The Use of the Idea of Loan Extraction to Produce a Skin Care Serum (Cosmetic) Containing a High Concentration of Bioactive Ingredients Isolated from Calendula officinalis L. Petals
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Sample Preparation
2.4. Formulations of Facial Serum
2.5. Determination of Bioactive Compounds by UPLC-ESI-MS/MS
2.6. Total Phenolic Content
2.7. Antioxidant Activity (DPPH Test)
2.8. Organoleptic Properties
2.9. Turbidity
2.10. Color Parameters of Extracts and Cosmetics (Face Serum) Containing Extracts
2.11. Spreadability Test
2.12. Viscosity
2.13. Determination of Irritant Potential—Zein Value
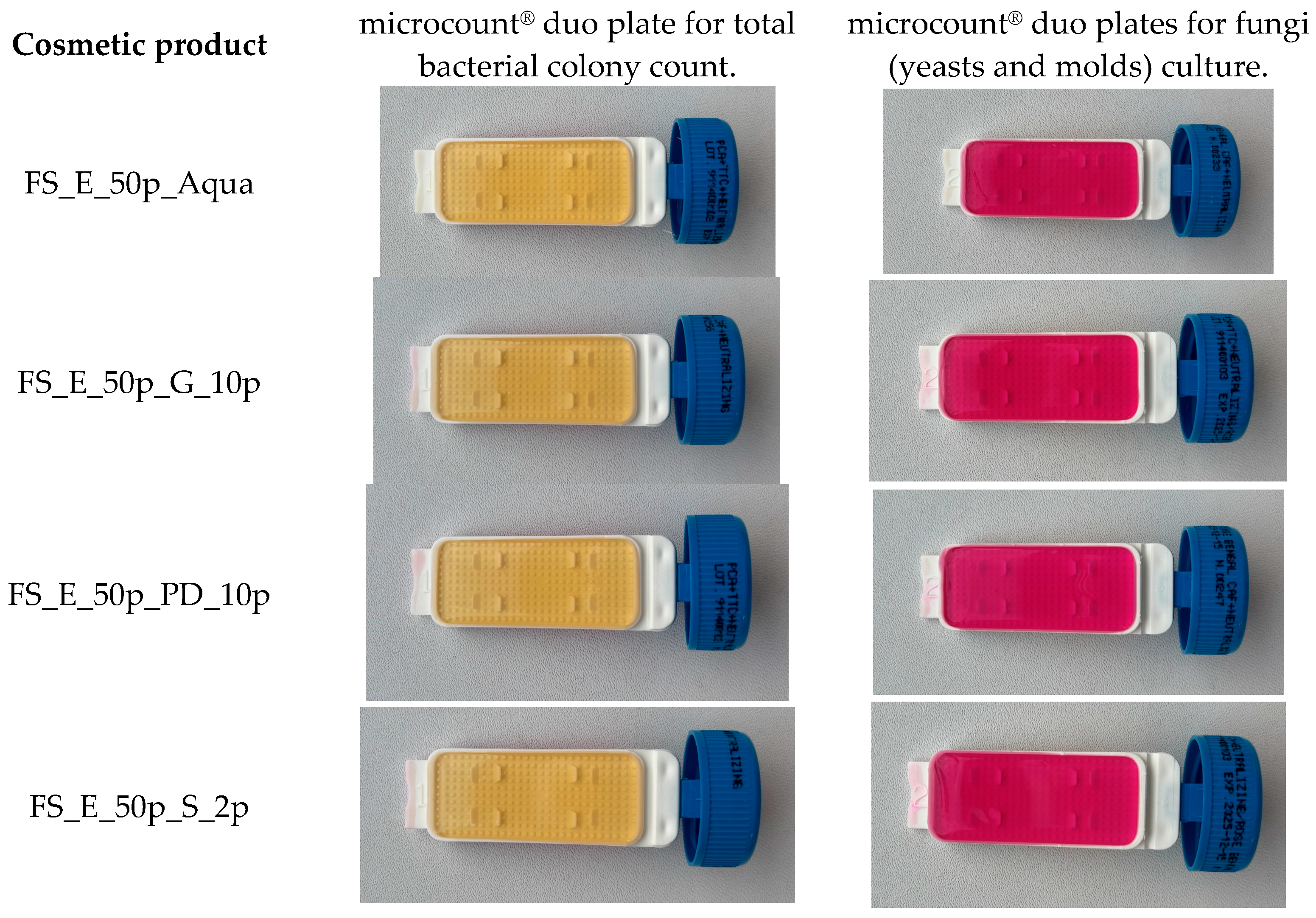
2.14. Microbiological Stability
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Extraction Process Applying the Idea of Using an Extraction Medium Based on Components Derived from the Final Formulation
3.2. Determination of Selected Compounds by UPLC-MS/MS
3.3. Total Phenolic Content (TPC) and Antioxidant Capacity (DPPH)
3.4. Characteristic of Model Cosmetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTU | Nephelometric turbidity unit |
| ESI | Electrospray ionization |
| LE | Loan extraction |
| TPC | Total phenolic content |
| FS | Face serum |
| E_CO_2p_Aqua | Calendula officinalis L. petal extract obtained using aqueous solution |
| E_CO_2p_G_10p | Calendula officinalis L. petal extract obtained using aqueous solution of glycerin |
| E_CO_2p_PD_10p | Calendula officinalis L. petal extract obtained using aqueous solution of propanediol |
| E_CO_2p_S_2p | Calendula officinalis L. petal extract obtained using aqueous solution of surfactants |
| FS_E_50p_Aqua | Face serum with 50% extract based on aqueous solution |
| FS_E_50p_G_10p | Face serum with 50% extract based on aqueous solution of glycerin |
| FS_E_50p_PD_10p | Face serum with 50% extract based on aqueous solution of propanediol |
| FS_E_50p_S_2p | Face serum with 50% extract based on aqueous solution of surfactants |
References
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Mian, M.; Silfvast-Kaiser, A.S.; Paek, S.Y.; Kivelevitch, D.; Menter, A. A review of the most common dermatologic conditions and their debilitating psychosocial impacts. Int. Arch. Intern. Med. 2019, 3, 18. [Google Scholar] [CrossRef]
- Naidoo, K.; Birch-Machin, M.A. Oxidative stress and ageing: The influence of environmental pollution, sunlight and diet on skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Hu, S.; Anand, P.; Laughter, M.; Maymone, M.B.C.; Dellavalle, R.P. Holistic dermatology: An evidence-based review of modifiable lifestyle factor associations with dermatologic disorders. J. Am. Acad. Dermatol. 2022, 86, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Camilleri, M.A.; Cricelli, L.; Mauriello, R.; Strazzullo, S. Consumer perceptions of sustainable products: A systematic literature review. Sustainability 2023, 15, 8923. [Google Scholar] [CrossRef]
