The Use of Myocardial Work in Athletes: A Novel Approach to Assess Cardiac Adaptations and Differentiate Physiological Remodeling from Pathology
Abstract
1. Introduction
1.1. Background
1.2. Methods
2. Myocardial Work: A Different Method to Evaluate Cardiac Function
- Global Work Index (GWI), mmHg %: total work performed by the left ventricle during mechanical systole, including isovolumic contraction and relaxation. It is visually represented by the area within the pressure-strain loop. Normal values were established in the EACVI NORRE study [30] and range between 1310 and 2538 mmHg % in females and 1270–2428 mmHg % in males;
- Global Constructive Work (GCW), mmHg %: work performed by the left ventricle that contributes to LVEF during systole. It corresponds to positive work (shortening) during systole and negative work (lengthening) during isovolumic relaxation. Normal ranges are 1543–2924 mmHg % in females and 1650–2807 mmHg % in males [30];
- Global Wasted Work (GWW), mmHg %: work performed by the left ventricle that does not contribute to LVEF during systole. It corresponds to negative work (longitudinal lengthening) during systole plus positive work (shortening) during isovolumetric relaxation. Normal values are 239 ± 39 mmHg % in females and 238 ± 33 mmHg % in males [30];
- Global Work Efficiency (GWE), %: ratio between constructive work and total (constructive and wasted) work. It is obtained by the ratio of GCW to the sum of GCW and GWW (GWE = GCW/[GCW + GWW], 0–100%). Normal values in healthy controls are 91 ± 1 mmHg % in females and 90 ± 1.6 mmHg % in males [29,30].
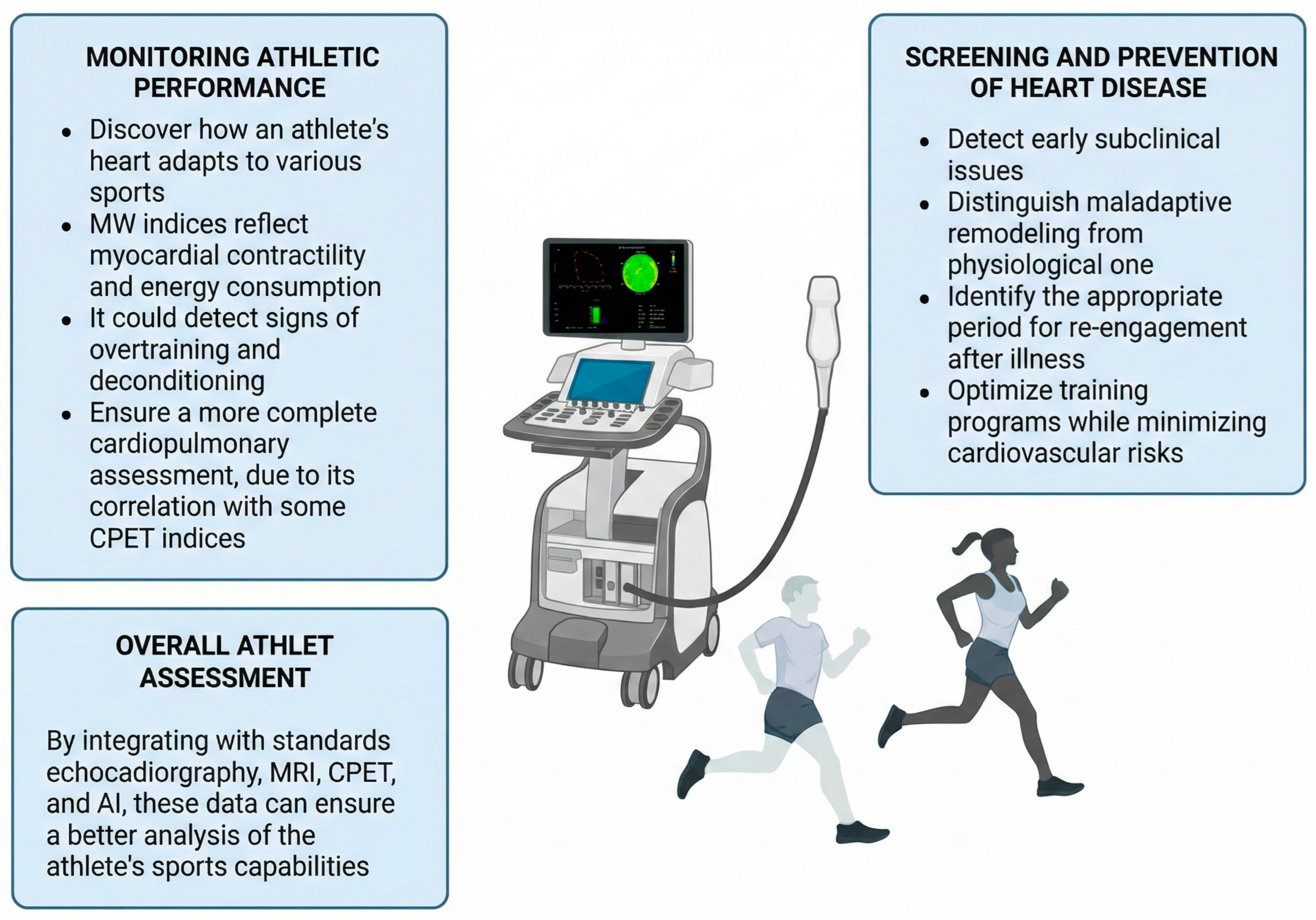
3. Myocardial Work and CPET: Markers of Athletic Performance
4. Myocardial Work and Cardiovascular Health in Athletes
5. MW in Different Athletic Populations
6. Future Directions and Limitations
6.1. Technical Limitations Related to the Echocardiographic Technique
6.2. Limitations Related to Arterial Pressure Measurement
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Segreti, A.; Celeski, M.; Guerra, E.; Crispino, S.P.; Vespasiano, F.; Buzzelli, L.; Fossati, C.; Papalia, R.; Pigozzi, F.; Grigioni, F. Effects of Environmental Conditions on Athlete’s Cardiovascular System. J. Clin. Med. 2024, 13, 4961. [Google Scholar] [CrossRef]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the Heart: The Good, the Bad, and the Ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Pluim, B.M.; Zwinderman, A.H.; Van Der Laarse, A.; Van Der Wall, E.E. The Athlete’s Heart: A Meta-Analysis of Cardiac Structure and Function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef]
