Defect Tailoring in HfO2/Si Films upon Post-Deposition Annealing and Ultraviolet Irradiation
Abstract
Featured Application
Abstract
1. Introduction
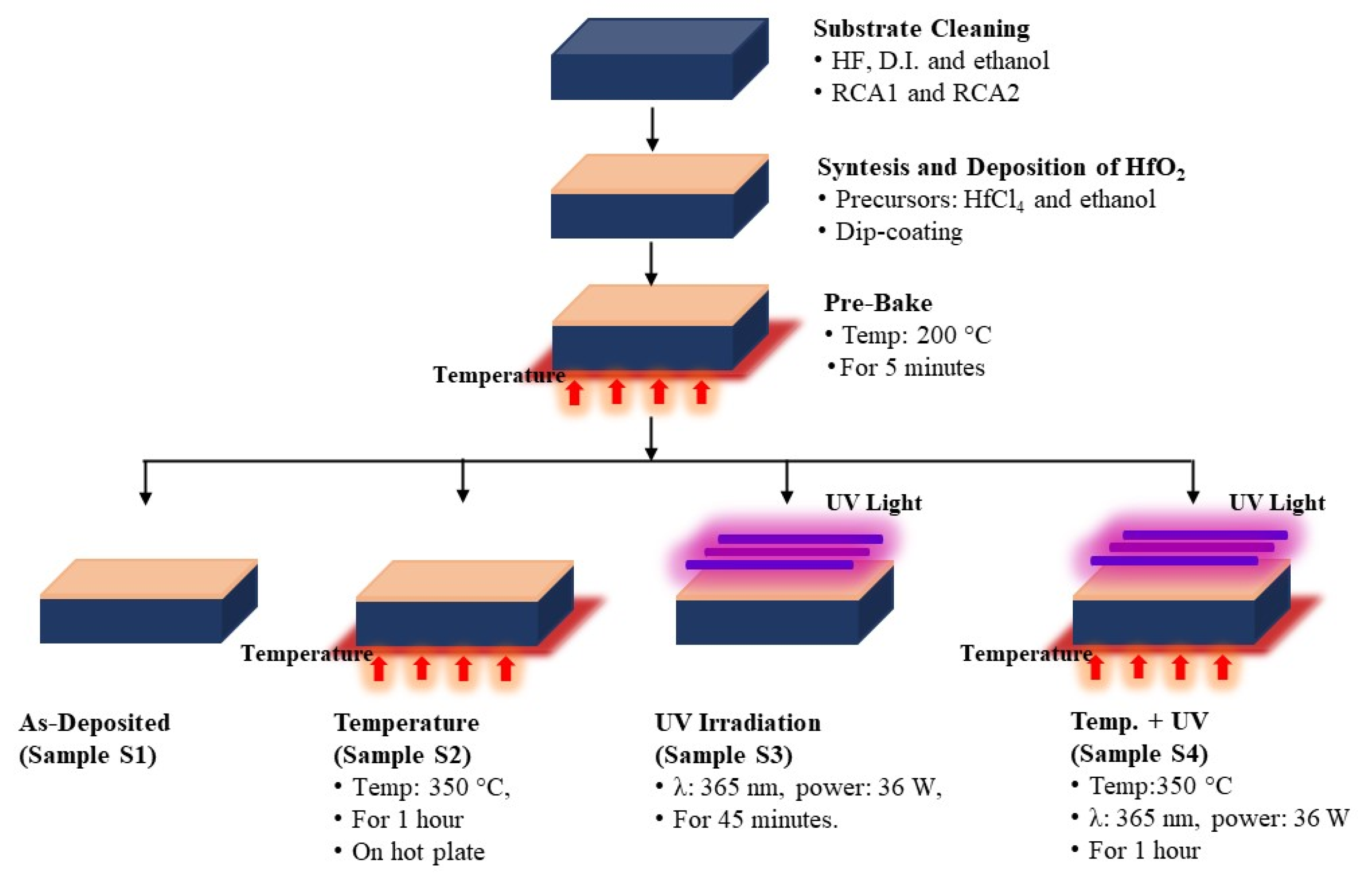
2. Materials and Methods
3. Results
3.1. Structural Characterization
3.1.1. X-ray Diffraction
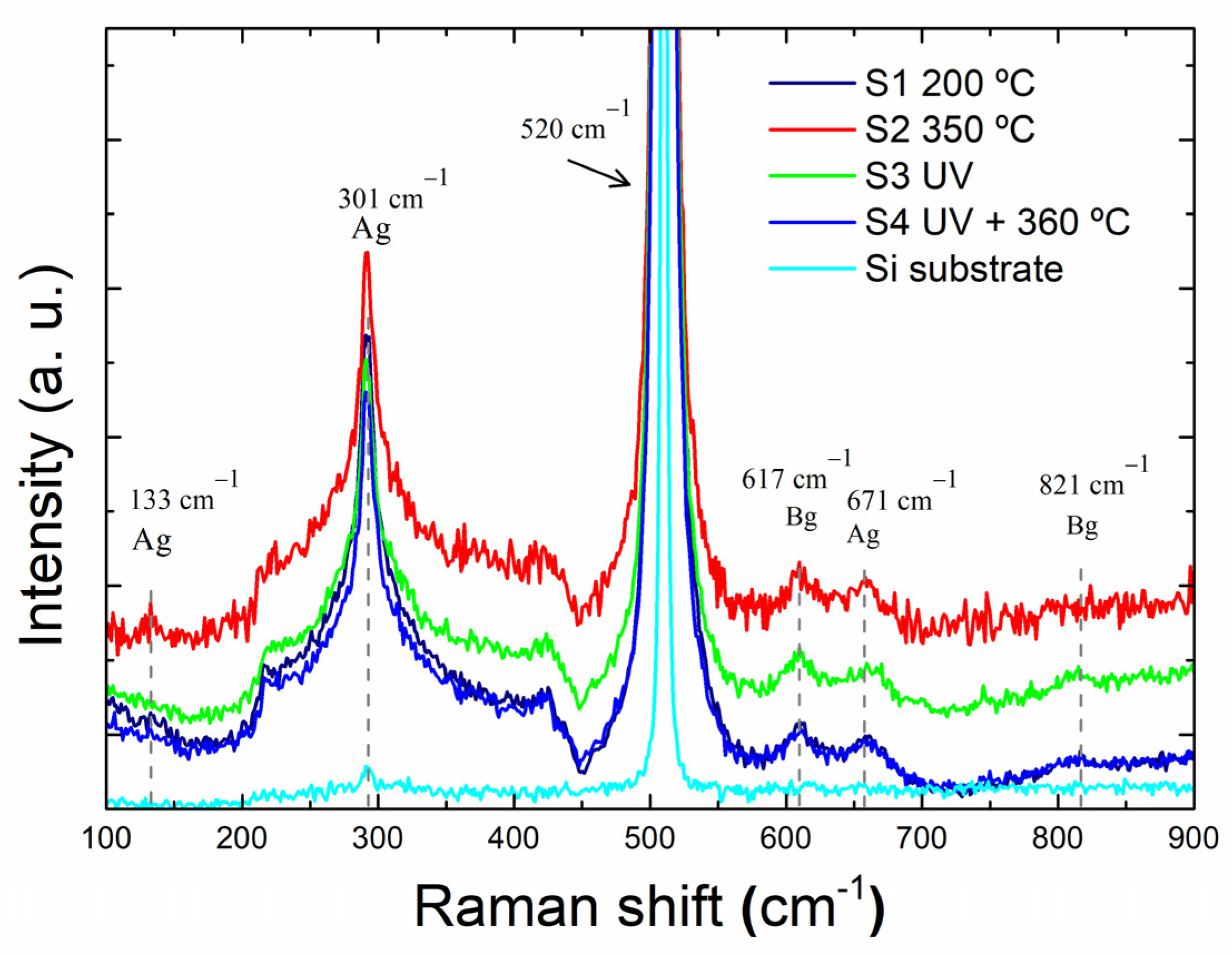
3.1.2. Raman Spectroscopy
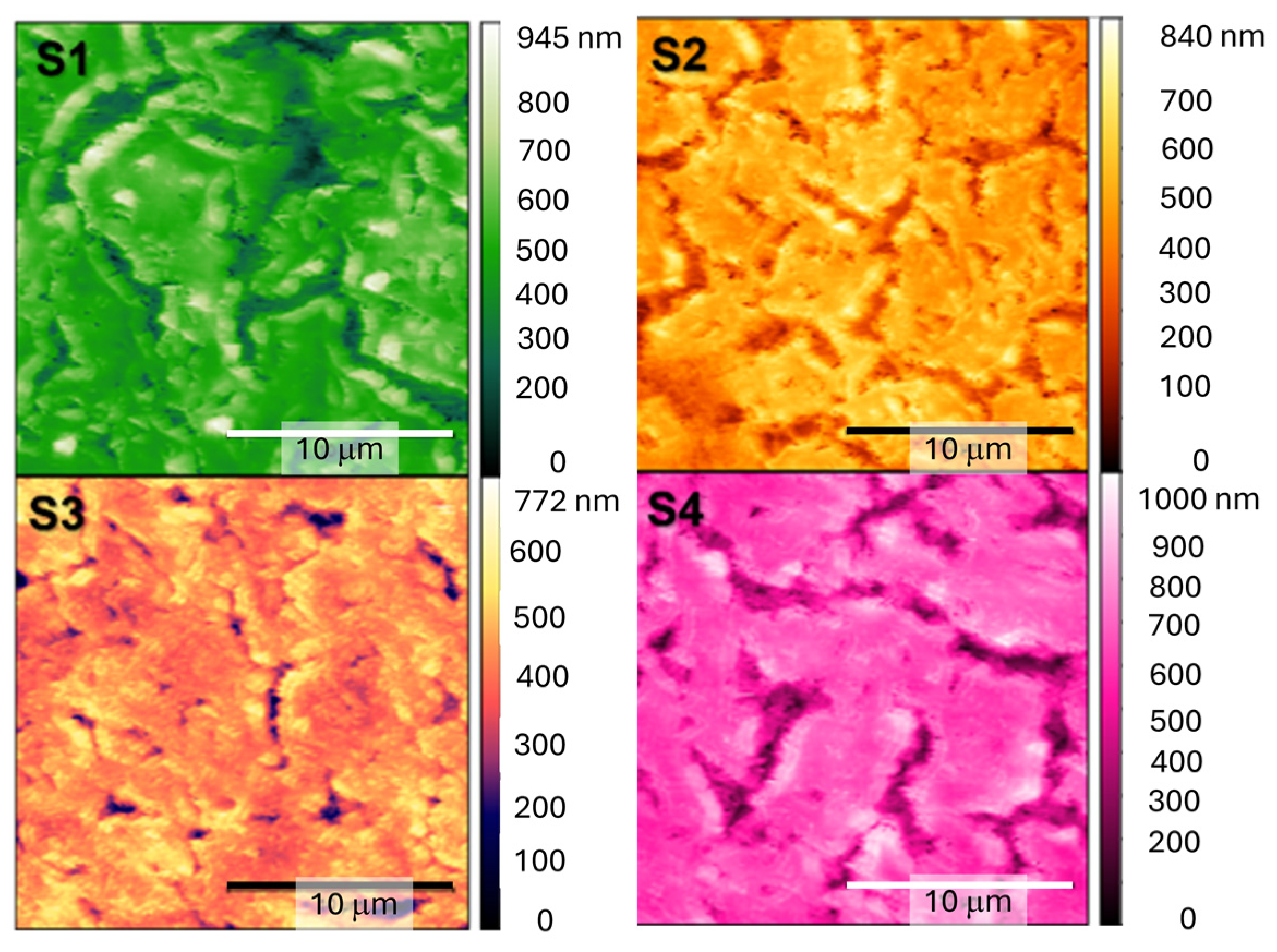
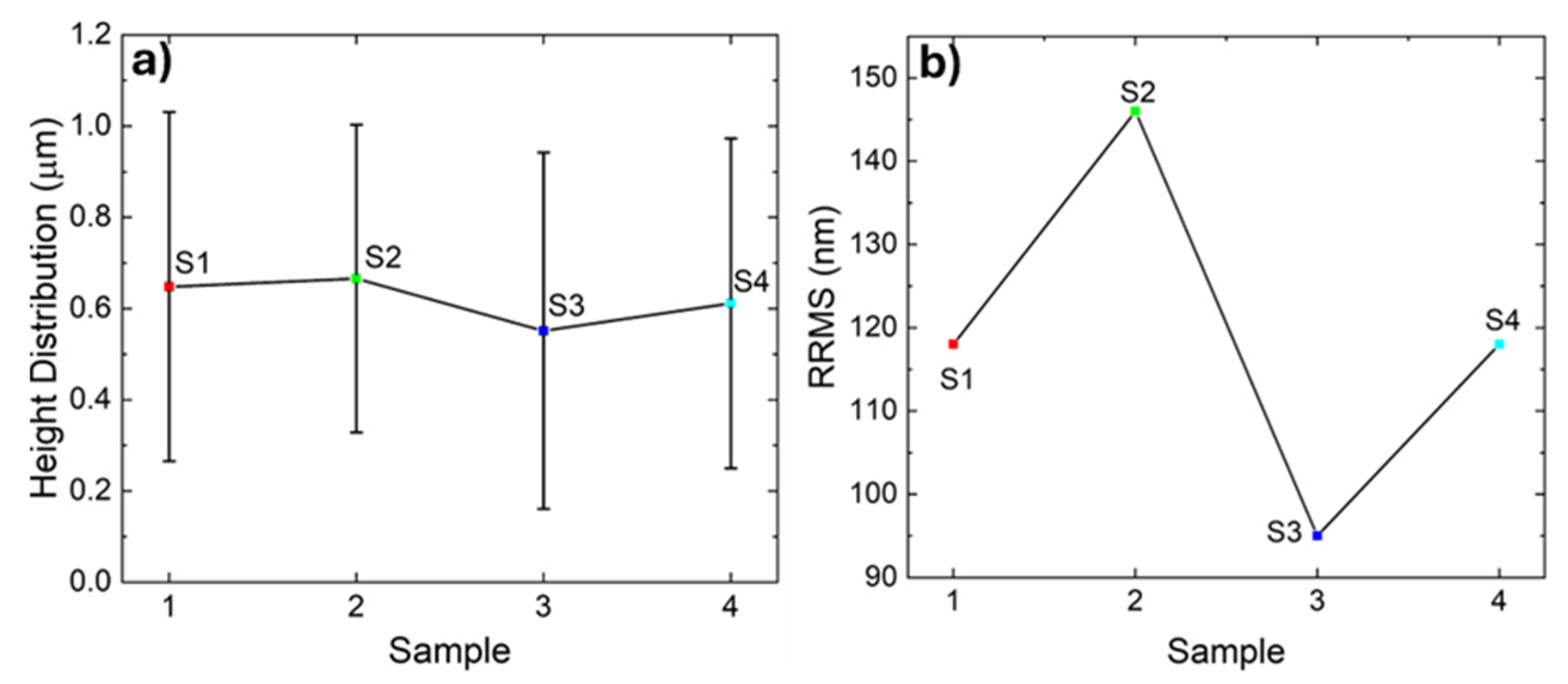
3.2. Film Morphology by Atomic Force Microscopy
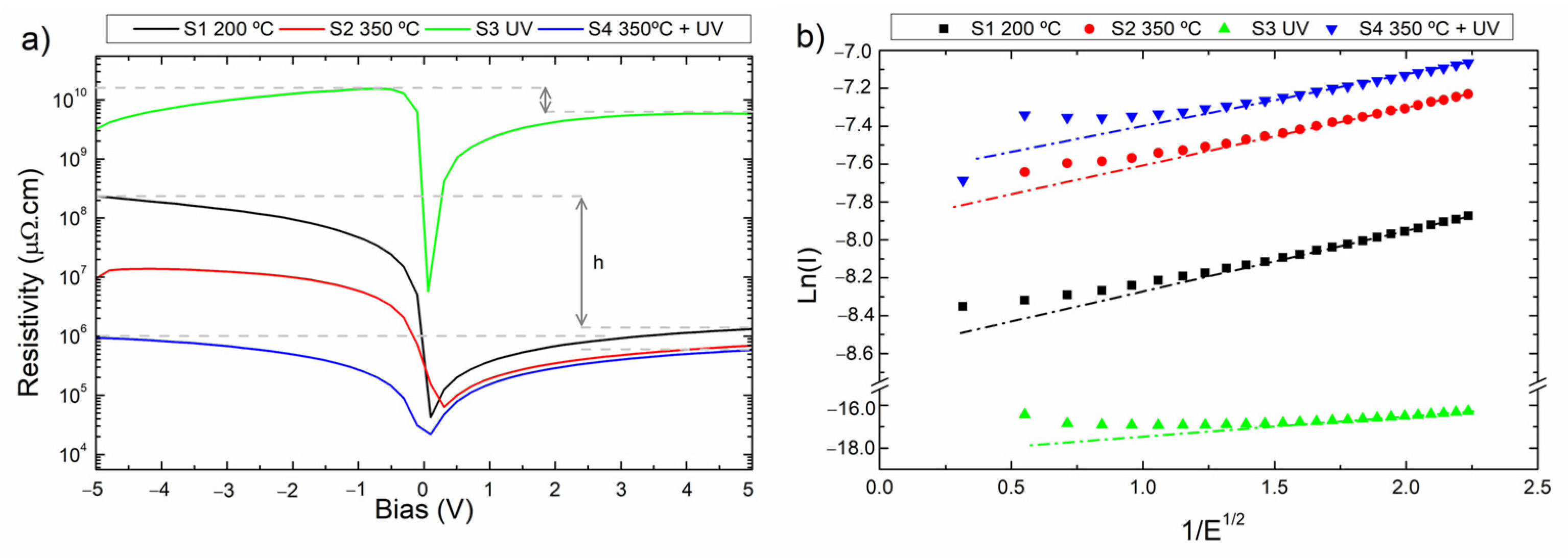
3.3. Electrical Characterization
3.4. Optoelectronic Properties
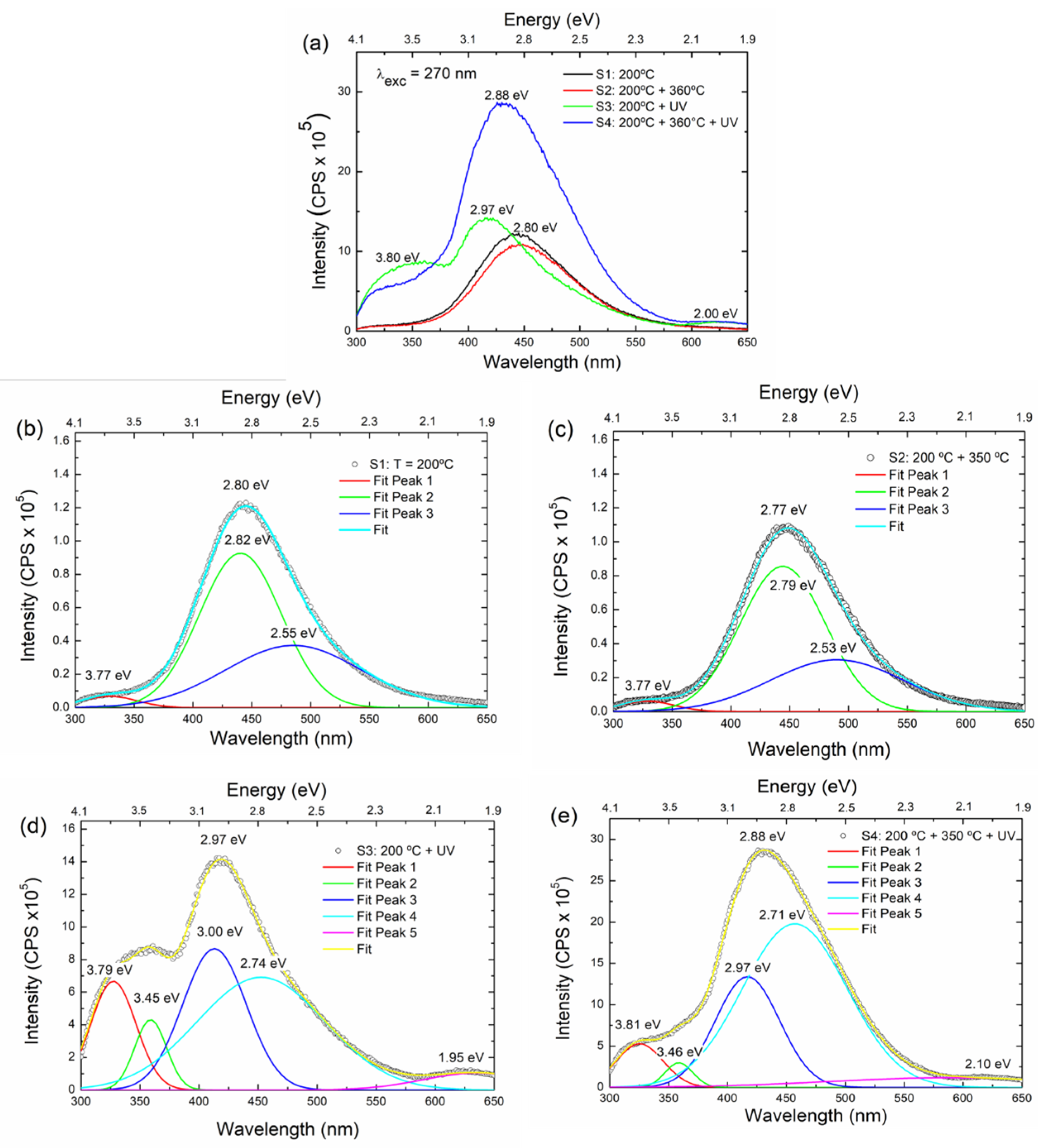
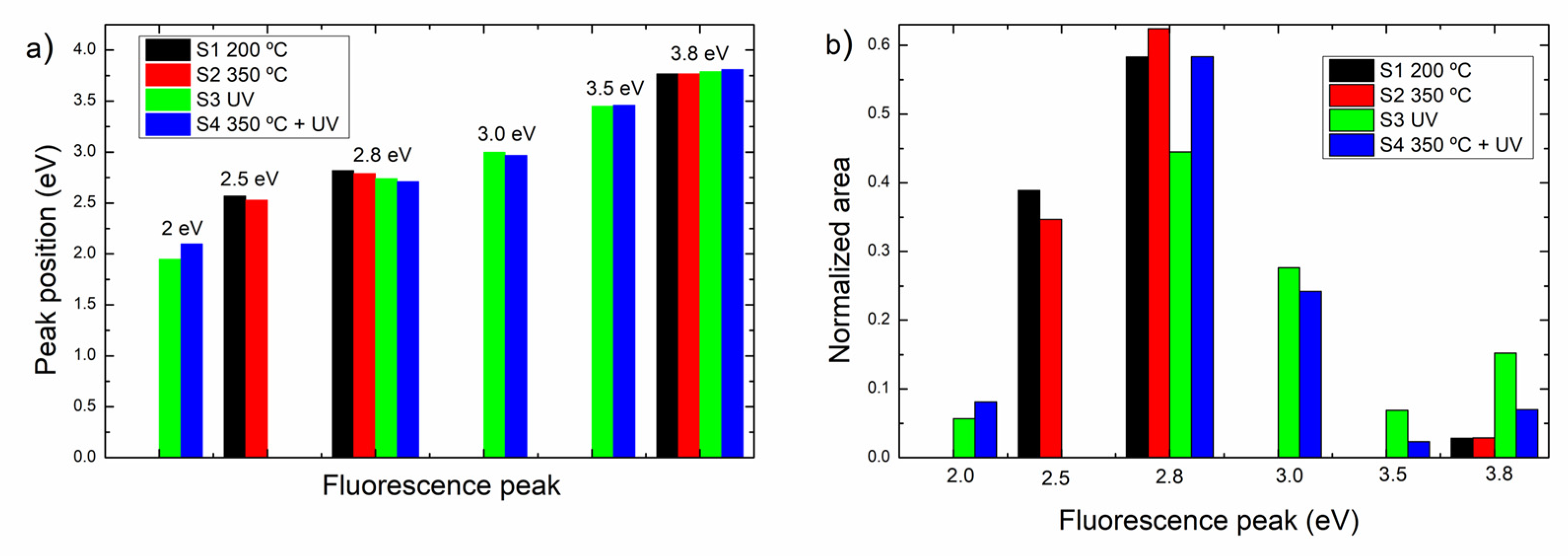
3.4.1. Optical Fluorescence
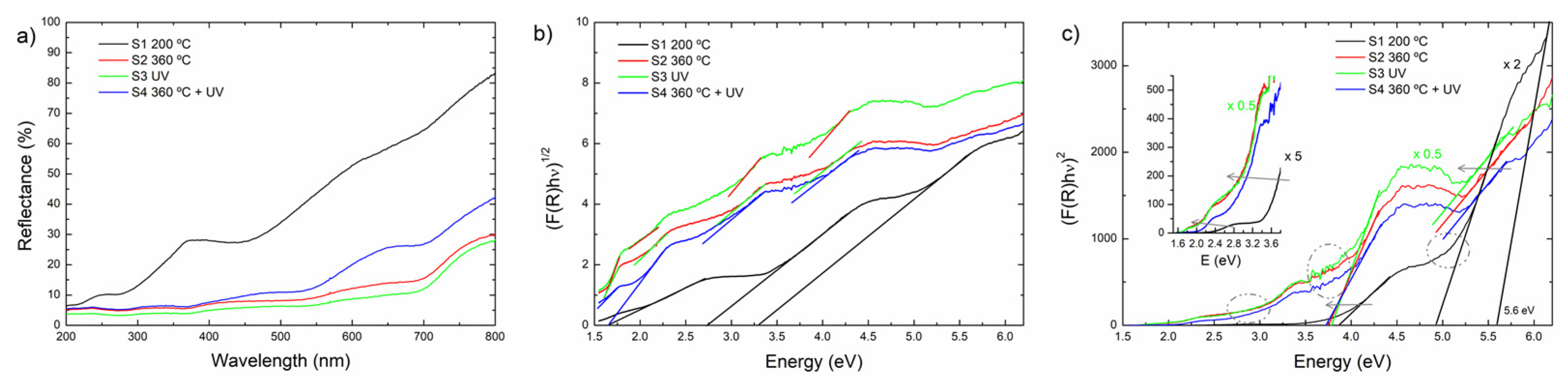
3.4.2. UV–Visible Spectroscopy
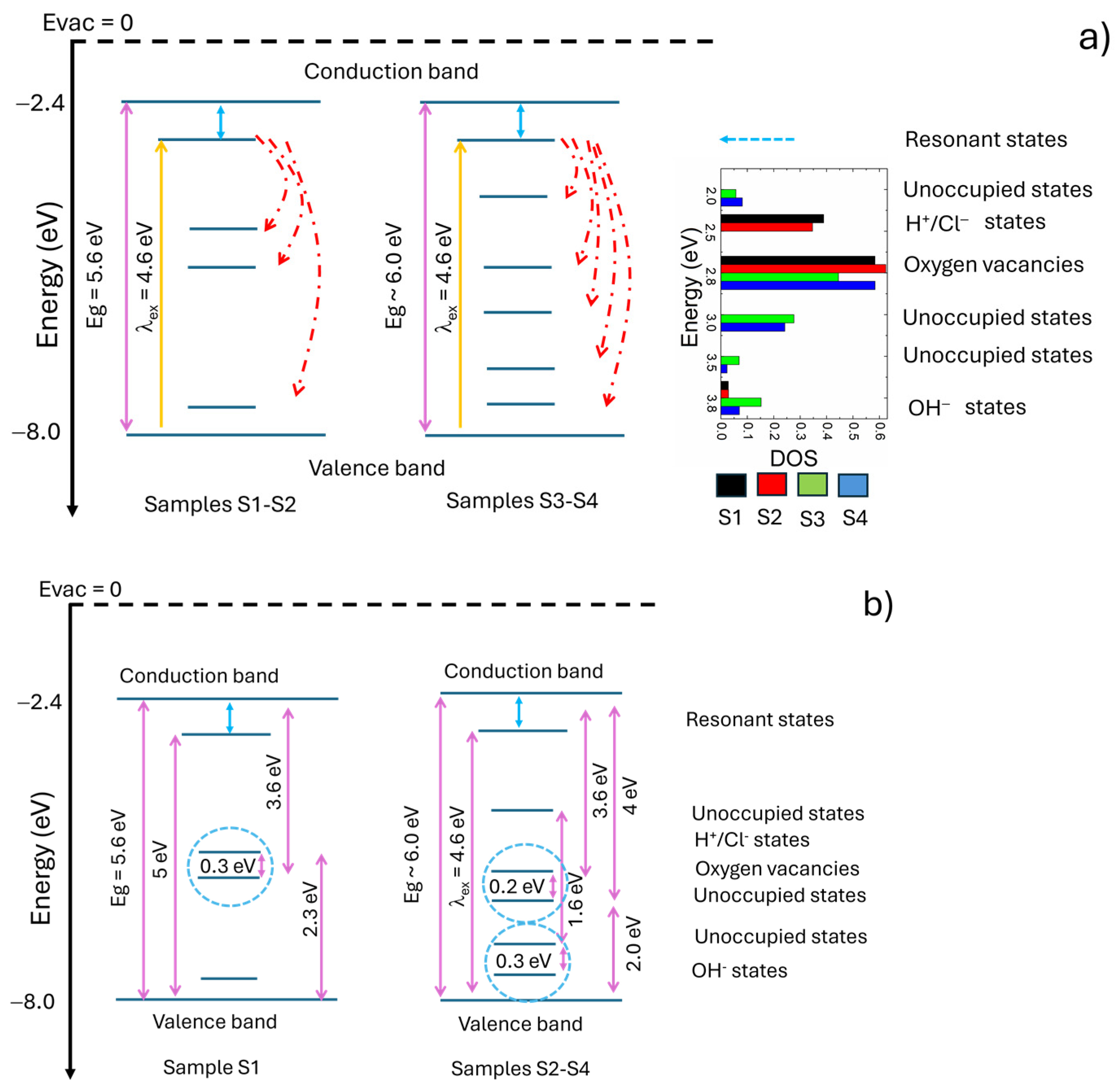
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, S.; Park, S.P.; Kim, Y.-G.; Kang, B.H.; Na, J.W. Low-temperature fabrication of an HfO2 passivation layer for amorphous indium–gallium–zinc oxide thin film transistors using a solution process. Sci. Rep. 2017, 7, 16265. