Complex Network Responses to Regulation of a Brain-Computer Interface During Semi-Naturalistic Behavior
Abstract
1. Introduction
2. Materials and Methods
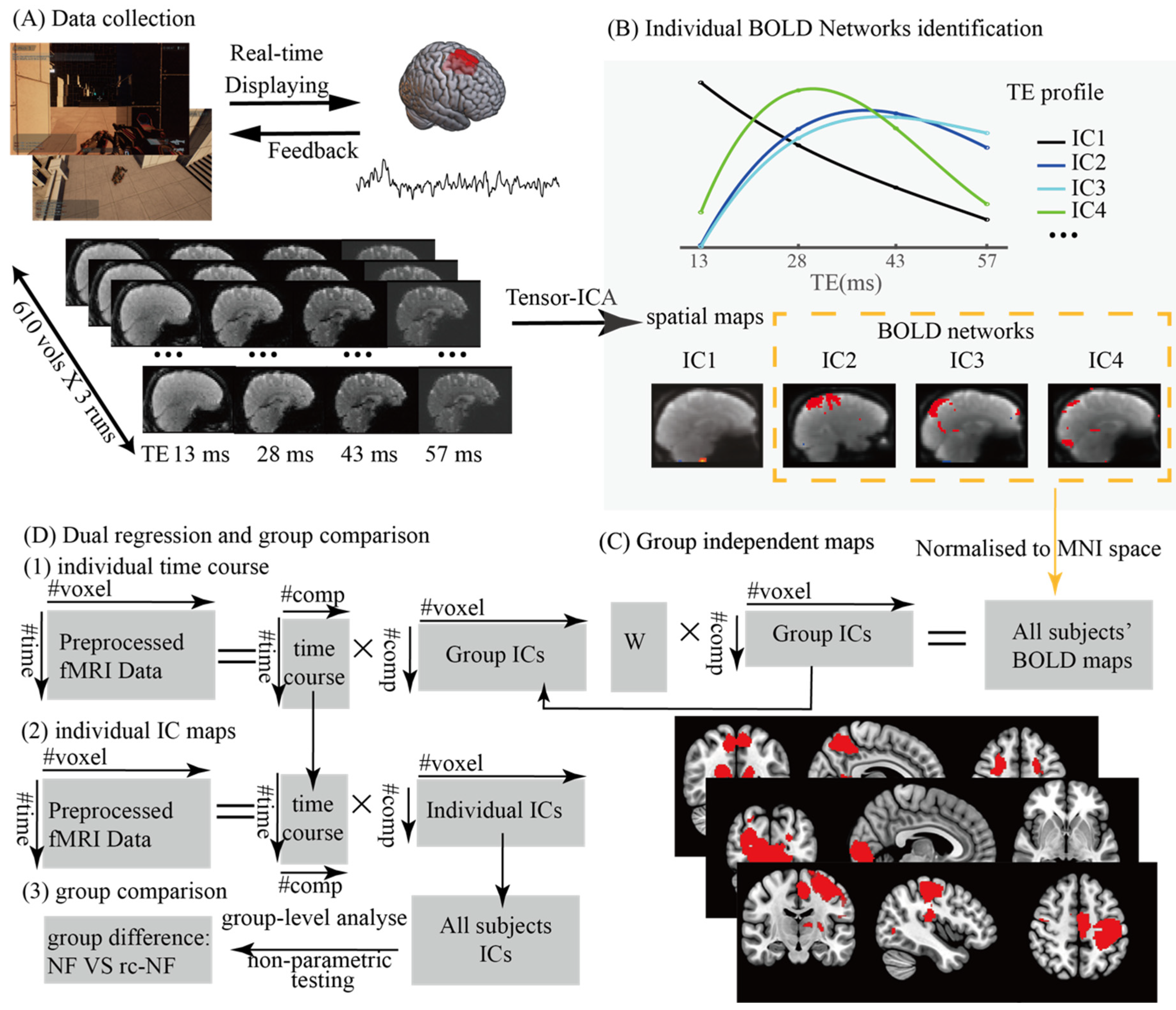
2.1. Participants and Experimental Design
2.2. MRI Data Acquisition and Preprocessing
2.3. Group Networks Under BCI Regulation and Dual Regression for Comparison
3. Results
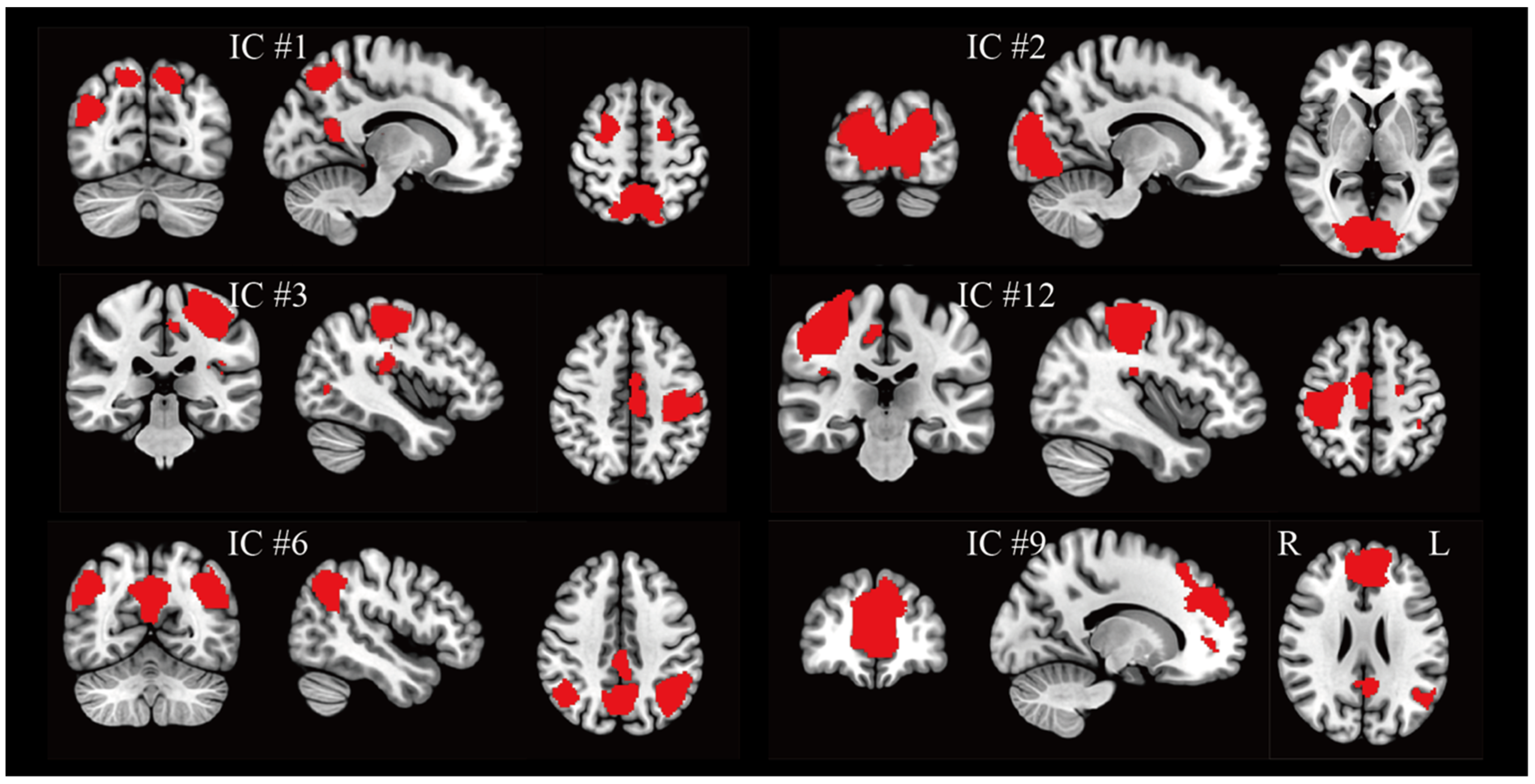
3.1. Group Networks Under BCI Regulation
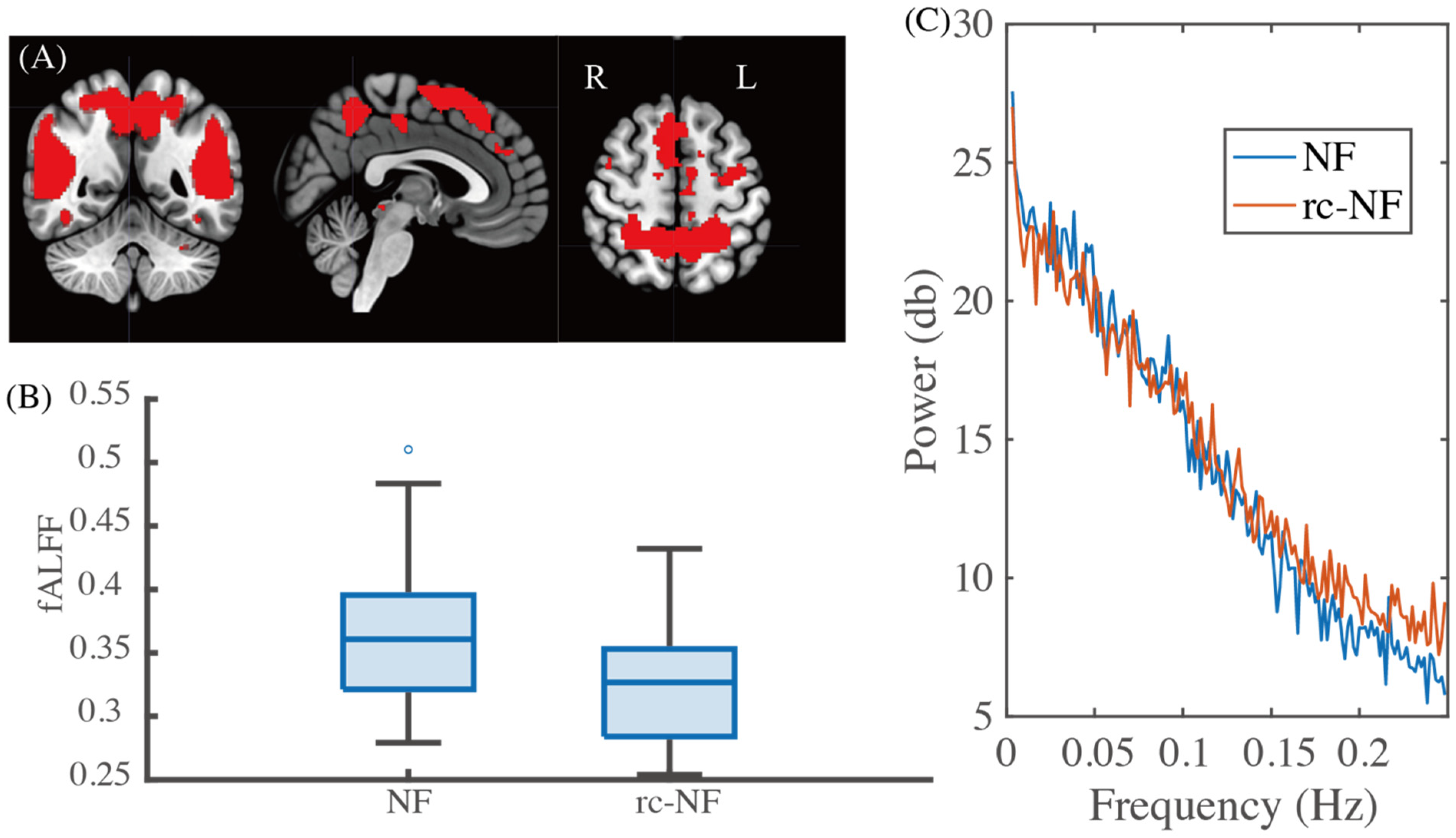
3.2. Group Difference in Network Dynamics
3.3. Group Differences in Spatial Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCI | Brain–computer interface |
| SMA | supplementary motor area |
| fALFF | fractional amplitude of low-frequency fluctuations |
| rt-fMRI | Real-time functional magnetic resonance imaging |
| BOLD | blood-oxygen-level-dependent |
| VE | virtual environment |
| ROI | Region-of-interest |
| ICA | Independent component analysis |
| NF | Neurofeedback |
References
- Zhang, H.; Jiao, L.; Yang, S.; Li, H.; Jiang, X.; Feng, J.; Zou, S.; Xu, Q.; Gu, J.; Wang, X.; et al. Brain–Computer Interfaces: The Innovative Key to Unlocking Neurological Conditions. Int. J. Surg. 2024, 110, 5745–5762. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Chen, X.; Gao, S. Interface, Interaction, and Intelligence in Generalized Brain–Computer Interfaces. Trends Cogn. Sci. 2021, 25, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-Loop Brain Training: The Science of Neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef]
- Sarkheil, P.; Zilverstand, A.; Kilian-Hütten, N.; Schneider, F.; Goebel, R.; Mathiak, K. fMRI Feedback Enhances Emotion Regulation as Evidenced by a Reduced Amygdala Response. Behav. Brain Res. 2015, 281, 326–332. [Google Scholar] [CrossRef]
- Zweerings, J.; Sarkheil, P.; Keller, M.; Dyck, M.; Klasen, M.; Becker, B.; Gaebler, A.J.; Ibrahim, C.N.; Turetsky, B.I.; Zvyagintsev, M.; et al. Rt-fMRI Neurofeedback-Guided Cognitive Reappraisal Training Modulates Amygdala Responsivity in Posttraumatic Stress Disorder. NeuroImage Clin. 2020, 28, 102483. [Google Scholar] [CrossRef]