- La Gerche, A.; Wasfy, M.M.; Brosnan, M.J.; Claessen, G.; Fatkin, D.; Heidbuchel, H.; Baggish, A.L.; Kovacic, J.C. The Athlete’s Heart—Challenges and Controversies. J. Am. Coll. Cardiol. 2022, 80, 1346–1362. [Google Scholar] [CrossRef]
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef]
- Di Gioia, G.; Ferrera, A.; Mango, F.; Maestrini, V.; Monosilio, S.; Pelliccia, A.; Squeo, M.R. The Spectrum of Eccentric Left Ventricular Hypertrophy in Endurance Sports Disciplines. Int. J. Cardiovasc. Imaging 2025, 41, 1407–1422. [Google Scholar] [CrossRef]
- D’Andrea, A.; Carbone, A.; Radmilovic, J.; Russo, V.; Fabiani, D.; Maio, M.D.; Ilardi, F.; Giallauria, F.; Caputo, A.; Cirillo, T.; et al. Myocardial Work Efficiency in Physiologic Left Ventricular Hypertrophy of Power Athletes. J. Cardiovasc. Echogr. 2022, 32, 154–159. [Google Scholar] [CrossRef]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef]
- Fanale, V.; Segreti, A.; Fossati, C.; Di Gioia, G.; Coletti, F.; Crispino, S.P.; Picarelli, F.; Antonelli Incalzi, R.; Papalia, R.; Pigozzi, F.; et al. Athlete’s ECG Made Easy: A Practical Guide to Surviving Everyday Clinical Practice. J. Cardiovasc. Dev. Dis. 2024, 11, 303. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Recommendations for Electrocardiographic Interpretation in Athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef]
- Celeski, M.; Segreti, A.; Crisci, F.; Cricco, R.; Piscione, M.; Di Gioia, G.; Nusca, A.; Fossati, C.; Pigozzi, F.; Ussia, G.P.; et al. The Role of Cardiac Troponin and Other Emerging Biomarkers Among Athletes and Beyond: Underlying Mechanisms, Differential Diagnosis, and Guide for Interpretation. Biomolecules 2024, 14, 1630. [Google Scholar] [CrossRef]
- Celeski, M.; Segreti, A.; Piscione, M.; Monticelli, L.M.; Di Gioia, G.; Fossati, C.; Ussia, G.P.; Pigozzi, F.; Grigioni, F. The Current Paradigm of Cardiac Troponin Increase Among Athletes. Monaldi Arch. Chest Dis. 2025, 95, 2878. [Google Scholar] [CrossRef]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial Work by Echocardiography: A Novel Method Ready for Clinical Testing. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- Suga, H.; Hayashi, T.; Suehiro, S.; Hisano, R.; Shirahata, M.; Ninomiya, I. Equal Oxygen Consumption Rates of Isovolumic and Ejecting Contractions with Equal Systolic Pressure-Volume Areas in Canine Left Ventricle. Circ. Res. 1981, 49, 1082–1091. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of Wasted Myocardial Work: A Novel Method to Quantify Energy Loss Due to Uncoordinated Left Ventricular Contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef]
- Marzlin, N.; Hays, A.G.; Peters, M.; Kaminski, A.; Roemer, S.; O’Leary, P.; Kroboth, S.; Harland, D.R.; Khandheria, B.K.; Tajik, A.J.; et al. Myocardial Work in Echocardiography. Circ. Cardiovasc. Imaging 2023, 16, 198–214. [Google Scholar] [CrossRef]
