Activity Against Human Pathogens of Two Polyunsaturated Aldehydes and Pheophorbide a
Abstract
1. Introduction
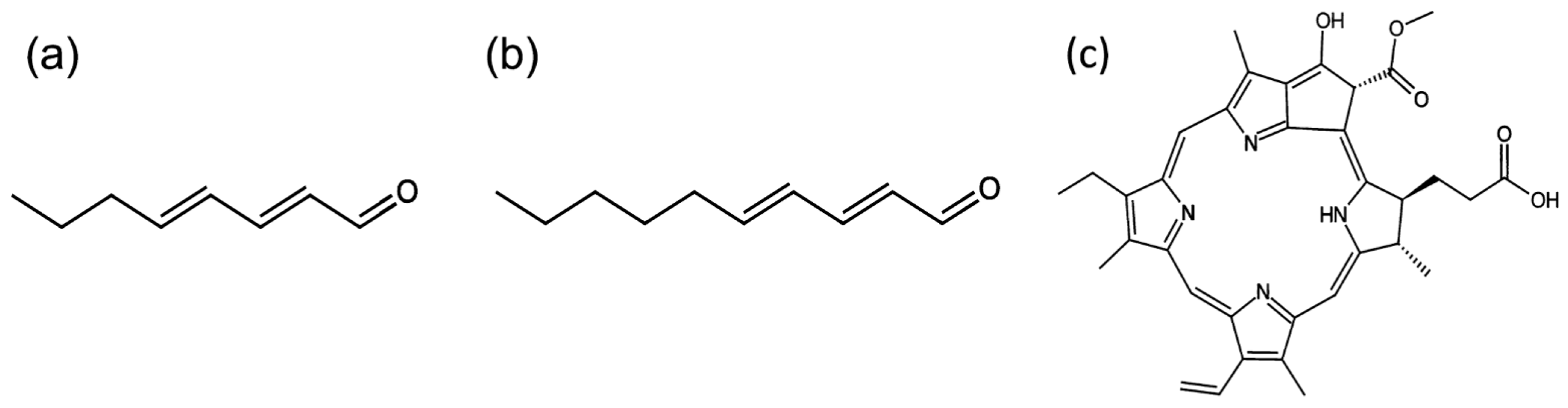
2. Materials and Methods
2.1. Testing Reagents
2.2. Antimicrobial Assays
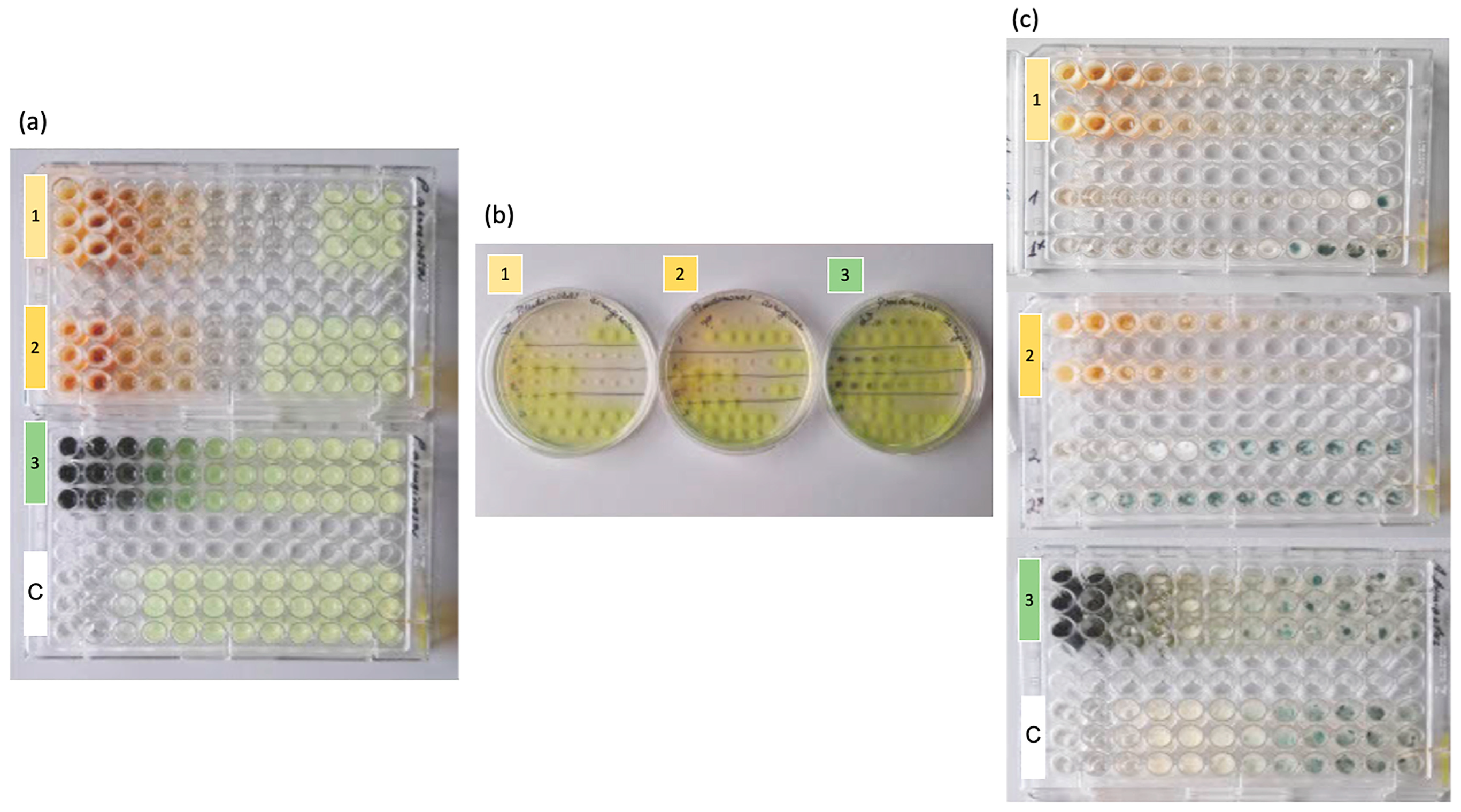
2.2.1. Minimal Inhibitory Concentration Assay (MIC)
2.2.2. Minimal Bactericidal/Fungicidal Concentration (MBC/MFC) Assay
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Suresh, K.; Anbalagan, S.; Ragini, Y.P.; Kadirvel, V. Investigating the Nutritional Viability of Marine-Derived Protein for Sustainable Future Development. Food Chem. 2024, 448, 139087. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Geada, P.; Pereira, R.N.; Teixeira, J.A. Microalgae Biomass–A Source of Sustainable Dietary Bioactive Compounds towards Improved Health and Well-Being. Food Chem. Adv. 2025, 6, 100926. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The Insidious Effect of Diatoms on Copepod Reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Sarno, D.; Kooistra, W.H.C.F.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in The Genus Skeletonema (Bacillariophyceae). Ii. An Assessment of The Taxonomy of S. costatum-Like Species with The Description of Four New Species. J. Phycol. 2005, 41, 151–176. [Google Scholar] [CrossRef]
- Lauritano, C.; Romano, G.; Roncalli, V.; Amoresano, A.; Fontanarosa, C.; Bastianini, M.; Braga, F.; Carotenuto, Y.; Ianora, A. New Oxylipins Produced at the End of a Diatom Bloom and Their Effects on Copepod Reproductive Success and Gene Expression Levels. Harmful Algae 2016, 55, 221–229. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde Suppression of Copepod Recruitment in Blooms of a Ubiquitous Planktonic Diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Castellano, I.; Ercolesi, E.; Romano, G.; Ianora, A.; Palumbo, A. The Diatom-Derived Aldehyde Decadienal Affects Life Cycle Transition in the Ascidian Ciona intestinalis through Nitric Oxide/ERK Signalling. Open Biol. 2015, 5, 140182. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of Oxylipin Pathways in Marine Diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- Pohnert, G. Diatom/Copepod Interactions in Plankton: The Indirect Chemical Defense of Unicellular Algae. ChemBioChem 2005, 6, 946–959. [Google Scholar] [CrossRef]
- Caldwell, G.S. The Influence of Bioactive Oxylipins from Marine Diatoms on Invertebrate Reproduction and Development. Mar. Drugs 2009, 7, 367–400. [Google Scholar] [CrossRef] [PubMed]
- Marrone, V.; Piscopo, M.; Romano, G.; Ianora, A.; Palumbo, A.; Costantini, M. Defensome against Toxic Diatom Aldehydes in the Sea Urchin Paracentrotus lividus. PLoS ONE 2012, 7, e31750. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, A.; Esposito, R.; Ianora, A.; Spagnuolo, A. Ciona Intestinalis as a Marine Model System to Study Some Key Developmental Genes Targeted by the Diatom-Derived Aldehyde Decadienal. Mar. Drugs 2015, 13, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Carotenuto, Y.; Vitiello, V.; Buttino, I.; Romano, G.; Hwang, J.