A Traveling Multi-Analyte Chemosensor Based on Wet-Chemical Colorimetry for Shipboard Seawater Analysis
Featured Application
Abstract
1. Introduction
2. Methods
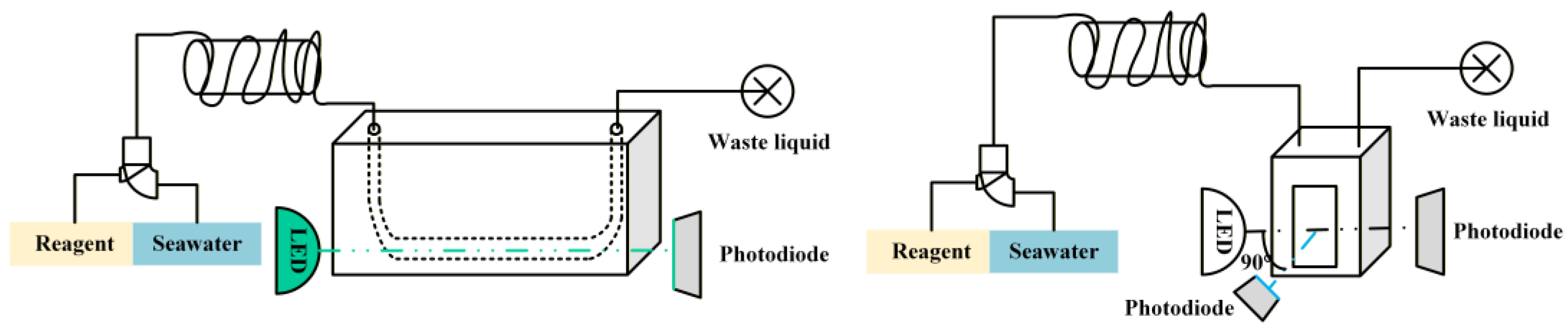
2.1. Instrument Measurement Principle
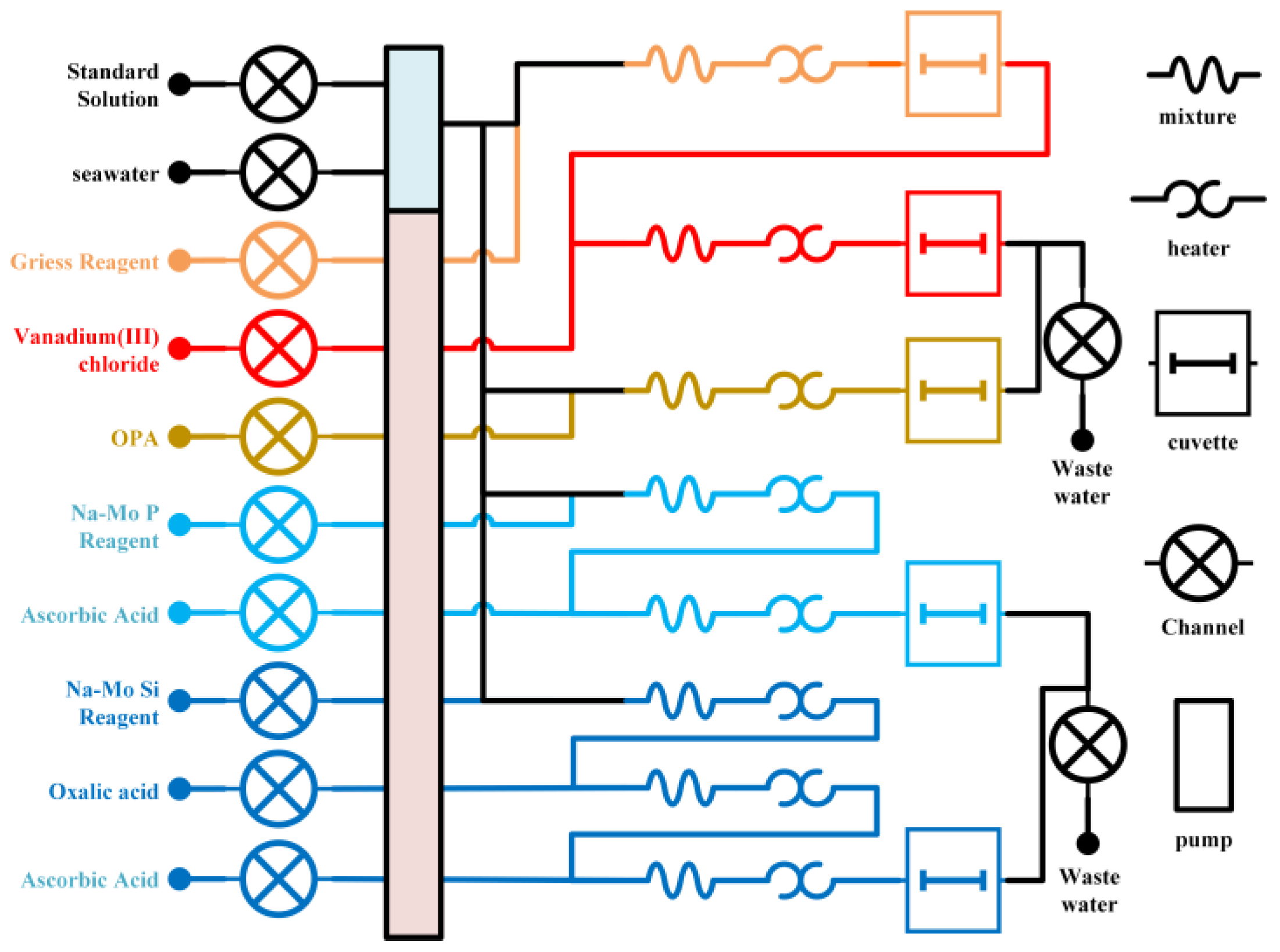
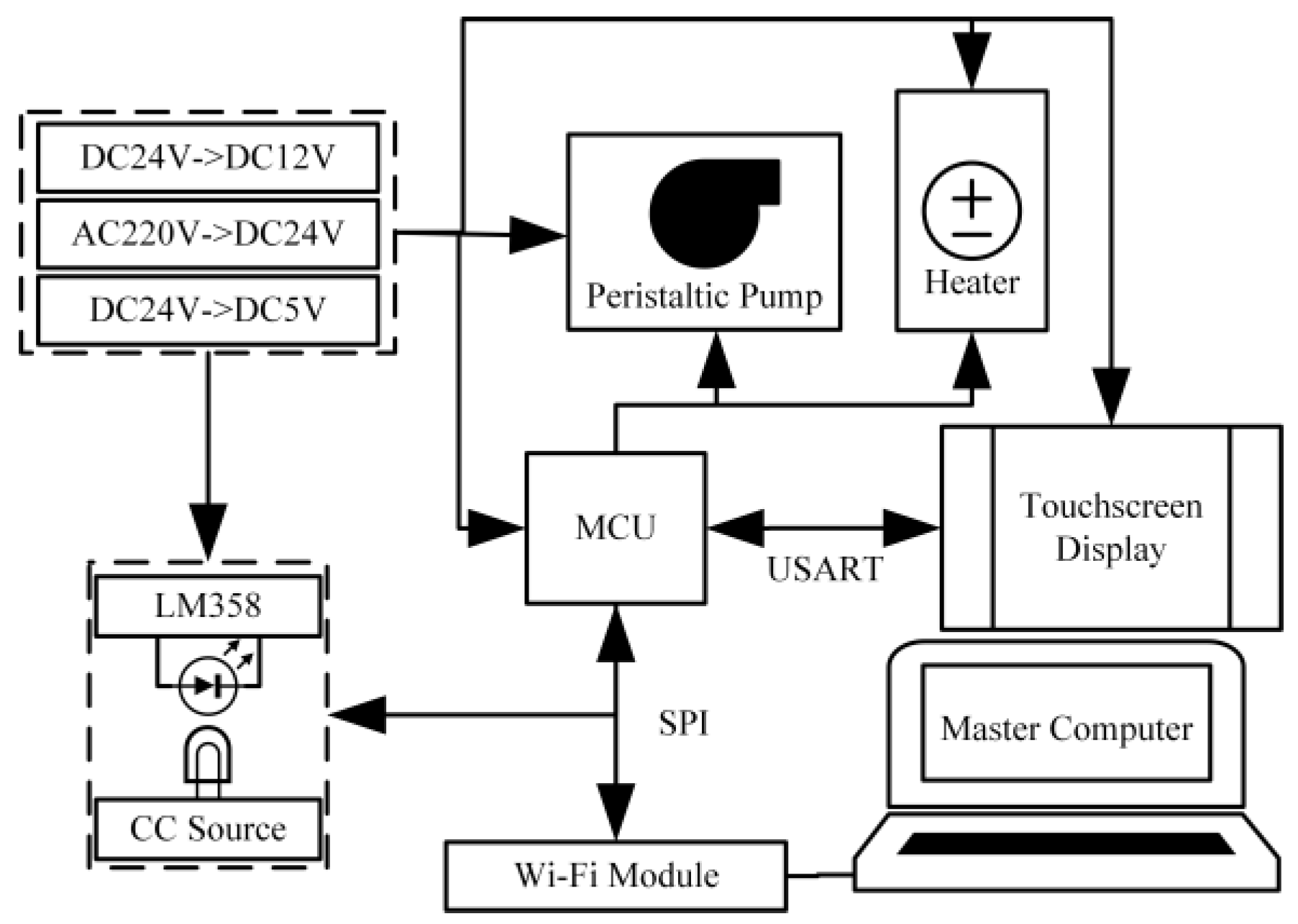
2.2. Flow Path Configuration
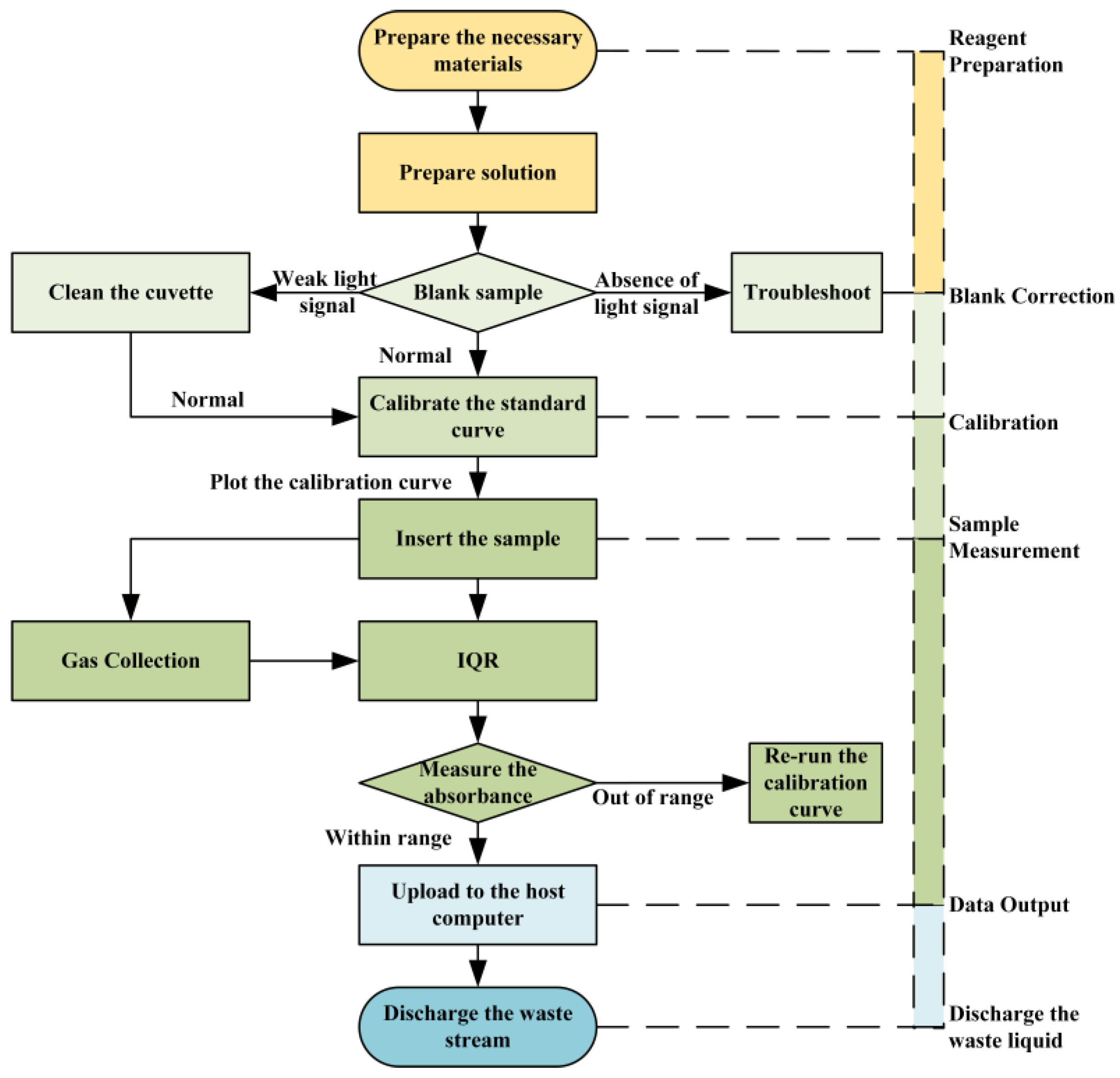
2.3. Instrument Workflow
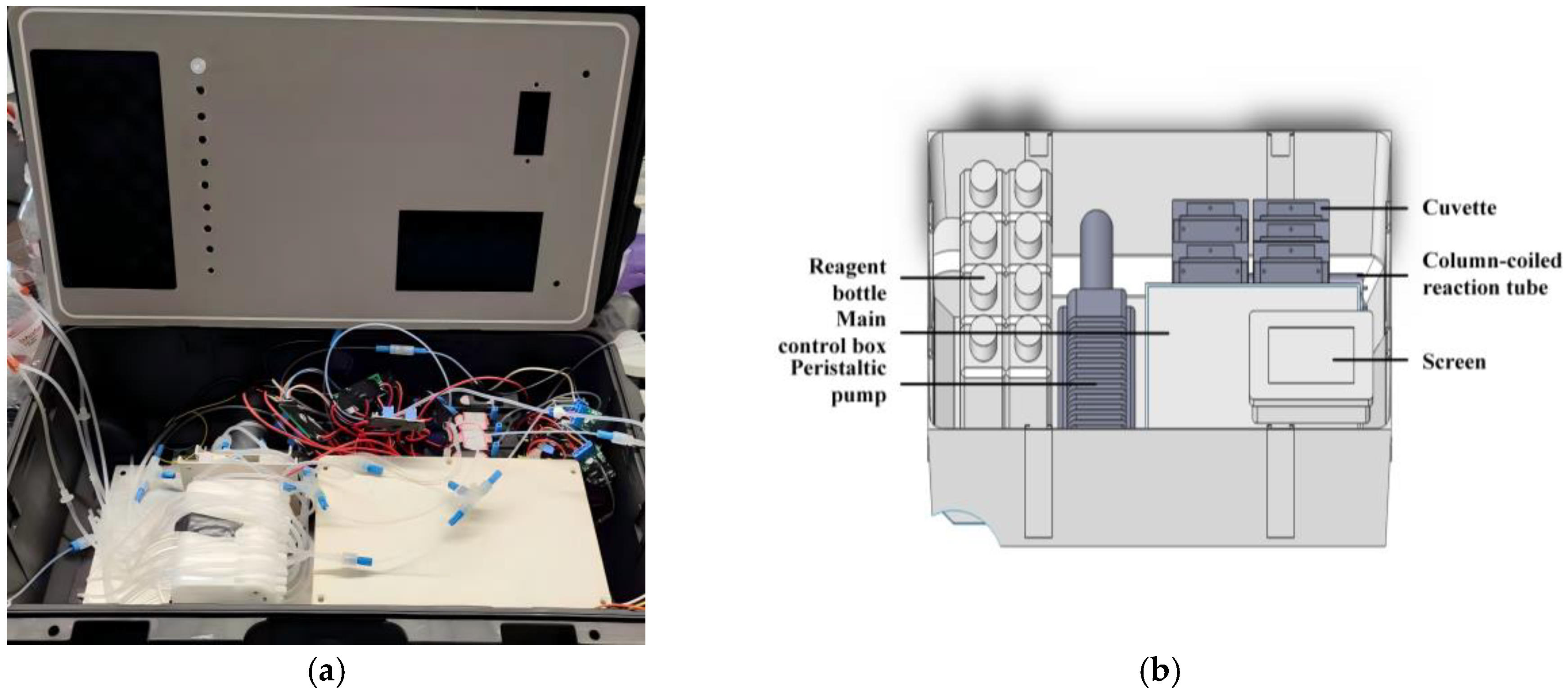
3. Instrument Configuration and Optimization
3.1. Reagent Unit
3.2. Chemical Reaction and Detection Unit
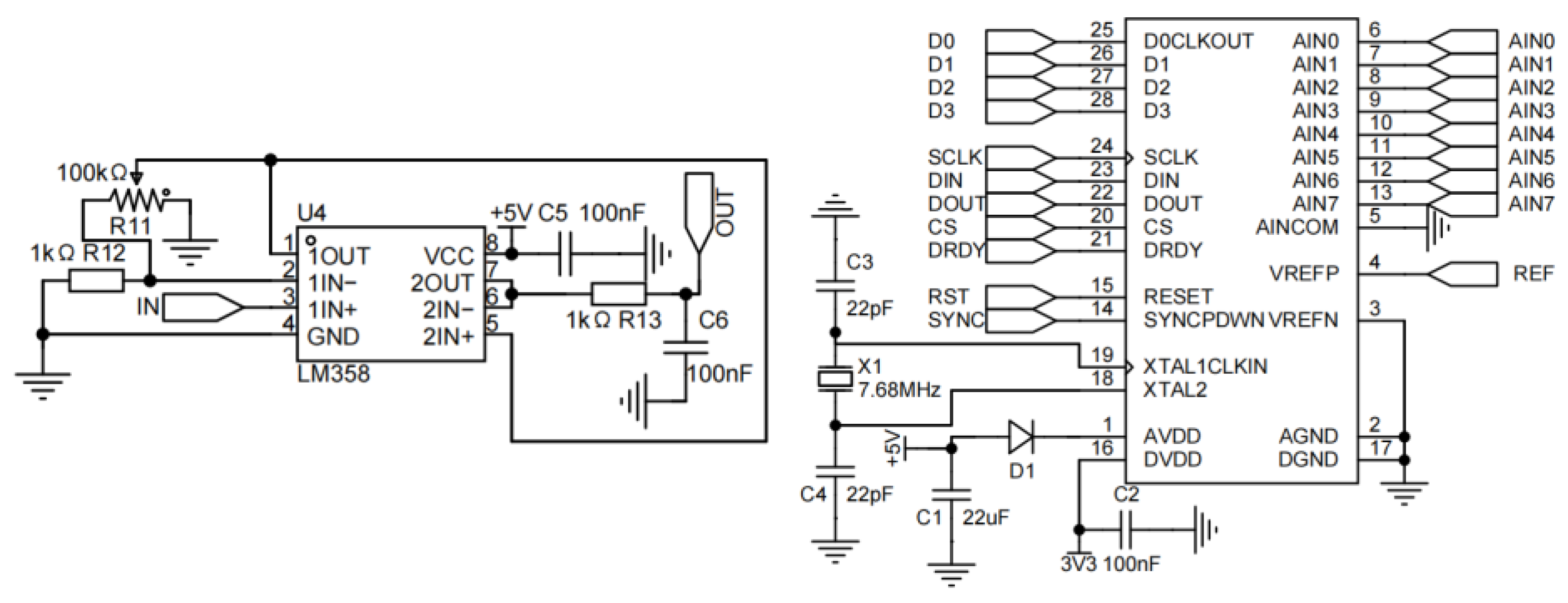
3.3. Control and Signal Processing Unit
3.4. Optimization of Reaction Reagents
3.5. Handling of Interference Factors in the Instrument
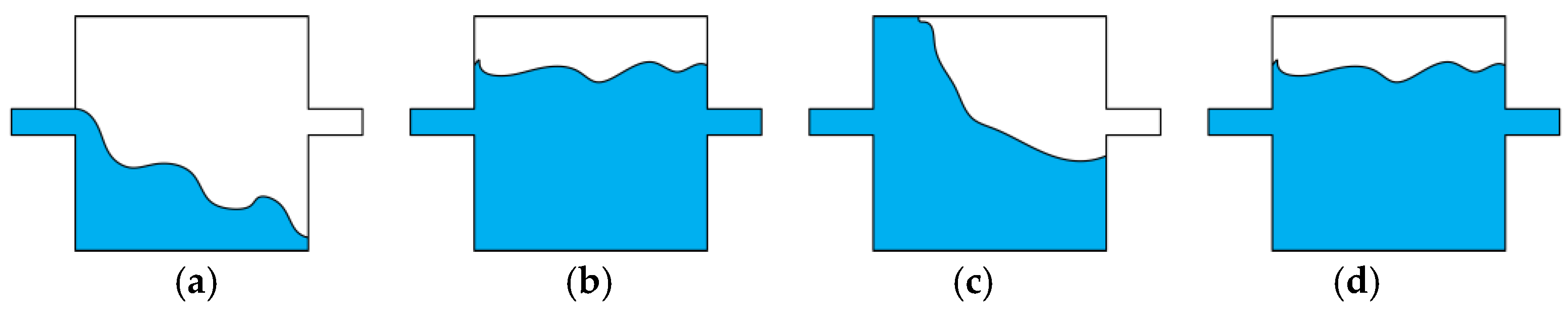
3.5.1. Impact of Bubbles During Solution Heating and Countermeasures
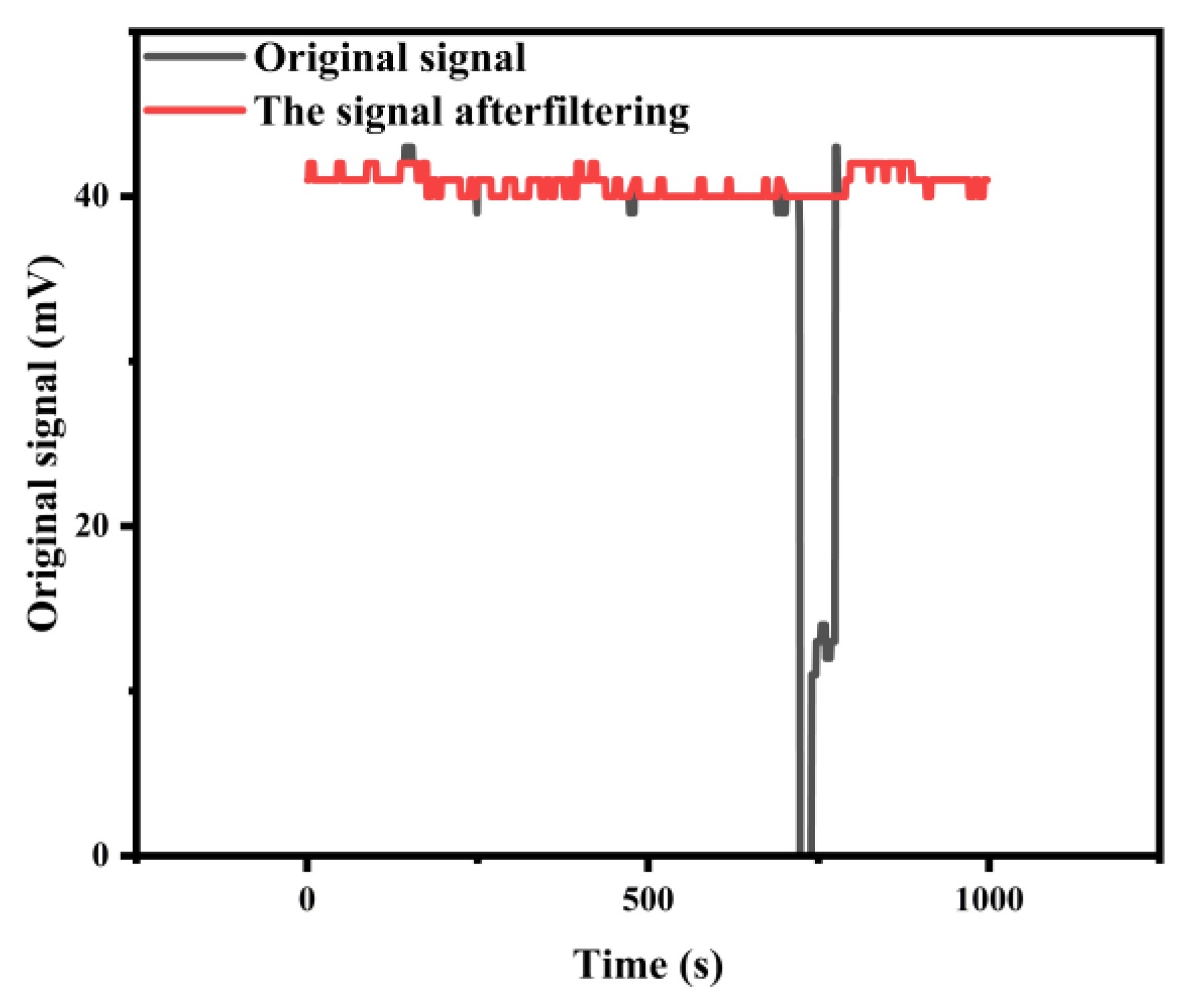
3.5.2. Impact of Bubbles on Silicon Photodiode Detection and the Corresponding Data Processing Method
4. Discussion
4.1. Instrument Performance Testing
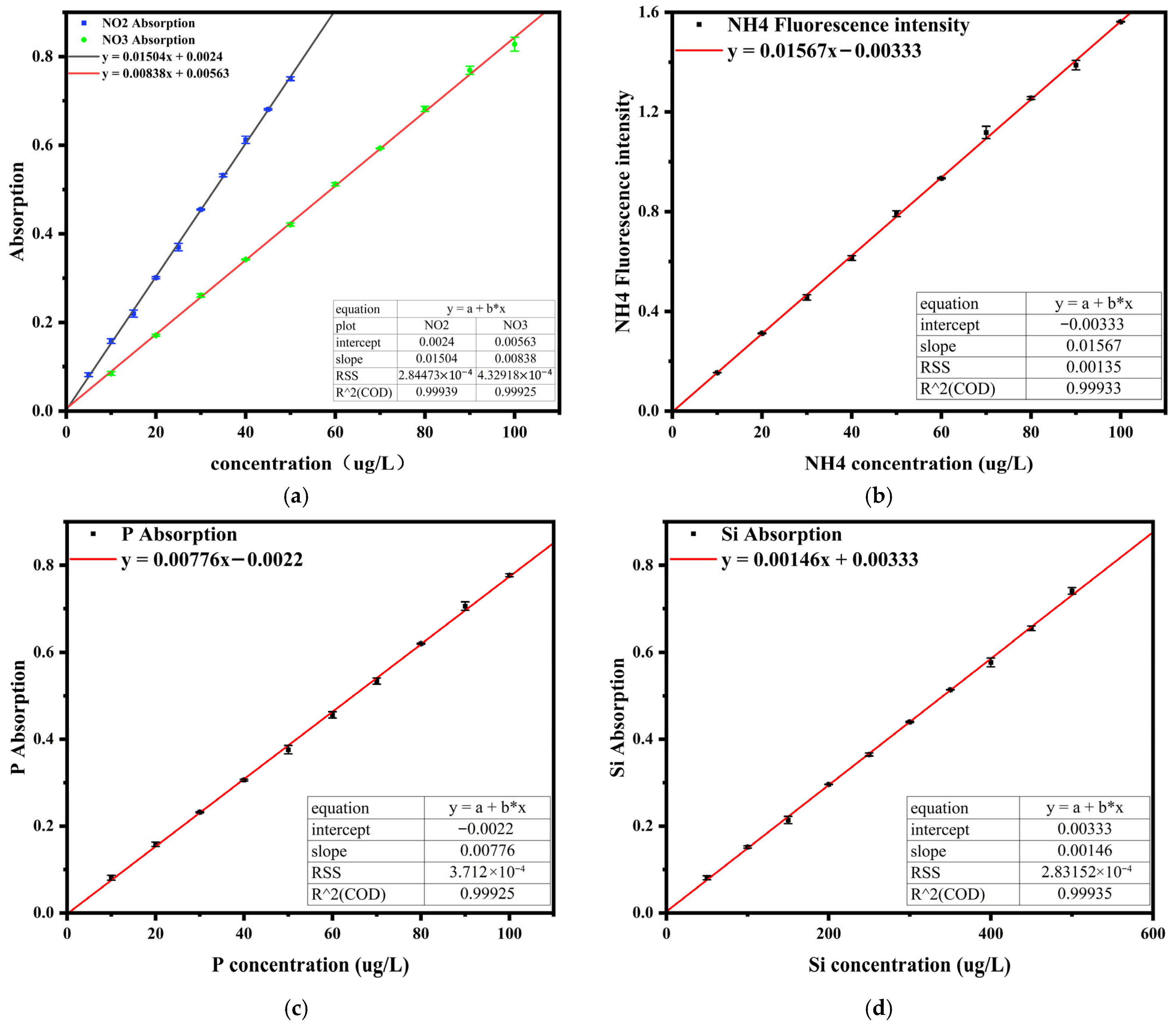
4.1.1. Linear Range and Detection Limit
4.1.2. Accuracy and Precision
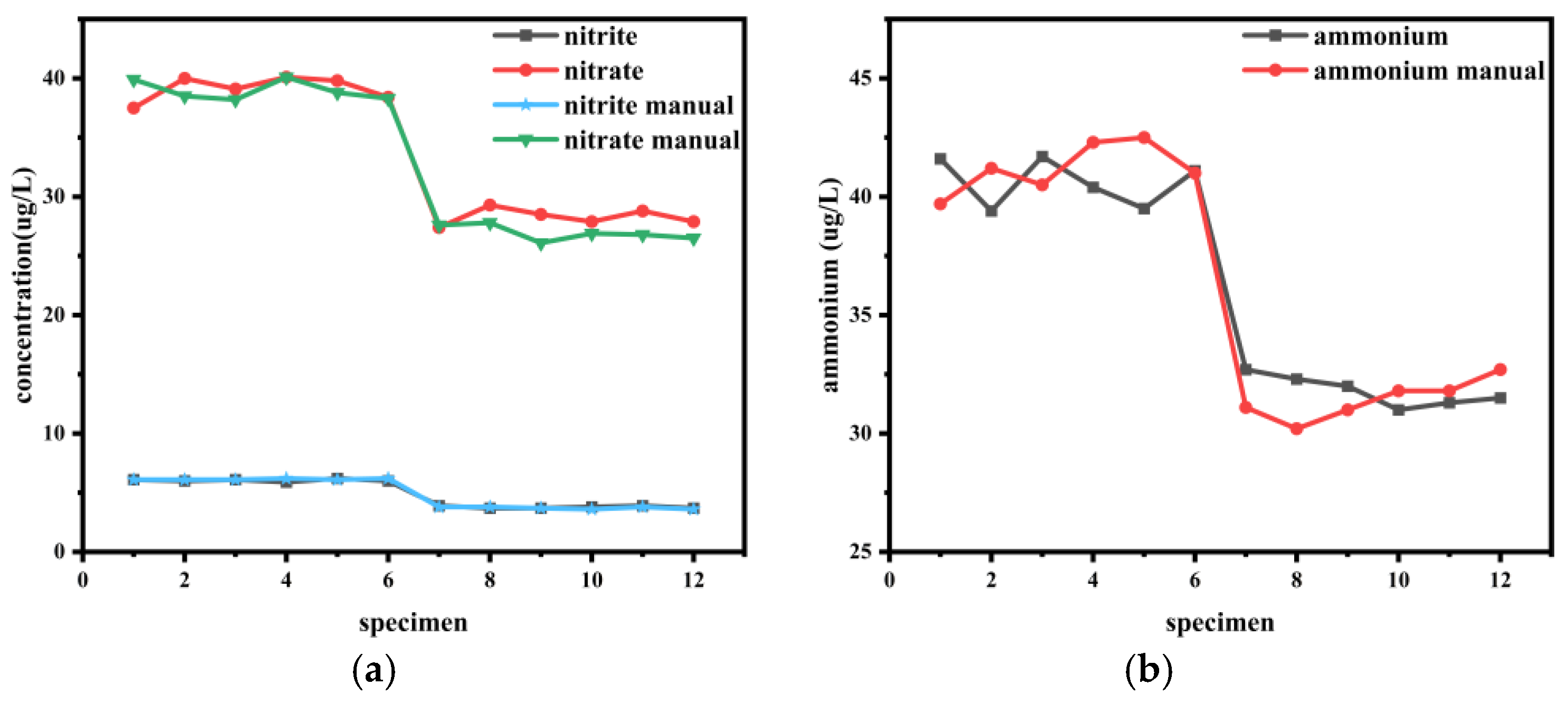
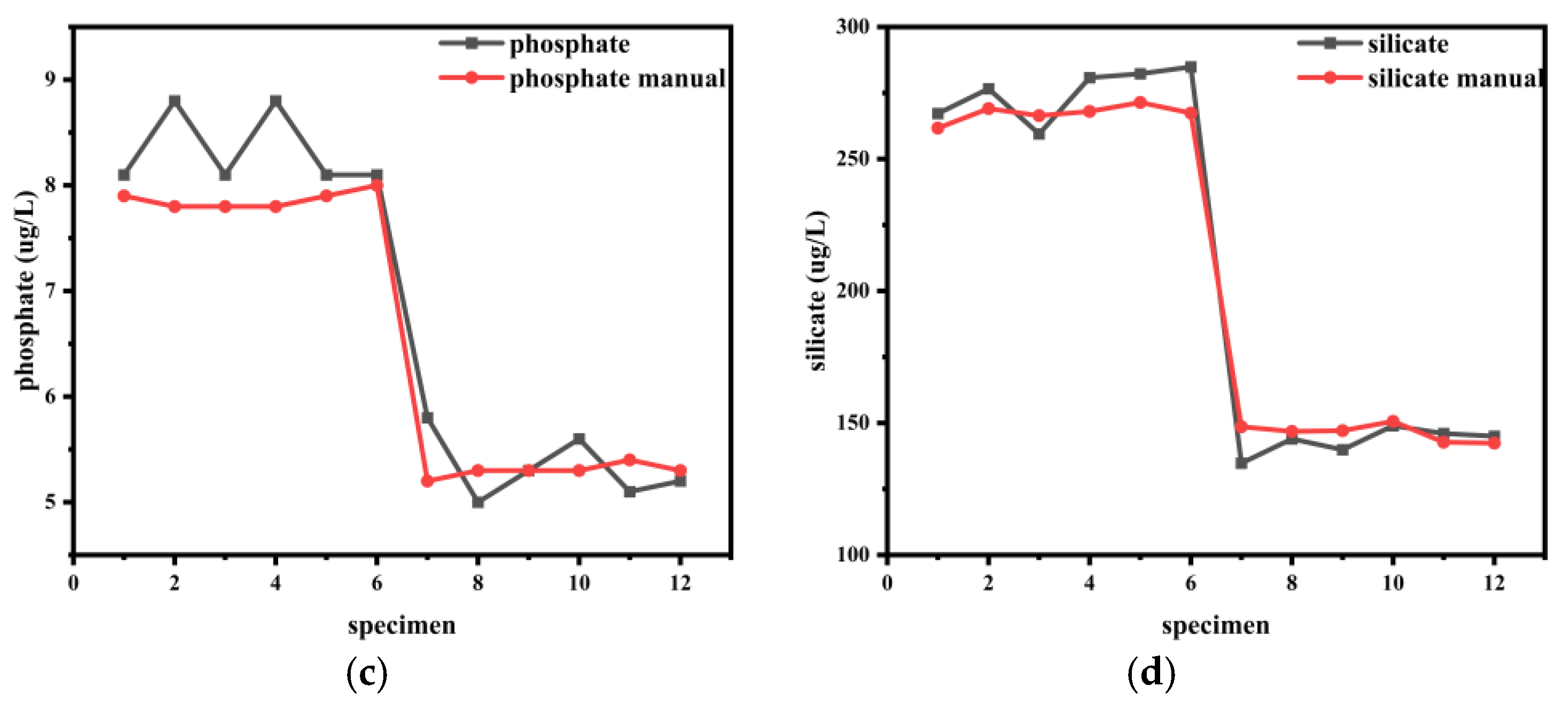
4.2. Comparison Between Manual Laboratory Analysis and Instrumental Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Designation in Text | Item Number | Producer |