- Oxborough, D.; Augustine, D.; Gati, S.; George, K.; Harkness, A.; Mathew, T.; Papadakis, M.; Ring, L.; Robinson, S.; Sandoval, J.; et al. A Guideline Update for the Practice of Echocardiography in the Cardiac Screening of Sports Participants: A Joint Policy Statement from the British Society of Echocardiography and Cardiac Risk in the Young. Echo Res. Pract. 2018, 5, G1–G10. [Google Scholar] [CrossRef]
- Palermi, S.; Serio, A.; Vecchiato, M.; Sirico, F.; Gambardella, F.; Ricci, F.; Iodice, F.; Radmilovic, J.; Russo, V.; D’Andrea, A. Potential Role of an Athlete-Focused Echocardiogram in Sports Eligibility. World J. Cardiol. 2021, 13, 271–297. [Google Scholar] [CrossRef]
- Segreti, A.; Celeski, M.; Monticelli, L.M.; Perillo, A.; Crispino, S.P.; Di Gioia, G.; Cammalleri, V.; Fossati, C.; Mega, S.; Papalia, R.; et al. Mitral and Tricuspid Valve Disease in Athletes. J. Clin. Med. 2023, 12, 3562. [Google Scholar] [CrossRef]
- Refoyo, E.; Troya, J.; De La Fuente, A.; Beltrán, A.; Celada, O.L.; Díaz-González, L.; Pedrero-Tomé, R.; García-Yébenes, M.; Villalón, J.M. Myocardial Work Index in Professional Football Players: A Novel Method for Assessment of Cardiac Adaptation. J. Clin. Med. 2023, 12, 3059. [Google Scholar] [CrossRef]
- Marwick, T.H. Ejection Fraction Pros and Cons. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Konstam, M.A.; Abboud, F.M. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial Strain Imaging: Review of General Principles, Validation, and Sources of Discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef]
- Bastos, M.B.; Burkhoff, D.; Maly, J.; Daemen, J.; Den Uil, C.A.; Ameloot, K.; Lenzen, M.; Mahfoud, F.; Zijlstra, F.; Schreuder, J.J.; et al. Invasive Left Ventricle Pressure–Volume Analysis: Overview and Practical Clinical Implications. Eur. Heart J. 2020, 41, 1286–1297. [Google Scholar] [CrossRef]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- Suga, H. Total Mechanical Energy of a Ventricle Model and Cardiac Oxygen Consumption. Am. J. Physiol. Heart Circ. Physiol. 1979, 236, H498–H505. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A Novel Clinical Method for Quantification of Regional Left Ventricular Pressure–Strain Loop Area: A Non-Invasive Index of Myocardial Work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Samset, E.; Healthcare, G. Evaluation of Segmental Myocardial Work in the Left Ventricle. Available online: https://www.gehealthcare.com/-/media/8cab29682ace4ed7841505f813001e33.pdf@line-2@?srsltid=AfmBOop7qrhKkrMsDpOIJvk-1r3YPNOWusaW334bL3mB6ke3wPE_yMiB (accessed on 1 July 2025).
