Effects of Soil Rhizobia and Drought on Plant–Vector–Pathogen Interactions on a Legume Host
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Experimental Design and Data Collection
2.3. Data Analysis
3. Results
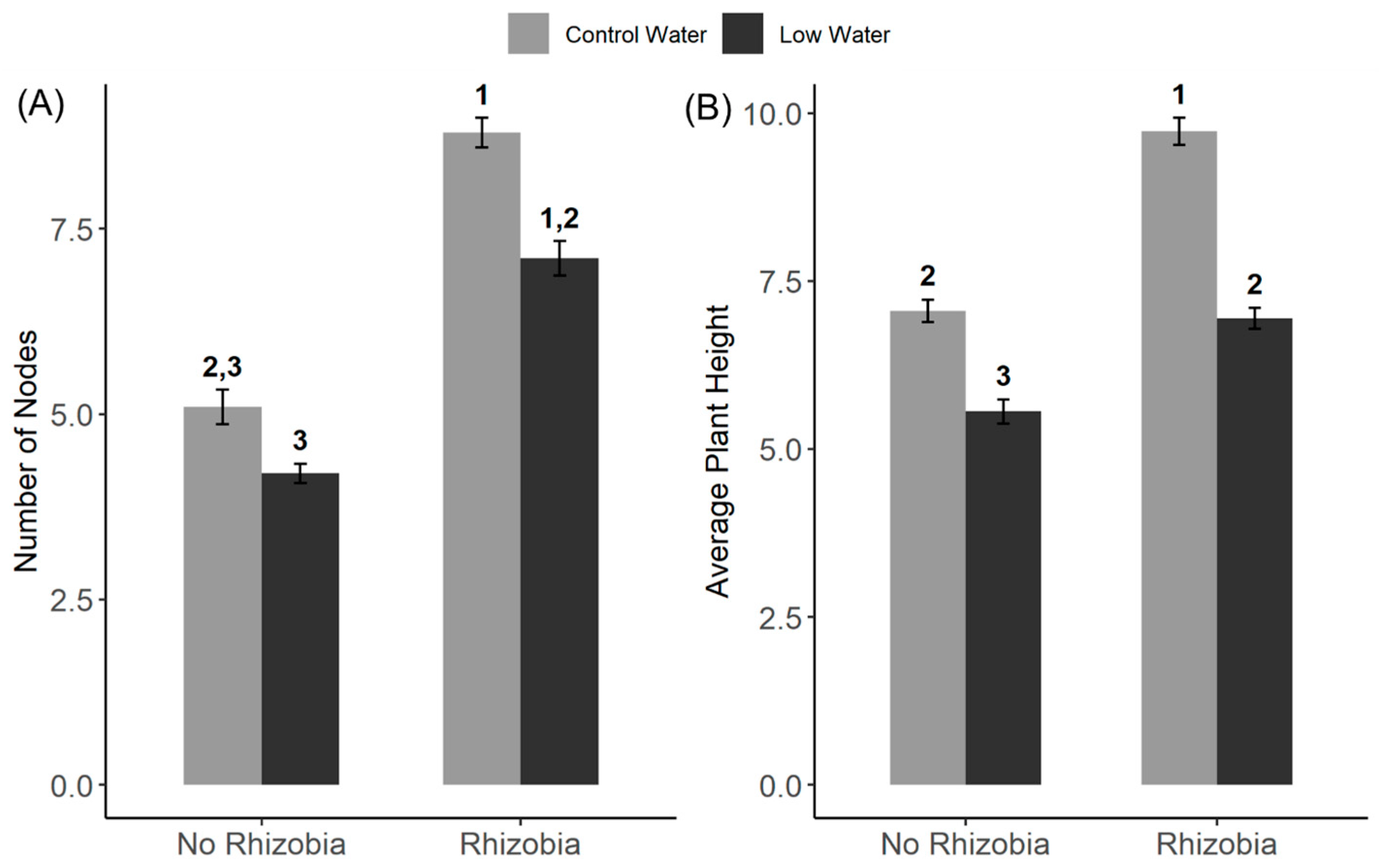
3.1. Effects of Rhizobia and Water Availability on Plant Traits
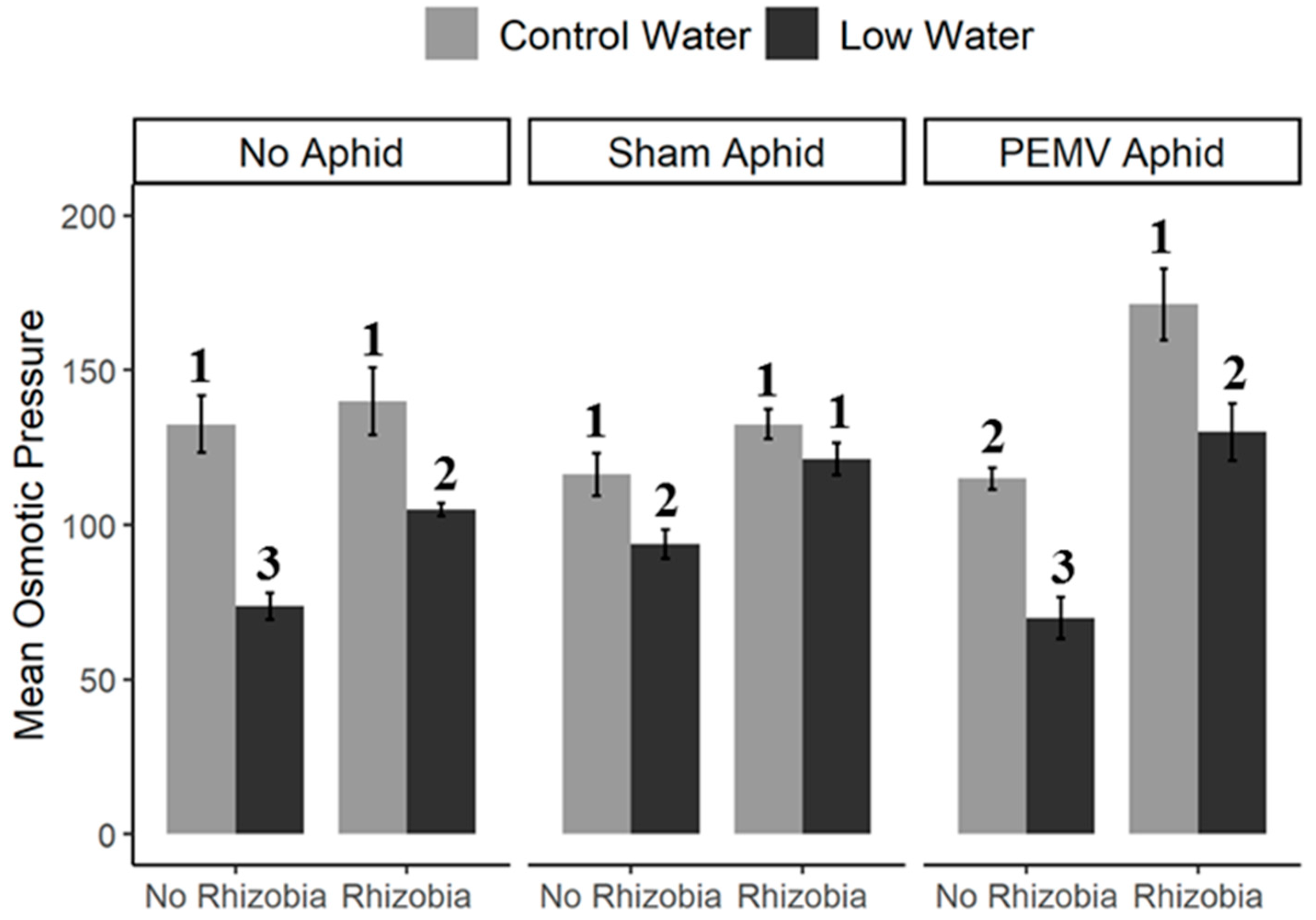
3.2. Effects of Soil Treatment and Drought on Viral Accumulation
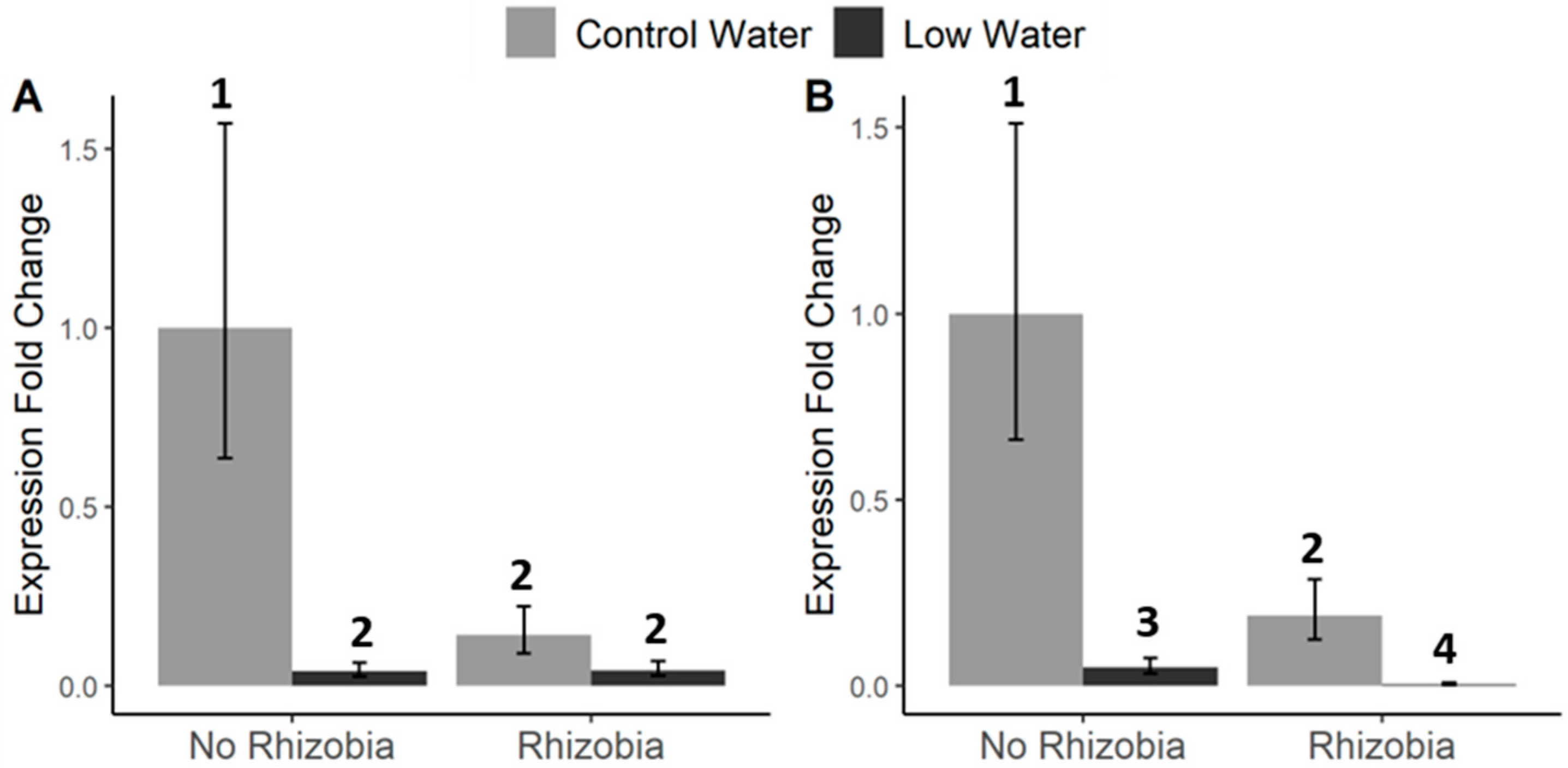
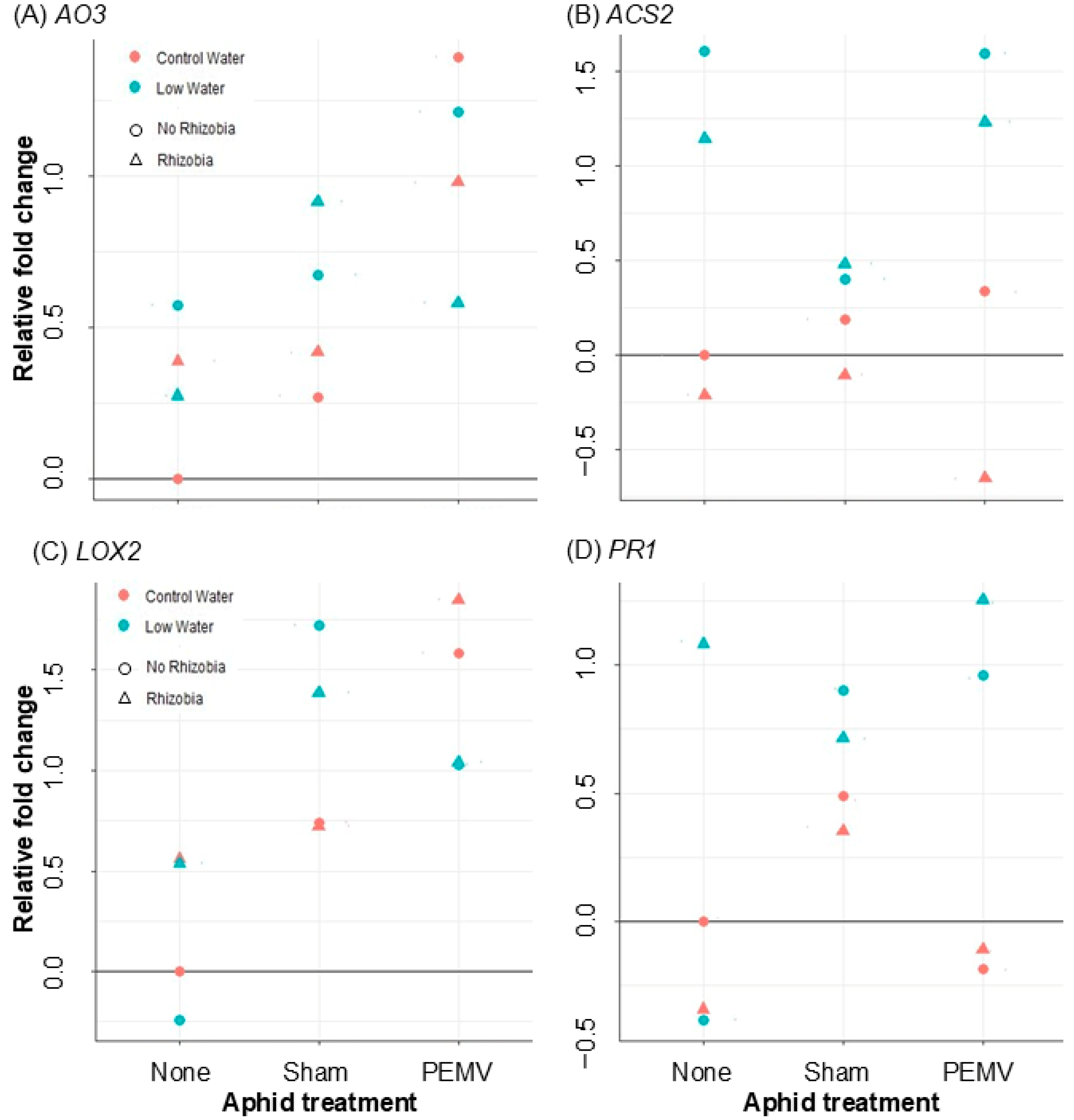
3.3. Effects of Rhizobia, Drought, Aphids, and PEMV on Plant Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Schädler, M.; Elias, J.D.; Millar, J.A.; Kautz, S. Friend or foe—Light availability determines the relationship between mycorrhizal fungi, rhizobia and lima bean (Phaseolus lunatus L.). PLoS ONE 2016, 11, e0154116. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Ulrich, D.E.; Sevanto, S.; Ryan, M.; Albright, M.B.; Johansen, R.B.; Dunbar, J.M. Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci. Rep. 2019, 9, 249. [Google Scholar] [CrossRef]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Kumar, H.; Dubey, R.C.; Maheshwari, D.K. Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.). Crop Prot. 2011, 30, 1396–1403. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Bera, S.; Casteel, C.L.; Crowder, D.W. Responses of pea plants to multiple antagonists are mediated by order of attack and phytohormone crosstalk. Mol. Ecol. 2021, 30, 4939–4948. [Google Scholar] [CrossRef]