|---|---|---|
| Sulfanilamide | S108473 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| HCl | H399657 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| NEDD | N105071 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Vanadium Chloride | V498277 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sodium Tetraborate | S112463 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sodium Sulfite | S112300 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| o-Phthaldialdehyde | P108633 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Ethanol | E329897 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sodium Molybdate | S104867 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Potassium Antimony Tartrate | P191240 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sulfuric Acid | S399848 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Ascorbic Acid | A103533 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sodium Dodecyl Sulfate | S108347 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Oxalic Acid | O107180 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
References
- Tivig, M.; Keller, D.P.; Oschlies, A. Riverine nutrient impact on global ocean nitrogen cycle feedbacks and marine primary production in an Earth system model. Biogeosciences 2024, 21, 4469–4493. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Tagliabue, A. Feedbacks between phytoplankton and nutrient cycles in a warming ocean. Nat. Geosci. 2024, 17, 495–502. [Google Scholar] [CrossRef]
- Hoel, P.; Bianchi, D.; Cavanaugh, K.C.; Freider, C.A.; Kessouri, F. Influence of anthropogenic nutrient sources on kelp canopies during a marine heat wave. Mar. Pollut. Bull. 2025, 216, 117788. [Google Scholar] [CrossRef]
- Anderson, S.I.; Franzè, G.; Kling, J.D.; Wilburn, P.; Kremer, C.T.; Deuer, S.M.; Litchman, E.; Hutchins, D.A.; Rynearson, T.A. The interactive effects of temperature and nutrients on a spring phytoplankton community. Limnol. Oceanogr. 2022, 67, 634–645. [Google Scholar] [CrossRef]
- Pang, M.; Liu, K.; Chen, B.; Zhang, X.; Gao, Z.; Xu, Z.; Tan, Y.; Yang, J.; Liu, H. Nutrient availability influences the thermal response of marine diatoms. Limnol. Oceanogr. 2024, 69, 2318–2331. [Google Scholar] [CrossRef]
- Thig, T.R.; Tackett, S.M.T.; Voolstra, C.R.; Ross, C.; Chaffron, S.; Durack, P.J.; Warmuth, L.M.; Sweet, M. Human-induced salinity changes impact marine organisms and ecosystems. Glob. Change Biol. 2023, 29, 4731–4749. [Google Scholar] [CrossRef]
- Su, H.; Zou, R.; Zhang, X.; Liang, Z.; Ye, R.; Liu, Y. Exploring the type and strength of nonlinearity in water quality responses to nutrient loading reduction in shallow eutrophic water bodies: Insights from a large number of numerical simulations. J. Environ. Manag. 2022, 313, 115000. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; White, J.R. On the calculation of carbon and nutrient transport to the oceans. Sci. Rep. 2025, 15, 8994. [Google Scholar] [CrossRef]
