DTI-Based Structural Connectome Analysis of SCLC Patients After Chemotherapy via Machine Learning
Abstract
1. Introduction
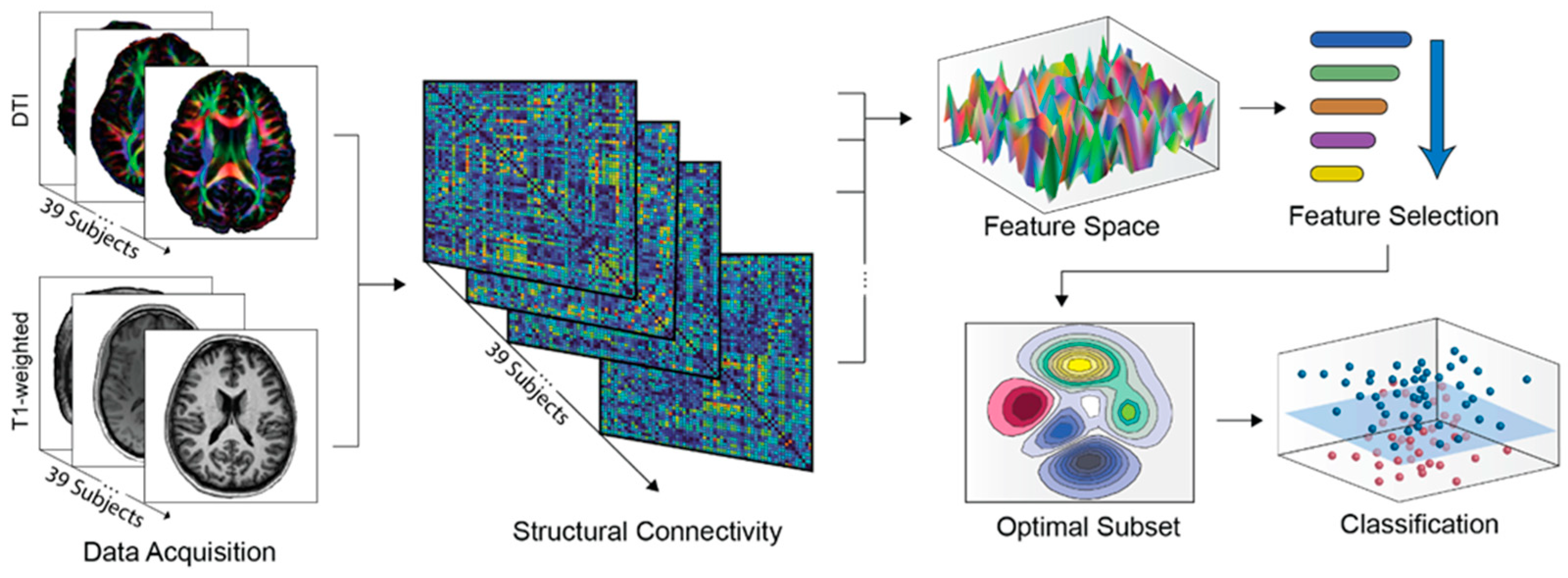
2. Materials and Methods
2.1. Subjects
2.2. Data Acquisition and Preprocessing
2.3. Structural Connectivity Estimation
2.4. Network-Based Feature Selection
2.5. Classification
2.6. Feature-Related Performance Validation
3. Results
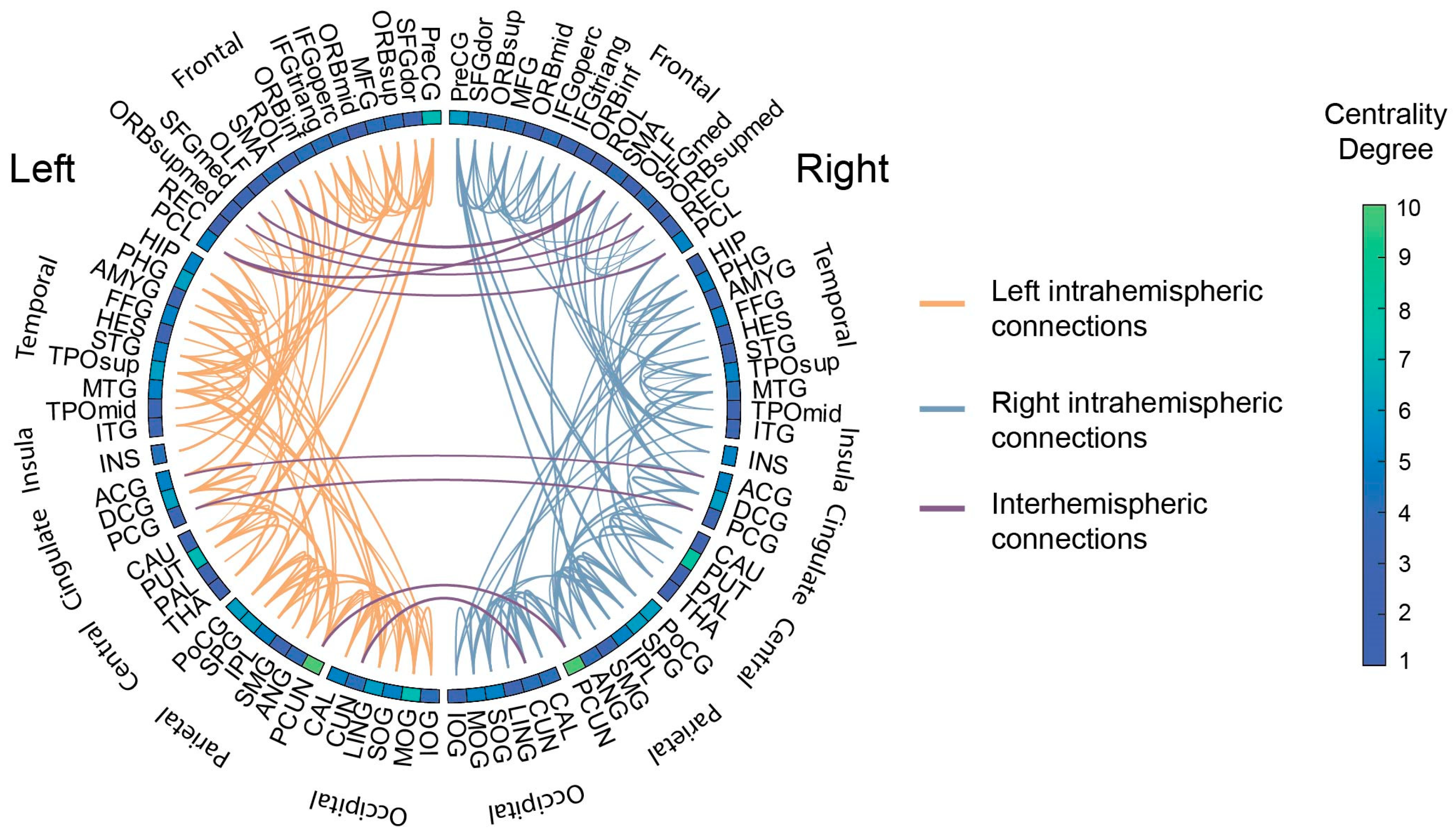
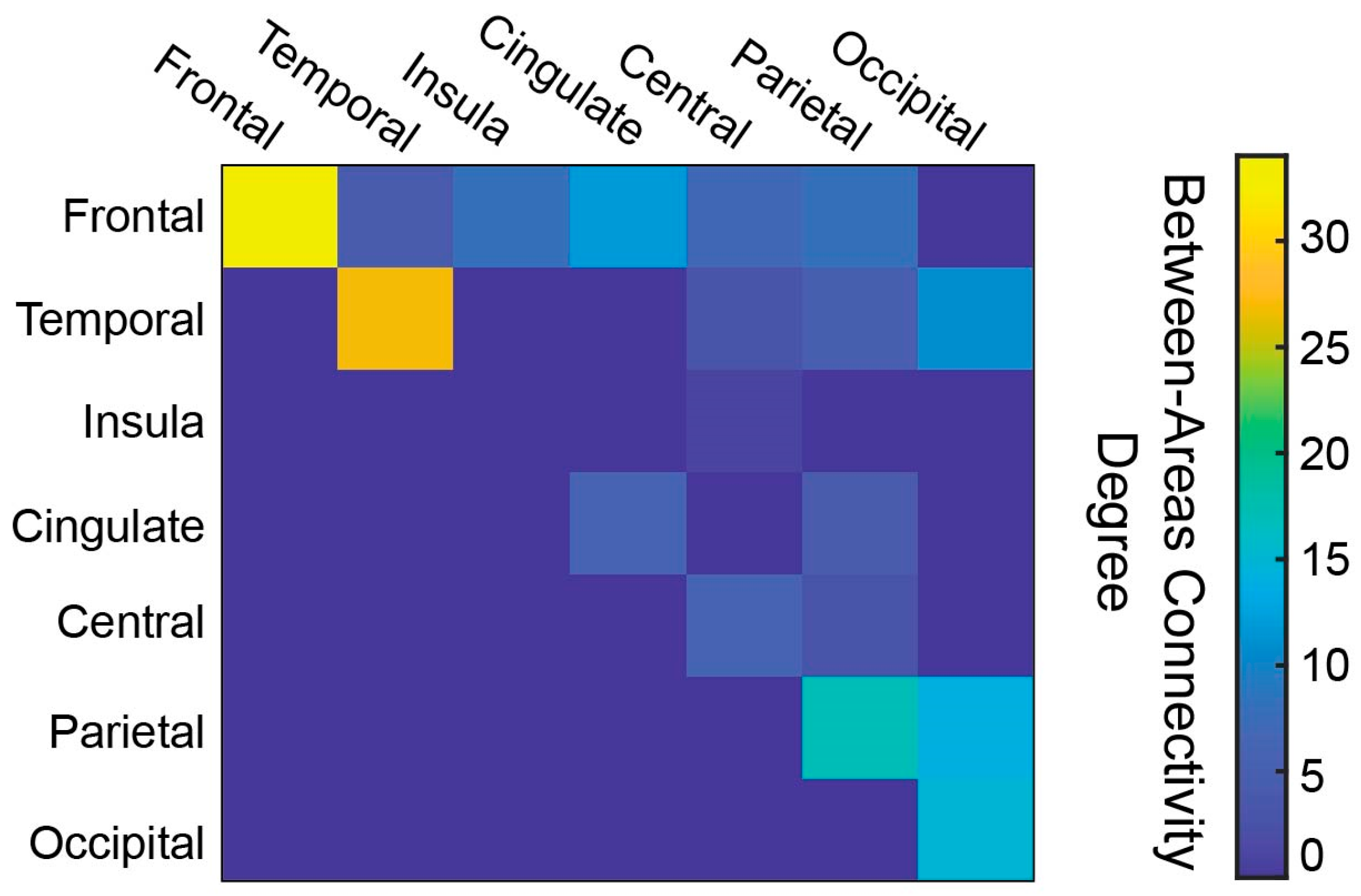
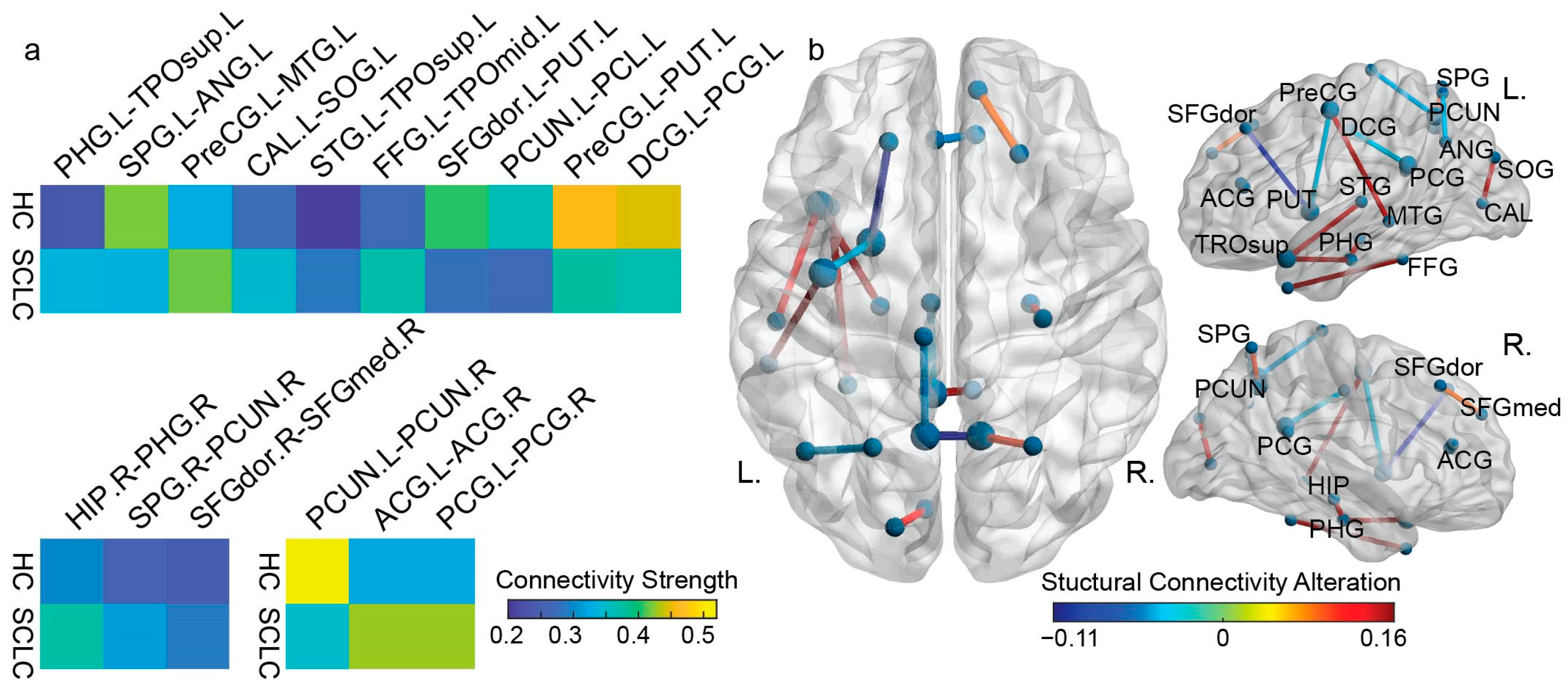
3.1. White Matter Connectivity Patterns
3.2. Classification Performance and Feature Robustness
3.3. ROC-Based Analytic Power Estimation
3.4. Interpretation of Discriminative Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAL | Automated Anatomical Labeling |
| ANOVA | Analysis of Variance |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| DMN | Default Mode Network |
| DTI | Diffusion Tensor Imaging |
| EPI | Echo-Planar Imaging |
| fMRI | Functional Magnetic Resonance Imaging |
| FA | Fractional Anisotropy |
| FACT | Fiber Assignment by Continuous Tracking |
| FOV | Field of View |
| GE | Gradient Echo |
| GMV | Gray Matter Volume |
| HC | Healthy Control |
| kNN | k-Nearest Neighbor |
| LAT | Left Anterior Temporal |
| LDA | Linear Discrimination Analysis |
| LOOCV | Leave-One-Out Cross Validation |
| MCI | Mild Cognitive Impairment |
| ML | Machine Learning |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| MVPA | Multivariate Pattern Analysis |
| NB | Naïve Bayes |
| NSCLC | Non-Small-Cell Lung Cancer |
| PANDA | Pipeline for Analyzing Brain Diffusion Images |
| PCI | Prophylactic Cranial Irradiation |
| rs-fMRI | Resting-State Functional Magnetic Resonance Imaging |
| RAT | Right Anterior Temporal |
| RBF | Radial Basis Function |
| RF | Random Forest |
| RFE-CBR | Recursive Feature Elimination algorithm with Correlation Bias Reduction |
| ROC | Receiver Operator Characteristic |
| ROI | Region of Interest |
| SCLC | Small-Cell Lung Cancer |
| SE | Standard Error |
| SMN | Sensorimotor Network |
| SVM | Support Vector Machine |
| SVR | Support Vector Regression |
| TE | Echo Time |
| TPN | Task-Positive Network |
| TR | Repetition Time |
| WBDT | Whole-Brain Deterministic Tractography |
Appendix A. Demographics
| Classifier | Controls (n = 14) | Patients (n = 20) | p-Value |
|---|---|---|---|
| Age (years) | 56.42 (7.62) | 54.81 (5.98) | 0.49 |
| Education (years) | 17 (6.44) | 13.31 (4.9) | 0.07 |
| Gender | 0.46 | ||
| Male | 8 (57%) | 13 (65%) | |
| Female | 6 (43%) | 7 (35%) | |
| Smoking | 2 (14%) | 12 (60%) | 0.002 |
| Stage | - | IIB—10 (50%) | |
| - | IIIA—8 (40%) | ||
| - | IIIB—2 (10%) | ||
| Regimen_1 1 | - | 15 (75%) | |
| Regimen_2 2 | - | 5 (25%) |
Appendix B. AAL Regions of Interest (ROIs)
| Region Name | Abbreviation | Area |
|---|---|---|
