Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spray Drying
2.3. Characterization of the Encapsulated SBO
2.3.1. Fatty Acid Composition
2.3.2. α-Tocopherol Content
2.3.3. Carotenoid Composition
2.3.4. Determination of Color Parameters
2.3.5. Bioaccessibility
2.3.6. Experimental Design and Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aaby, K.; Martinsen, B.K.; Borge, G.I.A.; Roen, D. Bioactive compounds and color of sea buckthorn (Hippophae rhamnoides L.) purees as affected by heat treatment and high-pressure homogenization. Int. J. Food Prop. 2020, 23, 651–664. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focsan, M.; Rugina, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Corrêa, L.C.; Lourenço, M.M.; Moldao-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 8914852. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef]
- Lee, S.J.; Wong, M. Nano- and Microencapsulation of Phytochemicals. Nano-Microencapsul. Foods 2014, 119–165. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. Wonderful) residues. Int. J. Food Sci. Tech. 2017, 52, 1452–1462. [Google Scholar] [CrossRef]
- Abd Hashib, S.; Abd Rahman, N.; Suzihaque, M.U.H.; Ibrahim, U.K.; Hanif, N.E. Effect of Slurry Concentration and Inlet Temperature towards Glass Temperature of Spray Dried Pineapple Powder. Procedia-Soc. Behav. Sci. 2015, 195, 2660–2667. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Schwarz, K. Microencapsulation of fish oil with -octenylsuccinate-derivatised starch:: Flow properties and oxidative stability. Eur. J. Lipid Sci. Tech. 2006, 108, 501–512. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.H.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils-A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Botrel, D.A.; Fernandes, R.V.D.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Calvo, P.; Hernández, T.; Lozano, M.; González-Gómez, D. Microencapsulation of extra-virgin olive oil by spray-drying: Influence of wall material and olive quality. Eur. J. Lipid Sci. Tech. 2010, 112, 852–858. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Atefi, M.; Nayebzadeh, K.; Mohammadi, A.; Mortazavian, A.M. Using ß-cyclodextrin and Arabic Gum as Wall Materials for Encapsulation of Saffron Essential Oil. Iran. J. Pharm. Res. 2017, 16, 93–102. [Google Scholar]
- Huang, Y.; Quan, P.; Wang, Y.W.; Zhang, D.S.; Zhang, M.W.; Li, R.; Jiang, N. Host-guest interaction of β-cyclodextrin with isomeric ursolic acid and oleanolic acid: Physicochemical characterization and molecular modeling study. J. Biomed. Res. 2017, 31, 395–407. [Google Scholar] [CrossRef]
- Culina, P.; Zoric, Z.; Garofulic, I.E.; Repajic, M.; Dragovic-Uzelac, V.; Pedisic, S. Optimization of the Spray-Drying Encapsulation of Sea Buckthorn Berry Oil. Foods 2023, 12, 2448. [Google Scholar] [CrossRef]
- ISO 12966-22017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 1 October 2022).
- ISO 12966-42014; Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Chromatography. ISO: Geneva, Switzerland, 2014.
- Balbino, S.; Repajic, M.; Obranovic, M.; Medved, A.M.; Tonkovic, P.; Dragovic-Uzelac, V. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aroma 2021, 25, 100326. [Google Scholar] [CrossRef]
- ISO 9936-1:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by Highperformance Liquid Chromatography. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 20 October 2022).
