Natural Antimicrobial Compounds in Veterinary Medicine: Focus on Companion Animals
Abstract
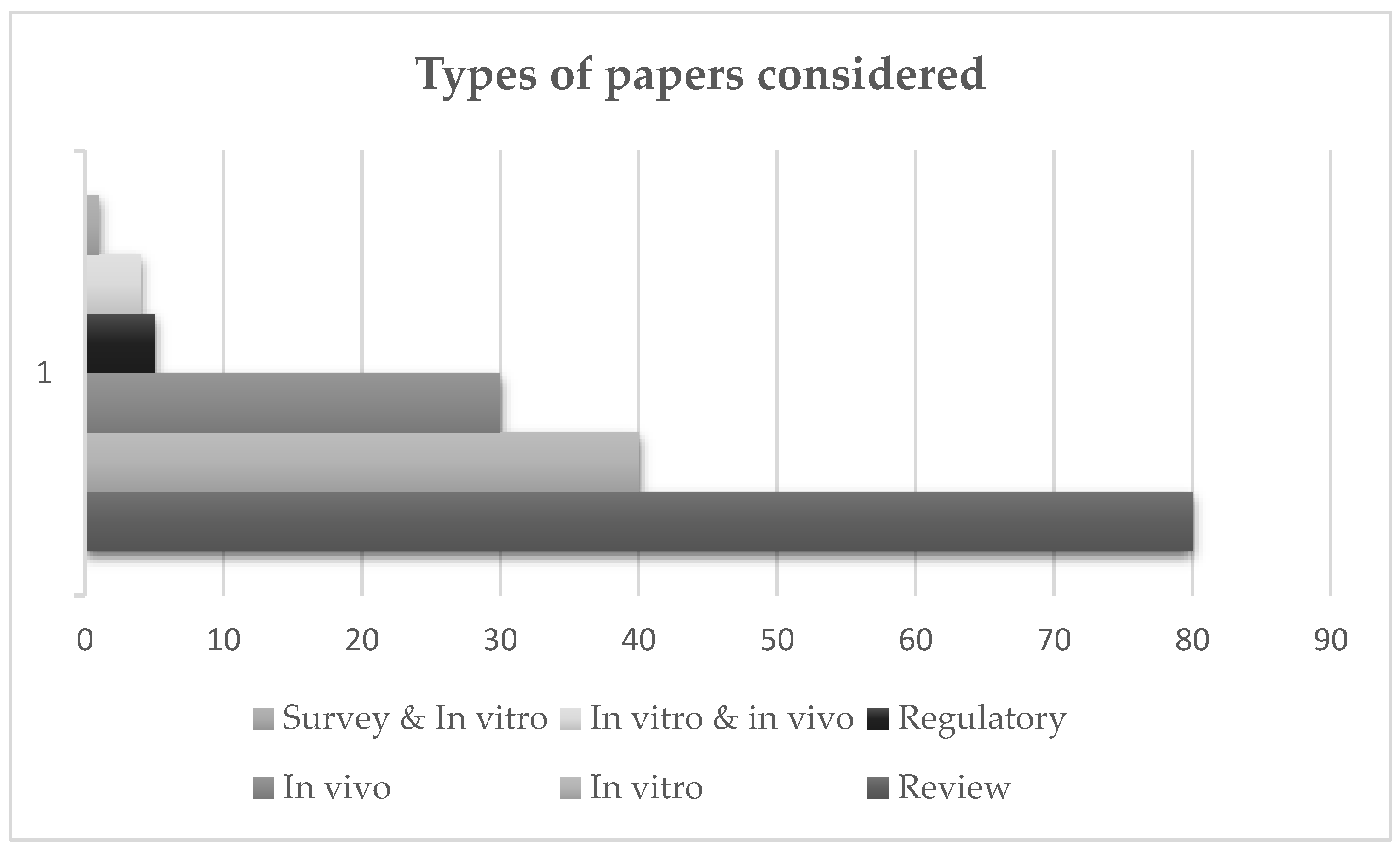
1. Introduction
- Therapeutic use: topical treatments for skin infections, otitis externa, oral infections, and wound management.
- Nutritional strategies: inclusion in pet diets as functional feed additives or nutraceuticals to promote gut health, modulate microbiota, and prevent gastrointestinal infections.