- Chisholm, P.J.; Charlton, A.; Anderson, R.M.; Oeller, L.; Reganold, J.P.; Crowder, D.W. Soil rhizobia promote plant yield by increasing tolerance to pests and pathogens under field conditions. Agric. Ecosyst. Environ. 2025, 109552, in press. [Google Scholar] [CrossRef]
- López, M.; Muñoz, N.; Lascano, H.R.; Izaguirre-Mayoral, M.L. The seed-borne Southern bean mosaic virus hinders the early events of nodulation and growth in Rhizobium-inoculated Phaseolus vulgaris L. Funct. Plant Biol. 2016, 44, 208–218. [Google Scholar] [CrossRef]
- Kunert, K.J.; Vorster, B.J.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Finke, D. Plant-vector-pathogen interactions in the context of drought stress. Front. Ecol. Evol. 2019, 7, 262. [Google Scholar] [CrossRef]
- Davis, T.S.; Bosque-Pérez, N.A.; Popova, I.; Eigenbrode, S.D. Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop. Front. Ecol. Evol. 2015, 3, 114. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, P.C.; Tak, T.; Rood, A.M.; van Brussel, A.A.; Kijne, J.W.; Boot, K.J. Salicylic acid inhibits indeterminate-type nodulation but not determinate-type nodulation. Mol. Plant Microbe Interact. 2003, 16, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G.; McAlvin, C.B.; Kim, S.Y.; Olivares, J.; Soto, M.J. Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 2006, 141, 1473–1481. [Google Scholar] [CrossRef]
- Cohen, A.; Basu, S.; Crowder, D.W. Drought stress affects interactions between potato plants, psyllid vectors, and a bacterial pathogen. FEMS Microbiol. Ecol. 2023, 99, 142. [Google Scholar] [CrossRef]
