Optimized Enzymatic Extraction of Phenolic Compounds from Verbascum nigrum L.: A Sustainable Approach for Enhanced Extraction of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
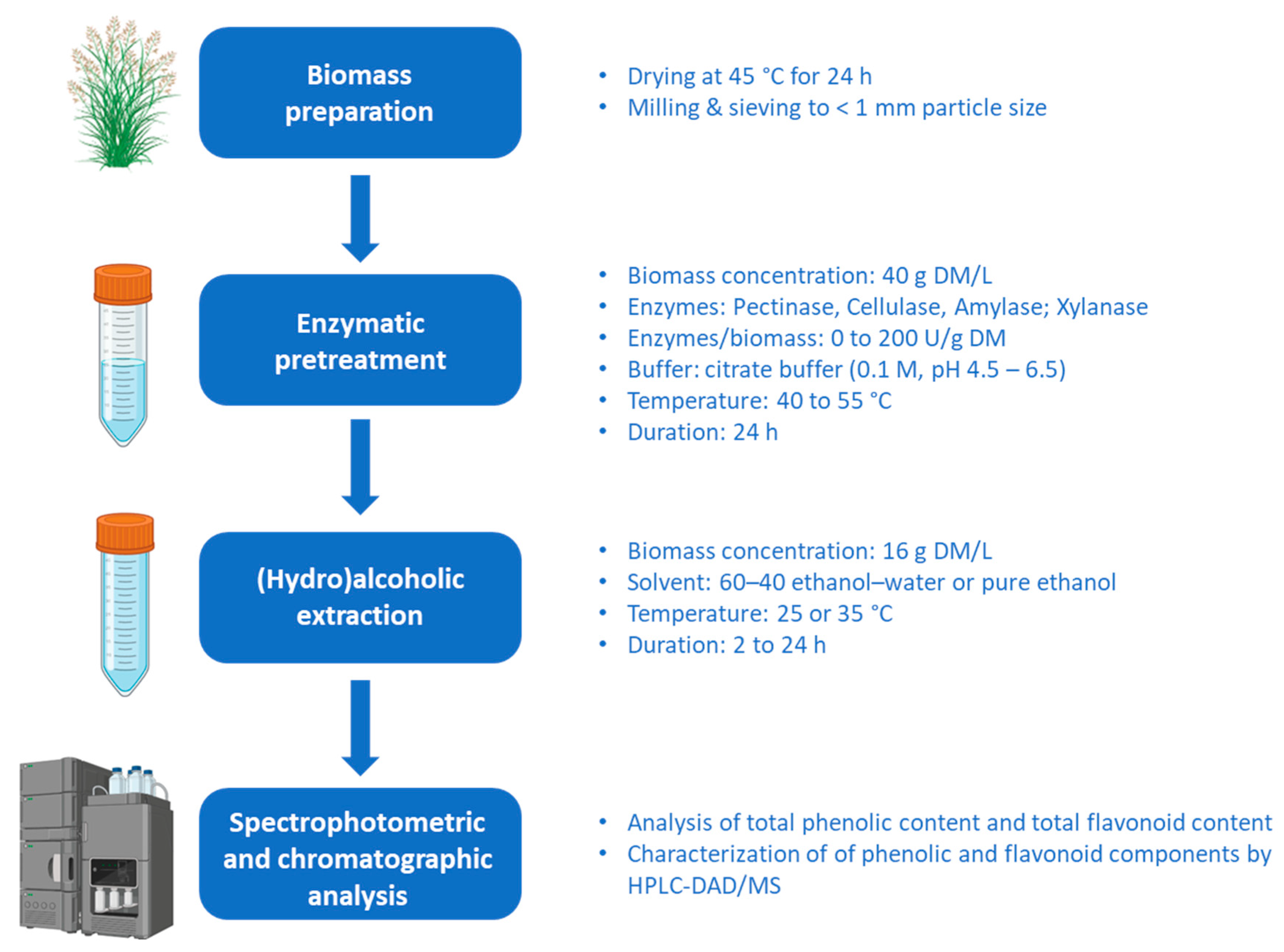
2.1. Plant Material Processing
2.2. Enzyme-Assisted Hydroalcoholic Extraction
2.2.1. Development of the Two-Step Extraction Method
- Pectinase (Cat No: DIS-1030; Lot No: ECD0021415; activity: 30,240 U/g)
- Cellulase (Cat No: DIS-1017; Lot No: ECD7011006; activity: 20,340 U/g)
- Mid-temperature refining α-amylase for beer (Cat No: BER-1513; Lot No: ECB3081602; Activity: 4230 U/g)
- Xylanase (Cat No: DIS-1032; Lot No: ECD2022310; activity: 20,280 U/g)
- Enzyme treatment: biomass concentration of 40 g DM/L. Temperature of 47.5 °C. Citrate buffer (0.1 M) at pH 5.5. Treatment time of 24 h. Enzyme/biomass mixture ratios of 15% and 30% were tested (corresponding to 100 and 200 U/g DM of biomass). Notably, high enzyme loads were studied compared to the 5–20 U/g of dry biomass previously reported [22,23,25] to ensure that enzyme concentration would not be limiting the outcome of the pretreatment (Figure 1).