- Preventive applications: coatings for veterinary devices, dental implants, and hygiene products.
2. Natural Antimicrobial Compounds: Classes and Mechanisms
2.1. Antimicrobial Peptides (AMPs)
- Cathelicidins and defensins: these are the primary AMPs utilized in dogs and cats. Canine beta-defensins (cBDs) and cathelicidins play roles in skin and mucosal defense [45].
- Lactoferricin and derivatives: lactoferricin is compound derived by lactoferrin hydrolysis. It has been demonstrated that it has potent antibacterial activity and has been evaluated for use in canine otitis and skin infections caused by Pseudomonas aeruginosa and Staphylococcus spp. Moreover, oral supplementation with lactoferrin has been associated with improved immune response and reduced incidence of gastrointestinal infections in dogs [85,86,87].
- Synthetic analogues: advances in peptide engineering have enabled the design of synthetic AMPs with enhanced stability and reduced cytotoxicity, paving the way for future veterinary applications [46].
2.2. Enzymatic Proteins with Antimicrobial Properties
- Stability and proteolytic susceptibility of proteins on skin and in exudates.
- Delivery and formulation to achieve effective concentrations at the infection site (including penetration of biofilms).
- Activity in complex biological matrices (e.g., proteases, ionic strength and pH of exudate).
- Safety/immunogenicity with repeated topical applications.
2.3. Plant-Derived Phytochemicals
2.4. Other Natural Compounds
3. Feed Additives and Nutraceuticals with Antimicrobial Activity in Companion Animals
3.1. Phytogenic Feed Additives in Pet Nutrition
3.2. Probiotics, Prebiotics, and Synbiotics
3.3. Functional Nutraceuticals with Antimicrobial Properties
- Spirulina (Arthrospira platensis) is a microalga that is rich in phycocyanin and bioactive peptides. Spirulina enhances immune response and exhibits antimicrobial effects against Gram-negative bacteria [102].
- Curcumin (Turmeric extract) shows anti-inflammatory and antimicrobial properties, although bioavailability remains a challenge. Feed integration of curcumin in dogs and cats is an area of growing interest, particularly because of its antioxidative and anti-inflammatory properties. Recent studies have shown that dietary curcumin, either alone or in formulations combined with other compounds, can reduce oxidative damage, support joint health, and improve some metabolic and biochemical markers in these species [58]. For example, in Beagle dogs fed a diet supplemented with curcumin (33 mg/kg feed), there was a reduction in lipid and protein oxidation and increased antioxidant enzyme activities. In dogs with diabetes mellitus, long-term curcuminoid supplementation (250 mg/day for 180 days) improved oxidative stress markers (e.g., higher glutathione/oxidized glutathione ratio) and affected proteomic profiles associated with insulin sensitivity, without observed adverse effects on liver or kidney parameters [59]. Furthermore, in dogs with osteoarthritis, a co-micronized formulation of palmitoyl–glucosamine with curcumin helped to maintain pain relief when tapering non-steroidal inflammatory drugs (NSAIDs), reducing pain scores and lameness severity over an 18-week period [60].
- Cranberry extract is commonly used in urinary health formulations for dogs and cats due to its anti-adhesive effects against Escherichia coli in the urinary tract [128]. Cats prone to recurrent urinary tract infections (UTIs) benefit from cranberry-derived proanthocyanidins; these were shown to prevent bacterial adhesion to the urothelium in a double-blind, placebo-controlled trial involving 60 cats. This study showed a 35% reduction in UTI recurrence over 6 months in the cranberry group compared to the placebo group [57].
4. Challenges and Limitations in Clinical Translation
5. Future Opportunities and Synergistic Strategies
- Essential oils and organic acids: Organic acids lower pH, enhancing the membrane-disrupting action of essential oils [152]. This has been successfully proven in poultry and may be potentially translated to companion animal medicine.
- Probiotics and phytochemicals: Probiotics can colonize the gut and produce bacteriocins, while phytochemicals inhibit pathogen adhesion, providing dual protection [49].