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic Reference Ranges for Normal Non-Invasive Myocardial Work Indices: Results from the EACVI NORRE Study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A New Approach to Assess Myocardial Work by Non-Invasive Left Ventricular Pressure–Strain Relations in Hypertension and Dilated Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Pencic, B.; Grassi, G.; Celic, V. Myocardial Work in Hypertensive Patients with and without Diabetes: An Echocardiographic Study. J. Clin. Hypertens. 2020, 22, 2121–2127. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Deng, Y.; Zhu, Y.; Sun, R.; Lu, S. Non-Invasive Global and Regional Myocardial Work Predicts High-Risk Stable Coronary Artery Disease Patients with Normal Segmental Wall Motion and Left Ventricular Function. Front. Cardiovasc. Med. 2021, 8, 711547. [Google Scholar] [CrossRef]
- Boe, E.; Russell, K.; Eek, C.; Eriksen, M.; Remme, E.W.; Smiseth, O.A.; Skulstad, H. Non-Invasive Myocardial Work Index Identifies Acute Coronary Occlusion in Patients with Non-ST-Segment Elevation-Acute Coronary Syndrome. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1247–1255. [Google Scholar] [CrossRef]
- Jin, W.; Wang, L.; Zhu, T.; Ma, Y.; Yu, C.; Zhang, F. Usefulness of Echocardiographic Myocardial Work in Evaluating the Microvascular Perfusion in STEMI Patients After Revascularization. BMC Cardiovasc. Disord. 2022, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Zhan, J.; Van Den Eynde, J.; Cordrey, K.; Long, R.; Danford, D.A.; Hays, A.G.; Barnes, B.T.; Kutty, S. Deterioration in Myocardial Work Indices Precedes Changes in Global Longitudinal Strain Following Anthracycline Chemotherapy. Int. J. Cardiol. 2022, 363, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Bao, W.; Xu, Y.; Yang, W.; Li, M.; Xu, M.; Zhang, Y.; Zhang, M. Assessment of Myocardial Work in Cancer Therapy-Related Cardiac Dysfunction and Analysis of CTRCD Prediction by Echocardiography. Front. Pharmacol. 2021, 12, 770580. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Thampinathan, B.; Somerset, E.; Shalmon, T.; Amir, E.; Steve Fan, C.-P.; Moon, S.; Abdel-Qadir, H.; Thevakumaran, Y.; Day, J.; et al. Diagnostic and Prognostic Value of Myocardial Work Indices for Identification of Cancer Therapy–Related Cardiotoxicity. JACC Cardiovasc. Imaging 2022, 15, 1361–1376. [Google Scholar] [CrossRef]
- Galli, E.; Vitel, E.; Schnell, F.; Le Rolle, V.; Hubert, A.; Lederlin, M.; Donal, E. Myocardial Constructive Work Is Impaired in Hypertrophic Cardiomyopathy and Predicts Left Ventricular Fibrosis. Echocardiography 2019, 36, 74–82. [Google Scholar] [CrossRef]
- Xiao, C.; Zhao, X.; Sun, L.; Zhang, F. Evaluation of Global and Regional Myocardial Work in Hypertrophic Cardiomyopathy Patients by Left Ventricular Pressure-Strain Loop. BMC Cardiovasc. Disord. 2023, 23, 479. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; van der Bijl, P.; El Mahdiui, M.; Bax, J.J.; Delgado, V.; Marsan, N.A. Myocardial Work in Nonobstructive Hypertrophic Cardiomyopathy: Implications for Outcome. J. Am. Soc. Echocardiogr. 2020, 33, 1201–1208. [Google Scholar] [CrossRef]
- Jacquemyn, X.; Dryer, R.; Cordrey, K.; Long, R.; Danford, D.A.; Kutty, S.; Barnes, B.T. Myocardial Work in Children with Hypertrophic Cardiomyopathy: Longitudinal Evaluation and Prognostic Implications. JACC Adv. 2025, 4, 101885. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.; Liu, Y.; Huang, D.; Hu, Y.; Wang, Y.; Ma, L.; Liu, L. Association Between Echocardiographic Non-Invasive Myocardial Work Indices and Myocardial Fibrosis in Patients with Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 704251. [Google Scholar] [CrossRef]