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Alvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Hostetler, G.L. Determination of Lutein, β-Carotene, and Lycopene in Infant Formula and Adult Nutritionals by Ultra-High Performance Liquid Chromatography: Collaborative Study, Final Action 2016.13 for β-Carotene and Lycopene Only. J. AOAC Int. 2020, 103, 818–832. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macro 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Karmowski, J.; Enke, K.; Killenberg, M.; Böhm, V. Interactions between lipophilic antioxidants measured by photochemiluminescence assay and α-tocopherol equivalent antioxidant capacity assay as well as the influence of matrix compounds on the lipophilic antioxidant capacity. LWT—Food Sci. Technol. 2015, 64, 817–823. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.J.; Yi, B.; Oh, S.; Lee, J. Effects of relative humidity on the antioxidant properties of α-tocopherol in stripped corn oil. Food Chem. 2015, 167, 191–196. [Google Scholar] [CrossRef]
- Ko, S.N.; Kim, C.J.; Kim, C.T.; Kim, Y.; Kim, I.H. Effects of tocopherols and tocotrienols on the inhibition of autoxidation of conjugated linoleic acid. Eur. J. Lipid Sci. Tech. 2010, 112, 496–501. [Google Scholar] [CrossRef]
- Xu, D.X.; Wang, X.Y.; Jiang, J.P.; Yuan, F.; Decker, E.A.; Gao, Y.X. Influence of pH, EDTA, α-tocopherol, and WPI oxidation on the degradation of β-carotene in WPI-stabilized oil-in-water emulsions. LWT—Food Sci. Technol. 2013, 54, 236–241. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Vitamin E research: Past, now and future. Free Radic. Bio Med. 2021, 177, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.A.; O’riordan, E.D.; O’sullivan, M. Microencapsulation and oxidative stability of spray-dried fish oil emulsions. J. Microencapsul. 2003, 20, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Shi, Y.; Han, L.P. Development and evaluation of microencapsulated peony seed oil prepared by spray drying: Oxidative stability and its release behavior during in-vitro digestion. J. Food Eng. 2018, 231, 1–9. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Microencapsulation by spray drying of omega-3 lipids extracted from oilseeds and microalgae: Effect on polyunsaturated fatty acid composition. LWT—Food Sci. Technol. 2021, 148, 111789. [Google Scholar] [CrossRef]