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.B.; Park, J.H.; Lee, K.H.; Lee, H.W.; Song, K.M.; Leec, S.J.; Baik, H.K. Solution-processed high-k HfO2 gate dielectric processed under softening temperature of polymer substrates. J. Mater. Chem. C 2013, 1, 1651–1658. [Google Scholar] [CrossRef]
- Kim, W.; Bang, J.-H.; Uhm, H.-S.; Lee, S.-H.; Park, J.-S. Effects of post plasma treatment on material properties and device characteristics in indium zinc oxide thin film transistors. Thin Solid Film. 2010, 519, 1573–1577. [Google Scholar] [CrossRef]
- Vargas, M.; Murphy, N.R.; Ramana, C.V. Structure and optical properties of nanocrystalline hafnium oxide thin films. Opt. Mater. 2014, 37, 621–628. [Google Scholar] [CrossRef]
- Tan, T.; Liu, Z.; Lu, H.; Liu, W.; Tian, H. Structure and optical properties of HfO2 thin films on silicon after rapid thermal annealing. Opt. Mater. 2010, 32, 432–435. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P. Photoluminescent cubic and monoclinic HfO2 nanoparticles: Effects of temperature and ambient. Mater. Res. Express 2014, 1, 015035. [Google Scholar] [CrossRef]