- AMPs and plant extracts: AMPs exert rapid bactericidal activity, while phytochemicals target virulence factors and biofilms [153].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.L. A History of Antimicrobial Drugs in Animals: Evolution and Revolution. J. Vet. Pharmacol. Ther. 2021, 44, 137–171. [Google Scholar] [CrossRef]
- Vercelli, C.; Amadori, M.; Gambino, G.; Re, G. A Review on the Most Frequently Used Methods to Detect Antibiotic Residues in Bovine Raw Milk. Int. Dairy J. 2023, 144, 105695. [Google Scholar] [CrossRef]
- Vercelli, C.; Gambino, G.; Amadori, M.; Re, G. Implications of Veterinary Medicine in the Comprehension and Stewardship of Antimicrobial Resistance Phenomenon: From the Origin till Nowadays. Vet. Anim. Sci. 2022, 16, 100249. [Google Scholar] [CrossRef] [PubMed]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet Animals as Reservoirs of Antimicrobial-Resistant Bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef]
- Singleton, D.A.; Sánchez-Vizcaíno, F.; Dawson, S.; Jones, P.H.; Noble, P.J.M.; Pinchbeck, G.L.; Williams, N.J.; Radford, A.D. Patterns of Antimicrobial Agent Prescription in a Sentinel Population of Canine and Feline Veterinary Practices in the United Kingdom. Vet. J. 2017, 224, 18–24. [Google Scholar] [CrossRef]
- Singleton, D.A.; Rayner, A.; Brant, B.; Smyth, S.; Noble, P.J.; Radford, A.D.; Pinchbeck, G.L. A Randomised Controlled Trial to Reduce Highest Priority Critically Important Antimicrobial Prescription in Companion Animals. Nat. Commun. 2021, 12, 1593. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Samanta, I. Antimicrobial Resistance in Agri-Food Chain and Companion Animals as a Re-Emerging Menace in Post-COVID Epoch: Low- and Middle-Income Countries Perspective and Mitigation Strategies. Front. Vet. Sci. 2020, 7, 620. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Muisa-Zikali, N.; Teta, C.; Musvuugwa, T.; Rzymski, P.; Abia, A.L.K. Insects, Rodents, and Pets as Reservoirs, Vectors, and Sentinels of Antimicrobial Resistance. Antibiotics 2021, 10, 68. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public Health Risk of Antimicrobial Resistance Transfer from Companion Animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Aworh, M.K.; Abiodun-Adewusi, O.; Mba, N.; Helwigh, B.; Hendriksen, R.S. Prevalence and Risk Factors for Faecal Carriage of Multidrug Resistant Escherichia coli among Slaughterhouse Workers. Sci. Rep. 2021, 11, 13362. [Google Scholar] [CrossRef]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D.; et al. Bacterial Zoonoses Transmitted by Household Pets: State-of-the-Art and Future Perspectives for Targeted Research and Policy Actions. J. Comp. Pathol. 2016, 155 (Suppl. S1), S27–S40. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Tompson, A.C.; Mateus, A.L.P.; Brodbelt, D.C.; Chandler, C.I.R. Understanding Antibiotic Use in Companion Animals: A Literature Review Identifying Avenues for Future Efforts. Front. Vet. Sci. 2021, 8, 719547. [Google Scholar] [CrossRef]
- Vercelli, C.; Della Ricca, M.; Re, M.; Gambino, G.; Re, G. Antibiotic Stewardship for Canine and Feline Acute Urinary Tract Infection: An Observational Study in a Small Animal Hospital in Northwest Italy. Antibiotics 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; May, H.E.; AbuOun, M.; Stubberfield, E.; Gilson, D.; Chau, K.K.; Crook, D.W.; Shaw, L.P.; Read, D.S.; Stoesser, N.; et al. A Longitudinal Study Reveals Persistence of Antimicrobial Resistance on Livestock Farms Is Not Due to Antimicrobial Usage Alone. Front. Microbiol. 2023, 14, 1070340. [Google Scholar] [CrossRef]
- Vercelli, C.; Łebkowska-Wieruszewska, B.; Barbero, R.; Lisowski, A.; Re, G.; Giorgi, M. Pharmacokinetics of Levofloxacin in Non-Lactating Goats and Evaluation of Drug Effects on Resistance in Coliform Rectal Flora. Res. Vet. Sci. 2020, 133, 283–288. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Decision 2013/652/EU on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria. Off. J. Eur. Union 2013, 303, 26–39. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013D0652 (accessed on 15 November 2025).