- Damerau, A.; Mustonen, S.A.; Ogrodowska, D.; Varjotie, L.; Brandt, W.; Laaksonen, O.; Tanska, M.; Linderborg, K.M. Food Fortification Using Spray-Dried Emulsions of Fish Oil Produced with Maltodextrin, Plant and Whey Proteins-Effect on Sensory Perception, Volatiles and Storage Stability. Molecules 2022, 27, 3553. [Google Scholar] [CrossRef]
- Calvo, P.; Castaño, A.L.; Lozano, M.; González-Gómez, D. Influence of the microencapsulation on the quality parameters and shelf-life of extra-virgin olive oil encapsulated in the presence of BHT and different capsule wall components. Food Res. Int. 2012, 45, 256–261. [Google Scholar] [CrossRef]
- Rubilar, M.; Morales, E.; Contreras, K.; Ceballos, C.; Acevedo, F.; Villarroel, M.; Shene, C. Development of a soup powder enriched with microencapsulated linseed oil as a source of omega-3 fatty acids. Eur. J. Lipid Sci. Tech. 2012, 114, 423–433. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W.; Czaplicki, S. The influence of emulsion drying on the fatty acid composition, bioactive compounds content and oxidative stability of encapsulated bio-oils. Cyta-J. Food 2019, 17, 949–959. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W. The Influence of Drying Process Conditions on the Physical Properties, Bioactive Compounds and Stability of Encapsulated Pumpkin Seed Oil. Food Bioprocess. Tech. 2017, 10, 1265–1280. [Google Scholar] [CrossRef]
- Shiga, H.; Yoshii, H.; Ohe, H.; Yasuda, M.; Furuta, T.; Kuwahara, H.; Ohkawara, M.; Linko, P. Encapsulation of shiitake (Lenthinus Edodes) flavors by spray drying. Biosci. Biotechnol. Biochem. 2004, 68, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Shiga, H.; Yoshii, H.; Nishiyama, T.; Furuta, T.; Forssele, P.; Poutanen, K.; Linko, P. Flavor encapsulation and release characteristics of spray-dried powder by the blended encapsulant of cyclodextrin and gum Arabic. Dry. Technol. 2001, 19, 1385–1395. [Google Scholar] [CrossRef]
