Comparative Study of the Bioactive Compound Content of Sweet Potato Varieties Grown in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Classification
2.2. Sample Preparation and Extraction
2.3. Reagents and Instrumentation
2.4. Determination of Flavonoid Content
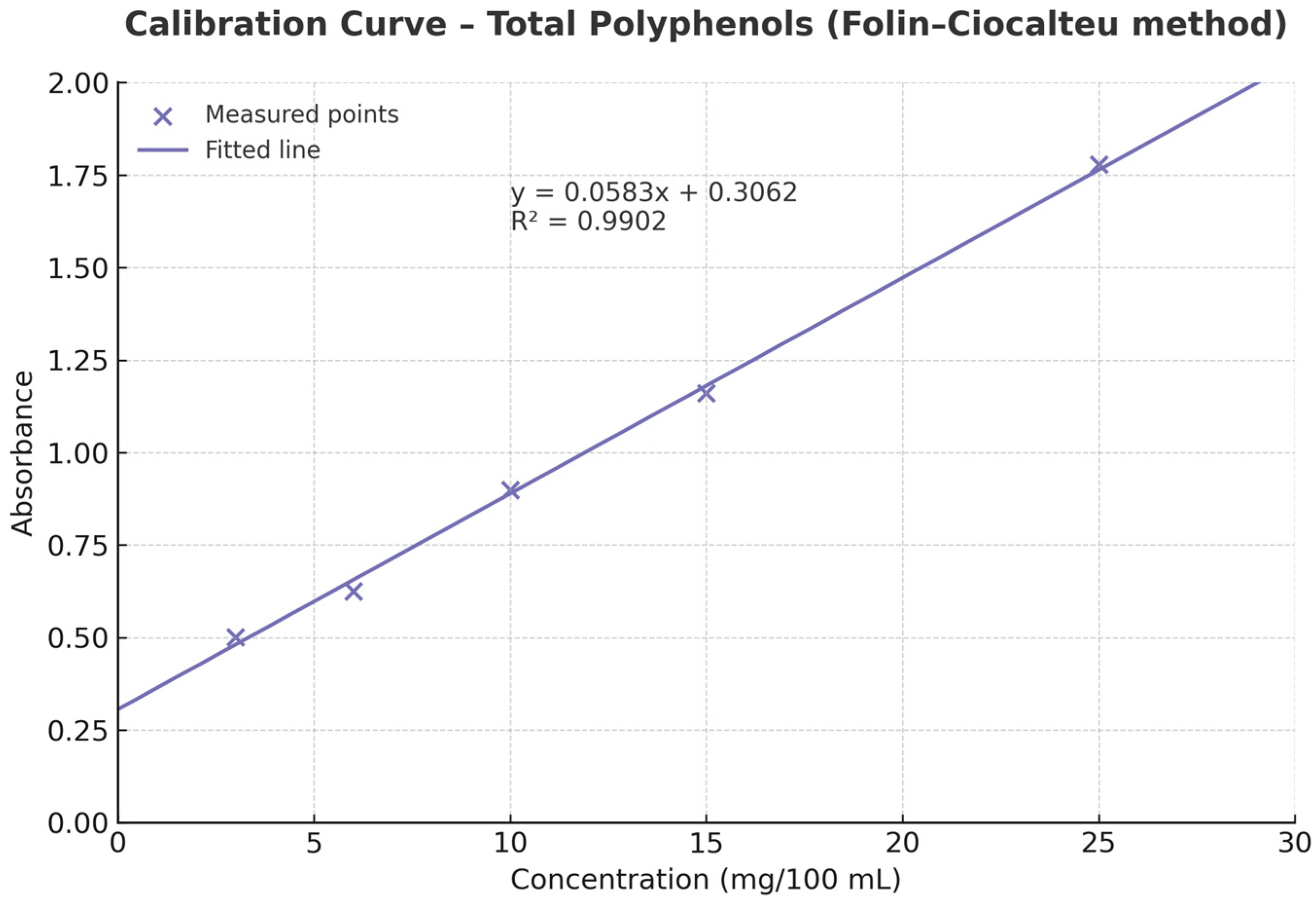
2.5. Determination of Phenolic Content
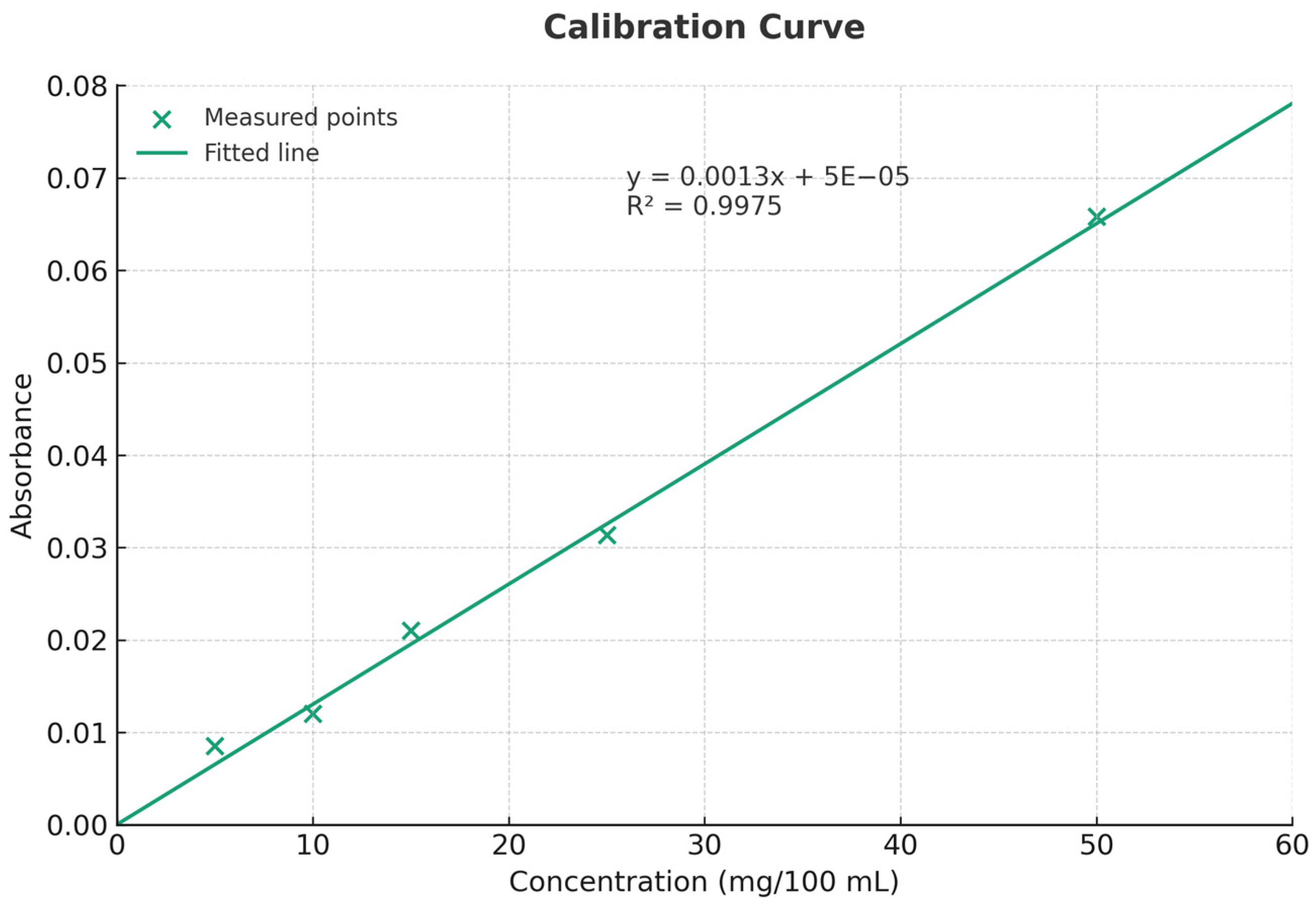
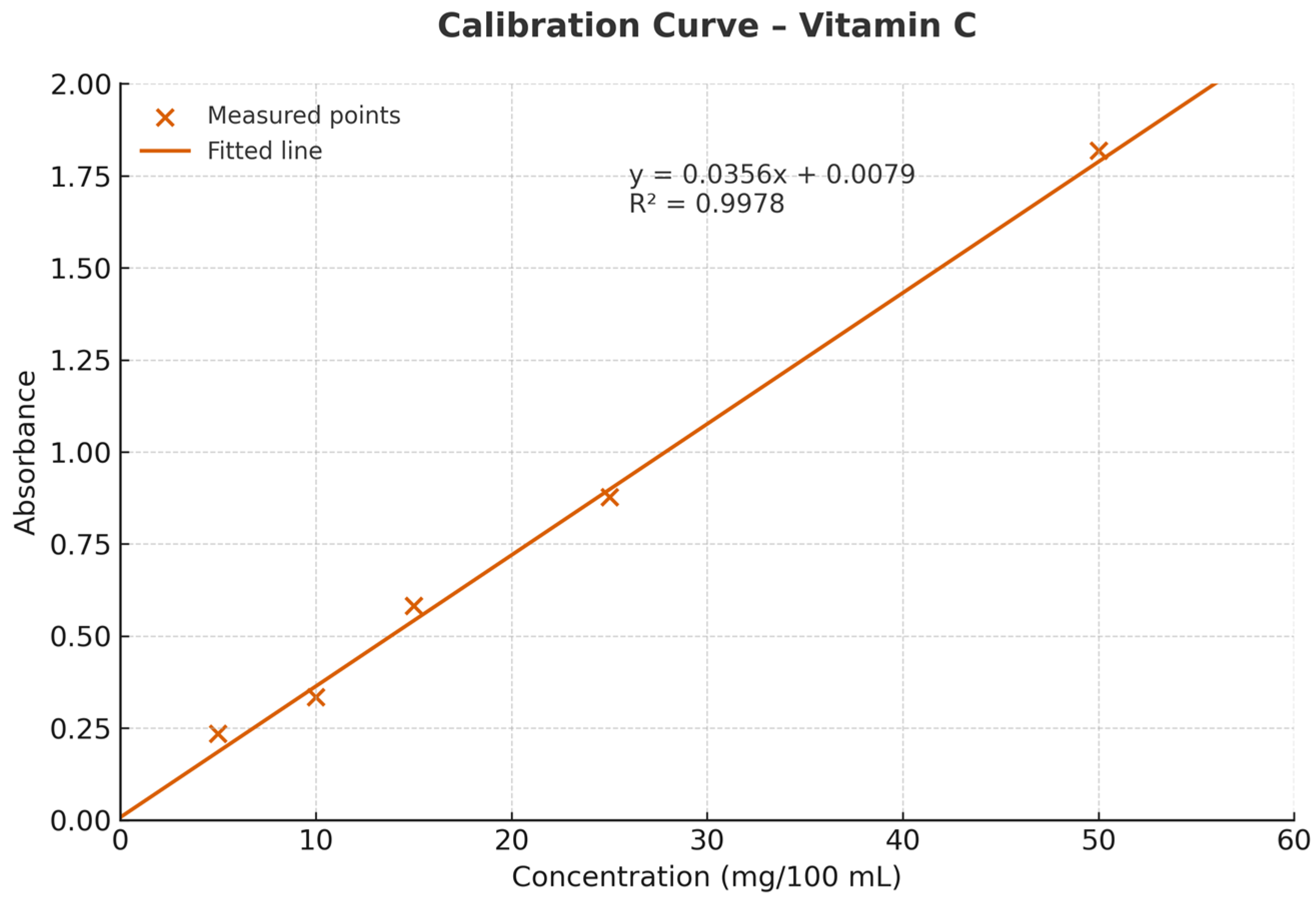
2.6. Determination of Vitamin C Content
2.7. Statistical Analysis
3. Results
3.1. Influence of Tuber Flesh Colour and Growing Region on Antioxidant Parameters
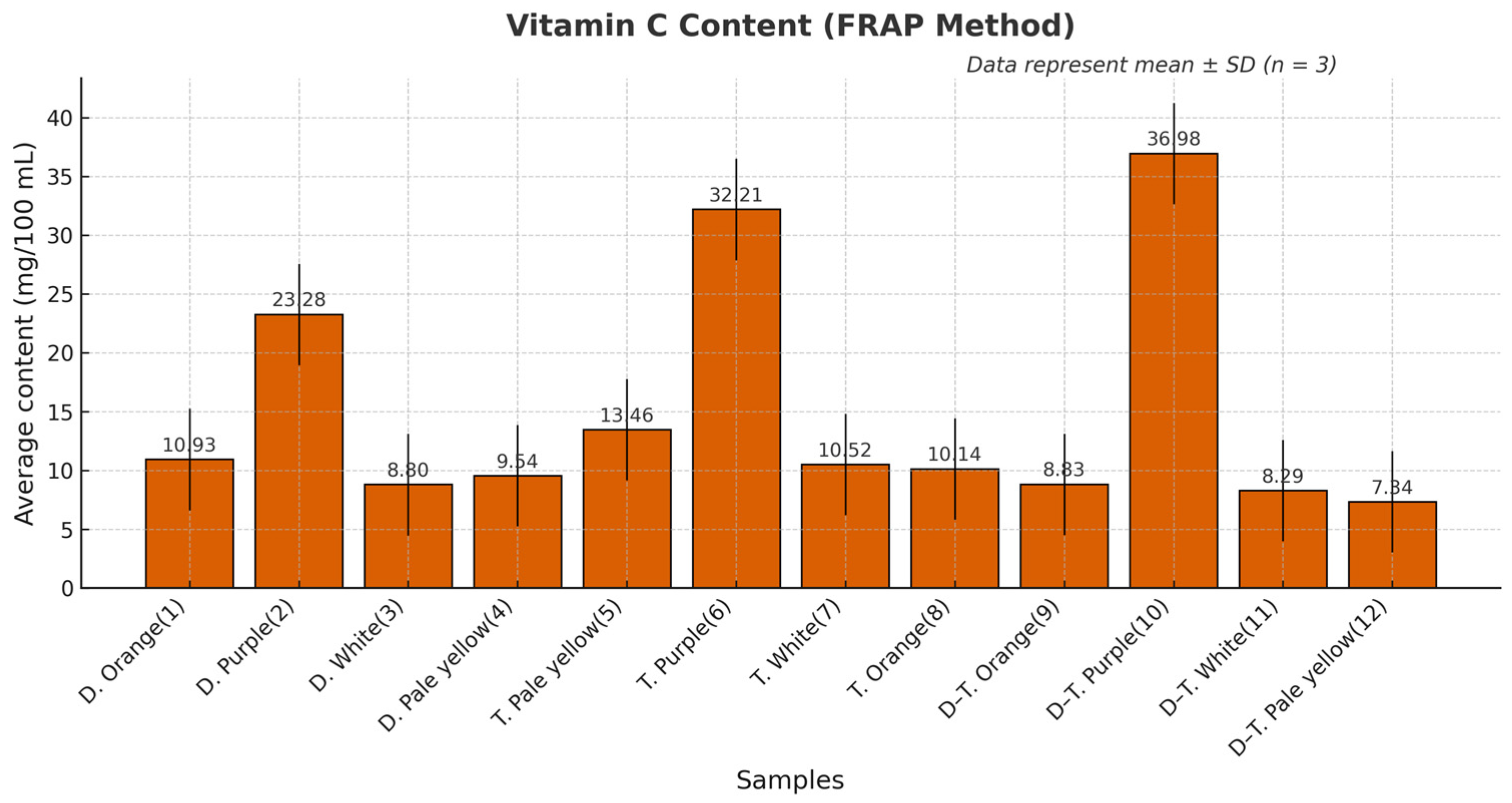
3.2. Total Vitamin C Content
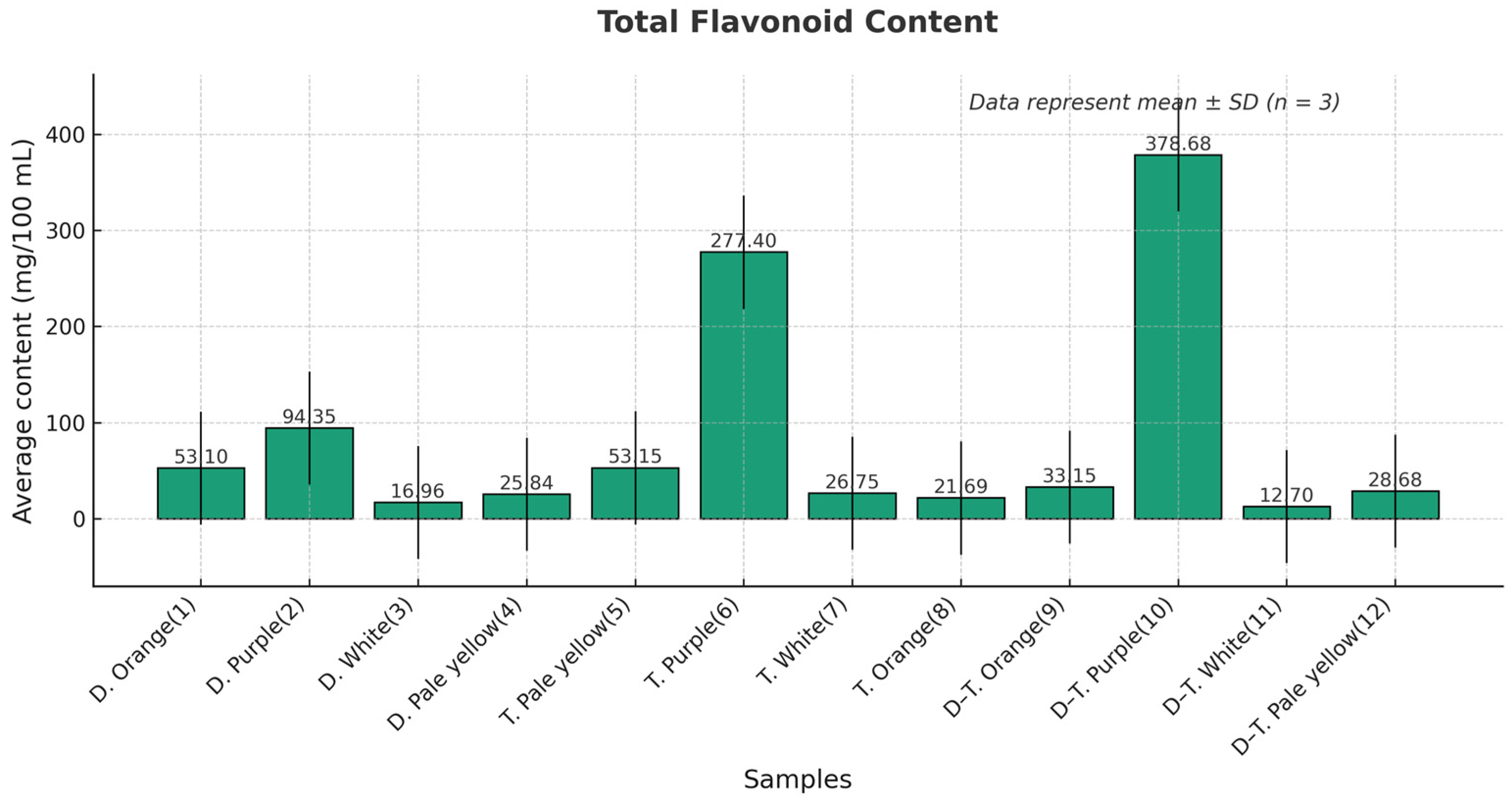
3.3. Total Flavonoid Content
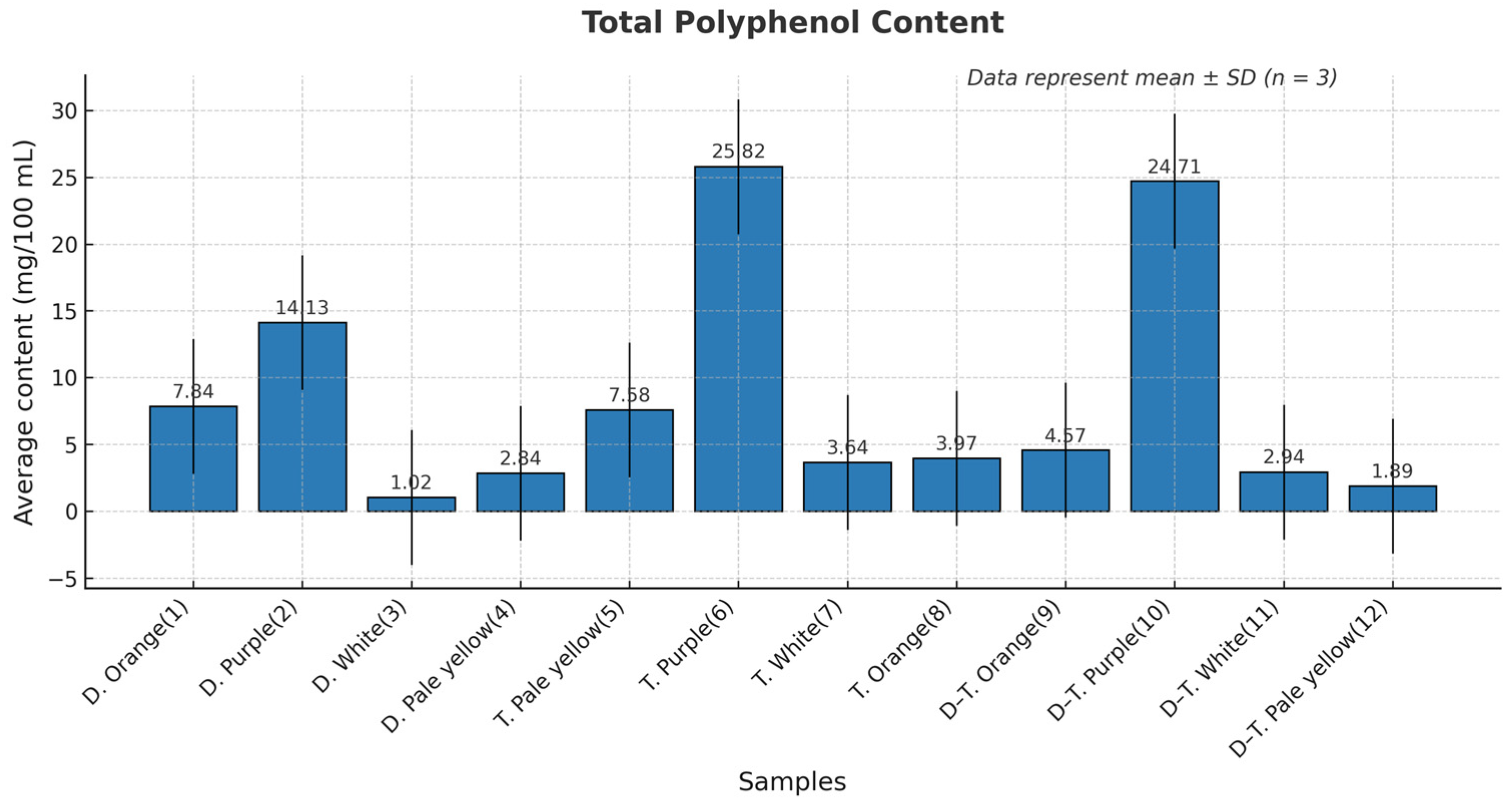
3.4. Total Phenolic Content