- Kumar, V. Perspective of natural products in skincare. Pharm. Pharmacol. Int. J. 2016, 4, 339–341. [Google Scholar] [CrossRef]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Robles Velasco, M.V.; Baby, A.R. Innovative approaches to an eco-friendly cosmetic industry: A review of sustainable ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Wichtl, M. (Ed.) Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basisi; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Vidal-Ollivier, E.; Elias, R.; Faure, F.; Babadjamian, A.; Crespin, F.; Balansard, G.; Boudon, G. Flavonol glycosides from Calendula officinalis flowers. Planta Medica 1989, 55, 73–74. [Google Scholar] [CrossRef]
- Wasli, A.L.; Wan Ismail, W.A.; Zamery, M.I. Review on pharmacological and potential cosmeceutical values of Centella asiatica (Pegaga). Int. J. Pharm. Nutraceut. Cosmet. Sci. 2022, 5, 13–32. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Buarque, F.S.; Gautério, G.V.; Coelho, M.A.Z.; Lemes, A.C.; Dias Ribeiro, B. Aqueous two-phase systems based on ionic liquids and deep eutectic solvents as a tool for the recovery of non-protein bioactive compounds—A review. Processes 2023, 11, 31. [Google Scholar] [CrossRef]
- Yara-Varon, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Wasilewski, T.; Seweryn, A.; Bujak, T. Supercritical carbon dioxide blackcurrant seed extract as an anti-irritant additive for hand dishwashing liquids. Green Chem. Lett. Rev. 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Wasilewski, T.; Hordyjewicz-Baran, Z.; Zarębska, M.; Stanek, N.; Zajszły-Turko, E.; Tomaka, M.; Bujak, T.; Nizioł-Łukaszewska, Z. Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics. Molecules 2022, 27, 2444. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, M.; Wang, G.; Wang, K.; Jian, K. The antioxidant activity of pomegranate seed polyphenols in vitro. Sci. Technol. Food Ind. 2018, 39, 17–20. [Google Scholar]
- Su, H.; Wei, J.; Bi, Y.; Hu, H.; Wang, Z. Optimization of ultrasonic-assisted extraction of polyphenols from Chinese Seabuckthorn berries and composition analysis. Food Ferment. Sci. Technol. 2017, 53, 34–41. [Google Scholar]
- Zhang, X.; Bei, Z.; Qiao, Y. The anti-oxidation effects of polyphenols in Lyciumbarbarum L. fresh flower. Feed. Ind. 2017, 38, 38–43. [Google Scholar]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, C.; Du, P.; Yu, Y.; Zhu, S.; Zheng, Y.; Wang, M. Research progress of polyphenols in food, extraction methods and detection techniques. Food Ferment Ind. 2019, 45, 231–238. [Google Scholar]