- Kumar, M.; Jeong, H.; Lee, D. Effect of UV/ozone plasma treatment on sol–gel-derived HfO2 thin films. Ceram. Int. 2017, 43, 1174–1179. [Google Scholar] [CrossRef]
- González, L.A.; Gálvez-Barboza, S.; Vento-Lujano, E.; Rodríguez-Galicia, J.L.; García-Cerda, L.A. Mn-modified HfO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2020, 46, 13466–13473. [Google Scholar] [CrossRef]
- Jayaraman, V.; Mahalingam, S.; Chinnathambi, S.; Pandian, G.N.; Prakasarao, A.; Ganesan, S.; Ramasamy, J.; Ayyaru, S.; Ahn, Y.-H. Facile Synthesis of Hafnium Oxide Nanoparticle Decorated on Graphene Nanosheet and Its Photocatalytic Degradation of Organic Pollutants under UV-Light Irradiation. Appl. Sci. 2022, 12, 11222. [Google Scholar] [CrossRef]
- Jeffery, A.A.; Chougule, S.S.; Hasan, I.; Alagarasan, J.K.; Sankar, P.R.; Somu, P.; Lin, M.-C.; Kumarasamy, K.; Ahn, Y.-H.; Murugasamy, J. Oxygen-Vacancy-Rich HfO2–x Nanoparticles Supported on Reduced Graphene Oxide for Electrocatalytic Hydrogen Evolution Reactions. ACS Appl. Nano Mater. 2023, 6, 23053–23063. [Google Scholar] [CrossRef]