- Timofte, D.; Broens, E.M.; Guardabassi, L.; Pomba, C.; Allerton, F.; Ikonomopoulos, J.; Overesch, G.; Damborg, P.; European Network for Optimization of Veterinary Antimicrobial Treatment (ENOVAT); ESCMID Study Group for Veterinary Microbiology (ESGVM); et al. Driving Laboratory Standardization of Bacterial Culture and Antimicrobial Susceptibility Testing in Veterinary Clinical Microbiology in Europe and Beyond. J. Clin. Microbiol. 2021, 59, e02572-20. [Google Scholar] [CrossRef]
- Mateus, A.; Brodbelt, D.C.; Barber, N.; Stärk, K.D. Antimicrobial Usage in Dogs and Cats in First Opinion Veterinary Practices in the UK. J. Small Anim. Pract. 2011, 52, 515–521. [Google Scholar] [CrossRef]
- Papich, M.G. Antimicrobial Agent Use in Small Animals: What Are the Prescribing Practices, Use of PK–PD Principles, and Extralabel Use in the United States? J. Vet. Pharmacol. Ther. 2021, 44, 238–249. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Medicines Agency (EMEA). Joint Scientific Report on Meticillin Resistant Staphylococcus aureus (MRSA) in Livestock, Companion Animals and Foods. EFSA J. 2009, 7, 301r. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/6 of the European Parliament and of the Council on Veterinary Medicinal Products. Off. J. Eur. Union. 2019, 4, 1–25. [Google Scholar]
- Middlemiss, C. Encouraging Responsible Antibiotic Use by Pet Owners. Vet. Rec. 2018, 182, 410. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In vitro and In vivo Evaluation of a New Phytotherapic Blend to Treat Acute Externa Otitis in Dogs. J. Vet. Pharmacol. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Maddox, C.W. Canine Antimicrobial Peptides Are Effective against Resistant Bacteria and Yeasts. Vet. Dermatol. 2014, 25, 35-e12. [Google Scholar] [CrossRef]
- Rizzo, A.; Piccinno, M.; Lillo, E.; Carbonari, A.; Jirillo, F.; Sciorsci, R.L. Antimicrobial Resistance and Current Alternatives in Veterinary Medicine. Vet. Med. Sci. 2023, 29, 312–322. [Google Scholar] [CrossRef]
- Valdez-Miramontes, C.E.; De Haro-Acosta, J.; Aréchiga-Flores, C.F.; Verdiguel-Fernández, L.; Rivas-Santiago, B. Antimicrobial peptides in domestic animals and their applications in veterinary medicine. Peptides 2021, 142, 170576. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of Microbiological, Histological, and Immunomodulatory Parameters in Response to Treatment with Either Combination Antibiotics or Probiotics in Dogs with Inflammatory Bowel Disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- Leonard, B.C.; Affolter, V.K.; Bevins, C.L. Antimicrobial Peptides: Agents of Border Protection for Companion Animals. Vet. Dermatol. 2012, 23, 177-e36. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, V.; Passamonti, F.; Rampacci, E. Antimicrobial Strategies Proposed for the Treatment of S. pseudintermedius and Other Dermato-Pathogenic Staphylococcus spp. in Companion Animals: A Narrative Review. Vet. Sci. 2024, 11, 311. [Google Scholar] [CrossRef]
- Di Marco, N.; Lucero-Estrada, C.; Pungitore, C.R. Aporphinoid Alkaloids as Antimicrobial Agents against Yersinia enterocolitica. Lett. Appl. Microbiol. 2019, 68, 437–445. [Google Scholar] [CrossRef]
- Faehnrich, B.; Pastor, A.; Heide, C.; Kröger, S.; Zentek, J. Effects of Isoquinoline Alkaloids from Macleaya cordata on Physiological, Immunological and Inflammatory Parameters in Healthy Beagles: Alkaloids in Dog Nutrition. J. Anim. Physiol. Anim. Nutr. 2019, 103, 661–667. [Google Scholar] [CrossRef]
- Cao, J.; Chen, M.; Xu, R.; Guo, M. Therapeutic Mechanisms of Berberine to Improve the Intestinal Barrier Function via Modulating Gut Microbiota, TLR4/NF-κB/MTORC Pathway and Autophagy in Cats. Front. Microbiol. 2022, 13, 961885. [Google Scholar] [CrossRef] [PubMed]