- Hu, Y.; Yan, B.; Chen, Z.S.; Wang, L.; Tang, W.; Huang, C. Recent Technologies for the Extraction and Separation of Polyphenols in Different Plants: A Review. J. Renew. Mater. 2022, 10, 1471–1490. [Google Scholar] [CrossRef]
- Chemat, F.; Li, Y.; Tomao, V.; Ginies, C.; Cravotto, G. Optimization of procedures for in-line extraction of lipids and polyphenols from grape seeds. Food Anal. Methods 2014, 7, 459–464. [Google Scholar] [CrossRef]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Abasi, S.; Amani Tehran, M.; Fairchild, M.D. Distance metrics for very large color differences. Color Res. Appl. 2020, 45, 208–223. [Google Scholar] [CrossRef]
- Khouchlaa, A.; El Baaboua, A.; El Moudden, H.; Lakhdar, F.; Bakrim, S.; El Menyiy, N.; Belmehdi, O.; Harhar, H.; El Omari, N.; Balahbib, A.; et al. Traditional Uses, Bioactive Compounds, and Pharmacological Investigations of Calendula arvensis L.: A Comprehensive Review. Adv. Pharm. Pharm. Sci. 2023, 2023, 2482544. [Google Scholar] [CrossRef] [PubMed]
- Ercan, L.; Doğru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Djerassi, D. Amino Acids and Peptides: Building Blocks for Skin Proteins. In Nutritional Cosmetics; Elsevier: Amsterdam, The Netherlands, 2009; pp. 287–317. [Google Scholar] [CrossRef]
- Gandhi, F.; Rosario, C.; Mishra, M.; Gandhi, M.d.H.; Kadam, P. Review on application of one of the most valuable players of amino acids: L-Lysine. Int. J. Pharm. Res. Appl. 2024, 9, 1337–1344. [Google Scholar] [CrossRef]
- Morávková, T.; Stern, P. Rheological and textural properties of cosmetic emulsions. Appl. Rheol. 2011, 21, 35189. [Google Scholar] [CrossRef]
- Kelley, C.D.; Krolick, A.; Brunner, L.; Burklund, A.; Kahn, D.; Ball, W.P.; Weber-Shirk, M. An Affordable Open-Source Turbidimeter. Sensors 2014, 14, 7142–7155. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. extracts as bioactive ingredients that increase safety of body wash cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef]
- Pezron, I.; Galet, L.; Clausse, D. Surface interaction between a protein monolayer and surfactants and its correlation with skin irritation by surfactants. J. Colloid Interface Sci. 1996, 180, 285–289. [Google Scholar] [CrossRef]



| Name According to INCI Nomenclature | Concentration, % [w/w] | ||||
|---|---|---|---|---|---|
| FS_E_0p | FS_E_50p_Aqua | FS_E_50p_G | FS_E_50p_PD | FS_E_50p_S | |
| Polyglyceryl-4 Laurate/Sebacate (and) Polyglyceryl-6 Caprylate/Caprate | 1 | 1 | 1 | 1 | - |
| Propanediol | 5 | 5 | 5 | - | 5 |
| Glycerin | 5 | 5 | - | 5 | 5 |
| Extract | 0 | 50 | 50 | 50 | 50 |
| Polyglyceryl-4 Laurate/Sebacate (and) Polyglyceryl-6 Caprylate/Caprate | - | - | - | - | 1 |
| Propanediol | - | - | - | 5 | - |
| Glycerin | - | - | 5 | - | - |
| Aqua | - | 49.8875 | 44.8875 | 44.8875 | 48.8875 |
| Sodium Benzoate, Potassium Sorbate | - | 0.1125 | 0.1125 | 0.1125 | 0.1125 |