- Chisholm, P.J.; Sertsuvalkul, N.; Casteel, C.L.; Crowder, D.W. Reciprocal plant-mediated interactions between a virus and a non-vector herbivore. Ecol. Rep. 2018, 99, 2139–2144. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Blundell, R.; Casteel, C.L.; Charlton, A.M.; Crowder, D.W. Reciprocal plant-mediated antagonism between a legume plant virus and soil rhizobia. Funct. Ecol. 2021, 35, 2045–2055. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Wang, W.; Wietsma, T.W.; Winkler, T.; Lange, I.; Jansson, C.; Lange, B.M.; McDowell, N.G. Metabolic shifts associated with drought-induced senescence in Brachypodium. Plant Sci. 2019, 289, 110278. [Google Scholar] [CrossRef]
- Yergaliyev, T.M.; Nurbekova, Z.; Mukiyanova, G.; Akbassova, A.; Sutula, M.; Zhangazin, S.; Bari, A.; Tleukulova, Z.; Shamekova, M.; Masalimov, Z.K.; et al. The involvement of ROS producing aldehyde oxidase in plant response to Tombusvirus infection. Plant Physiol. Biochem. 2016, 109, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kulaeva, O.A.; Zhernakov, A.I.; Afonin, A.M.; Boikov, S.S.; Sulima, A.S.; Tikhonovich, I.A.; Zhukov, V.A. Pea Marker Database (PMD)—A new online database combining known pea (Pisum sativum L.) gene-based markers. PLoS ONE 2017, 12, e0186713. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Principles of Soil and Plant Water Relations; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Soft. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Aremu, B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019, 19, 159. [Google Scholar] [CrossRef]