- Mulukutla, A.; Shreshtha, R.; Deb, V.; Chatterjee, P.; Jain, U.; Chauhan, N. Recent Advances in Antimicrobial Peptide-Based Therapy. Bioorg. Chem. 2024, 145, 107151. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by Alpha-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Silva, N.C.; Sarmento, B.; Pintado, M. The Importance of Antimicrobial Peptides and Their Potential for Therapeutic Use in Ophthalmology. Int. J. Antimicrob. Agents 2013, 41, 5–10. [Google Scholar] [CrossRef]
- Liu, T.; She, R.; Wang, K.; Bao, H.; Zhang, Y.; Luo, D.; Hu, Y.; Ding, Y.; Wang, D.; Peng, K. Effects of Rabbit Sacculus Rotundus Antimicrobial Peptides on the Intestinal Mucosal Immunity in Chickens. Poult. Sci. 2008, 87, 250–254. [Google Scholar] [CrossRef]
- Bao, H.; She, R.; Liu, T.; Zhang, Y.; Peng, K.S.; Luo, D.; Yue, Z.; Ding, Y.; Hu, Y.; Liu, W.; et al. Effects of Pig Antibacterial Peptides on Growth Performance and Intestine Mucosal Immunity of Broiler Chickens. Poult. Sci. 2009, 88, 291–297. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial Peptides: Promising Alternatives in the Post-Feeding Antibiotic Era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Cote, C.K.; Blanco, I.I.; Hunter, M.; Shoe, J.L.; Klimko, C.P.; Panchal, R.G.; Welkos, S.L. Combinations of Early Generation Antibiotics and Antimicrobial Peptides Are Effective against a Broad Spectrum of Bacterial Biothreat Agents. Microb. Pathog. 2020, 142, 104050. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.; Aslam, M.A.; Rahman, S.U.; Sindhu, Z.U.; Sajid, S.; Zafar, N.; Razaq, M.; Kanwar, R.; Amanullah. A Review of Antimicrobial Peptides: Its Function, Mode of Action and Therapeutic Potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Santoro, D.; Bunick, D.; Graves, T.K.; Segre, M. Evaluation of Canine Antimicrobial Peptides in Infected and Noninfected Chronic Atopic Skin. Vet. Dermatol. 2013, 24, 39–47.e10. [Google Scholar] [CrossRef]
- Leonard, B.C.; Marks, S.L.; Outerbridge, C.A.; Affolter, V.K.; Kananurak, A.; Young, A.; Moore, P.F.; Bannasch, D.L.; Bevins, C.L. Activity, Expression and Genetic Variation of Canine β-Defensin 103: A Multifunctional Antimicrobial Peptide in the Skin of Domestic Dogs. J. Innate Immun. 2012, 4, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.E.; Hwang, C.Y. Antimicrobial Peptide Reduces Cytotoxicity and Inflammation in Canine Epidermal Keratinocyte Progenitor Cells Induced by Pseudomonas aeruginosa Infection. Vet. Sci. 2024, 11, 235. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Garbacz, K.; Neubauer, D.; Kamysz, W. In vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma. Animals 2020, 10, 470. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic action of antimicrobial peptides and antibiotics: Current understanding and future directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef]
- Onuma, M.; Ataka, K.; Murakami, A. Evaluating the Safety and Functionality of a Novel Compound Containing Prebiotics, Probiotics, and Postbiotics in Healthy Cats and Dogs. Open Vet. J. 2025, 15, 1969–1981. [Google Scholar] [CrossRef]
- Schmitz, S.S. Evidence-Based Use of Biotics in the Management of Gastrointestinal Disorders in Dogs and Cats. Vet. Rec. 2024, 195, 26–32. [Google Scholar] [CrossRef]
- Schmitz, S.S. Value of Probiotics in Canine and Feline Gastroenterology. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 171–217. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, Z. Gut probiotics and health of dogs and cats: Benefits, applications, and underlying mechanisms. Microorganisms 2023, 11, 2452. [Google Scholar] [CrossRef]