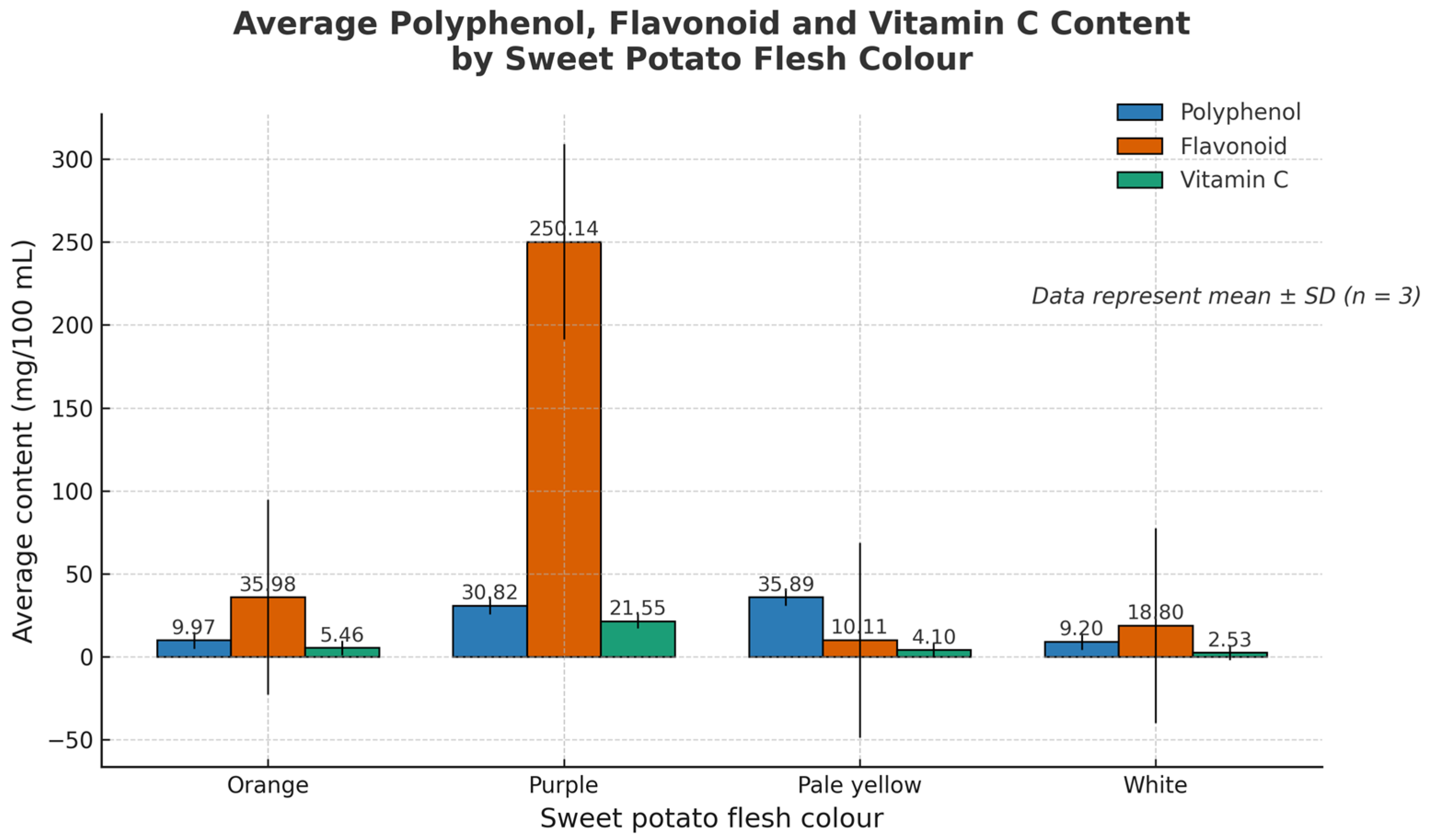
3.5. Summary of Antioxidant Composition by Flesh Colour
3.6. Statistical Validation: Tukey’s HSD Post Hoc Analysis
3.6.1. Phenolic Content
3.6.2. Flavonoid Content
3.6.3. Vitamin C Content
4. Discussion
4.1. Comparative Analysis of Antioxidant Composition Across Cultivars and Regions
4.2. Methodological Considerations and Future Research Directions
4.3. Local Relevance and Functional Food Applications in Central Europe
4.4. Human Health Implications of Bioactive Compound Profiles
5. Conclusions
6. Limitations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAE | Ascorbic Acid Equivalents |
| ANOVA | Analysis of Variance |
| D | Transdanubian region |
| D-T | Danube–Tisza Interfluve |
| FRAP | Ferric Reducing Antioxidant Power |