-S.; Ianora, A. Effects of the Oxylipin-Producing Diatom Skeletonema marinoi on Gene Expression Levels of the Calanoid Copepod Calanus sinicus. Mar. Genom. 2015, 24, 89–94. [Google Scholar] [CrossRef]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-Derived Polyunsaturated Aldehydes Activate Cell Death in Human Cancer Cell Lines but Not Normal Cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef]
- Simon, C.A.; Bentley, M.G.; Caldwell, G.S. 2,4-Decadienal: Exploring a Novel Approach for the Control of Polychaete Pests on Cultured Abalone. Aquaculture 2010, 310, 52–60. [Google Scholar] [CrossRef]
- Ribalet, F.; Intertaglia, L.; Lebaron, P.; Casotti, R. Differential Effect of Three Polyunsaturated Aldehydes on Marine Bacterial Isolates. Aquat. Toxicol. 2008, 86, 249–255. [Google Scholar] [CrossRef]
- Matsuda, K.; Shimoda, Y.; Tanaka, A.; Ito, H. Chlorophyll a Is a Favorable Substrate for Chlamydomonas Mg-Dechelatase Encoded by STAY-GREEN. Plant Physiol. Biochem. 2016, 109, 365–373. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Schuitmaker, J.J.; Baas, P.; Van Leengoed, H.L.L.M.; Van Der Meulen, F.W.; Star, W.M.; Van Zandwijk, N. Photodynamic Therapy: A Promising New Modality for the Treatment of Cancer. J. Photochem. Photobiol. B Biol. 1996, 34, 3–12. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Riccio, G.; Ianora, A.; Lauritano, C. The Diatom Cylindrotheca Closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications. Appl. Sci. 2023, 13, 2590. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Xu, B.; Dong, Q.; Jia, K.; Lin, Z.; Wang, X.; Liu, Y.; Qin, X. Prevalence, Antibiotic Resistance, Resistance and Virulence Determinants of Campylobacter jejuni in China: A Systematic Review and Meta-Analysis. One Health 2025, 20, 100990. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal Properties and Mechanisms of Three Volatile Aldehydes (Octanal, Nonanal and Decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Bisignano, G.; LaganÃ, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In Vitro Antibacterial Activity of Some Aliphatic Aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Shi, X.-L.; Yang, J.; Zhang, Y.; Qin, P.; Zhou, H.-Y.; Chen, Y.-Z. The Photoactivated Antifungal Activity and Possible Mode of Action of Sodium Pheophorbide a on Diaporthe mahothocarpus Causing Leaf Spot Blight in Camellia oleifera. Front. Microbiol. 2024, 15, 1403478. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Tawara-Matsuda, J.; Lorenz, P.; Mueller, P.; Zenewicz, L.; Lewis, K. 5‘-Methoxyhydnocarpin-D and Pheophorbide A: Berberis Species Components That Potentiate Berberine Growth Inhibition of Resistant Staphylococcus aureus. J. Nat. Prod. 2000, 63, 1146–1149. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Dharmaratne, P.; Wang, B.; Lau, K.M.; Lee, C.C.; Cheung, D.W.S.; Chan, J.Y.W.; Yue, G.G.L.; Lau, C.B.S.; Wong, C.K.; et al. Hypericin and Pheophorbide a Mediated Photodynamic Therapy Fighting MRSA Wound Infections: A Translational Study from In Vitro to In Vivo. Pharmaceutics 2021, 13, 1399. [Google Scholar] [CrossRef]