- Wilson, S.M.; Swanson, K.S. The influence of ‘biotics’ on the gut microbiome of dogs and cats. Vet. Rec. 2024, 195, 2–12. [Google Scholar] [CrossRef]
- Whittemore, J.C.; Moyers, T.D.; Price, J.M. Randomized, controlled, crossover trial of prevention of antibiotic-induced gastrointestinal signs using a synbiotic mixture in healthy research dogs. J. Vet. Intern. Med. 2019, 33, 1619–1626. [Google Scholar] [CrossRef]
- Bai, L.; Takagi, S.; Ando, T.; Yoneyama, H.; Ito, K.; Mizugai, H.; Isogai, E. Antimicrobial Activity of Tea Catechin against Canine Oral Bacteria and the Functional Mechanisms. J. Vet. Med. Sci. 2016, 78, 1439–1445. [Google Scholar] [CrossRef]
- Carvajal-Campos, A.; Trebossen, L.; Jeusette, I.; Mayot, G.; Torre, C.; Fragua, V.; Fernandez, A.; Di Martino, P. The effect of cranberry (Vaccinium macrocarpon) consumption on Escherichia coli adherence to feline uroepithelial cells in a blind randomised cross-over trial in cats. J. Vet. Res. 2024, 68, 583–587. [Google Scholar] [CrossRef]
- Campigotto, G.; Alba, D.F.; Sulzbach, M.M.; Dos Santos, D.S.; Souza, C.F.; Baldissera, M.D.; Gundel, S.; Ourique, A.F.; Zimmer, F.; Petrolli, T.G.; et al. Dog food production using curcumin as antioxidant: Effects of intake on animal growth, health and feed conservation. Arch. Anim. Nutr. 2020, 74, 397–413. [Google Scholar] [CrossRef]
- Suemanotham, N.; Photcharatinnakorn, P.; Chantong, B.; Buranasinsup, S.; Phochantachinda, S.; Sakcamduang, W.; Reamtong, O.; Thiangtrongjit, T. Curcuminoid supplementation in canine diabetes mellitus and its complications using proteomic analysis. Front. Vet. Sci. 2022, 9, 1057972. [Google Scholar] [CrossRef]
- Della Rocca, G.; Schievano, C.; Di Salvo, A.; Conti, M.B.; Della Valle, M.F. Palmitoyl-glucosamine co-micronized with curcumin for maintenance of meloxicam-induced pain relief in dogs with osteoarthritis pain. BMC Vet. Res. 2023, 19, 37. [Google Scholar] [CrossRef]
- Nakamura, T.; Kitana, J.; Fujiki, J.; Takase, M.; Iyori, K.; Simoike, K.; Iwano, H. Lytic Activity of Polyvalent Staphylococcal Bacteriophage PhiSA012 and Its Endolysin Lys-PhiSA012 against Antibiotic-Resistant Staphylococcal Clinical Isolates from Canine Skin Infection Sites. Front. Med. 2020, 7, 234. [Google Scholar] [CrossRef]
- Haddad Kashani, H.; Schmelcher, M.; Sabzalipoor, H.; Seyed Hosseini, E.; Moniri, R. Recombinant Endolysins as Potential Therapeutics against Antibiotic-Resistant Staphylococcus aureus: Current Status of Research and Novel Delivery Strategies. Clin. Microbiol. Rev. 2018, 31, e00071-17. [Google Scholar] [CrossRef]
- López, V.; Pavela, R.; Gómez-Rincón, C.; Les, F.; Bartolucci, F.; Galiffa, V.; Petrelli, R.; Cappellacci, L.; Maggi, F.; Canale, A.; et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules 2019, 24, 2563. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial Activity of Thyme Oil, Oregano Oil, Thymol and Carvacrol against Sensitive and Resistant Microbial Isolates from Dogs with Otitis Externa. Vet. Dermatol. 2019, 30, 524–e159. [Google Scholar] [CrossRef]
- Mickienė, R.; Bakutis, B.; Baliukonienė, V. Antimicrobial Activity of Two Essential Oils. Ann. Agric. Environ. Med. 2011, 18, 139–144. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Tea Tree Oil: Contact Allergy and Chemical Composition. Contact Dermatitis 2016, 75, 129–143. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; De Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.; Vullo, V.; Ragno, R. High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Camargo, S.; Oliveira, L. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 22. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Juergens, U.R.; Stober, M.; Vetter, H. Inhibition of Cytokine Production and Arachidonic Acid Metabolism by Eucalyptol (1,8-Cineole) in Human Blood Monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Najar, B.; Pistelli, L.; Mancianti, F. Antibacterial and Antifungal Activity of Essential Oils against Pathogens Responsible for Otitis Externa in Dogs and Cats. Medicines 2017, 4, 21. [Google Scholar] [CrossRef]