| FW | Fresh Weight |
| GAE | Gallic Acid Equivalents |
| HSD | Honest Significant Difference |
| R2 | Determination coefficient |
| QE | Quercetin Equivalents |
| SD | Standard Deviation |
| T | Tiszántúl region |
| TPTZ | 2,4,6-Tri(2-pyridyl)-s-triazine |
| UV-Vis | Ultraviolet–Visible Spectrophotometry |
References
- Yuan, B.; Yang, X.Q.; Kou, M.; Lu, C.Y.; Wang, Y.Y.; Peng, J.; Chen, P.; Jiang, J.H. Selenylation of Polysaccharide from the Sweet Potato and Evaluation of Antioxidant, Antitumor, and Antidiabetic Activities. J. Agric. Food Chem. 2017, 65, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L. A batáta és termesztése: Az édesburgonya Magyarországon. Kertészet és Szőlészet 1991, 40, 16–17. [Google Scholar]
- Sun, Y.; Pan, Z.; Yang, C.; Jia, Z.; Guo, X. Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea batatas) Storage Roots. Molecules 2019, 24, 4476. [Google Scholar] [CrossRef]
- Rock, C.L. Carotenoids: Biology and Treatment. Pharmacol. Ther. 2019, 175, 36–44. [Google Scholar] [CrossRef]
- van Jaarsveld, P.J.; Faber, M.; Tanumihardjo, S.A.; Nestel, P.; Lombard, C.J.; Spinnler Benadé, A.J. β-Carotene–Rich Orange-Fleshed Sweet Potato Improves the Vitamin A Status of Primary School Children Assessed with the Modified-Relative-Dose-Response Test. Am. J. Clin. Nutr. 2005, 81, 1080–1087. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.). Horticulturae 2024, 10, 629. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, Antioxidant Activity and Hepatoprotective Effect of Purple Sweetpotato Polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef]