- Extraction after enzyme treatment: biomass concentration of 16 g DM/L. Temperature of 25 or 35 °C. Solvent: ethanol/water (60:40 v/v) or pure ethanol. Extraction time of 2, 5, or 24 h.
2.2.2. Optimization of the Enzymatic Reaction Conditions
2.2.3. Optimization of the Enzymatic Cocktail Composition
2.2.4. Hydroalcoholic Extraction
2.3. Determining the Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Chromatography
2.4.1. HPLC-DAD
2.4.2. HPLC-DAD/MS
2.4.3. UHPLC-DAD/MS/MS
2.5. Statistical Analysis
3. Results
3.1. Development of the Extraction Method
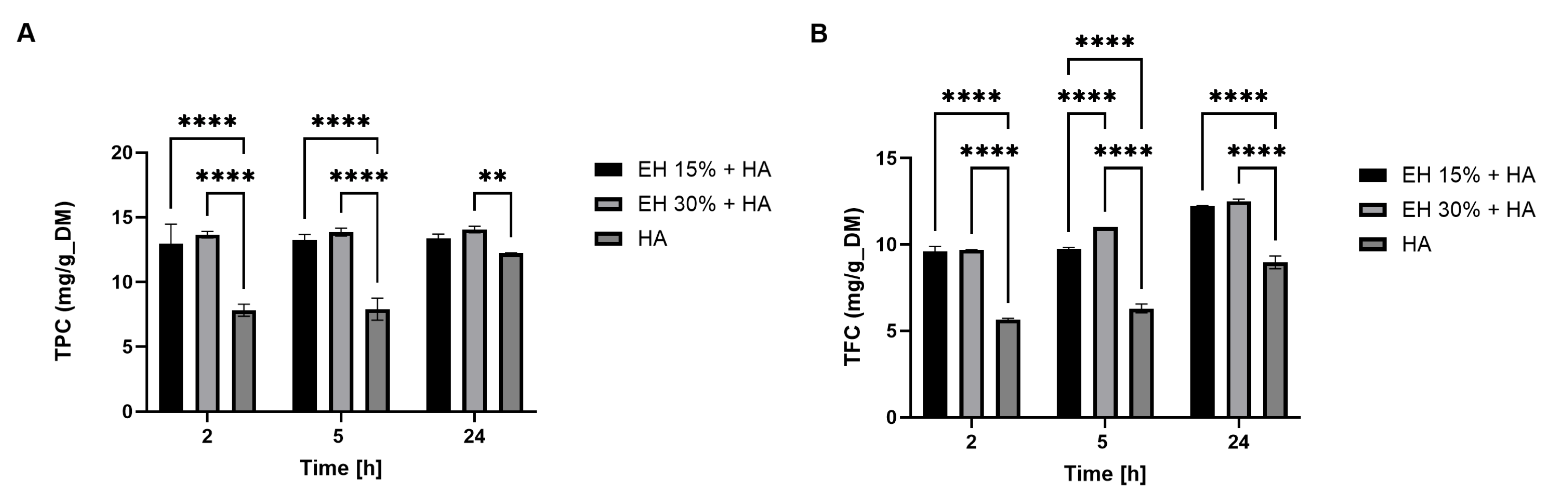
3.1.1. Preliminary Analyses of the Products Extracted
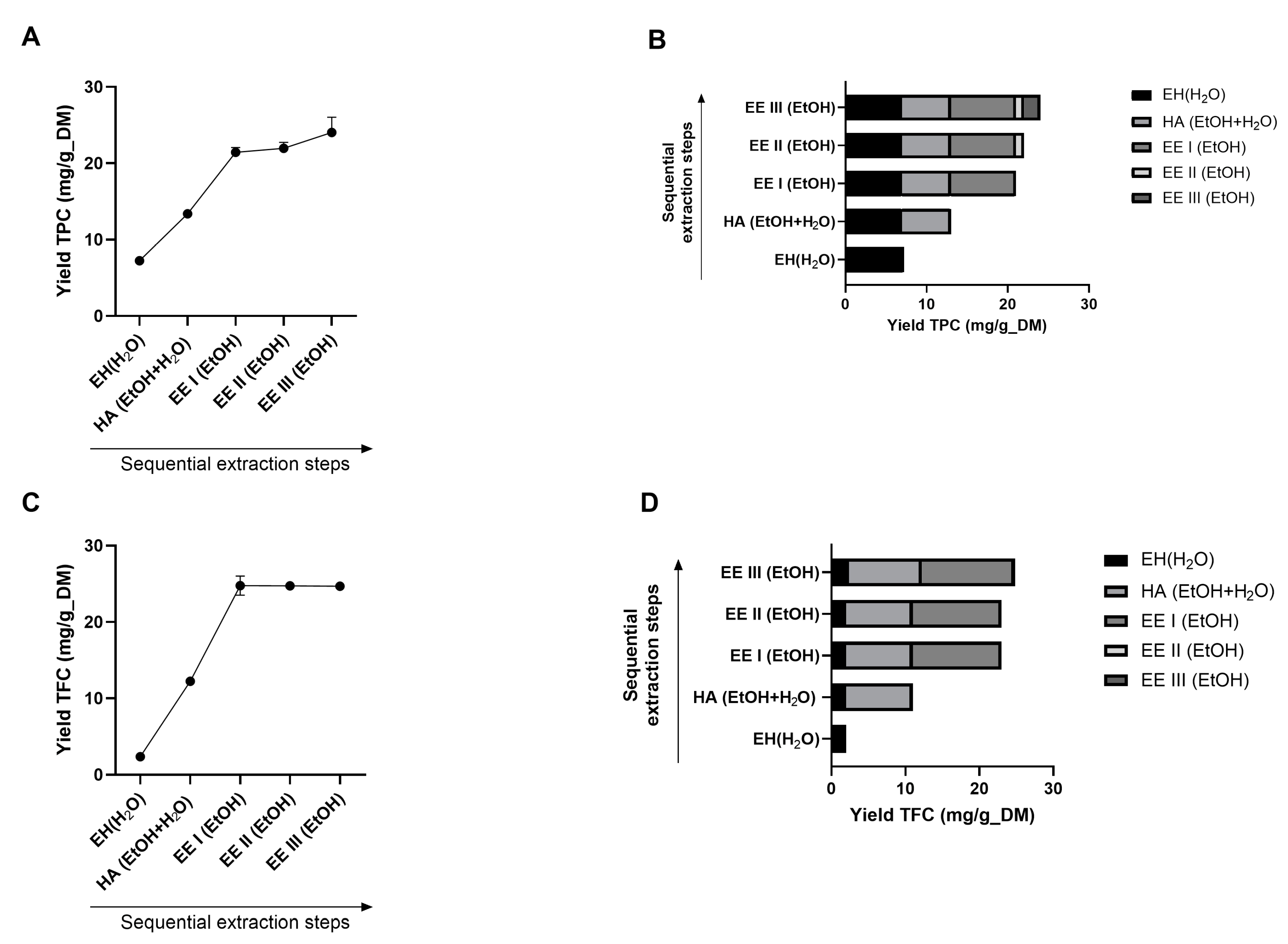
3.1.2. Evaluation of the Best Process Sequence
3.2. Optimization of the Enzymatic Treatment
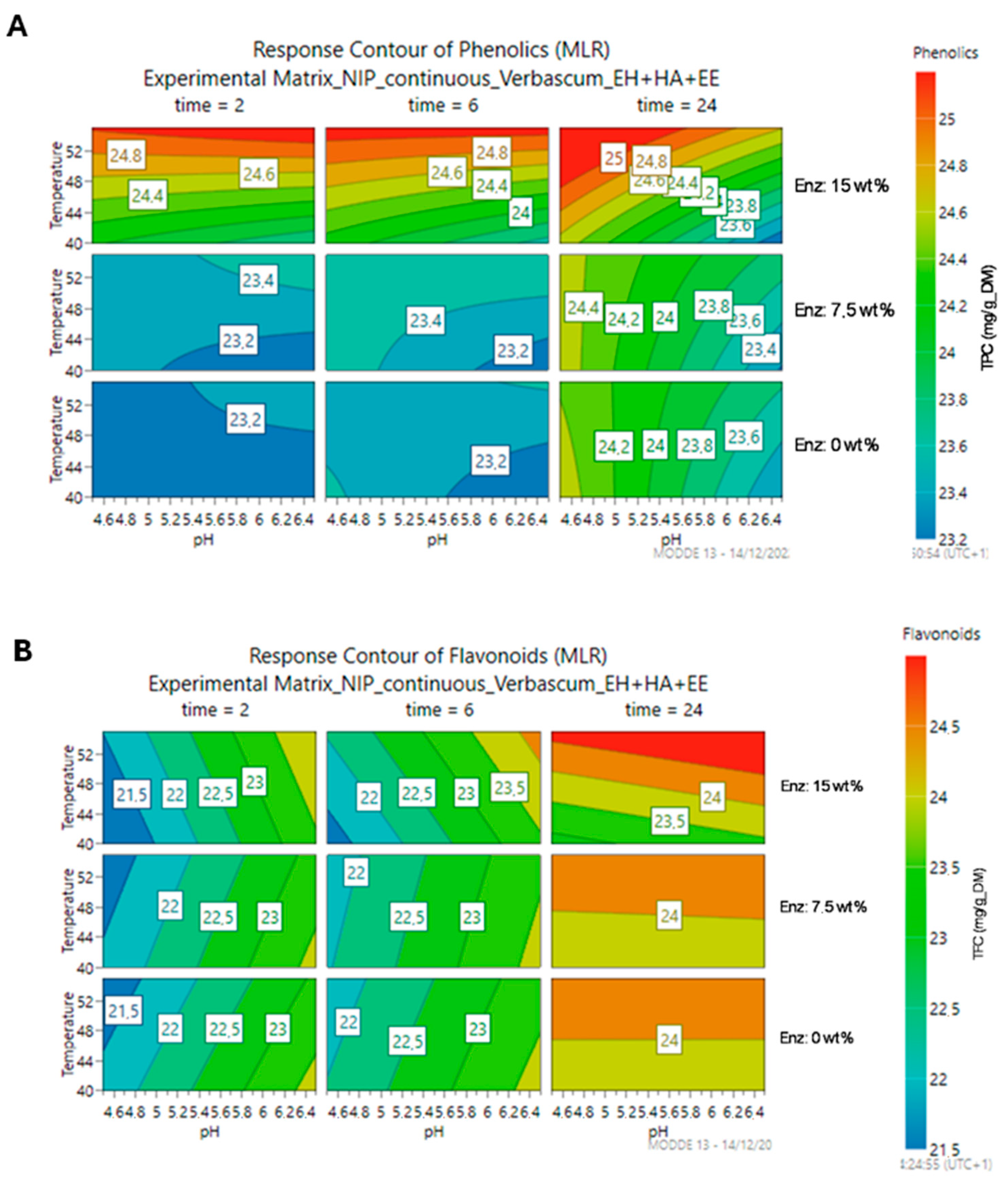
3.2.1. Optimization of the Enzymatic Reaction Conditions
3.2.2. Optimization of the Enzymatic Cocktail Composition
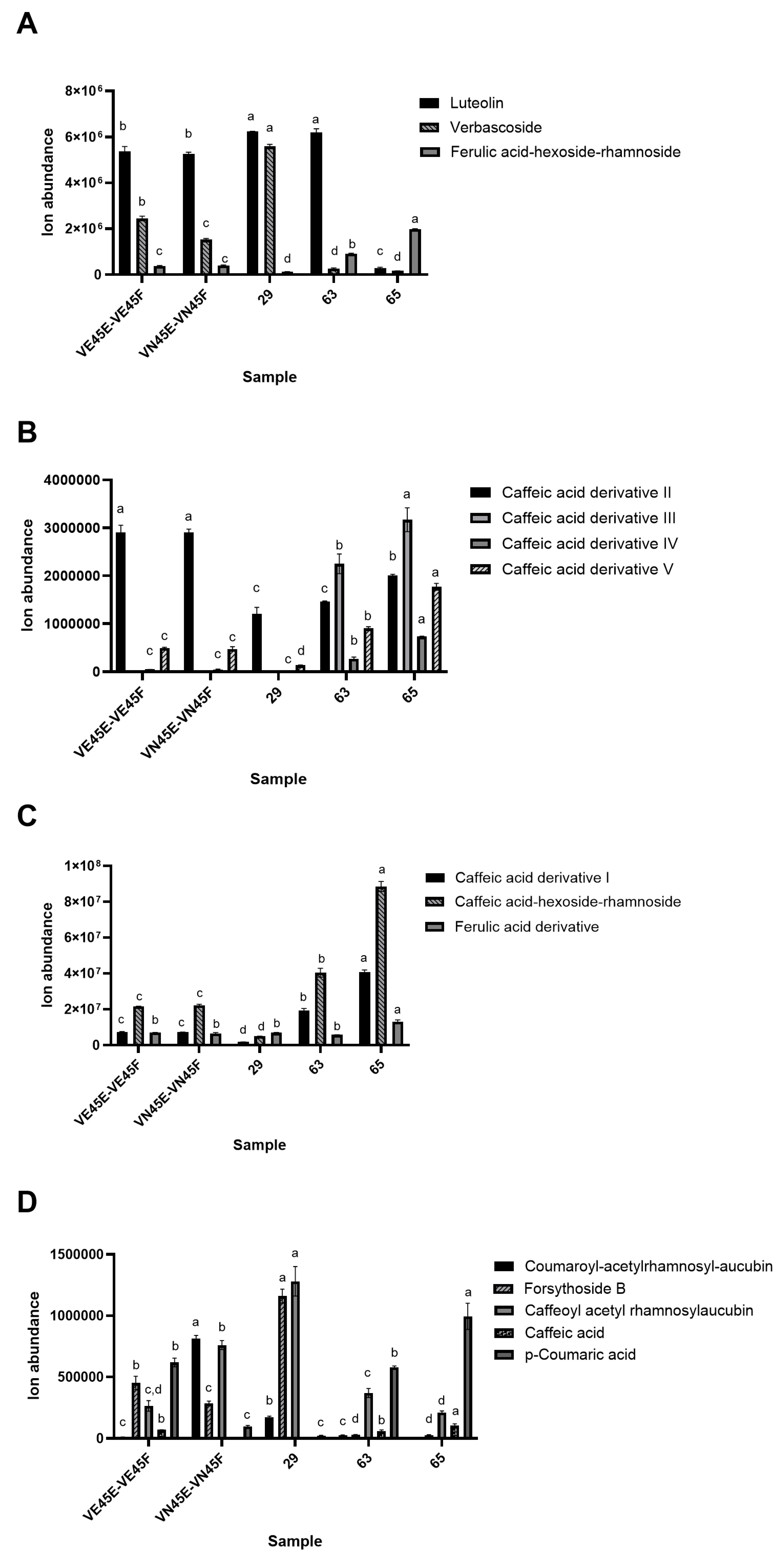
3.3. Characterization of the (Poly)Phenolic Compounds of V. nigrum Extracts
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Builders, P.F.; Builders, P.F. Introductory Chapter: Introduction to Herbal Medicine. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar]
- Moghadam, E.T.; Yazdanian, M.; Tahmasebi, E.; Tebyanian, H.; Ranjbar, R.; Yazdanian, A.; Seifalian, A.; Tafazoli, A. Current Herbal Medicine as an Alternative Treatment in Dentistry: In Vitro, in Vivo and Clinical Studies. Eur. J. Pharmacol. 2020, 889, 173665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z. Prevention and Treatment of Viral Respiratory Infections by Traditional Chinese Herbs. Chin. Med. J. 2014, 127, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, K.; Wu, T. Chinese Medicinal Herbs for Acute Bronchitis. Cochrane Database Syst. Rev. 2012, 2, CD004560. [Google Scholar] [CrossRef]
- Fernando, W.G.D. Plants: An International Scientific Open Access Journal to Publish All Facets of Plants, Their Functions and Interactions with the Environment and Other Living Organisms. Plants 2012, 1, 1–5. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural Plant Chemicals: Sources of Industrial and Medicinal Materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef]