| Precentral gyrus | PreCG | Frontal Lobe |
| Superior frontal gyrus, dorsolateral | SFGdor | Frontal Lobe |
| Superior frontal gyrus, orbital part | ORBsup | Frontal Lobe |
| Middle frontal gyrus | MFG | Frontal Lobe |
| Middle frontal gyrus, orbital part | ORBmid | Frontal Lobe |
| Inferior frontal gyrus, opercular part | IFGoperc | Frontal Lobe |
| Inferior frontal gyrus, triangular part | IFGtriang | Frontal Lobe |
| Inferior frontal gyrus, orbital part | ORBinf | Frontal Lobe |
| Rolandic operculum | ROL | Frontal Lobe |
| Supplementary motor area | SMA | Frontal Lobe |
| Olfactory cortex | OLF | Frontal Lobe |
| Superior frontal gyrus, medial | SFGmed | Frontal Lobe |
| Superior frontal gyrus, medial orbital | ORBsupmed | Frontal Lobe |
| Gyrus rectus | REC | Frontal Lobe |
| Insula | INS | Insula Gyri |
| Anterior cingulate, paracingulate gyri | ACG | Cingulate Gyri |
| Median cingulate, paracingulate gyri | DCG | Cingulate Gyri |
| Posterior cingulate gyrus | PCG | Cingulate Gyri |
| Hippocampus | HIP | Temporal Lobe |
| Parahippocampal gyrus | PHG | Temporal Lobe |
| Amygdala | AMYG | Temporal Lobe |
| Calcarine fissure | CAL | Occipital Lobe |
| Cuneus | CUN | Occipital Lobe |
| Lingual gyrus | LING | Occipital Lobe |
| Superior occipital gyrus | SOG | Occipital Lobe |
| Middle occipital gyrus | MOG | Occipital Lobe |
| Inferior occipital gyrus | IOG | Occipital Lobe |
| Fusiform gyrus | FFG | Temporal Lobe |
| Postcentral gyrus | PoCG | Parietal Lobe |
| Superior parietal gyrus | SPG | Parietal Lobe |
| Inferior parietal lobule | IPL | Parietal Lobe |
| Supramarginal gyrus | SMG | Parietal Lobe |
| Angular gyrus | ANG | Parietal Lobe |
| Precuneus | PCUN | Parietal Lobe |
| Paracentral lobule | PCL | Frontal Lobe |
| Caudate nucleus | CAU | Central Structures |
| Lenticular nucleus, putamen | PUT | Central Structures |
| Lenticular nucleus, pallidum | PAL | Central Structures |
| Thalamus | THA | Central Structures |
| Heschl gyrus | HES | Temporal Lobe |
| Superior temporal gyrus | STG | Temporal Lobe |
| Temporal pole (superior) | TPOsup | Temporal Lobe |
| Middle temporal gyrus | MTG | Temporal Lobe |
| Temporal pole (middle) | TPOmid | Temporal Lobe |
| Inferior temporal gyrus | ITG | Temporal Lobe |
Appendix C. ROC-Based Analytic Power Estimation
References
- Tian, Y.; Li, Q.; Yang, Z.; Zhang, S.; Xu, J.; Wang, Z.; Bai, H.; Duan, J.; Zheng, B.; Li, W.; et al. Single-Cell Transcriptomic Profiling Reveals the Tumor Heterogeneity of Small-Cell Lung Cancer. Signal Transduct. Target. Ther. 2022, 7, 346. [Google Scholar] [CrossRef]
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical Insights into Small Cell Lung Cancer: Tumor Heterogeneity, Diagnosis, Therapy, and Future Directions. CA Cancer J. Clin. 2023, 73, 620–652. [Google Scholar] [CrossRef]