- Hedwig, F.; Nemchyna, O.; Stein, J.; Knosalla, C.; Merke, N.; Knebel, F.; Hagendorff, A.; Schoenrath, F.; Falk, V.; Knierim, J. Myocardial Work Assessment for the Prediction of Prognosis in Advanced Heart Failure. Front. Cardiovasc. Med. 2021, 8, 691611. [Google Scholar] [CrossRef] [PubMed]
- Ródenas-Alesina, E.; Lozano-Torres, J.; Badia-Molins, C.; Tobías-Castillo, P.E.; Rodríguez-Palomares, J.F.; Ferreira-González, I. Association Between Global Myocardial Work Index and Outcomes in Nonischemic Dilated Cardiomyopathy. J. Cardiovasc. Med. 2025, 26, 547–551. [Google Scholar] [CrossRef]
- Vrettos, A.; Monteiro, R.P.; Triantafyllou, M.; Gul, U.; Bhattacharyya, S.; Lopes, L.R.; Antonopoulos, A.; Protonotarios, A.; Lloyd, G.; Gossios, T.; et al. Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis. Diagnostics 2025, 15, 2363. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Feng, X.; Guo, T.; Liu, G.; Wu, D.; Liu, Y.; Lai, J.; Liu, Y.; Lin, X.; et al. Prognostic Implications of Multiple Chamber Longitudinal Strains and Myocardial Work in Restrictive Cardiomyopathy. Sci. Rep. 2025, 15, 12504. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Petek, B.J.; Gustus, S.K.; Wasfy, M.M. Cardiopulmonary Exercise Testing in Athletes: Expect the Unexpected. Curr. Treat. Options Cardio. Med. 2021, 23, 49. [Google Scholar] [CrossRef]
- Segreti, A.; Picarelli, F.; Di Gioia, G.; Coletti, F.; Crispino, S.P.; Fanale, V.; Fossati, C.; Antonelli Incalzi, R.; Pigozzi, F.; Grigioni, F. Athlete’s Heart or Heart Disease in the Athlete? Evaluation by Cardiopulmonary Exercise Testing. J. Sports Med. Phys. Fit. 2023, 63, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Heidbüchel, H.; Corrado, D.; Sharma, S.; Börjesson, M. Criteria and Considerations Relative to Safe Participation in Sport for Athletes with Cardiac Abnormalities. In The ESC Textbook of Sports Cardiology; Pelliccia, A., Heidbuchel, H., Corrado, D., Börjesson, M., Sharma, S., Pelliccia, A., Heidbuchel, H., Corrado, D., Borjesson, M., Sharma, S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Arena, R.; Ozemek, C. Intracardiac Multimorbidity: Assessing Right Ventricular Function in Left-Sided Heart Failure Through Cardiopulmonary Exercise Testing. Expert. Rev. Cardiovasc. Ther. 2019, 17, 331–333. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Dong, L.; Gao, B. Echocardiographic Myocardial Work in Pre-Adolescent Male Basketball Players: A Comparison with Cardiopulmonary Exercise Test-Derived Aerobic Capacity. Front. Physiol. 2022, 13, 913623. [Google Scholar] [CrossRef] [PubMed]
- Tokodi, M.; Oláh, A.; Fábián, A.; Lakatos, B.K.; Hizoh, I.; Ruppert, M.; Sayour, A.A.; Barta, B.A.; Kiss, O.; Sydó, N.; et al. Novel Insights into the Athlete’s Heart: Is Myocardial Work the New Champion of Systolic Function? Eur. Heart J. Cardiovasc. Imaging 2022, 23, 188–197. [Google Scholar] [CrossRef]
- Segreti, A.; Fossati, C.; Monticelli, L.M.; Valente, D.; Polito, D.; Guerra, E.; Zampoli, A.; Albimonti, G.; Zampogna, B.; Vasta, S.; et al. Changes in Cardiopulmonary Capacity Parameters After Surgery: A Pilot Study Exploring the Link Between Heart Function and Knee Surgery. J. Funct. Morphol. Kinesiol. 2024, 9, 172. [Google Scholar] [CrossRef]
- Erevik, C.B.; Kleiven, Ø.; Frøysa, V.; Bjørkavoll-Bergseth, M.; Chivulescu, M.; Klæboe, L.G.; Dejgaard, L.; Auestad, B.; Skadberg, Ø.; Melberg, T.; et al. Myocardial Inefficiency Is an Early Indicator of Exercise-Induced Myocardial Fatigue. Front. Cardiovasc. Med. 2023, 9, 1081664. [Google Scholar] [CrossRef]