- Partanen, R.; Ahro, M.; Hakala, M.; Kallio, H.; Forssell, P. Microencapsulation of caraway extract in β-cyclodextrin and modified starches. Eur. Food Res. Technol. 2002, 214, 242–247. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Rudzinska, M.; Siger, A.; Bartkowiak-Broda, I. A high drying temperature causes degradation of sterols and tocopherols in yellow-seeded oils. Eur. J. Lipid Sci. Tech. 2015, 117, 483–490. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W.; Czaplicki, S. Impact of the Encapsulation Process by Spray- and Freeze-Drying on the Properties and Composition of Powders Obtained from Cold-Pressed Seed Oils with Various Unsaturated Fatty Acids. Pol. J. Food Nutr. Sci. 2020, 70, 241–252. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Ferreira, L.; Peixoto, D.; Silva, F.; Saores, M.J.; Zeinali, M.; Zafar, H.; Mascarenhas-Melo, F.; Raza, F.; Mazzola, P.G.; et al. Cyclodextrins as an encapsulation molecular strategy for volatile organic compounds—Pharmaceutical applications. Colloid. Surf. B 2022, 218, 112758. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Marques, G.R.; Borges, S.V.; Botrel, D.A. Effect of solids content and oil load on the microencapsulation process of rosemary essential oil. Ind. Crops Prod. 2014, 58, 173–181. [Google Scholar] [CrossRef]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.R.F.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.B.; Hong, J.H.; Chung, J.H.; Kim, S.S.; Lee, W.J.; Yoon, J. Optimization of pine flavor microencapsulation by spray drying. Food Sci. Biotechnol. 2005, 14, 747–751. [Google Scholar]
- Barbosa, M.I.M.J.; Borsarelli, C.D.; Mercadante, A.Z. Light stability of spray-dried bixin encapsulated with different edible polysaccharide preparations. Food Res. Int. 2005, 38, 989–994. [Google Scholar] [CrossRef]
- Rascón, M.P.; Beristain, C.I.; García, H.S.; Salgado, M.A. Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and Soy protein isolate as wall materials. LWT—Food Sci. Technol. 2011, 44, 549–557. [Google Scholar] [CrossRef]
- Chen, W.; Chiu, H.T.; Feng, Z.; Maes, E.; Serventi, L. Effect of Spray-Drying and Freeze-Drying on the Composition, Physical Properties, and Sensory Quality of Pea Processing Water (Liluva). Foods 2021, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, J.; Guan, Y.F.; Zhao, G.H. Effect of carriers on physicochemical properties, antioxidant activities and biological components of spray-dried purple sweet potato flours. LWT—Food Sci. Technol. 2013, 51, 348–355. [Google Scholar] [CrossRef]