- Ghosh, D. Quality Issues of Herbal Medicines: Internal and External Factors. Int. J. Complement. Altern. Med. 2018, 11, 67–69. [Google Scholar] [CrossRef]
- Kubra, R.I.; Kumar, D.; Rao, J.M.L. Emerging Trends in Microwave Processing of Spices and Herbs. Crit. Rev. Food Sci. Nutr. 2016, 56, 2160–2173. [Google Scholar] [CrossRef]
- Blanco-Salas, J.; Hortigón-Vinagre, M.P.; Morales-Jadán, D.; Ruiz-Téllez, T. Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology 2021, 10, 618. [Google Scholar] [CrossRef]
- Süntar, I.; Tatli, I.I.; Küpeli Akkol, E.; Keleş, H.; Kahraman, Ç.; Akdemir, Z. An Ethnopharmacological Study on Verbascum Species: From Conventional Wound Healing Use to Scientific Verification. J. Ethnopharmacol. 2010, 132, 408–413. [Google Scholar] [CrossRef]
- Donn, P.; Barciela, P.; Perez-Vazquez, A.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Bioactive Compounds of Verbascum sinuatum L.: Health Benefits and Potential as New Ingredients for Industrial Applications. Biomolecules 2023, 13, 427. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Jaafar, H.Z.E. Common Mullein, Pharmacological and Chemical Aspects. Rev. Bras. Farmacogn. 2013, 23, 948–959. [Google Scholar] [CrossRef]
- El Gizawy, H.A.E.H.; Hussein, M.A.; Abdel-Sattar, E. Biological Activities, Isolated Compounds and HPLC Profile of Verbascum Nubicum. Pharm. Biol. 2019, 57, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, Z.S.; Tatli, I.I.; Bedir, E.; Khan, I.A. Antioxidant Flavonoids from Verbascum Salviifolium Boiss. Fabad J. Pharm. Sci. 2004, 28, 71–75. [Google Scholar]
- Symoniuk, E.; Marczak, Z.; Brzezińska, R.; Janowicz, M.; Ksibi, N. Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils. Foods 2023, 12, 2391. [Google Scholar] [CrossRef]
- Tatli, I.I.; Akdemir, Z.Ş. Traditional Uses and Biological Activities of Verbascum Species. Fabad J. Pharm. Sci. 2006, 31, 85–96. [Google Scholar]
- Armatu, A.; Bodirlau, R.; Nechita, C.B.; Niculaua, M.; Teaca, C.A.; Ichim, M.; Spiridon, I. Characterization of Biological Active Compounds from Verbascum Phlomoides by Chromatography Techniques. I. Gas Chromatography. Rom. Biotechnol. Lett. 2011, 16, 6297–6304. [Google Scholar]