- Ionescu, C.; Samide, A.; Tigae, C. Trend in Detection of Anthocyanins from Fresh Fruits and the Influence of Some Factors on Their Stability Impacting Human Health: Kinetic Study Assisted by UV-Vis Spectrophotometry. Antioxidants 2025, 14, 227. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV–Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Mu, J.; Xu, J.; Wang, L.; Chen, C.; Chen, P. Anti-Inflammatory Effects of Purple Sweet Potato Anthocyanin Extract in DSS-Induced Colitis: Modulation of Commensal Bacteria and Attenuated Bacterial Intestinal Infection. Food Funct. 2021, 12, 11503–11514. [Google Scholar] [CrossRef] [PubMed]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R. Oxidative Stress and Antioxidant Strategies: Relationships and Cellular Pathways for Human Health. Cells 2024, 13, 1871. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. Dietary Polyphenols and Health: Recent Advances. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 95–102. [Google Scholar]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. A Review of Recent Studies on the Effects of Polyphenols on Brain Health and Neurodegeneration. Nutrients 2019, 11, 777. [Google Scholar]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.; Yang, B. Improved Analysis of Anthocyanins and Vitamin C in Blue-Purple Potato Cultivars. Food Chem. 2018, 242, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Kelemen, J.; Nagy, I. Műszeres Analitika; Medicina Könyvkiadó: Budapest, Hungary, 2014; Available online: https://pea.lib.pte.hu/handle/pea/44888 (accessed on 19 June 2025).