- Steindl, A.; Schlieter, F.; Klikovits, T.; Leber, E.; Gatterbauer, B.; Frischer, J.M.; Dieckmann, K.; Widhalm, G.; Zöchbauer-Müller, S.; Hoda, M.A.R.; et al. Prognostic Assessment in Patients with Newly Diagnosed Small Cell Lung Cancer Brain Metastases: Results from a Real-Life Cohort. J. Neurooncol. 2019, 145, 85–95. [Google Scholar] [CrossRef]
- Saltos, A.; Shafique, M.; Chiappori, A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front. Oncol. 2020, 10, 1074. [Google Scholar] [CrossRef]
- Rittberg, R.; Banerji, S.; Kim, J.O.; Rathod, S.; Dawe, D.E. Treatment and Prevention of Brain Metastases in Small Cell Lung Cancer. Am. J. Clin. Oncol. 2021, 44, 629–638. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, Y.; Zheng, X.; Zhao, Y.; Sun, G. Small-Cell Lung Cancer Brain Metastasis: From Molecular Mechanisms to Diagnosis and Treatment. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Hu, Y.; Zhang, Y.; Lin, Z.; Zhao, Z.; Jiao, S. Prophylactic Cranial Irradiation Could Improve Overall Survival in Patients with Extensive Small Cell Lung Cancer: A Retrospective Study. Strahlenther. Onkol. 2016, 192, 905–912. [Google Scholar] [CrossRef]
- Tang, L.; Tian, G.; Li, N. Current Dilemma and Future Directions over Prophylactic Cranial Irradiation in SCLC: A Systematic Review in MRI and Immunotherapy Era. Front. Oncol. 2024, 14, 1382220. [Google Scholar] [CrossRef]
- Simó, M.; Vaquero, L.; Alemany, M.; Padrones, S.; Caravaca, A.; Padró, A.; Navarro, A.; Palmero, R.; Nadal, E.; Bruna, J. P01.04.B LONG-TERM COGNITIVE TOXICITY IN SMALL CELL LUNG CANCER POPULATION: EARLY CLINICAL, NEUROIMAGING, AND SEROLOGICAL BIOMARKERS. Neuro-Oncology 2023, 25, ii26–ii27. [Google Scholar] [CrossRef]
- Vaquero, L.; Rodríguez-Fornells, A.; Pera-Jambrina, M.Á.; Bruna, J.; Simó, M. Plasticity in Bilateral Hippocampi after a 3-Month Physical Activity Programme in Lung Cancer Patients. Eur. J. Neurol. 2021, 28, 1324–1333. [Google Scholar] [CrossRef]
- Bassett, D.S.; Sporns, O. Network Neuroscience. Nat. Neurosci. 2017, 20, 353–364. [Google Scholar] [CrossRef]
- Bromis, K.; Gkiatis, K.; Karanasiou, I.; Matsopoulos, G.; Karavasilis, E.; Papathanasiou, M.; Efstathopoulos, E.; Kelekis, N.; Kouloulias, V. Altered Brain Functional Connectivity in Small-Cell Lung Cancer Patients after Chemotherapy Treatment: A Resting-State fMRI Study. Comput. Math. Methods Med. 2017, 2017, 1403940. [Google Scholar] [CrossRef]
- Simó, M.; Rifà-Ros, X.; Vaquero, L.; Ripollés, P.; Cayuela, N.; Jové, J.; Navarro, A.; Cardenal, F.; Bruna, J.; Rodríguez-Fornells, A. Brain Functional Connectivity in Lung Cancer Population: An Exploratory Study. Brain Imaging Behav. 2018, 12, 369–382. [Google Scholar] [CrossRef]
- Simó, M.; Root, J.C.; Vaquero, L.; Ripollés, P.; Jové, J.; Ahles, T.; Navarro, A.; Cardenal, F.; Bruna, J.; Rodríguez-Fornells, A. Cognitive and Brain Structural Changes in a Lung Cancer Population. J. Thorac. Oncol. 2015, 10, 38–45. [Google Scholar] [CrossRef]