- Kandels, J.; Stöbe, S.; Kogel, A.; Hepp, P.; Riepenhof, H.; Droste, J.N.; Stoeggl, T.; Marshall, R.P.; Rudolph, U.; Laufs, U.; et al. Effect of Maximum Exercise on Left Ventricular Deformation and Its Correlation with Cardiopulmonary Exercise Capacity in Competitive Athletes. Echo Res. Pract. 2023, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Cameli, M.; Carbone, A.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; D’Ascenzi, F.; Bossone, E.; Galderisi, M.; et al. Speckle Tracking Evaluation in Endurance Athletes: The “Optimal” Myocardial Work. Int. J. Cardiovasc. Imaging 2020, 36, 1679–1688. [Google Scholar] [CrossRef]
- Di Gioia, G.; Ferrera, A.; Mango, F.; Ortolina, D.; Maestrini, V.; Monosilio, S.; Paoletti, G.; Lemme, E.; Squeo, M.R.; Pelliccia, A. Correlation Between Myocardial Work Indices and Main Echocardiographic and Cardiopulmonary Exercise Stress Test Parameters in Olympic Endurance Athletes. Eur. J. Appl. Physiol. 2025, 125, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Borzì, D.D.; Saladino, S.; Losi, V.; Faro, D.C.; Monte, I.P. Strain and Myocardial Work Index During Echo Exercise to Evaluate Myocardial Function in Athletes. J. Cardiovasc. Echogr. 2022, 32, 82–88. [Google Scholar] [CrossRef]
- Avula, H.R.; Leong, T.K.; Lee, K.K.; Sung, S.H.; Go, A.S. Long-Term Outcomes of Adults with Heart Failure by Left Ventricular Systolic Function Status. Am. J. Cardiol. 2018, 122, 1008–1016. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial Strain Imaging: How Useful Is It in Clinical Decision Making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial Strain of the Left Ventricle by Speckle Tracking Echocardiography: From Physics to Clinical Practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Moya, A.; Buytaert, D.; Penicka, M.; Bartunek, J.; Vanderheyden, M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J. Am. Soc. Echocardiogr. 2023, 36, 1027–1042. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Özden Tok, Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef]
- Huang, L.; Ye, L.; Zhang, H.; Zhang, Q.; Ding, G.; Li, C.; Deng, Y.; Yin, L.; Wang, Y. Characteristics of Myocardial Work During Exercise Stress Echocardiography in Healthy Adults. Front. Cardiovasc. Med. 2025, 12, 1511464. [Google Scholar] [CrossRef] [PubMed]
- Mont, L.; Elosua, R.; Brugada, J. Endurance Sport Practice as a Risk Factor for Atrial Fibrillation and Atrial Flutter. Europace 2008, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; De Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited—Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Left Atrial Function in Elite Athletes: A Meta-analysis of Two-dimensional Speckle Tracking Echocardiographic Studies. Clin. Cardiol. 2019, 42, 579–587. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Li, L.; Chen, Y.; Li, Z.; Liu, S.; Hua, S. Assessment of Left Ventricular Systolic Function by Non-Invasive Pressure-Strain Loop Area in Young Male Strength Athletes. Cardiovasc. Ultrasound 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Jain, R.; Burkule, N.; Olet, S.; Khandheria, B.K. Myocardial Work Index: A Novel Method for Assessment of Myocardial Function in South Asian Recreational Athletes. J. Patient-Centered Res. Rev. 2020, 7, 147–156. [Google Scholar] [CrossRef]
- Da Luz, S.R.; Okawa, R.T.P.; Lopes, W.A. Myocardial Work and Left Ventricular Function: Implications for Aerobic Capacity and Performance in Professional Soccer Athletes. Sport Sci. Health 2025, 21, 1889–1898. [Google Scholar] [CrossRef]
- Grandperrin, A.; Schnell, F.; Donal, E.; Galli, E.; Hedon, C.; Cazorla, O.; Nottin, S. Specific Alterations of Regional Myocardial Work in Strength-Trained Athletes Using Anabolic Androgenic Steroids Compared to Athletes with Genetic Hypertrophic Cardiomyopathy. J. Sport Health Sci. 2023, 12, 477–485. [Google Scholar] [CrossRef]