- Condurache-Bota, S.; Tigau, N.; Drasovean, R. Explicit application of Swanepoel’s method for the analysis of Sb2O3 films. J. Sci. Arts 2010, 2, 335–340. [Google Scholar]
- Araiza, J.d.J.; Álvarez-Fraga, L.; Gago, R.; Sánchez, O. Surface morphology and optical properties of hafnium oxide thin films produced by magnetron sputtering. Materials 2023, 16, 5331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, G.; Liu, A.; Shin, B.; Shan, F. Solution-processed hafnium oxide dielectric thin films for thin-film transistors applications. Ceram. Int. 2015, 41, 13218–13223. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Pham, D.P.; Yeo, M.S.; Rhee, H.; Kim, Y.-S.; Cho, E.-C.; Yi, J. Combination of ultraviolet exposure and thermal post-treatment to obtain high quality HfO2 thin films. Ceram. Int. 2021, 47, 9643–9650. [Google Scholar] [CrossRef]
- Jancovic, P.; Hudec, B.; Dobrocka, E.; Dérer, J.; Fedor, J.; Fröhlich, K. Resistive switching in HfO2-based atomic layer deposition grown metal–insulator–metal structures. Appl. Surf. Sci. 2014, 312, 112–116. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Deng, N.; Shih, C.-C.; Tseng, Y.-T.; Chang, T.-C.; Chang, K.-C.; Wang, M.-H.; Chen, W.-C.; Zheng, H.-X.; Wu, H.; et al. Conduction Mechanism and Improved Endurance in HfO2-Based RRAM with Nitridation Treatment. Nanoscale Res. Lett. 2017, 12, 574. [Google Scholar] [CrossRef]
- Mercado, C.; Seeley, Z.; Bandyopadhyay, A.; Bose, S.; McHale, J.L. Photoluminescence of Dense Nanocrystalline Titanium Dioxide Thin Films: Effect of Doping and Thickness and Relation to Gas Sensing. ACS Appl. Mater. Interfaces 2011, 3, 2281–2288. [Google Scholar] [CrossRef]
- Kong, M.; Li, B.; Guo, C.; Zeng, P.; Wei, M.; He, W. The Optical Absorption and Photoluminescence Characteristics of Evaporated and IAD HfO2 Thin Films. Coatings 2019, 9, 307. [Google Scholar] [CrossRef]