- Mentzelopoulos, A.; Gkiatis, K.; Karanasiou, I.; Karavasilis, E.; Papathanasiou, M.; Efstathopoulos, E.; Kelekis, N.; Kouloulias, V.; Matsopoulos, G.K. Chemotherapy-Induced Brain Effects in Small-Cell Lung Cancer Patients: A Multimodal MRI Study. Brain Topogr. 2021, 34, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus Machine Learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef]
- Wein, S.; Deco, G.; Tomé, A.M.; Goldhacker, M.; Malloni, W.M.; Greenlee, M.W.; Lang, E.W. Brain Connectivity Studies on Structure-Function Relationships: A Short Survey with an Emphasis on Machine Learning. Comput. Intell. Neurosci. 2021, 2021, 5573740. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.M.; Harrod, J.B.; Subramanian, S.; Robinson, M.; Chang, K.; Cetin-Karayumak, S.; Dalca, A.V.; Eickhoff, S.; Fox, M.; Franke, L.; et al. How Machine Learning Is Powering Neuroimaging to Improve Brain Health. Neuroinformatics 2022, 20, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, Y.; Wang, J.; Liu, Y.; Yang, Y.; Wang, Z.; Xi, Q. Machine Learning Based on Functional and Structural Connectivity in Mild Cognitive Impairment. Magn. Reson. Imaging 2024, 109, 10–17. [Google Scholar] [CrossRef]
- Billeci, L.; Badolato, A.; Bachi, L.; Tonacci, A. Machine Learning for the Classification of Alzheimer’s Disease and Its Prodromal Stage Using Brain Diffusion Tensor Imaging Data: A Systematic Review. Processes 2020, 8, 1071. [Google Scholar] [CrossRef]
- Huang, X.; He, Q.; Ruan, X.; Li, Y.; Kuang, Z.; Wang, M.; Guo, R.; Bu, S.; Wang, Z.; Yu, S.; et al. Structural Connectivity from DTI to Predict Mild Cognitive Impairment in de Novo Parkinson’s Disease. NeuroImage Clin. 2024, 41, 103548. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Kakkos, I.; Matsopoulos, G.K.; Yuan, J.; Suckling, J.; Xu, L.; Cao, S.; Chen, W.; Hu, X.; et al. Inferring the Individual Psychopathologic Deficits with Structural Connectivity in a Longitudinal Cohort of Schizophrenia. IEEE J. Biomed. Health Inform. 2022, 26, 2536–2546. [Google Scholar] [CrossRef]
- Kamiya, K.; Amemiya, S.; Suzuki, Y.; Kunii, N.; Kawai, K.; Mori, H.; Kunimatsu, A.; Saito, N.; Aoki, S.; Ohtomo, K. Machine Learning of DTI Structural Brain Connectomes for Lateralization of Temporal Lobe Epilepsy. Magn. Reson. Med. Sci. 2016, 15, 121–129. [Google Scholar] [CrossRef]
- Hu, X.; Varkanitsa, M.; Kropp, E.; Betke, M.; Ishwar, P.; Kiran, S. Aphasia Severity Prediction Using a Multi-Modal Machine Learning Approach. NeuroImage 2025, 317, 121300. [Google Scholar] [CrossRef]
- Mateos-Pérez, J.M.; Dadar, M.; Lacalle-Aurioles, M.; Iturria-Medina, Y.; Zeighami, Y.; Evans, A.C. Structural Neuroimaging as Clinical Predictor: A Review of Machine Learning Applications. NeuroImage Clin. 2018, 20, 506–522. [Google Scholar] [CrossRef]