- Hamilton, D.S.; Perron, M.M.G.; Bond, T.C.; Bowie, A.R.; Buchholz, R.R.; Guieu, C.; Ito, A.; Maenhaut, W.; Myriokefalitakis, S.; Olgun, N.; et al. Earth, Wind, Fire, and Pollution: Aerosol Nutrient Sources and Impacts on Ocean Biogeochemistry. Annu. Rev. Mar. Sci. 2022, 14, 303–330. [Google Scholar] [CrossRef] [PubMed]
- Brandes, J.A.; Devol, A.H.; Deutsch, C. New Developments in the Marine Nitrogen Cycle. Chem. Rev. 2007, 107, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Dian, W.; Jinhui, Z.; Haonan, W.; Guixian, L.; Xiurong, H.; Tiantian, G. Effect of different measurement methods on data quality of nutrients in seawater. Xiamen Daxue Xuebao (Ziran Kexue Ban) 2020, 59, 93–98. [Google Scholar]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, W.; Miao, H.; Han, X. Effects and improvements of different reagents preservation methods on the determination of phosphate in seawater by phosphomolybdenum blue spectrophotometric method. Mar. Pollut. Bull. 2019, 139, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Hnelt, A.M.R.; Martin, P.R.; Haderlein, S.B. Limitations of the molybdenum blue method for phosphate quantification in the presence of organophosphonates. Anal. Bioanal. Chem. 2025, 417, 3103–3111. [Google Scholar] [CrossRef]