- Kim, H.J.; Lee, Y.M.; Kim, Y.K.; Park, H.J.; Lee, S.Y.; Kim, J.-H. Metabolomic Approach for Evaluating Anthocyanin Metabolism and Its Relation to Antioxidant Activity in Pigmented Rice. J. Agric. Food Chem. 2013, 61, 11222–11228. [Google Scholar] [CrossRef] [PubMed]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- ISO 3819:2015; Laboratory glassware—Beakers. ISO: Basel, Switzerland, 2015.
- Csepregi, K. Levelek Alkalmazkodása a Napfényhez—Polifenolos Vegyületek Lehetséges Szerepei. Ph.D. Thesis, University of Pécs, Faculty of Sciences, Doctoral School of Biology and Sport Biology, Pécs, Hungary, 2017. Available online: https://pea.lib.pte.hu/handle/pea/16261 (accessed on 19 June 2025).
- Ferguson, L.R.; Schlothauer, R.C. Role of Plant Bioactives in Nutrition and Health. Food Funct. 2014, 5, 614–628. [Google Scholar]
- Franková, H.; Musilová, J.; Árvay, J.; Šnirc, M.; Jančo, I.; Lidiková, J.; Vollmannová, A. Changes in Antioxidant Properties and Phenolics in Sweet Potatoes (Ipomoea batatas L.) Due to Heat Treatments. Molecules 2022, 27, 1884. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, B.W.; Lee, H.U.; Lee, Y.Y.; Kim, M.H.; Lee, J.Y.; Lee, B.K.; Woo, K.S.; Kim, H.J. Phenolic Compounds and Antioxidant Activity in Sweet Potato after Heat Treatment. J. Sci. Food Agric. 2019, 99, 6833–6840. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical Characterization and Antioxidant Properties of Ethanolic Extract and Its Fractions from Sweet Potato (Ipomoea batatas L.) Leaves. Foods 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic–Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Insanu, M.; Amalia, R.; Fidrianny, I. Potential Antioxidative Activity of Waste Product of Purple Sweet Potato (Ipomoea batatas Lam.). Pak. J. Biol. Sci. 2022, 25, 681–687. [Google Scholar] [CrossRef]
- Tanaka, M.; Ishiguro, K.; Oki, T.; Okuno, S. Functional Components in Sweetpotato and Their Genetic Improvement. Breed. Sci. 2017, 67, 52–61. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef]
- Liao, M.; Zou, B.; Chen, J.; Yao, Z.; Huang, L.; Luo, Z.; Wang, Z. Effect of Domestic Cooking Methods on the Anthocyanins and Antioxidant Activity of Deeply Purple-Fleshed Sweetpotato GZ9. Heliyon 2019, 5, e01515. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Effect of Processing on the Antioxidant Properties of Fruit and Vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Vu, S.T.; Van Loey, A.; Hendrickx, M. Degree of Processing Affects Both the Extractability and the Structure of Pectin in Carrots. Food Chem. 2006, 97, 452–457. [Google Scholar]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of Processing on the Bioavailability of Polyphenols and Carotenoids. Food Chem. 2018, 240, 941–945. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid Interface Sci. 2020, 275, 102076. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- López-Rubio, A.; Gavara, R.; Lagaron, J.M. Overview of Microencapsulation Techniques for Improving the Stability and Bioavailability of Bioactive Compounds in Food Systems. Food Hydrocoll. 2006, 20, 1–23. [Google Scholar]
- Singh, M.N.; Hemant, K.S.; Ram, M.; Shivakumar, H.G. Microencapsulation: A Promising Technique for Controlled Drug Delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed] [PubMed Central]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Transcriptomic and Metabolomic Profiling Reveals the Role of Anthocyanin Biosynthesis Genes in Purple-Fleshed Sweet Potato. BMC Genom. 2020, 21, 343. [Google Scholar]
- Ji, C.Y.; Kim, Y.H.; Kim, H.S.; Ke, Q.; Kim, G.W.; Park, S.C.; Lee, H.S.; Jeong, J.C.; Kwak, S.S. Molecular Characterization of Tocopherol Biosynthetic Genes in Sweetpotato That Respond to Stress and Activate the Tocopherol Production in Tobacco. Plant Physiol. Biochem. 2016, 106, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry Polyphenols and Cardiovascular Health. J. Sci. Food Agric. 2014, 94, 1236–1243. [Google Scholar]
- Kehrer, J.P.; Klotz, L.O. Free Radicals and Related Reactive Species as Mediators of Tissue Injury and Disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Happ, E. Innovatív MarketingkommunikáCiós Megoldások a Turizmusban—Okostelefonos Alkalmazások Lehetőségei. Turizmus ízei: V. Nemzetközi Turizmus Konferencia 2013: Tanulmányok. 2013. Available online: https://www.researchgate.net/publication/372230953 (accessed on 19 June 2025).