- Shahbaz, F.; Akhter, N.; Shahid, M.; Riaz, M.; Anjum, F.; Hussain, F. Ultrasound Assisted Extraction and Characterization of Bioactives from Verbascum Thapsus Roots to Evaluate Their Antioxidant and Medicinal Potential. Dose-Response 2022, 20, 15593258221097665. [Google Scholar] [CrossRef]
- Mihajlovski, K.R.; Milić, M.D. The Role of Plant Cell Wall Degrading Enzymes in Biorefinery Development. In Lignocellulose Bioconversion Through White Biotechnology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; Volume 5, pp. 99–135. [Google Scholar]
- Chakraborty, D.; Chatterjee, S.; Althuri, A.; Palani, S.G.; Venkata Mohan, S. Sustainable Enzymatic Treatment of Organic Waste in a Framework of Circular Economy. Bioresour. Technol. 2023, 370, 128487. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Le-Tan, H.; Fauster, T.; Vladic, J.; Gerhardt, T.; Haas, K.; Jaeger, H. Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Appl. Sci. 2021, 11, 8238. [Google Scholar] [CrossRef]
- Morreeuw, Z.P.; Ríos-González, L.J.; Salinas-Salazar, C.; Melchor-Martínez, E.M.; Ascacio-Valdés, J.A.; Parra-Saldívar, R.; Iqbal, H.M.N.; Reyes, A.G. Early Optimization Stages of Agave lechuguilla Bagasse Processing toward Biorefinement: Drying Procedure and Enzymatic Hydrolysis for Flavonoid Extraction. Molecules 2021, 26, 7292. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Ayuso-Yuste, M.C.; Bernalte, M.J.; Gil, M.V.; Delgado-Adàmez, J. Cellulase-assisted extraction of phenolic compounds from rice bran (Oryza sativa L.): Process optimization and characterization. J. Food Meas. Charact. 2021, 15, 1719–1726. [Google Scholar] [CrossRef]
- Kurmudle, N.; Kagliwal, D.L.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Porgali, E.; Büyüktuncel, E. Determination of Phenolic Composition and Antioxidant Capacity of Native Red Wines by High Performance Liquid Chromatography and Spectrophotometric Methods. Food Res. Int. 2012, 45, 145–154. [Google Scholar] [CrossRef]