- Li, H.; Song, Y.; Zhou, B.; Xu, H. Nitrite: From Application to Detection and Development. Appl. Sci. 2024, 14, 9027. [Google Scholar] [CrossRef]
- Niu, W.; Li, H.; Guo, X.; Chen, J.; Shi, X.; Zhu, Y. Field determination of nitrate in seawater using a novel on-line coppered cadmium column: A comparison study with the vanadium reduction method. Front. Mar. Sci. 2023, 10, 1138734. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper Nanoparticles Confined in a Silica Nanochannel Film for the Electrochemical Detection of Nitrate Ions in Water Samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, K.; Zhu, X.; Su, J.; Wu, C. An Improved Algorithm for Measuring Nitrate Concentrations in Seawater Based on Deep-Ultraviolet Spectrophotometry: A Case Study of the Aoshan Bay Seawater and Western Pacific Seawater. Sensors 2021, 21, 965. [Google Scholar] [CrossRef]
- Sakamoto, C.; Johnson, K.; Coletti, L.; Maurer, T.; Massion, G.; Pennington, J.T.; Plant, J.; Jannasch, H.; Chavez, F. Hourly In Situ Nitrate on a Coastal Mooring: A 15-Year Record and Insights into New Production. Oceanography 2017, 30, 114–127. [Google Scholar] [CrossRef]
- Meyer, A.M.; Oliveri, E.; Kautenburger, R.; Hein, C.; Kickelbick, G.; Beck, H.P. In situ real-time monitoring of ammonium, potassium, chloride and nitrate in small and medium-sized rivers using ion-selective-electrodes—A case study of feasibility. Environ. Sci. Adv. 2025, 4, 1238–1249. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Weeser, B.R.; Rufino, M.C.; Breuer, L. Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts? Sensors 2020, 20, 859. [Google Scholar] [CrossRef]
- Altahan, M.F.; Esposito, M.; Achterberg, E.P. Improvement of On-Site Sensor for Simultaneous Determination of Phosphate, Silicic Acid, Nitrate plus Nitrite in Seawater. Sensors 2022, 22, 3479. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xiang, J.; Lin, D.; Fu, L.; Li, B.; Chen, L. Applications of Microfluidic Technology in Marine Analysis and Monitoring. Chin. J. Anal. Chem. 2023, 51, 1545–1556. [Google Scholar]
- Wang, L.; Huang, T.; Du, C.; Guo, X. Comparison of different continuous in-situ observation systems in seawater. J. Trop. Oceanogr. 2021, 40, 103–113. [Google Scholar]
- van der Molen, J.; Ruardij, P.; Greenwood, N. Potential environmental impact of tidal energy extraction in the Pentland Firth at large spatial scales: Results of a biogeochemical model. Biogeosciences 2016, 13, 2593–2609. [Google Scholar] [CrossRef]
- Fang, T.; Li, H.; Bo, G.; Lin, K.; Yuan, D.; Ma, J. On-site detection of nitrate plus nitrite in natural water samples using smartphone-based detection. Microchem. J. 2021, 165, 106117. [Google Scholar] [CrossRef]