- Dutta, S.; Mishra, A.K.; Kumar, B.D. Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol. Biochem. 2008, 40, 452–461. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Yvon, M.; Vile, D.; Brault, V.; Blanc, S.; van Munster, M. Drought reduces transmission of Turnip yellows virus, an insect-vectored circulative virus. Virus Res. 2017, 241, 131–136. [Google Scholar] [CrossRef]
- van Munster, M.; Yvon, M.; Vile, D.; Dader, B.; Fereres, A.; Blanc, S. Water deficit enhances the transmission of plant viruses by insect vectors. PLoS ONE 2017, 12, e0174398. [Google Scholar] [CrossRef]
- Nachappa, P.; Culkin, C.T.; Saya, P.M.; Han, J.; Nalam, V.J. Water stress modulates soybean aphid performance, feeding behavior, and virus transmission in soybean. Front. Plant Sci. 2016, 7, 552. [Google Scholar] [CrossRef] [PubMed]
- Bergès, S.E.; Vile, D.; Vazquez-Rovere, C.; Blanc, S.; Yvon, M.; Bédiée, A.; Rolland, G.; Dauzat, M.; Van Munster, M. Interactions between drought and plant genotype change epidemiological traits of Cauliflower mosaic virus. Front. Plant Sci. 2018, 9, 703. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Kalo, P.; Yendrek, C.; Sun, J.; Liang, Y.; Marsh, J.F.; Harris, J.M.; Oldroyd, G.E. Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 2008, 20, 2681–2695. [Google Scholar] [CrossRef]