- Gu, L.; Su, Y.; Zhang, M.; Chang, C.; Li, J.; McClements, D.J.; Yang, Y. Protection of β-carotene from chemical degradation in emulsion-based delivery systems using antioxidant interfacial complexes: Catechin-egg white protein conjugates. Food Res. Int. 2017, 96, 84–93. [Google Scholar] [CrossRef]
- McClements, D.J.; Peng, S.F. Current status in our understanding of physicochemical basis of bioaccessibility. Curr. Opin. Food Sci. 2020, 31, 57–62. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. A Comparative Study of Encapsulation of β-Carotene via Spray-Drying and Freeze-Drying Techniques Using Pullulan and Whey Protein Isolate as Wall Material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef]
- Li, R.Y.; Tan, Y.B.; Dai, T.T.; Zhang, R.J.; Fu, G.M.; Wan, Y.; Liu, C.M.; McClements, D.J. Bioaccessibility and stability of β-carotene encapsulated in plant-based emulsions: Impact of emulsifier type and tannic acid. Food Funct. 2019, 10, 7239–7252. [Google Scholar] [CrossRef]
- Sun, X.W.; Xu, Y.; Zhao, L.L.; Yan, H.X.; Wang, S.H.; Wang, D.F. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef]
- Eun, J.B.; Maruf, A.; Das, P.R.; Nam, S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. 2019, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of Fats and Oils on the Bioaccessibility of Carotenoids and Vitamin E in Vegetables. Biosci. Biotech. Bioch 2013, 77, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Terán, K.; Grootaert, C.; Ortiz, J.; Donoso, S.; Ruales, J.; Van Bockstaele, F.; Van Camp, J.; Van de Wiele, T. In vitro bioaccessibility and uptake of β-carotene from encapsulated carotenoids from mango by-products in a coupled gastrointestinal digestion/Caco-2 cell model. Food Res. Int. 2023, 164, 112301. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, C.E.; Focsan, M.; Ciont, C.; Bunea, A.; Rugina, D.; Pintea, A. Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads. Nutrients 2024, 16, 2726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Zhang, R.J.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
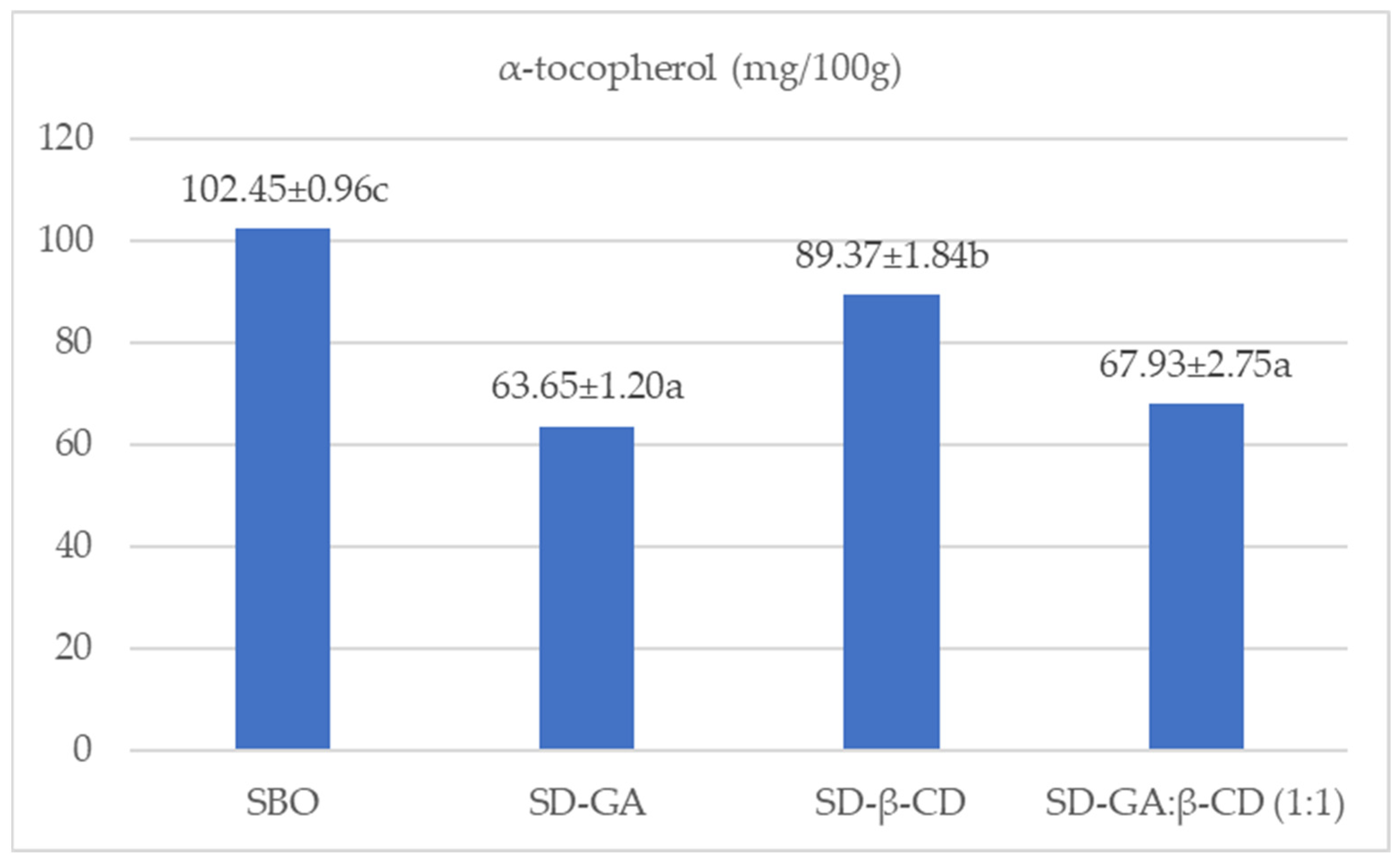
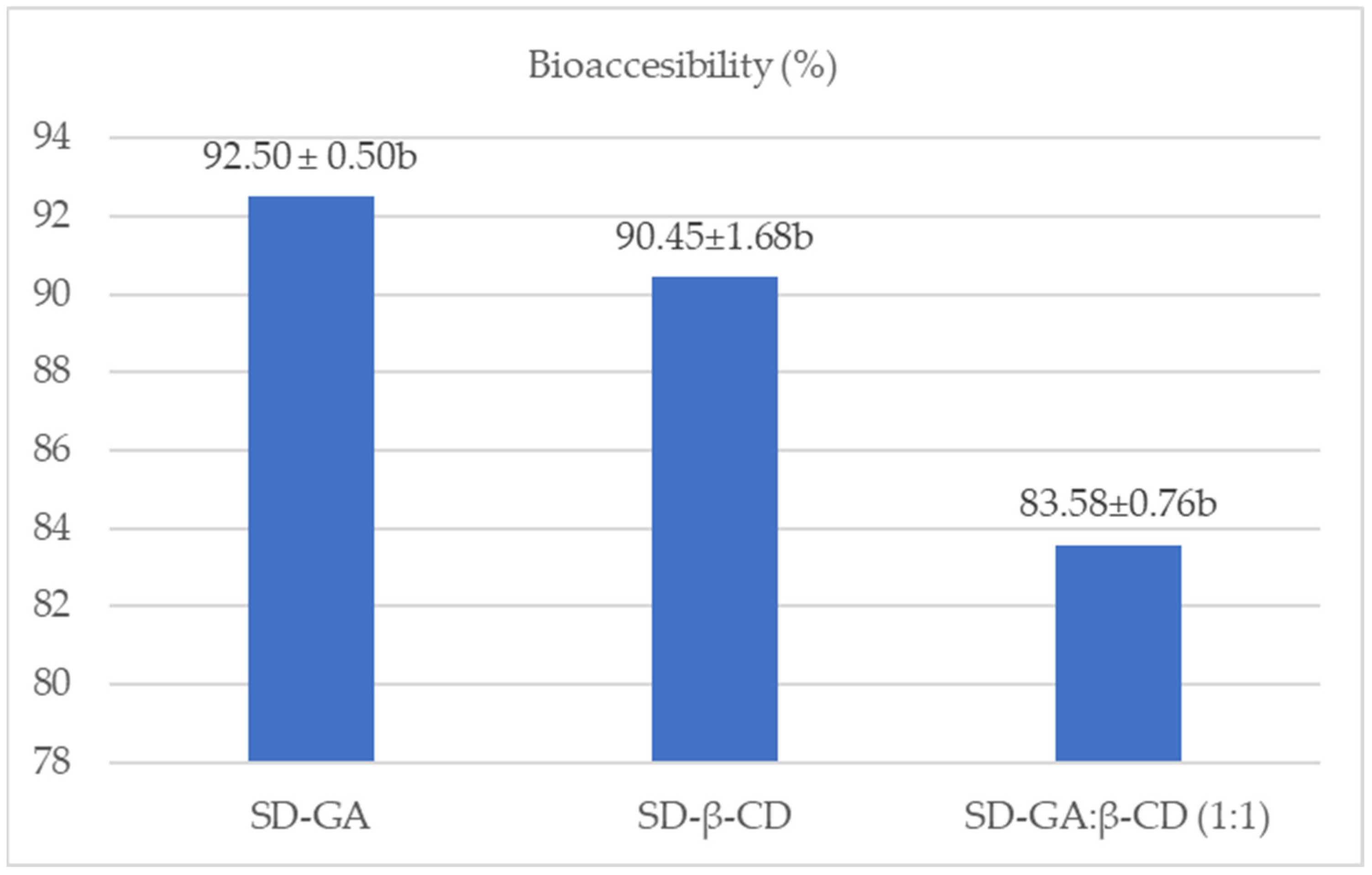
| Fatty Acids | SBO | SD-GA | SD-β-CD | SD GA:β-CD (1:1) | |
|---|---|---|---|---|---|
| Saturated (%) | |||||