- Aarik, J.; Mandar, H.; Kirm, M.; Pung, L. Optical characterization of HfO2 thin films grown by atomic layer deposition. Thin Solid Film. 2004, 466, 41–47. [Google Scholar] [CrossRef]
- Papernov, S.; Brunsman, M.D.; Oliver, J.B.; Hoffman, B.N.; Kozlov, A.A.; Demos, S.G.; Shvydky, A.; Cavalcante, F.H.M.; Yang, L.; Demoni, C.S.; et al. Optical properties of oxygen vacancies in HfO2 thin films studied by absorption and luminescence spectroscopy. Opt. Express 2018, 26, 17608–17623. [Google Scholar] [CrossRef]
- Ibrahim, A.; Al-Ani, S.K.J. Models of optical absorption in amorphous semiconductors at the absorption edge -a revie w and re-evaluation. Czechoslov. J. Phys. 1994, 44, 785–797. [Google Scholar] [CrossRef]
- Shilov, A.O.; Savchenko, S.S.; Vokhmintsev, A.S.; Gritsenko, V.A.; Weinstein, I.A. Thermal quenching of self-trapped exciton luminescence in nanostructured hafnia. J. Lumin. 2022, 247, 118908. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Davydov, A.V.; Chandler-Horowitz, D.; Frank, M.M. Sub-bandgap defect states in polycrystalline hafnium oxide and their suppression by admixture of silicon. Appl. Phys. Lett. 2005, 87, 192903. [Google Scholar] [CrossRef]