| Sodium Benzoate Potassium Sorbate | 0.225 | 0.1125 | 0.1125 | 0.1125 | 0.1125 |
| Xanthan Gum | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Aqua | to 100 | to 100 | to 100 | to 100 | to 100 |
| Extraction Medium | Viscosity mPa·s | Density g/cm3 | pH | Spreadability % | Turbidity NTU |
|---|---|---|---|---|---|
| Aqua | 0.899 ± 0.003 | 1.000 ± 0.002 | 7.0 | 79.4 | 0.2 ± 0.1 |
| G_10p | 0.985 ± 0.002 | 1.026 ± 0.002 | 6.7 | 76.2 | 0.3 ± 0.1 |
| PD_10p | 1.247 ± 0.002 | 1.010 ± 0.002 | 7.0 | 77.0 | 0.4 ± 0.1 |
| S_2p | 1.011 ± 0.005 | 1.006 ± 0.002 | 6.4 | 61.4 | 2.2 ± 0.2 |
| Sample | Viscosity mPa·s | Turbidity NTU | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|---|---|
| E_CO_2p_Aqua | 0.986 ± 0.001 | 14 ± 0.5 | 26.28 | −1.5 | 13.18 | 13.27 | 31.84 |
| E_CO_2p_G_10p | 1.304 ± 0.002 | 13 ± 0.5 | 28.44 | −1.73 | 14.04 | 14.15 | 13.60 |
| E_CO_2p_PD_10p | 1.283 ± 0.001 | 15 ± 0.5 | 26.84 | −0.79 | 14.25 | 14.27 | 13.88 |
| E_CO_2p_S_2p | 1.326 ± 0.001 | 209 ± 5.0 | 71.37 | −1.17 | 24.14 | 24.17 | 50.25 |
| Compound | MRM, Q1 > Q3 m/z | E_CO_2p_ Aqua | E_CO_2p_ S_2p | E_CO_2p_PD_10p | E_CO_2p_ G_10p |
|---|---|---|---|---|---|
| mg/L | |||||
| Tartaric acid | 148.9 > 87.0, 148.9 > 73.0 | 105 a ± 5 | 9.9 b ± 0.4 | 11.7 b ± 0.6 | 13.2 b ± 0.4 |
| DL-malic acid | 132.9 > 114.9, 132.9 > 71.0 | 468 a ± 9 | 770 b ± 10 | 884 c ± 8 | 933 d ± 13 |
| Fumaric acid | 114.8 > 70.9, 114.8 > 26.9 | 8.9 a ± 0.2 | 4.4 b ± 0.2 | 4.2 b ± 0.1 | 4.5 b ± 0.2 |
| Sum of organic acids | 582 | 784 | 950 | 900 | |
| Quinic acid | 190.9 > 84.9, 190.9 > 93.0 | 14.9 a ± 0.3 | 23.4 b ± 0.6 | 31.3 c ± 0.7 | 34.0 c ± 0.4 |
| Rutin | 608.9 > 299.9, 608.9 > 270.9 | 1.6 a ± 0.3 | 2.8 b ± 0.2 | 4.5 c ± 0.7 | 3.1 b ± 0.1 |
| Sum of phenolic compounds | 16.5 | 26.1 | 37.1 | 35.7 | |
| L-Valine | 118.1 > 72.0, 118.8 > 55.0 | 11.4 b ± 0.1 | 9.6 a ± 0.1 | 12.5 c ± 0.3 | 10.0 a,b ± 0.2 |
| L-Leucine | 132.1 > 86.0, 132.1 > 44.0 | 8.5 b ± 0.1 | 6.6 a ± 0.1 | 9.4 c ± 0.6 | 8.9 b ± 0.1 |
| L-Histidine | 156.1 > 82.9, 156.1 > 74.0 | 0.71 a ± 0.01 | 0.72 a ± 0.01 | 0.71 a ± 0.02 | 0.71 a ± 0.02 |
| L-Threonine | 120.3 > 74.0, 120.3 > 56.0 | 5.6 b ± 0.1 | 5.4 a ± 0.1 | 6.2 c ± 0.1 | 6.0 c ± 0.1 |
| L-Lysine | 147.1 > 84.0, 147.1 > 130.0 | 19.1 b ± 0.1 | 17.9 a ± 0.4 | 20.5 c ± 0.4 | 20.5 c ± 0.3 |
| L-Phenylalanine | 163.9 > 147.0, 163.9 > 103.0 | 6.1 a ± 0.1 | 7.5 b ± 0.4 | 9.2 c ± 0.9 | 9.6 c ± 0.4 |
| L-Aspartic acid | 131.9 > 88.0, 131.9 > 114.9 | 7.5 a ± 0.9 | 9.4 b ± 1.1 | 13.8 c ± 1.0 | 16.0 d ± 1.6 |
| Sum of amino acids | 58.8 | 57.1 | 71.6 | 72.2 | |
| Glucose | 178.9 > 58.9, 178.9 > 88.9 | 20.2 a ± 0.6 | 25.2 b ± 0.4 | 32.1 c ± 1.4 | 38.2 d ± 1.1 |
| Sucrose | 340.9 > 179.0, 340.9 > 179.0 | 1.5 b ± 0.1 | 1.3 a ± 0.2 | 4.4 c ± 0.9 | 4.6 c ± 0.1 |