- Mel’nikov, M.Y.; Bezmaternykh, D.D.; Savelov, A.A.; Petrovskiy, E.D.; Kozlova, L.I.; Natarova, K.A.; Larina, T.D.; Andamova, T.M.; Zvyagintsev, M.; Shtark, M.B.; et al. Real-Time fMRI Neurofeedback Compared to Cognitive Behavioral Therapy in a Pilot Study for the Treatment of Mild and Moderate Depression. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1139–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Becker, B.; Kendrick, K.M.; Zhang, Q.; Yao, S. Self-Navigating the “Island of Reil”: A Systematic Review of Real-Time fMRI Neurofeedback Training of Insula Activity. Transl. Psychiatry 2025, 15, 170. [Google Scholar] [CrossRef]
- Pindi, P.; Houenou, J.; Piguet, C.; Favre, P. Real-Time fMRI Neurofeedback as a New Treatment for Psychiatric Disorders: A Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110605. [Google Scholar] [CrossRef]
- Baqapuri, H.I.; Roes, L.D.; Zvyagintsev, M.; Ramadan, S.; Kell, M.; Roecher, E.; Zweerings, J.; Klasen, M.; Gur, R.C.; Mathiak, K. A Novel Brain–Computer Interface Virtual Environment for Neurofeedback During Functional MRI. Front. Neurosci. 2021, 14, 593854. [Google Scholar] [CrossRef] [PubMed]
- Watve, A.; Haugg, A.; Frei, N.; Koush, Y.; Willinger, D.; Bruehl, A.B.; Stämpfli, P.; Scharnowski, F.; Sladky, R. Facing Emotions: Real-Time fMRI-Based Neurofeedback Using Dynamic Emotional Faces to Modulate Amygdala Activity. Front. Neurosci. 2024, 17, 1286665. [Google Scholar] [CrossRef]
- Du, B.; Cheng, X.; Duan, Y.; Ning, H. fMRI Brain Decoding and Its Applications in Brain–Computer Interface: A Survey. Brain Sci. 2022, 12, 228. [Google Scholar] [CrossRef]
- Wen, D.; Wei, Z.; Zhou, Y.; Li, G.; Zhang, X.; Han, W. Deep Learning Methods to Process fMRI Data and Their Application in the Diagnosis of Cognitive Impairment: A Brief Overview and Our Opinion. Front. Neuroinform. 2018, 12, 23. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Z.; Ma, L. SED-GPT: A Non-Invasive Method for Long-Sequence Fine-Grained Semantics and Emotions Decoding. Appl. Sci. 2025, 15, 11100. [Google Scholar] [CrossRef]