| Myristic | p = 0.07 | 0.15 ± 0.01 a | 0.18 ± 0.00 a | 0.17 ± 0.00 a | 0.16 ± 0.00 a |
| Pentadecanoic acid | p = 0.94 | 0.07 ± 0.01 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a |
| Palmitic | p = 0.36 | 25.44 ± 1.05 a | 25.20 ± 0.00 a | 25.49 ± 0.00 a | 25.38 ± 0.01 a |
| Heptadecanoic acid | p = 0.27 | 0.11 ± 0.01 a | 0.14 ± 0.00 a | 0.15 ± 0.00 a | 0.14 ± 0.00 a |
| Stearic | p = 0.83 | 1.59 ± 0.02 a | 1.59 ± 0.00 a | 1.63 ± 0.00 a | 1.66 ± 0.00 a |
| Arachidic acid | p = 0.08 | 0.28 ± 0.02 a | 0.24 ± 0.00 a | 0.28 ± 0.00 a | 0.29 ± 0.00 a |
| Docosanoic acid | p = 0.65 | 0.12 ± 0.01 a | 0.13 ± 0.00 a | 0.13 ± 0.00 a | 0.12 ± 0.01 a |
| SUM: | p = 0.20 | 27.76 ± 0.06 a | 27.55 ± 0.00 a | 27.92 ± 0.01 a | 27.81 ± 0.01 a |
| Unsaturated (%) | |||||
| Monounsaturated (%) | |||||
| Palmitoleic acid | p = 0.27 | 31.85 ± 1.15 a | 31.67 ± 0.01 a | 31.26 ± 0.00 a | 30.94 ± 0.01 a |
| Oleic acid | p = 0.84 | 16.38 ± 1.04 a | 16.36 ± 0.02 a | 16.57 ± 0.01 a | 16.53 ± 0.01 a |
| Vaccenic acid | p = 0.74 | 7.43 ± 1.03 a | 7.40 ± 0.01 a | 7.28 ± 0.00 a | 7.24 ± 0.00 a |
| SUM: | p < 0.01 | 55.66 ± 0.09 b | 55.44 ± 0.02 b | 55.11 ± 0.00 a | 54.71 ± 0.00 a |
| Polyunsaturated (%) | |||||
| Linoleic acid | p = 0.35 | 11.20 ± 0.45 a | 11.32 ± 0.01 a | 11.75 ± 0.00 a | 12.01 ± 0.00 a |
| α-Linolenic acid | p = 0.43 | 5.24 ± 0.14 a | 5.35 ± 0.00 a | 5.17 ± 0.00 a | 5.14 ± 0.00 a |
| SUM: | p = 0.28 | 16.44 ± 0.45 a | 16.67 ± 0.02 a | 16.93 ± 0.00 a | 17.15 ± 0.00 a |
| Carotenoids | SBO | SD-GA | SD-β-CD | SD-GA:β-CD (1:1) | |
|---|---|---|---|---|---|
| Zeaxanthin | p < 0.05 | 8.77 ± 0.02 c | 3.62 ± 0.09 a | 4.42 ± 0.09 b | 3.46 ± 0.13 a |
| β-cryptoxanthin | p < 0.01 | 6.85 ± 0.03 d | 1.45 ± 0.12 a | 3.26 ± 0.01 c | 2.56 ± 0.07 b |
| γ-carotene | p < 0.01 | 0.18 ± 0.00 c | 0.13 ± 0.00 a | 0.16 ± 0.00 b | 0.12 ± 0.00 a |
| cis γ-carotene | p < 0.01 | 1.18 ± 0.00 c | 0.68 ± 0.03 a | 0.97 ± 0.01 b | 0.77 ± 0.02 a |
| β-carotene | p < 0.05 | 2.09 ± 0.01 c | 0.55 ± 0.01 a | 2.04 ± 0.00 c | 1.32 ± 0.06 b |
| β-cryptoxanthin palmitate | p < 0.01 | 0.14 ± 0.00 d | 0.03 ± 0.00 a | 0.07 ± 0.00 c | 0.05 ± 0.00 b |
| Zeaxanthin-myristate | p < 0.01 | 28.65 ± 0.09 d | 9.51 ± 0.11 a | 23.44 ± 0.51 c | 16.62 ± 0.55 b |
| Lutein-palmitate | p < 0.01 | 3.30 ± 0.10 c | 0.78 ± 0.00 a | 3.12 ± 0.10 b,c | 2.21 ± 0.34 b |