- Tax, C.M.W.; Bastiani, M.; Veraart, J.; Garyfallidis, E.; Okan Irfanoglu, M. What’s New and What’s next in Diffusion MRI Preprocessing. NeuroImage 2022, 249, 118830. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a New Parcellation of the Orbitofrontal Cortex in the Automated Anatomical Labeling Atlas. NeuroImage 2015, 122, 1–5. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.; Janke, A.L.; Budge, M.M.; Smith, D.; Pruessner, J.; Collins, D.L. Symmetric Atlasing and Model Based Segmentation: An Application to the Hippocampus in Older Adults. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006; Larsen, R., Nielsen, M., Sporring, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 58–66. [Google Scholar]
- Mori, S.; Crain, B.J.; Chacko, V.P.; Van Zijl, P.C.M. Three-Dimensional Tracking of Axonal Projections in the Brain by Magnetic Resonance Imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Choi, Y.-H.; Lee, J.-M. A Physarum Centrality Measure of the Human Brain Network. Sci. Rep. 2019, 9, 5907. [Google Scholar] [CrossRef]
- Cha, J.H.; Choi, Y.-H.; Lee, J.-M.; Lee, J.Y.; Park, H.-K.; Kim, J.; Kim, I.-K.; Lee, H.J. Altered Structural Brain Networks at Term-Equivalent Age in Preterm Infants with Grade 1 Intraventricular Hemorrhage. Ital. J. Pediatr. 2020, 46, 43. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A Pipeline Toolbox for Analyzing Brain Diffusion Images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Kakkos, I.; Dimitrakopoulos, G.N.; Sun, Y.; Yuan, J.; Matsopoulos, G.K.; Bezerianos, A.; Sun, Y. EEG Fingerprints of Task-Independent Mental Workload Discrimination. IEEE J. Biomed. Health Inform. 2021, 25, 3824–3833. [Google Scholar] [CrossRef]
- Toloşi, L.; Lengauer, T. Classification with Correlated Features: Unreliability of Feature Ranking and Solutions. Bioinformatics 2011, 27, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, I.; Ventouras, E.M.; Asvestas, P.A.; Karanasiou, I.S.; Matsopoulos, G.K. A Condition-Independent Framework for the Classification of Error-Related Brain Activity. Med. Biol. Eng. Comput. 2020, 58, 573–587. [Google Scholar] [CrossRef]
- Golland, P.; Liang, F.; Mukherjee, S.; Panchenko, D. Permutation Tests for Classification. In Proceedings of the Learning Theory; Auer, P., Meir, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 501–515. [Google Scholar]
- Graf, R.; Zeldovich, M.; Friedrich, S. Comparing Linear Discriminant Analysis and Supervised Learning Algorithms for Binary Classification—A Method Comparison Study. Biometrical J. 2024, 66, 2200098. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A Method of Comparing the Areas under Receiver Operating Characteristic Curves Derived from the Same Cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Pendergrass, J.C.; Targum, S.D.; Harrison, J.E. Cognitive Impairment Associated with Cancer. Innov. Clin. Neurosci. 