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; López-Badillo, C.M.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Green Bean, Pea and Mesquite Whole Pod Flours Nutritional and Functional Properties and Their Effect on Sourdough Bread. Foods 2021, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. Optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Lemus-Conejo, A.; Rivero-Pino, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Nutritional composition and biological activity of narrow-leafed lupins (Lupinus angustifolius L.) hydrolysates and seeds. Food Chem. 2023, 420, 136104. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Phenolic Substance Characterization and Chemical and Cell-Based Antioxidant Activities of 11 Lentils Grown in the Northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Barakat, H.; Alshimali, S.I.; Almutairi, A.S.; Alkhurayji, R.I.; Almutiri, S.M.; Aljutaily, T.; Algheshairy, R.M.; Alhomaid, R.M.; Aljalis, R.A.; Alkhidhr, M.F. Antioxidative potential and ameliorative effects of green lentil (Lens culinaris M.) sprouts against CCl4-induced oxidative stress in rats. Front. Nutr. 2022, 9, 1029793. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Perazzoli, G.; Sánchez González, C.; Llopis, J.; Cantarero, S.; Goua, M.; Bermano, G.; Prados, J.; Melguizo, C.; et al. Germination Improves the Polyphenolic Profile and Functional Value of Mung Bean (Vigna radiata L.). Antioxidants 2020, 9, 746. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; Saraiva de Carvalho, I.; Paśko, P. Determination of essential minerals and trace elements in edible sprouts from different botanical families—Application of Chemometric analysis. Foods 2022, 11, 371. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://aurosan.de/images/mediathek/servicematerial/EUCAST_RefStaemme_Sollwerte.pdf (accessed on 29 December 2024).
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Oliveira, M.B.P.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control. 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Valli, S.A.; Gowrie, S.U. Synergistic effect of phytoconstituents in mixed sprouts-an approach towards therapeutic applications. J. Chem. Pharm. Res. 2017, 9, 95–112. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).