- Floren, A.; Naylor, B.; Miikkulainen, R.; Ress, D. Accurately Decoding Visual Information from fMRI Data Obtained in a Realistic Virtual Environment. Front. Hum. Neurosci. 2015, 9, 327. [Google Scholar] [CrossRef]
- Guo, J.; Yi, C.; Li, F.; Xu, P.; Tian, Y. MindLDM: Reconstruct Visual Stimuli from fMRI Using Latent Diffusion Model. In Proceedings of the 2024 IEEE International Conference on Computational Intelligence and Virtual Environments for Measurement Systems and Applications (CIVEMSA), Xi’an, China, 14–16 June 2024; pp. 1–6. [Google Scholar]
- Rithwik, P.; Benzy, V.K.; Vinod, A.P. High Accuracy Decoding of Motor Imagery Directions from EEG-Based Brain Computer Interface Using Filter Bank Spatially Regularised Common Spatial Pattern Method. Biomed. Signal Process. Control 2022, 72, 103241. [Google Scholar] [CrossRef]
- Liu, F.; Meamardoost, S.; Gunawan, R.; Komiyama, T.; Mewes, C.; Zhang, Y.; Hwang, E.; Wang, L. Deep Learning for Neural Decoding in Motor Cortex. J. Neural Eng. 2022, 19, 056021. [Google Scholar] [CrossRef] [PubMed]
- Candelori, B.; Bardella, G.; Spinelli, I.; Ramawat, S.; Pani, P.; Ferraina, S.; Scardapane, S. Spatio-Temporal Transformers for Decoding Neural Movement Control. J. Neural Eng. 2025, 22, 016023. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Wood, G.; Berger, L.M. Controlling Virtual Reality With Brain Signals: State of the Art of Using VR-Based Feedback in Neurofeedback Applications. Appl. Psychophysiol. Biofeedback 2024. [Google Scholar] [CrossRef]
- Piszcz, A.; Rojek, I.; Mikołajewski, D. Impact of Virtual Reality on Brain–Computer Interface Performance in IoT Control—Review of Current State of Knowledge. Appl. Sci. 2024, 14, 10541. [Google Scholar] [CrossRef]
- Castanho, L.; Martinho, D.V.; Saial, A.C.; Gouveia, B.R.; Gouveia, É.R.; Ribeiro, F. The Efficacy of Virtual Reality-Based EEG Neurofeedback in Health-Related Symptoms Relief: A Systematic Review. Appl. Psychophysiol. Biofeedback 2025. [Google Scholar] [CrossRef]