2018, 15, 36–44. [Google Scholar]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-Related Cognitive Impairment: An Update on State of the Art, Detection, and Management Strategies in Cancer Survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Wang, L.; Hu, S.; Yao, Z.; Xue, M.; Lu, Z.; Xiao-ju, Z.; Ding, Y. Correlation between Cancer-Related Cognitive Impairment and Resting Cerebral Glucose Metabolism in Patients with Ovarian Cancer. Heliyon 2024, 10, e34106. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N. Intra- and Inter-Hemispheric Connectivity Supporting Hemispheric Specialization. In Micro-, Meso- and Macro-Connectomics of the Brain; Kennedy, H., Van Essen, D.C., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-27776-9. [Google Scholar]
- Bullmore, E.; Sporns, O. Complex Brain Networks: Graph Theoretical Analysis of Structural and Functional Systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Aceña, V.; Martín de Diego, I.; Fernández, R.R.; Moguerza, J.M. Minimally Overfitted Learners: A General Framework for Ensemble Learning. Knowl.-Based Syst. 2022, 254, 109669. [Google Scholar] [CrossRef]
- Jollans, L.; Boyle, R.; Artiges, E.; Banaschewski, T.; Desrivières, S.; Grigis, A.; Martinot, J.-L.; Paus, T.; Smolka, M.N.; Walter, H.; et al. Quantifying Performance of Machine Learning Methods for Neuroimaging Data. Neuroimage 2019, 199, 351–365. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S. Visual Interpretability for Deep Learning: A Survey. Front. Inf. Technol. Electron. Eng. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Montavon, G.; Samek, W.; Müller, K.-R. Methods for Interpreting and Understanding Deep Neural Networks. Digit. Signal Process. 2018, 73, 1–15. [Google Scholar] [CrossRef]
- Alelyani, S. Stable Bagging Feature Selection on Medical Data. J. Big Data 2021, 8, 11. [Google Scholar] [CrossRef]
- Khaire, U.M.; Dhanalakshmi, R. Stability of Feature Selection Algorithm: A Review. J. King Saud. Univ.-Comput. Inf. Sci. 2022, 34, 1060–1073. [Google Scholar] [CrossRef]
- Mentzelopoulos, A.; Karanasiou, I.; Papathanasiou, M.; Kelekis, N.; Kouloulias, V.; Matsopoulos, G.K. A Comparative Analysis of White Matter Structural Networks on SCLC Patients After Chemotherapy. Brain Topogr. 2022, 35, 352–362. [Google Scholar] [CrossRef]
- Manan, A.A.; Yahya, N.A.; Taib, N.H.M.; Idris, Z.; Manan, H.A. The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review. Cancers 2023, 15, 3326. [Google Scholar] [CrossRef]
- Saward, J.B.; Ellis, E.G.; Cobden, A.L.; Caeyenberghs, K. Mapping Cognitive Deficits in Cancer Patients after Chemotherapy: An Activation Likelihood Estimation Meta-Analysis of Task-Related fMRI Studies. Brain Imaging Behav. 2022, 16, 2320–2334. [Google Scholar] [CrossRef]