- Fang, T.; Bo, G.; Zhang, Z.; Ma, J. Real-Time Underway Mapping of Nutrient Concentrations of Surface Seawater Using an Autonomous Flow Analyzer. Anal. Chem. 2022, 94, 11307–11314. [Google Scholar] [CrossRef]
- Vishnikin, A.; Hedjazi, M.; Al-Shwaiyat, M.; Skok, A.; Bazel, Y. Consecutive spectrophotometric determination of phosphate and silicate in a sequential injection lab-at-valve flow system. Anal. Chim. Acta 2023, 1273, 341464. [Google Scholar] [CrossRef]
- Hani, O.E.; Karrat, A.; Digua, K.; Amine, A. Development of a simplified spectrophotometric method for nitrite determination in water samples. Spectrochim. Acta A 2022, 267, 120574. [Google Scholar] [CrossRef]
- García-Robledo, E.; Corzo, A.; Papaspyrou, S. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar. Chem. 2014, 162, 30–36. [Google Scholar] [CrossRef]
- Guo, X.; Chen, J.; Shen, Y.; Li, H.; Zhu, Y. Evolution of the fluorometric method for the measurement of ammonium/ammonia in natural waters: A review. TrAC-Trends Anal. Chem. 2024, 171, 117519. [Google Scholar] [CrossRef]
- Ilyushin, B.B. On Applicability of IQR Method for Filtering of Experimental Data. J. Eng. Thermophys. 2024, 33, 1–8. [Google Scholar] [CrossRef]
- SAC. The Specification for Marine Monitoring Part 2: Data Processing and Quality Control of Analysis. Standardization Administration of the People’s Republic of China. 2007. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=2A832569DF947259C8793864F584618F (accessed on 12 August 2025).