- Pinheiro, J.; de Almeida, R.S.; Marques, A. Emotional Self-Regulation, Virtual Reality and Neurofeedback. Comput. Hum. Behav. Rep. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Li, K.; Huang, W.; Gao, W.; Guan, Z.; Huang, Q.; Yu, J.-G.; Yu, Z.L.; Li, Y. An Electroencephalography-Based Brain-Computer Interface for Emotion Regulation With Virtual Reality Neurofeedback. IEEE Trans. Cogn. Dev. Syst. 2024, 16, 1405–1417. [Google Scholar] [CrossRef]
- Cong, F.; Puoliväli, T.; Alluri, V.; Sipola, T.; Burunat, I.; Toiviainen, P.; Nandi, A.K.; Brattico, E.; Ristaniemi, T. Key Issues in Decomposing fMRI during Naturalistic and Continuous Music Experience with Independent Component Analysis. J. Neurosci. Methods 2014, 223, 74–84. [Google Scholar] [CrossRef]
- Feng, T.; Gaebler, A.J.; Keller, M.; Zweerings, J.; Li, H.; Cong, F.; Mathiak, K. Basic Stimulus Processing Alterations from Top-down Cognitive Control in Depression Drive Independent Temporal Components of Multi-Echo Naturalistic fMRI Data. Transl. Psychiatry 2025, 15, 171. [Google Scholar] [CrossRef]
- Koush, Y.; Masala, N.; Scharnowski, F.; Van De Ville, D. Data-Driven Tensor Independent Component Analysis for Model-Based Connectivity Neurofeedback. NeuroImage 2019, 184, 214–226. [Google Scholar] [CrossRef]
- Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct Patterns of Brain Activity in Young Carriers of the APOE-Ε4 Allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, L.D.; Smith, S.M.; Öngür, D.; Beckmann, C.F. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front. Neurosci. 2017, 11, 115. [Google Scholar] [CrossRef]
- Salman, M.S.; Du, Y.; Lin, D.; Fu, Z.; Fedorov, A.; Damaraju, E.; Sui, J.; Chen, J.; Mayer, A.R.; Posse, S.; et al. Group ICA for Identifying Biomarkers in Schizophrenia: ‘Adaptive’ Networks via Spatially Constrained ICA Show More Sensitivity to Group Differences than Spatio-Temporal Regression. NeuroImage Clin. 2019, 22, 101747. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Silva, R.F.; Adalı, T.; Rachakonda, S. Comparison of PCA Approaches for Very Large Group ICA. NeuroImage 2015, 118, 662–666. [Google Scholar] [CrossRef]
- Hu, G.; Waters, A.B.; Aslan, S.; Frederick, B.; Cong, F.; Nickerson, L.D. Snowball ICA: A Model Order Free Independent Component Analysis Strategy for Functional Magnetic Resonance Imaging Data. Front. Neurosci. 2020, 14, 569657. [Google Scholar] [CrossRef]