- Bardakci, H.; Celep, E.; Gözet, T.; Kurt-Celep, I.; Deniz, I.; Şen-Utsukarci, B.; Akaydin, G. A Comparative Investigation on Phenolic Composition, Antioxidant and Antimicrobial Potentials of Salvia heldreichiana Boiss. Ex Bentham Extracts. S. Afr. J. Bot. 2019, 125, 72–80. [Google Scholar] [CrossRef]
- Kimel, K.; Godlewska, S.; Krauze-Baranowska, M.; PobŁocka-Olech, L. Hplc-Dad-Esi/Ms Analysis of Arnica Tm Constituents. Acta Pol. Pharm. Drug Res. 2019, 76, 1015–1027. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From Tradition to Health: Chemical and Bioactive Characterization of Five Traditional Plants. Molecules 2022, 27, 6495. [Google Scholar] [CrossRef]
- Amini, S.; Hassani, A.; Alirezalu, A.; Maleki, R. Phenolic and flavonoid compounds and antioxidant activity in flowers of nine endemic Verbascum species from Iran. J. Sci. Food Agric. 2022, 102, 3250–3258. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef]
- Gini, T.G.; Jothi, G.J. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt. J. Basic. Appl. Sci. 2018, 5, 197–203. [Google Scholar]
- Iliescu, I.A.; Peter, S.; Albert, I.; Skalicka-Woźniak, K.; Miron, A.; Luca, S.V.; Wolfram, E. Verbascum nigrum: Cytotoxicity Evaluation in A431 Epidermoid Carcinoma Cells and Untargeted LC-HR-MS/MS Metabolite Profiling. Chem. Biodivers. 2020, 17, e2000644. [Google Scholar] [CrossRef]
- Luca, S.V.; Miron, A.; Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Gherghel, D.; Ciocarlan, N.; Skalicka-Woźniak, K. HPLC-DAD-ESI-Q-TOF-MS/MS Profiling of Verbascum Ovalifolium Donn Ex Sims and Evaluation of Its Antioxidant and Cytogenotoxic Activities. Phytochem. Anal. 2019, 30, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Kviesis, J.; Lazdina, D.; Valdovska, A.; Vallejo, F.; Lacis, G. Lignocellulose-Degrading Enzymes: A Biotechnology Platform for Ferulic Acid Production from Agro-Industrial Side Streams. Foods 2021, 10, 3056. [Google Scholar] [CrossRef]
- Angeloni, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Maggi, F.; Caprioli, G.; Kaplan, A.; Bahşi, M.; Çakılcıoğlu, U.; Bouyahya, A.; et al. An insight into Verbascum bombyciferum extracts: Different extraction methodologies, biological abilities and chemical profiles. Ind. Crops Prod. 2021, 161, 113201. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Sadeer, N.; Maggi, F.; Caprioli, G.; Angeloni, S.; Mollica, A.; Stefanucci, A.; Ak, G.; et al. Evaluation of chemical constituents and biological properties of two endemic Verbascum species. Process Biochem. 2021, 108, 110–120. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of Agricultural Lignocellulosic Plant Byproducts through Enzymatic and Enzyme-Assisted Extraction of High-Value-Added Compounds: A Review. ACS Sustain. Chem. Eng. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Guo, Y.; Gong, P.; Pei, S.; Wang, D.; Wang, P.; Wang, M.; Chen, F. Advances in Chemical Composition, Extraction Techniques, Analytical Methods, and Biological Activity of Astragali Radix. Molecules 2022, 27, 1058. [Google Scholar] [CrossRef] [PubMed]
- Machado, X.T.d.O.; Portugal, I.B.M.; Padilha, C.V.d.S.; Ferreira Padilha, F.; dos Santos Lima, M. New Trends in the Use of Enzymes for the Recovery of Polyphenols in Grape Byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar]
- Dursun, İ.; Felek, İ.; Çobanoğlu, D.N. Analyzing the Antioxidant Activity and Fatty Acid Composition of Monofloral Mullein (Verbascum sp.) Pollen Oil Obtained via Various Extraction Techniques. Chem. Biodivers. 2024, 21, e202400117. [Google Scholar] [CrossRef]
- Legesse, A.B.; Emire, S.A.; Tadesse, M.G.; Dadi, D.W.; Kassa, S.K.; Oyinloye, T.M.; Yoon, W.B. Optimization of Ultrasound-Assisted Extraction of Verbascum Sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity. Foods 2024, 13, 1255. [Google Scholar] [CrossRef]
- Shakour, Z.T.A.; Fayek, N.M.; Farag, M.A. How Do Biocatalysis and Biotransformation Affect Citrus Dietary Flavonoids Chemistry and Bioactivity? A Review. Crit. Rev. Biotechnol. 2020, 40, 689–714. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors Affecting Intake, Metabolism and Health Benefits of Phenolic Acids: Do We Understand Individual Variability? Eur. J. Nutr. 2019, 59, 1275–1293. [Google Scholar] [CrossRef]