| Nutrient | Principle | Detection Wavelength (nm) |
|---|---|---|
| Nitrite | N-(1-Naphthyl)ethylenediamine spectrophotometry(NEDD) | 540 |
| Nitrate | Vanadium chloride reduction coupled with N-(1-Naphthyl)ethylenediamine spectrophotometry | 540 |
| Ammonium | o-Phthaldialdehyde (OPA) fluorometry | Ex: 360; Em: 400–480 |
| Phosphate | Phosphomolybdenum blue spectrophotometry | 880 |
| Silicate | Silicomolybdenum blue spectrophotometry | 810 |
| Nutrient | Reagent Name and Formulation |
|---|---|
| Nitrite | Sulfanilamide Solution: Sulfanilamide 4 g/L, HCl 1.8 mol/L. NEDD Solution: NEDD 0.4 g/L. Mixed Solution: Sulfanilamide Solution and NEDD Solution mixed at a volume ratio of 1:1. |
| Nitrate | Mixed Solution: The same as the mixed solution of nitrite in the table. Vanadium Chloride Solution: Vanadium Chloride 8 g/L, HCl 1.0 mol/L. |
| Ammonium | OPA Mixed Solution: Sodium Tetraborate 20 g/L, Sodium Sulfite 0.24 g/L, o-Phthaldialdehyde 4 g/L, Ethanol 8% (v/v). |
| Phosphate | Sodium Molybdate Solution: Sodium Molybdate 28 g/L, Potassium Antimony Tartrate 0.48 g/L, Sulfuric Acid 2 mol/L. |
| Ascorbic Acid Solution: Ascorbic Acid 50 g/L, Sodium Dodecyl Sulfate (SDS) 2.5 g/L. | |
| Silicate | Acidic Sodium Molybdate Solution: Sodium Molybdate 15.5 g/L, Sulfuric Acid 0.5 mol/L. Oxalic Acid Solution: Oxalic Acid 75 g/L. Ascorbic Acid Solution: Ascorbic Acid 50 g/L, Sodium Dodecyl Sulfate (SDS) 2.5 g/L. |
| Parameter | Linear Range (μg/L) | Regression Equation | R2 | LOD (μg/L) |
|---|---|---|---|---|
| NO2− | 5–50 | y = 0.01504x + 0.0024 | 0.9994 | 2.34 |
| NO3− | 10–100 | y = 0.00838x + 0.00563 | 0.9993 | 4.65 |
| NH4+ | 10–100 | y = 0.01567x − 0.00333 | 0.9993 | 2.94 |
| PO43− | 10–100 | y = 0.00776x − 0.0022 | 0.9994 | 3.46 |
| SiO32− | 50–500 | y = 0.00146x + 0.00333 | 0.9993 | 9.40 |
| Parameter | Concentration (μg/L) | Measurement 1 (μg/L) | Measurement 2 (μg/L) | Measurement 3 (μg/L) | Mean Value (μg/L) | δ (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| NO2− | 15 | 14.46 | 14.88 | 14.75 | 14.70 | −2.02 | 1.46 |
| 40 | 39.71 | 39.14 | 39.20 | 39.35 | −1.63 | 0.80 | |
| NO3− | 30 | 29.62 | 29.97 | 29.23 | 29.61 | −1.31 | 1.25 |
| 80 | 78.89 | 77.87 | 78.24 | 78.33 | −2.08 | 0.66 | |
| PO43− | 30 | 29.95 | 31.72 | 30.97 | 30.97 | 3.22 | 2.95 |
| 80 | 85.92 | 83.36 | 84.34 | 84.54 | 5.68 | 1.53 | |
| SiO32− | 150 | 150.66 | 145.1 | 146.83 | 147.53 | −1.65 | 1.93 |
| 400 | 415.14 | 420.33 | 408.30 | 414.59 | 3.65 | 1.46 | |
| NH4+ | 30 | 29.5 | 27.81 | 28.73 | 28.68 | −4.40 | 2.95 |
| 80 | 75.12 | 78.84 | 79.83 | 77.93 | −2.59 | 3.19 |
| Analyzer | Detection Method | LOD (μg/L) | Time | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| NO2− | NO3− | PO43− | SiO32− | NH4+ | ||||
| CuNPs/NH2-VMSF/ITO | ultraviolet spectroscopy | 143.0 | - | - | - | - | 5 min | 17 |
| SIA-LAV | Wet-chemical analysis | - | - | 5.0 | 3.8 | - | 12 min | 28 |
| Smartphone-based detector | Wet-chemical analysis | - | 9.3 | - | - | - | 15 min | 26 |
| AutoLAB multi-nutrient analyzer | Wet-chemical analysis | 16.1 | 27.9 | 17.1 | 9.0 | - | 66 min | 22 |
| This equipment | Wet-chemical analysis | 2.34 | 4.65 | 3.46 | 9.40 | 2.94 | 20 min | This |
| Parameter | Station 1 Average (μg/L) | Station 2 Average (μg/L) | Absolute Error (μg/L) | Relative Error (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Auto | Manual | Auto | Manual | Station 1 | Station 2 | Station 1 | Station 2 | |
| NO2− | 6.05 | 6.13 | 3.78 | 3.72 | 0.08 | 0.07 | −1.36 | 1.79 |
| NO3− | 39.15 | 38.97 | 28.30 | 26.95 | 0.18 | 1.35 | 0.47 | 5.01 |
| NH4+ | 40.62 | 41.20 | 31.80 | 31.43 | 0.58 | 0.37 | −1.42 | 1.17 |
| PO43− | 8.33 | 7.87 | 5.33 | 5.30 | 0.47 | 0.03 | 5.93 | 0.63 |
| SiO32− | 275.22 | 267.33 | 143.12 | 146.37 | 7.88 | 3.25 | 2.95 | −2.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, Y.; Zhang, J.; Wang, S.; Wang, H. A Traveling Multi-Analyte Chemosensor Based on Wet-Chemical Colorimetry for Shipboard Seawater Analysis. Appl. Sci. 2025, 15, 12861. https://doi.org/10.3390/app152412861
Wang J, Wu Y, Zhang J, Wang S, Wang H. A Traveling Multi-Analyte Chemosensor Based on Wet-Chemical Colorimetry for Shipboard Seawater Analysis. Applied Sciences. 2025; 15(24):12861. https://doi.org/10.3390/app152412861
Chicago/Turabian StyleWang, Jianzhang, Yingxia Wu, Jian Zhang, Shengli Wang, and Hongliang Wang. 2025. "A Traveling Multi-Analyte Chemosensor Based on Wet-Chemical Colorimetry for Shipboard Seawater Analysis" Applied Sciences 15, no. 24: 12861. https://doi.org/10.3390/app152412861
APA StyleWang, J., Wu, Y., Zhang, J., Wang, S., & Wang, H. (2025). A Traveling Multi-Analyte Chemosensor Based on Wet-Chemical Colorimetry for Shipboard Seawater Analysis. Applied Sciences, 15(24), 12861. https://doi.org/10.3390/app152412861