- Gillam, L.D.; Marcoff, L. Echocardiography: Past, Present, and Future. Circ. Cardiovasc. Imaging 2024, 17, e016517. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Li, M.; Gao, X.; Yin, L.; Qi, R.; Wu, R.S.; Chen, X.; Ma, Y.; Shi, K.; et al. A Wearable Cardiac Ultrasound Imager. Nature 2023, 613, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Alsharqi, M.; Woodward, W.J.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial Intelligence and Echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Kusunose, K. AI in Echocardiography: State-of-the-Art Automated Measurement Techniques and Clinical Applications. JMA J. 2025, 8, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sahashi, Y.; Ouyang, D.; Okura, H.; Kagiyama, N. AI-Echocardiography: Current Status and Future Direction. J. Cardiol. 2025, 85, 458–464. [Google Scholar] [CrossRef]
- Galderisi, M.; Cardim, N.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; Edvardsen, T.; Freitas, A.; Habib, G.; et al. The Multi-Modality Cardiac Imaging Approach to the Athlete’s Heart: An Expert Consensus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 353–353r. [Google Scholar] [CrossRef]
- Liao, M.; Pan, J.; Liao, T.; Liu, X.; Wang, L. Transthoracic Echocardiographic Assessment of Ventricular Function in Functional Single Ventricle: A Comprehensive Review. Cardiovasc. Ultrasound 2025, 23, 9. [Google Scholar] [CrossRef]
- Lembo, M.; Santoro, C.; Casciano, O.; Capone, V.; Fedele, T.; Luciano, F.; Canonico, M.; Buonauro, A.; Esposito, R.; Galderisi, M. Impact of Diastolic Blood Pressure on Speckle Tracking Derived Myocardial Work Components in a Population of Normotensive and Untreated Hypertensive Patients. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.2700. [Google Scholar] [CrossRef]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial Work Assessment in Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Global Longitudinal Strain (GLS) | Myocardial Work (MW) |
|---|---|---|
| Principle | Detects myocardial longitudinal deformation (strain) during systole | Quantifies myocardial performance and energy consumption via pressure–strain loops (PSLs). |
| Methodology | Tracks myocardial speckles to assess LV deformation in the longitudinal direction, expressed as a negative percentage (−%). | Integrates speckle-tracking strain data with estimated LV pressure to calculate myocardial work (mmHg·%). |
| Parameters | LV global longitudinal strain (GLS). |
|
| Load dependency | Highly preload- and afterload-dependent, limiting reliability under variable hemodynamic conditions. | Less load-dependent, as LV pressure is incorporated into calculations. |
| Detection of Physiological Adaptations | Interpretation for differentiating physiological remodeling in athletes is limited by load dependency. | Quantifies myocardial efficiency and oxygen consumption. Emerging evidence supports its use in distinguishing physiological adaptations from pathology. |
| Sensitivity to detect subclinical dysfunctions | Detects subclinical systolic dysfunction earlier than LVEF. | Identifies subclinical dysfunction through GWW and GWE changes, often before GLS or LVEF abnormalities. |
| Correlation with CPET | Weak correlation between GLS and CPET-derived VO2/kg due to load dependence. | Moderate correlations between MW indices and CPET-derived VO2/kg in both semi-recumbent ergometer and treadmill protocols. |
| Limits | Operator-dependent; strongly influenced by load conditions. Limited capacity to estimate energy expenditure. | Operator-dependent; requires specialized software and trained operators. Reference benchmarks still need to be established. |
| Study | Type of Study and Population | Findings |
|---|---|---|