- Lykkesfeldt, J.; Carr, A.C. Vitamin C. Adv. Nutr. 2024, 15, 100155. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499877/ (accessed on 25 March 2025).
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Esteve, M.J.; Frígola, A. Effect of Processing on Retention of Polyphenols in Fruit Juice: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 935–950. [Google Scholar]
- Holst, B.; Williamson, G. Nutrients and Phytochemicals: From Bioavailability to Bioefficacy. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Csaba, G. Az Emberi Élettartam Megnövelésének Lehetőségei [Possibilities for Prolonging Human Lifespan]. Orv. Hetil. 2018, 159, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Araya-Cloutier, C.; Vincken, J.P.; van Ederen, R.; Gruppen, H. Bioavailability Studies and Challenges of Phenolic Compounds. In The Health Benefits of Fruits and Vegetables; Skinner, M., Hunter, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
| Comparison | Mean Difference (mg/100 mL) | HSD Critical Value | Significance |
|---|---|---|---|
| Purple vs. Orange | 20.86 | 11.35 | Significant |
| Purple vs. Pale Yellow | 20.71 | 11.35 | Significant |
| Purple vs. White | 21.62 | 11.35 | Significant |
| Orange vs. Pale Yellow | 0.14 | 11.35 | Not significant |
| Orange vs. White | 0.76 | 11.35 | Not significant |
| Pale Yellow vs. White | 0.62 | 11.35 | Not significant |
| Comparison | Mean Difference (mg/100 mL) | HSD Critical Value | Significance |
|---|---|---|---|
| Purple vs. Orange | 214.15 | 211.05 | Significant |
| Purple vs. Pale Yellow | 214.25 | 211.05 | Significant |
| Purple vs. White | 231.34 | 211.05 | Significant |
| Orange vs. Pale Yellow | 0.18 | 211.05 | Not significant |
| Orange vs. White | 17.27 | 211.05 | Not significant |
| Pale Yellow vs. White | 17.09 | 211.05 | Not significant |
| Comparison | Mean Difference (mg/100 mL) | HSD Critical Value | Significance |
|---|---|---|---|
| Purple vs. Orange | 16.09 | 2.18 | Significant |
| Purple vs. Pale Yellow | 17.45 | 2.18 | Significant |
| Purple vs. White | 19.02 | 2.18 | Significant |
| Orange vs. Pale Yellow | 1.36 | 2.18 | Not significant |
| Orange vs. White | 2.93 | 2.18 | Not significant |
| Pale Yellow vs. White | 1.57 | 2.18 | Not significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
József, T.; Végh, E.; Császár, J.; Stromájer, G.P.; Stromájer-Rácz, T. Comparative Study of the Bioactive Compound Content of Sweet Potato Varieties Grown in Hungary. Appl. Sci. 2025, 15, 12537. https://doi.org/10.3390/app152312537
József T, Végh E, Császár J, Stromájer GP, Stromájer-Rácz T. Comparative Study of the Bioactive Compound Content of Sweet Potato Varieties Grown in Hungary. Applied Sciences. 2025; 15(23):12537. https://doi.org/10.3390/app152312537
Chicago/Turabian StyleJózsef, Tibor, Emese Végh, Judit Császár, Gábor Pál Stromájer, and Tímea Stromájer-Rácz. 2025. "Comparative Study of the Bioactive Compound Content of Sweet Potato Varieties Grown in Hungary" Applied Sciences 15, no. 23: 12537. https://doi.org/10.3390/app152312537
APA StyleJózsef, T., Végh, E., Császár, J., Stromájer, G. P., & Stromájer-Rácz, T. (2025). Comparative Study of the Bioactive Compound Content of Sweet Potato Varieties Grown in Hungary. Applied Sciences, 15(23), 12537. https://doi.org/10.3390/app152312537