- Mulholland, M.M.; Stuifbergen, A.; Schutz, A.D.L.T.; Rocha, O.Y.F.; Blayney, D.W.; Kesler, S.R. Evidence of Compensatory Neural Hyperactivity in a Subgroup of Chemotherapy-Treated Breast Cancer Survivors and Its Association with Brain Aging. medRxiv 2024. [Google Scholar] [CrossRef]
- Oliva, G.; Giustiniani, A.; Danesin, L.; Burgio, F.; Arcara, G.; Conte, P. Cognitive Impairment Following Breast Cancer Treatments: An Umbrella Review. Oncologist 2024, 29, e848–e863. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Du, W.; Cao, J.; Jiang, Y.; Song, D.; Yu, D.; Zhang, J.; Guo, J.; Xie, X.; Xie, L.; et al. Relationship between δ-Catenin Expression and Whole-Brain Small-World Network in Breast Cancer Patients before Chemotherapy. Sci. Rep. 2024, 14, 31119. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wang, L.; Chen, X.; Liu, F.; Ruan, F.; Zhang, J.; Shen, L.; Yu, Y. Modulation of Interhemispheric Functional Coordination in Breast Cancer Patients Receiving Chemotherapy. Front. Psychol. 2020, 11, 1689. [Google Scholar] [CrossRef]
- Vasaghi Gharamaleki, M.; Mousavi, S.Z.; Owrangi, M.; Gholamzadeh, M.J.; Kamali, A.-M.; Dehghani, M.; Chakrabarti, P.; Nami, M. Neural Correlates in Functional Brain Mapping among Breast Cancer Survivors Receiving Different Chemotherapy Regimens: A qEEG/HEG-Based Investigation. Jpn. J. Clin. Oncol. 2022, 52, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, X.D.; Zheng, G.; Zhang, L.J. Chemotherapy-Induced Brain Changes in Breast Cancer Survivors: Evaluation with Multimodality Magnetic Resonance Imaging. Brain Imaging Behav. 2019, 13, 1799–1814. [Google Scholar] [CrossRef]
- McDonald, B.C. Structural Neuroimaging Findings Related to Adult Non-CNS Cancer and Treatment: Review, Integration, and Implications for Treatment of Cognitive Dysfunction. Neurotherapeutics 2021, 18, 792–810. [Google Scholar] [CrossRef]
- McDonald, C.R.; White, N.S.; Farid, N.; Lai, G.; Kuperman, J.M.; Bartsch, H.; Hagler, D.J.; Kesari, S.; Carter, B.S.; Chen, C.C.; et al. Recovery of White Matter Tracts in Regions of Peritumoral FLAIR Hyperintensity with Use of Restriction Spectrum Imaging. AJNR Am. J. Neuroradiol. 2013, 34, 1157–1163. [Google Scholar] [CrossRef]
- Ahn, S.J.; Kwon, H.; Kim, J.W.; Park, G.; Park, M.; Joo, B.; Suh, S.H.; Chang, Y.S.; Lee, J.-M. Hippocampal Metastasis Rate Based on Non-Small Lung Cancer TNM Stage and Molecular Markers. Front. Oncol. 2022, 12, 781818. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Li, Z.-H.; Zhang, Z.-P.; He, Y.-F.; Shang, B.-L.; Xu, X.-F.; Ding, Y.-Y.; Cheng, Y.-Q. Cognitive Changes Are Associated with Increased Blood-Brain Barrier Leakage in Non-Brain Metastases Lung Cancer Patients. Brain Imaging Behav. 2023, 17, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.H.S.; Sleurs, C.; Gavrila Laic, R.A.; Amidi, A.; Chen, B.T.; Deprez, S.; McDonald, B.C. Neuroimaging Studies of Cognitive Dysfunction Following Cancer and Treatment. J. Clin. Exp. Neuropsychol. 