- Puchkova, T.V.; Khapchaeva, S.A.; Zotov, V.S.; Lukyanov, A.A.; Solovchenko, A.E. Marine and freshwater microalgae as a sustainable source of cosmeceuticals. Mar. Biol. J. 2021, 6, 67–81. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, A.; Bala, S.; Satheesh, N.; Nile, A.S.; Nile, S.H. Microalgae in the Food-Health Nexus: Exploring Species Diversity, High-Value Bioproducts, Health Benefits, and Sustainable Market Potential. Bioresour. Technol. 2025, 427, 132424. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Carabin, I.G. Generally Recognized as Safe (GRAS): History and Description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef]
- De Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; De La Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the Microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and Their Activity as TNF-α Inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.-T.; Rosahl, S.; et al. Evaluation of the Antimicrobial Activities of Plant Oxylipins Supports Their Involvement in Defense against Pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef]
- Martínez, E.; Hamberg, M.; Busquets, M.; Díaz, P.; Manresa, A.; Oliw, E.H. Biochemical Characterization of the Oxygenation of Unsaturated Fatty Acids by the Dioxygenase and Hydroperoxide Isomerase of Pseudomonas aeruginosa 42A2. J. Biol. Chem. 2010, 285, 9339–9345. [Google Scholar] [CrossRef]
- Martínez, E.; Cosnahan, R.K.; Wu, M.; Gadila, S.K.; Quick, E.B.; Mobley, J.A.; Campos-Gómez, J. Oxylipins Mediate Cell-to-Cell Communication in Pseudomonas aeruginosa. Commun. Biol. 2019, 2, 66. [Google Scholar] [CrossRef]
- Martínez, E.; Campos-Gómez, J. Oxylipins Produced by Pseudomonas aeruginosa Promote Biofilm Formation and Virulence. Nat. Commun. 2016, 7, 13823. [Google Scholar] [CrossRef]
- Bean, H.D.; Rees, C.A.; Hill, J.E. Comparative Analysis of the Volatile Metabolomes of Pseudomonas aeruginosa Clinical Isolates. J. Breath. Res. 2016, 10, 047102. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.-R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef]
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Hata, K.; Horii, T.; Miyazaki, M.; Watanabe, N.; Okubo, M.; Sonoda, J.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; et al. Efficacy of Oral E1210, a New Broad-Spectrum Antifungal with a Novel Mechanism of Action, in Murine Models of Candidiasis, Aspergillosis, and Fusariosis. Antimicrob. Agents Chemother. 2011, 55, 4543–4551. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Kontoyiannis, D.P.; Cornely, O.A.; Perfect, J.R.; Walsh, T.J. Novel Agents and Drug Targets to Meet the Challenges of Resistant Fungi. J. Infect. Dis. 2017, 216, S474–S483. [Google Scholar] [CrossRef]