| Fructose | 179.9 > 59.0, 179.9 > 90.0 | 17.8 a ± 1.1 | 18.5 a ± 1.3 | 22.8 b ± 1.2 | 30.7 c ± 1.2 |
| Mannose | 178.8 > 58.9, 178.8 > 88.9 | 16.0 a ± 0.7 | 22.4 b ± 1.4 | 26.3 c ± 0.9 | 27.7 c ± 1.0 |
| Sum of sugars | 55.5 | 67.3 | 101.2 | 85.5 | |
| Extraction Medium | TPC [mg GAE/L] ± SD | DPPH [mg TE/L] ± SD |
|---|---|---|
| E_CO_2p_Aqua | 317 a ± 6 | 162 a ± 5 |
| E_CO_2p_G_10p | 346 b ± 4 | 167 a,b ± 1 |
| E_CO_2p_PD_10p | 365 c ± 3 | 171 b ± 3 |
| E_CO_2p_S_2p | 375 c ± 6 | 170 b ± 4 |
| Sample | Viscosity mPa·s | Density g/cm3 | Spreadability % | Turbidity NTU | Zein Number mgN/100 mL |
|---|---|---|---|---|---|
| FS_E_50p_Aqua | 4393 ± 36 | 1.287 | 18.0 | 211 ± 10 | 17.5 ± 0.5 |
| FS_E_50p_G_10p | 3480 ± 63 | 1.284 | 18.9 | 302 ± 12 | 21.0 ± 0.6 |
| FS_E_50p_PD_10p | 3157 ± 32 | 1.336 | 18.3 | 240 ± 10 | 16.8 ± 0.5 |
| FS_E_50p_S_2p | 4374 ± 159 | 1.283 | 18.5 | 590 ± 15 | 20.3 ± 0.5 |
| Sample | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| FS_E_50p_Aqua | 78.52 | 0.2 | 22.87 | 22.87 | 54.76 |
| FS_E_50p_G_10p | 81.79 | −0.22 | 22.15 | 22.15 | 55.73 |
| FS_E_50p_PD_10p | 79.97 | −0.14 | 22.12 | 22.12 | 57.39 |
| FS_E_50p_S_2p | 80.69 | 0.07 | 23.66 | 23.66 | 57.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzechowicz, W.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Stanek-Wandzel, N.; Malorna, K.; Fleszer, J. The Use of the Idea of Loan Extraction to Produce a Skin Care Serum (Cosmetic) Containing a High Concentration of Bioactive Ingredients Isolated from Calendula officinalis L. Petals. Appl. Sci. 2025, 15, 12444. https://doi.org/10.3390/app152312444
Orzechowicz W, Wasilewski T, Hordyjewicz-Baran Z, Stanek-Wandzel N, Malorna K, Fleszer J. The Use of the Idea of Loan Extraction to Produce a Skin Care Serum (Cosmetic) Containing a High Concentration of Bioactive Ingredients Isolated from Calendula officinalis L. Petals. Applied Sciences. 2025; 15(23):12444. https://doi.org/10.3390/app152312444
Chicago/Turabian StyleOrzechowicz, Wiktoria, Tomasz Wasilewski, Zofia Hordyjewicz-Baran, Natalia Stanek-Wandzel, Katarzyna Malorna, and Joanna Fleszer. 2025. "The Use of the Idea of Loan Extraction to Produce a Skin Care Serum (Cosmetic) Containing a High Concentration of Bioactive Ingredients Isolated from Calendula officinalis L. Petals" Applied Sciences 15, no. 23: 12444. https://doi.org/10.3390/app152312444
APA StyleOrzechowicz, W., Wasilewski, T., Hordyjewicz-Baran, Z., Stanek-Wandzel, N., Malorna, K., & Fleszer, J. (2025). The Use of the Idea of Loan Extraction to Produce a Skin Care Serum (Cosmetic) Containing a High Concentration of Bioactive Ingredients Isolated from Calendula officinalis L. Petals. Applied Sciences, 15(23), 12444. https://doi.org/10.3390/app152312444