- Qin, H.; He, L.; Huang, R. The coordination of ethylene and other hormones in primary root development. Front. Plant Sci. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Arraes, F.B.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.; Albuquerque, E.V.; Marin, S.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015, 15, 1. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 1. [Google Scholar] [CrossRef]
- Monte, I. Jasmonates and salicylic acid: Evolution of defense hormones in land plants. Curr. Opin. Plant Biol. 2023, 76, 102470. [Google Scholar] [CrossRef]
- Mishra, G.; Mohapatra, S.K.; Rout, G.R. Plant membrane transporters function under abiotic stresses: A review. Planta 2024, 260, 125. [Google Scholar] [CrossRef]
- Verdugo, J.A.; Sauge, M.H.; Lacroze, J.P.; Francis, F.; Ramirez, C.C. Drought-stress and plant resistance affect herbivore performance and proteome: The case of the green peach aphid Myzus persicae (Hemiptera: Aphididae). Physiol. Entomol. 2015, 40, 265–276. [Google Scholar] [CrossRef]
- Kansman, J.T.; Basu, S.; Casteel, C.L.; Crowder, D.W.; Lee, B.W.; Nihranz, C.T.; Finke, D.L. Plant water stress reduces aphid performance: Exploring mechanisms driven by water stress intensity. Front. Ecol. Evol. 2022, 10, 846908. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Bartels, D. Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing Rhizobium strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef]
| Gene | Primer Sequences | NCBI No. | Amplicon Size (bp) |
|---|---|---|---|
| PEMV-1 FP PEMV-1 RP | GCAATCCTACAGGACCTTCATA CTCATCGTCTTCCGTGTCATC | HM439775.1 | 121 |
| PEMV-2 FP PEMV-2 RP | TGCTAGGAGAGGTGGAGATATG GCAATTGAGTAGGGTGGGTAAA | JF713436.1 | 130 |