| Zeaxanthin-pamitate | p < 0.01 | 31.01 ± 0.04 d | 16.83 ± 0.35 a | 24.78 ± 0.66 c | 19.14 ± 0.06 b |
| Lutein di-myristate | p < 0.05 | 10.39 ± 0.02 c | 2.40 ± 0.19 a | 4.23 ± 0.01 b | 2.96 ± 0.24 a |
| Zeaxanthin-palmitate-myristate | p < 0.01 | 36.22 ± 0.03 b | 27.01 ± 0.51 a | 27.18 ± 0.24 a | 27.25 ± 0.97 a |
| lutein di palmitate | p < 0.01 | 24.17 ± 0.03 d | 16.00 ± 0.16 a | 22.36 ± 0.19 c | 19.15 ± 0.28 b |
| Zeaxanthin-di-palmitate | p < 0.01 | 58.08 ± 0.03 d | 34.53 ± 0.14 a | 54.61 ± 0.32 c | 46.29 ± 0.01 b |
| Lutein palmitate stearate | p < 0.01 | 4.37 ± 0.02 d | 1.16 ± 0.06 a | 3.12 ± 0.00 c | 1.94 ± 0.01 b |
| SUM: | p < 0.01 | 215.40 ± 0.18 d | 114.68 ± 0.90 a | 173.76 ± 0.22 b | 143.84 ± 0.95 c |
| Source of Variation | L* | a* | b* | H* | C* |
|---|---|---|---|---|---|
| p = 0.55 | p = 0.61 | p = 0.12 | p = 0.21 | p = 0.14 | |
| SD-GA | 86.53 ± 1.12 a | 11.26 ± 0.98 a | 43.98 ± 1.24 a | 75.67 ± 0.81 a | 45.40 ± 1.14 a |
| SD-β-CD | 88.51 ± 1.22 a | 11.25 ± 0.97 a | 38.56 ± 1.31 a | 73.77 ± 0.81 a | 40.17 ± 0.99 a |
| SD-GA:β-CD (1:1) | 87.05 ± 1.26 a | 12.44 ± 0.69 a | 42.19 ± 1.28 a | 73.58 ± 0.39 a | 43.99 ± 1.81 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čulina, P.; Balbino, S.; Vitali Čepo, D.; Golub, N.; Elez Garofulić, I.; Dragović-Uzelac, V.; You, L.; Pedisić, S. Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials. Appl. Sci. 2025, 15, 1194. https://doi.org/10.3390/app15031194
Čulina P, Balbino S, Vitali Čepo D, Golub N, Elez Garofulić I, Dragović-Uzelac V, You L, Pedisić S. Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials. Applied Sciences. 2025; 15(3):1194. https://doi.org/10.3390/app15031194
Chicago/Turabian StyleČulina, Patricija, Sandra Balbino, Dubravka Vitali Čepo, Nikolina Golub, Ivona Elez Garofulić, Verica Dragović-Uzelac, Lijun You, and Sandra Pedisić. 2025. "Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials" Applied Sciences 15, no. 3: 1194. https://doi.org/10.3390/app15031194
APA StyleČulina, P., Balbino, S., Vitali Čepo, D., Golub, N., Elez Garofulić, I., Dragović-Uzelac, V., You, L., & Pedisić, S. (2025). Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials. Applied Sciences, 15(3), 1194. https://doi.org/10.3390/app15031194