- Feng, T.; Baqapuri, H.I.; Zweerings, J.; Li, H.; Cong, F.; Mathiak, K. Characterizing the Distribution of Neural and Non-Neural Components in Multi-Echo EPI Data across Echo Times Based on Tensor-ICA. NeuroImage 2025, 311, 121199. [Google Scholar] [CrossRef]
- Bhavsar, S.; Zvyagintsev, M.; Mathiak, K. BOLD Sensitivity and SNR Characteristics of Parallel Imaging-Accelerated Single-Shot Multi-Echo EPI for fMRI. NeuroImage 2014, 84, 65–75. [Google Scholar] [CrossRef]
- Erhardt, E.B.; Rachakonda, S.; Bedrick, E.J.; Allen, E.A.; Adali, T.; Calhoun, V.D. Comparison of Multi-Subject ICA Methods for Analysis of fMRI Data. Hum. Brain Mapp. 2011, 32, 2075–2095. [Google Scholar] [CrossRef]
- Zou, Q.-H.; Zhu, C.-Z.; Yang, Y.; Zuo, X.-N.; Long, X.-Y.; Cao, Q.-J.; Wang, Y.-F.; Zang, Y.-F. An Improved Approach to Detection of Amplitude of Low-Frequency Fluctuation (ALFF) for Resting-State fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Menon, V. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Cole, M.W.; Reynolds, J.R.; Power, J.D.; Repovs, G.; Anticevic, A.; Braver, T.S. Multi-Task Connectivity Reveals Flexible Hubs for Adaptive Task Control. Nat. Neurosci. 2013, 16, 1348–1355. [Google Scholar] [CrossRef]
- Sulzer, J.; Haller, S.; Scharnowski, F.; Weiskopf, N.; Birbaumer, N.; Blefari, M.L.; Bruehl, A.B.; Cohen, L.G.; deCharms, R.C.; Gassert, R.; et al. Real-Time fMRI Neurofeedback: Progress and Challenges. NeuroImage 2013, 76, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.; Levitin, D.J.; Menon, V. A Critical Role for the Right Fronto-Insular Cortex in Switching between Central-Executive and Default-Mode Networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Korb, F.M.; Goschke, T.; Zwosta, K. Salience Network Resting-State Functional Connectivity Predicts Self-Controlled Decision-Making. Sci. Rep. 2025, 15, 16332. [Google Scholar] [CrossRef]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The Role of the Salience Network in Cognitive and Affective Deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional Role of the Supplementary and Pre-Supplementary Motor Areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.; Breakspear, M.; Guo, C. Naturalistic Stimuli in Neuroscience: Critically Acclaimed. Trends Cogn. Sci. 2019, 23, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Mathiak, K.; Weber, R. Toward Brain Correlates of Natural Behavior: fMRI during Violent Video Games. Hum. Brain Mapp. 2006, 27, 948–956. [Google Scholar] [CrossRef] [PubMed]

| Cluster # | Brain Label | Peaks’ MNI Coordinates | t | kE | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Left inferior parietal | −48 | −46 | 36 | 6.17 | 2532 |
| Left postcentral | −40 | −36 | 50 | 5.74 | ||
| Left inferior parietal | −46 | −44 | 52 | 5.32 | ||
| 2 | Left middle frontal | −26 | 36 | 26 | 5.78 | 6704 |
| Left superior frontal | 20 | −30 | 38 | 5.71 | ||
| Left superior frontal | −26 | −2 | −54 | 5.53 | ||
| 3 | Left calcarine | 16 | −80 | 8 | 5.25 | 1354 |
| Left middle occipital | −44 | −68 | 0 | 4.54 | ||
| Left inferior occipital | −34 | −74 | −8 | 3.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Baqapuri, H.I.; Zweerings, J.; Mathiak, K. Complex Network Responses to Regulation of a Brain-Computer Interface During Semi-Naturalistic Behavior. Appl. Sci. 2025, 15, 12583. https://doi.org/10.3390/app152312583
Feng T, Baqapuri HI, Zweerings J, Mathiak K. Complex Network Responses to Regulation of a Brain-Computer Interface During Semi-Naturalistic Behavior. Applied Sciences. 2025; 15(23):12583. https://doi.org/10.3390/app152312583
Chicago/Turabian StyleFeng, Tengfei, Halim Ibrahim Baqapuri, Jana Zweerings, and Klaus Mathiak. 2025. "Complex Network Responses to Regulation of a Brain-Computer Interface During Semi-Naturalistic Behavior" Applied Sciences 15, no. 23: 12583. https://doi.org/10.3390/app152312583
APA StyleFeng, T., Baqapuri, H. I., Zweerings, J., & Mathiak, K. (2025). Complex Network Responses to Regulation of a Brain-Computer Interface During Semi-Naturalistic Behavior. Applied Sciences, 15(23), 12583. https://doi.org/10.3390/app152312583