| Refoyo et al., 2023 [21] | Prospective, single-center cohort study 97 people: 49 professional football players and 48 controls. The mean age is 30.48 ± 7.20 years old. The number of males and females is not known. |
|
| Tokodi et al., 2022 [55] | Mixed: Animal model + Cross-sectional human study 40 people: 20 elite swimmers (50% males, 50% females) and 20 healthy sedentary controls (50% males, 50% females). The mean age of swimmers was 20 ± 5 years and the control group’s mean age was 22 ± 3 years. |
|
| D’Andrea et al., 2020 [59] | Cross-sectional observational study 350 EA: males are 58.5% of the total, females are 41.5%. 150 healthy controls: 85 male (57.4%) and 65 female. The mean age was 31.6 ± 4.2 years. |
|
| Zhao et al., 2022 [54] | Cross-sectional observational study 20 pre-adolescent male basketball players. The mean age was 9.7 ± 1.1 year. |
|
| Borzì et al., 2022 [61] | Observational 30 healthy males divided into three groups of 10: sedentary, EA and PA. The mean age was 26.9 ± 6.3 years. |
|
| Sengupta et al., 2020 [72] | Cross-sectional observational 24 recreational athletes with a mean age of 41.8 ± 7.4 years. 23 of 24 are males (98%). |
|
| D’Andrea et al., 2022 [8] | Cross-sectional with stress testing 250 PA: 155 males (62%) and 95 females. 180 age-and sex-comparable healthy controls. Mean age: 33.6 ± 4.8 years. |
|
| Da Luz et al., 2025 [73] | Cross-sectional observational 75 professional soccer athletes and 23 recreational athletes, between 18 and 35 years. The number of males and females is not known. |
|
| Di Gioia et al., 2025 [60] | Cross-sectional with CPET and stress echo 306 Olympic EA, 170 (55.5%) males. Mean age: 26.3 ± 4.3 years old. |
|
| Grandperrin et al., 2023 [74] | Cross-sectional comparative study 24 strength-trained asymptomatic athletes using anabolic androgenic steroids (AAS) (age: 32.3 ± 7.7). 22 athletes diagnosed with HCM (age: 34.8 ± 12.5). 20 healthy control athletes (34.5 ± 7.7). The number of males and females is not known. |
|
| Limitations | Description of Limitation | Strategies |
|---|---|---|
| Quality of echocardiographic images |
|
|
| Blood pressure measurement |
|
|
| Dependence on 2D models |
|
|
| Vendor-specific algorithm |
|
|
| Clinical applicability |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mega, S.; Fossati, C.; Segreti, A.; Cricco, R.; Lazarevic, Z.; Carpenito, M.; Coletti, F.; Valeri, J.; Lemme, E.; Pigozzi, F.; et al. The Use of Myocardial Work in Athletes: A Novel Approach to Assess Cardiac Adaptations and Differentiate Physiological Remodeling from Pathology. Appl. Sci. 2025, 15, 12490. https://doi.org/10.3390/app152312490
Mega S, Fossati C, Segreti A, Cricco R, Lazarevic Z, Carpenito M, Coletti F, Valeri J, Lemme E, Pigozzi F, et al. The Use of Myocardial Work in Athletes: A Novel Approach to Assess Cardiac Adaptations and Differentiate Physiological Remodeling from Pathology. Applied Sciences. 2025; 15(23):12490. https://doi.org/10.3390/app152312490
Chicago/Turabian StyleMega, Simona, Chiara Fossati, Andrea Segreti, Riccardo Cricco, Zlatan Lazarevic, Myriam Carpenito, Federica Coletti, Jacopo Valeri, Erika Lemme, Fabio Pigozzi, and et al. 2025. "The Use of Myocardial Work in Athletes: A Novel Approach to Assess Cardiac Adaptations and Differentiate Physiological Remodeling from Pathology" Applied Sciences 15, no. 23: 12490. https://doi.org/10.3390/app152312490
APA StyleMega, S., Fossati, C., Segreti, A., Cricco, R., Lazarevic, Z., Carpenito, M., Coletti, F., Valeri, J., Lemme, E., Pigozzi, F., & Grigioni, F. (2025). The Use of Myocardial Work in Athletes: A Novel Approach to Assess Cardiac Adaptations and Differentiate Physiological Remodeling from Pathology. Applied Sciences, 15(23), 12490. https://doi.org/10.3390/app152312490