- Balasubramaniam, V.G.; Ayyappan, P.; Sathvika, S.; Antony, U. Effect of Enzyme Pretreatment in the Ultrasound Assisted Extraction of Finger Millet Polyphenols. J. Food Sci. Technol. 2019, 56, 1583–1594. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and Ultrasound-Assisted Extractions of Polyphenols from Citrus sinensis (Cv. Malta) Peel: A Comparative Study. J. Food Process Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
| Exp No | pH | Temperature (°C) | Time (h) | Enz. (wt%) |
|---|---|---|---|---|
| 1 | 6.5 | 40 | 2 | 0 |
| 2 | 5.5 | 55 | 2 | 0 |
| 3 | 4.5 | 40 | 24 | 0 |
| 4 | 6.5 | 55 | 24 | 0 |
| 5 | 4.5 | 55 | 2 | 15 |
| 6 | 6.5 | 55 | 2 | 15 |
| 7 | 5.5 | 40 | 2 | 15 |
| 8 | 4.5 | 40 | 24 | 15 |
| 9 | 6.5 | 40 | 24 | 15 |
| 10 | 5.5 | 55 | 24 | 15 |
| 11 | 4.5 | 40 | 2 | 7.5 |
| 12 | 6.5 | 40 | 24 | 7.5 |
| 13 | 4.5 | 55 | 24 | 7.5 |
| 14 | 6.5 | 47.5 | 5 | 7.5 |
| 15 | 5.5 | 47.5 | 5 | 7.5 |
| 16 | 6.5 | 40 | 2 | 0 |
| 17 | 5.5 | 55 | 2 | 0 |
| 18 | 4.5 | 40 | 24 | 0 |
| 19 | 6.5 | 55 | 24 | 0 |
| 20 | 4.5 | 55 | 2 | 15 |
| 21 | 6.5 | 55 | 2 | 15 |
| 22 | 5.5 | 40 | 2 | 15 |
| 23 | 4.5 | 40 | 24 | 15 |
| 24 | 6.5 | 40 | 24 | 15 |
| 25 | 5.5 | 55 | 24 | 15 |
| 26 | 4.5 | 40 | 2 | 7.5 |
| 27 | 6.5 | 40 | 24 | 7.5 |
| 28 | 4.5 | 55 | 24 | 7.5 |
| 29 | 6.5 | 47.5 | 5 | 7.5 |
| 30 | 5.5 | 47.5 | 5 | 7.5 |
| Sample Code | Biomass | Pretreatment |
|---|---|---|
| VE45E | Verbascum nigrum | EH + HA |
| VE45F | Verbascum nigrum | EH + HA |
| 63 | Verbascum nigrum | EH + HA |
| 65 | Verbascum nigrum | EH |
| VN45E | Verbascum nigrum | H + HA |
| VN45F | Verbascum nigrum | H + HA |
| 29 | Verbascum nigrum | HA |
| Compound | R.T. | λmax (nm) | ESI Mode | [M-H]−/[M]+ (m/z) | MS2 Fragment Ions (m/z) |
|---|---|---|---|---|---|
| p-Coumaric acid * | 4.83 | 309 | - | 163 | 119 |
| Caffeic acid * | 4.28 | 323 | - | 179 | 135, 107 |
| Unknown I | 0.68 | 220 | - | 278 | 113, 193, 158, 160 |
| Luteolin * | 5.90 | 345 | - | 285 | 133, 151, 175, 199, 217, 213 |
| Caffeic acid-hexoside-rhamnoside | 3.53 | 328 | - | 487 | 179, 135, 161 |
| Ferulic acid-hexoside-rhamnoside | 4.20 | 303 | - | 501 | 193, 134, 149 |
| Unknown III | 2.09 | 220 | - | 555 | 129, 157, 139, 365 |
| Caffeic acid derivative I | 3.50 | 330 | - | 619 | 179, 135 |
| Caffeic acid derivative II | 4.97 | 324 | - | 621 | 161, 179, 135 |