2025, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ni, J.; Yan, F.; Yin, N.; Li, X.; Ma, R.; Wu, J.; Zhou, G.; Feng, J. Functional Changes of the Prefrontal Cortex, Insula, Caudate and Associated Cognitive Impairment (Chemobrain) in NSCLC Patients Receiving Different Chemotherapy Regimen. Front. Oncol. 2022, 12, 1027515. [Google Scholar] [CrossRef] [PubMed]
- Ivanoska, I.; Trivodaliev, K.; Kalajdziski, S.; Zanin, M. Statistical and Machine Learning Link Selection Methods for Brain Functional Networks: Review and Comparison. Brain Sci. 2021, 11, 735. [Google Scholar] [CrossRef]
- Tsegaye, B.; Snell, K.I.E.; Archer, L.; Kirtley, S.; Riley, R.D.; Sperrin, M.; Van Calster, B.; Collins, G.S.; Dhiman, P. Larger Sample Sizes Are Needed When Developing a Clinical Prediction Model Using Machine Learning in Oncology: Methodological Systematic Review. J. Clin. Epidemiol. 2025, 180, 111675. [Google Scholar] [CrossRef]
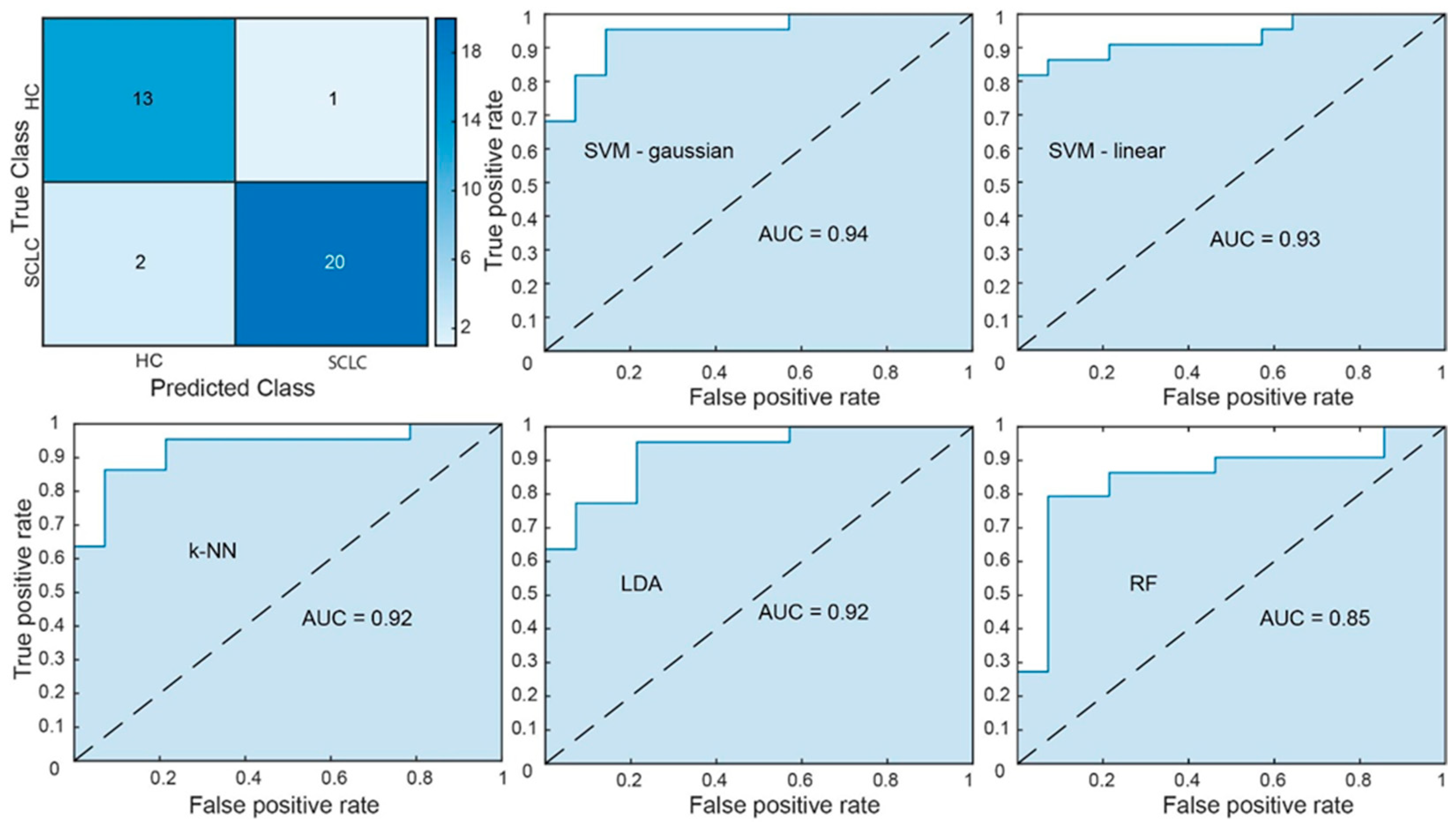
| Classifier | Accuracy | Sensitivity | Specificity | F1-Score | Area Under the ROC Curve |
|---|---|---|---|---|---|
| SVM-gaussian | 0.92 1 | 0.93 | 0.91 | 0.92 | 0.94 1 |
| SVM-linear | 0.82 1 | 0.79 | 0.86 | 0.82 | 0.93 1 |
| k-NN | 0.77 2 | 0.71 | 0.82 | 0.76 | 0.92 1 |
| LDA | 0.78 2 | 0.79 | 0.77 | 0.78 | 0.92 1 |
| RF | 0.75 2 | 0.73 | 0.76 | 0.74 | 0.85 2 |
| Soft-Voting Ensemble | 0.86 1 | 0.88 | 0.85 | 0.86 | 0.90 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miloulis, S.T.; Kakkos, I.; Zorzos, I.; Vezakis, I.A.; Kontopodis, E.; Petropoulou, O.; Ventouras, E.M.; Sun, Y.; Matsopoulos, G.K. DTI-Based Structural Connectome Analysis of SCLC Patients After Chemotherapy via Machine Learning. Appl. Sci. 2025, 15, 12458. https://doi.org/10.3390/app152312458
Miloulis ST, Kakkos I, Zorzos I, Vezakis IA, Kontopodis E, Petropoulou O, Ventouras EM, Sun Y, Matsopoulos GK. DTI-Based Structural Connectome Analysis of SCLC Patients After Chemotherapy via Machine Learning. Applied Sciences. 2025; 15(23):12458. https://doi.org/10.3390/app152312458
Chicago/Turabian StyleMiloulis, Stavros Theofanis, Ioannis Kakkos, Ioannis Zorzos, Ioannis A. Vezakis, Eleftherios Kontopodis, Ourania Petropoulou, Errikos M. Ventouras, Yu Sun, and George K. Matsopoulos. 2025. "DTI-Based Structural Connectome Analysis of SCLC Patients After Chemotherapy via Machine Learning" Applied Sciences 15, no. 23: 12458. https://doi.org/10.3390/app152312458
APA StyleMiloulis, S. T., Kakkos, I., Zorzos, I., Vezakis, I. A., Kontopodis, E., Petropoulou, O., Ventouras, E. M., Sun, Y., & Matsopoulos, G. K. (2025). DTI-Based Structural Connectome Analysis of SCLC Patients After Chemotherapy via Machine Learning. Applied Sciences, 15(23), 12458. https://doi.org/10.3390/app152312458