- Cisneros-Morales, M.C.; Aita, C.R. The effect of nanocrystallite size in monoclinic HfO2 films on lattice expansion and near-edge optical absorption. Appl. Phys. Lett. 2010, 96, 191904. [Google Scholar] [CrossRef]
- Perevalov, T.V.; Aliev, V.S.; Gritsenko, V.A.; Saraev, A.A.; Kaichev, V.V.; Ivanova, E.V.; Zamoryanskaya, M.V. The origin of 2.7 eV luminescence and 5.2 eV excitation band in hafnium oxide. Appl. Phys. Lett. 2014, 104, 071904. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Su, H. The transport properties of oxygen vacancy-related polaron-like bound state in HfOx. Sci. Rep. 2013, 3, 3246. [Google Scholar] [CrossRef]
- Wu, J. Impact of oxygen vacancies on monoclinic hafnium oxide and band alignment with semiconductors. Mater. Today Commun. 2020, 25, 101482. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Rodríguez, S.; González-Moreno, F.I.; Woo-García, R.M.; Herrera-May, A.L.; López-Huerta, F.; Caballero-Briones, F. Defect Tailoring in HfO2/Si Films upon Post-Deposition Annealing and Ultraviolet Irradiation. Appl. Sci. 2025, 15, 1573. https://doi.org/10.3390/app15031573
Salas-Rodríguez S, González-Moreno FI, Woo-García RM, Herrera-May AL, López-Huerta F, Caballero-Briones F. Defect Tailoring in HfO2/Si Films upon Post-Deposition Annealing and Ultraviolet Irradiation. Applied Sciences. 2025; 15(3):1573. https://doi.org/10.3390/app15031573
Chicago/Turabian StyleSalas-Rodríguez, Silvestre, Fernanda I. González-Moreno, Rosa M. Woo-García, Agustín L. Herrera-May, Francisco López-Huerta, and Felipe Caballero-Briones. 2025. "Defect Tailoring in HfO2/Si Films upon Post-Deposition Annealing and Ultraviolet Irradiation" Applied Sciences 15, no. 3: 1573. https://doi.org/10.3390/app15031573
APA StyleSalas-Rodríguez, S., González-Moreno, F. I., Woo-García, R. M., Herrera-May, A. L., López-Huerta, F., & Caballero-Briones, F. (2025). Defect Tailoring in HfO2/Si Films upon Post-Deposition Annealing and Ultraviolet Irradiation. Applied Sciences, 15(3), 1573. https://doi.org/10.3390/app15031573