| Caffeic acid derivative III | 5.07 | 324 | - | 621 | 161, 179, 135 |
| Verbascoside * | 4.86 | 315 | - | 623 | 161, 461, 135, 179, 133 |
| Caffeic acid derivative IV | 3.15 | 328 | - | 635 | 473, 291, 309, 141, 179, 135 |
| Caffeic acid derivative V | 4.56 | 328 | - | 667 | 179, 343, 161, 135 |
| Unknown IV | 6.73 | 241, 361 | - | 673 | 249, 209, 307, 231, 221, 163 |
| Coumaroyl-acetylrhamnosyl-aucubin | 5.34 | 266 | - | 679 | 187, 145, 163, 205, 309, 119, 499, 161 |
| Caffeoyl-acetylrhamnosyl-aucubin | 5.08 | 333 | - | 695 | 161, 203, 135, 179, 325, 653, 515 |
| Forsythoside B | 4.61 | 329 | - | 755 | 593, 161, 133, 179, 135, 447, 125 |
| Ferulic acid derivative | 5.21 | 323 | - | 783 | 175, 193, 149, 125, 160, 134 |
| Unknown V | 2.41 | 220 | - | 929 | 455, 353, 689, 809, 293, 851, 767, 191, 113 |
| Unknown VI | 2.86 | 340 | - | 987 | 467, 365, 701, 323, 779, 353, 305, 191, 941 |
| Unknown VII | 3.81 | 252 | + | 557 | 131, 203, 395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brienza, F.; Calani, L.; Bresciani, L.; Mena, P.; Rapacioli, S. Optimized Enzymatic Extraction of Phenolic Compounds from Verbascum nigrum L.: A Sustainable Approach for Enhanced Extraction of Bioactive Compounds. Appl. Sci. 2025, 15, 1405. https://doi.org/10.3390/app15031405
Brienza F, Calani L, Bresciani L, Mena P, Rapacioli S. Optimized Enzymatic Extraction of Phenolic Compounds from Verbascum nigrum L.: A Sustainable Approach for Enhanced Extraction of Bioactive Compounds. Applied Sciences. 2025; 15(3):1405. https://doi.org/10.3390/app15031405
Chicago/Turabian StyleBrienza, Filippo, Luca Calani, Letizia Bresciani, Pedro Mena, and Silvia Rapacioli. 2025. "Optimized Enzymatic Extraction of Phenolic Compounds from Verbascum nigrum L.: A Sustainable Approach for Enhanced Extraction of Bioactive Compounds" Applied Sciences 15, no. 3: 1405. https://doi.org/10.3390/app15031405
APA StyleBrienza, F., Calani, L., Bresciani, L., Mena, P., & Rapacioli, S. (2025). Optimized Enzymatic Extraction of Phenolic Compounds from Verbascum nigrum L.: A Sustainable Approach for Enhanced Extraction of Bioactive Compounds. Applied Sciences, 15(3), 1405. https://doi.org/10.3390/app15031405