- Buil, J.B.; Rijs, A.J.M.M.; Meis, J.F.; Birch, M.; Law, D.; Melchers, W.J.G.; Verweij, P.E. In Vitro Activity of the Novel Antifungal Compound F901318 against Difficult-to-Treat Aspergillus Isolates. J. Antimicrob. Chemother. 2017, 72, 2548–2552. [Google Scholar] [CrossRef]
- Nakamura, I.; Ohsumi, K.; Takeda, S.; Katsumata, K.; Matsumoto, S.; Akamatsu, S.; Mitori, H.; Nakai, T. ASP2397 Is a Novel Natural Compound That Exhibits Rapid and Potent Fungicidal Activity against Aspergillus Species through a Specific Transporter. Antimicrob. Agents Chemother. 2019, 63, e02689-18. [Google Scholar] [CrossRef]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.M.; James, K.D.; Krishnan, B.R. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; Bartizal, K.; Miesel, L.; Huang, H.H.; You, W.T.; Miesel, L. Efficacy of CD101, a Novel Echinocandin Antifungal, in a Mouse Model of Disseminated Aspergillosis. In Proceedings of the 7th Advances Against Aspergillosis Conference, Manchester, UK, 3–5 March 2016. [Google Scholar]
- Colley, T.; Sharma, C.; Alanio, A.; Kimura, G.; Daly, L.; Nakaoki, T.; Nishimoto, Y.; Bretagne, S.; Kizawa, Y.; Strong, P.; et al. Anti-Fungal Activity of a Novel Triazole, PC1244, against Emerging Azole-Resistant Aspergillus fumigatus and Other Species of Aspergillus. J. Antimicrob. Chemother. 2019, 74, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
| Sample | Escherichia coli MSCL 332 | Pseudomonas aeruginosa MSCL 331 | Staphylococcus aureus MSCL 334 | Candida albicans MSCL 378 | Aspergillus fumigatus MSCL 1323 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | MIC | MFC | |
| 2,4-octadienal | 0.122 | 0.122 | 1.95 | 1.95 | 0.122 | 0.244 | 0.031 | 0.122 | 0.001 | 0.004 |
| trans,trans-2,4-decadienal | 0.122 | 0.244 | 3.90 | 7.80 | 0.244 | 0.488 | 0.122 | 0.244 | 0.244 | 0.244 |
| Pheophorbide a | 0.625 | 0.625 | 0.625 | 0.625 | >0.625 * | >0.625 * | >0.313 * | >0.313 * | >0.313 * | >0.625 * |
| Gentamicin | 1.00 | 4.00 | 0.25 | 4.00 | 0.25 | 4.00 | ND | ND | ND | ND |
| Fluconazole | ND | ND | ND | ND | ND | ND | 32 | >256 * | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Nikolajeva, V.; Lauritano, C. Activity Against Human Pathogens of Two Polyunsaturated Aldehydes and Pheophorbide a. Appl. Sci. 2025, 15, 13221. https://doi.org/10.3390/app152413221
Coppola A, Nikolajeva V, Lauritano C. Activity Against Human Pathogens of Two Polyunsaturated Aldehydes and Pheophorbide a. Applied Sciences. 2025; 15(24):13221. https://doi.org/10.3390/app152413221
Chicago/Turabian StyleCoppola, Alessandro, Vizma Nikolajeva, and Chiara Lauritano. 2025. "Activity Against Human Pathogens of Two Polyunsaturated Aldehydes and Pheophorbide a" Applied Sciences 15, no. 24: 13221. https://doi.org/10.3390/app152413221
APA StyleCoppola, A., Nikolajeva, V., & Lauritano, C. (2025). Activity Against Human Pathogens of Two Polyunsaturated Aldehydes and Pheophorbide a. Applied Sciences, 15(24), 13221. https://doi.org/10.3390/app152413221