| PsPR1 FP PsPR1 RP | TGGGGCAGTGGTGACATAAC TGCGCCAAACAACCTGAGTA | LT635896 | 178 |
| PsLox2 FP PsLox2 RP | GCAACCAAGTGACGAAGTCTA GGAGACCCGATTGTAAGGTATTT | PsCam 059875 | 95 |
| PsACS2 FP PsACS2 RP | GGCATAGTAATTTGAGGTTGAGCC GCCCCAACATTTAAAGGACCTATTA | AF016459 | 103 |
| PsAO3 FP PsAO3 RP | TTATAGGACACAGGCTAGCTCAGCA TGACACAAGCTTATTCAGCATGACA | EF491600.1 | 127 |
| Psβ-tubulin FP Psβ-tubulin RP | GTAACCCAAGCTTTGGTGATC ACTGAGAGTCCTGTACTGCT | X54844.1 | 203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, P.; Basu, S.; Lee, B.W.; Baerlocher, C.W.; Oeller, L.; Crowder, D.W. Effects of Soil Rhizobia and Drought on Plant–Vector–Pathogen Interactions on a Legume Host. Appl. Sci. 2025, 15, 12442. https://doi.org/10.3390/app152312442
Malhotra P, Basu S, Lee BW, Baerlocher CW, Oeller L, Crowder DW. Effects of Soil Rhizobia and Drought on Plant–Vector–Pathogen Interactions on a Legume Host. Applied Sciences. 2025; 15(23):12442. https://doi.org/10.3390/app152312442
Chicago/Turabian StyleMalhotra, Pooja, Saumik Basu, Benjamin W. Lee, Chase W. Baerlocher, Liesl Oeller, and David W. Crowder. 2025. "Effects of Soil Rhizobia and Drought on Plant–Vector–Pathogen Interactions on a Legume Host" Applied Sciences 15, no. 23: 12442. https://doi.org/10.3390/app152312442
APA StyleMalhotra, P., Basu, S., Lee, B. W., Baerlocher, C. W., Oeller, L., & Crowder, D. W. (2025). Effects of Soil Rhizobia and Drought on Plant–Vector–Pathogen Interactions on a Legume Host. Applied Sciences, 15(23), 12442. https://doi.org/10.3390/app152312442