- Gómez-García, M.; Madrigal, I.; Puente, H.; Mencía-Ares, Ó.; Argüello, H.; Carvajal, A.; Fregeneda-Grandes, J.M. In vitro Activity of Essential Oils against Microbial Isolates from Otitis Externa Cases in Dogs. Nat. Prod. Res. 2022, 36, 4552–4556. [Google Scholar] [CrossRef]
- Tadee, P.; Chansakaow, S.; Tipduangta, P.; Tadee, P.; Khaodang, P.; Chukiatsiri, K. Essential Oil Pharmaceuticals for Killing Ectoparasites on Dogs. J. Vet. Sci. 2024, 25, e5. [Google Scholar] [CrossRef]
- Nardoni, S.; Costanzo, A.G.; Mugnaini, L.; Pisseri, F.; Rocchigiani, G.; Papini, R.; Mancianti, F. Open-Field Study Comparing an Essential Oil-Based Shampoo with Miconazole/Chlorhexidine for Haircoat Disinfection in Cats with Spontaneous Microsporiasis. J. Feline Med. Surg. 2016, 19, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Villar, D.; Knight, M.J.; Hansen, S.R.; Buck, W.B. Toxicity of Melaleuca Oil and Related Essential Oils Applied Topically on Dogs and Cats. Vet. Hum. Toxicol. 1994, 36, 139–142. [Google Scholar]
- Schlieck, T.M.M.; Petrolli, T.G.; Bissacotti, B.F.; Copetti, P.M.; Bottari, N.B.; Morsch, V.M.; da Silva, A.S. Addition of a Blend of Essential Oils (Clove, Rosemary and Oregano) and Vitamin E to Replace Conventional Chemical Antioxidants in Dog Feed: Effects on Food Quality and Health of Beagles. Arch. Anim. Nutr. 2021, 75, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.G.; McLean, M.K.; Khan, S.A. Adverse Reactions from Essential Oil-Containing Natural Flea Products Exempted from Environmental Protection Agency Regulations in Dogs and Cats. J. Vet. Emerg. Crit. Care 2012, 22, 470–475. [Google Scholar] [CrossRef]
- Mráz, P.; Kopecký, M.; Hasoňová, L.; Hoštičková, I.; Vaníčková, A.; Perná, K.; Žabka, M.; Hýbl, M. Antibacterial activity and chemical composition of popular plant essential oils and their positive interactions in combination. Molecules 2025, 30, 1864. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Fitzi, J.; Hellmann, K.; Wegener, T.; Bucher, S.; Saller, R. Topical tea tree oil effective in canine localised pruritic dermatitis: A multi-centre randomised double-blind controlled clinical trial in veterinary practice. Dtsch. Tierärztl. Wochenschr. 2024, 111, 408–414. [Google Scholar]
- Móritz, A.V.; Kovács, H.; Jerzsele, Á.; Psáder, R.; Farkas, O. Flavonoids in Mitigating the Adverse Effects of Canine Endotoxemia. Front. Vet. Sci. 2024, 11, 1396870. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- do Nascimento, J.B.; da Costa, J.G.M. Flavonoids: A Review of Antibacterial Activity against Gram-Negative Bacteria. Int. J. Microbiol. 2025, 2025, 9961121. [Google Scholar] [CrossRef]
- Moulari, B.; Pellequer, Y.; Chaumont, J.P.; Guillaume, Y.C.; Millet, J. In vitro Antimicrobial Activity of the Leaf Extract of Harungana madagascariensis Lam. Ex Poir. (Hypericaceae) against Strains Causing Otitis Externa in Dogs and Cats. Acta Vet. Hung. 2007, 55, 97–105. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef]
- Corona, A.; Vercelli, A.; Bruni, N.; Guidi, E.; Cornegliani, L. In vitro Activity of Lactoferricin Solution against Malassezia pachydermatis from Otitis Externa in Dogs and Cats. Vet. Dermatol. 2021, 32, 316-e86. [Google Scholar] [CrossRef]
- Berlov, M.N.; Korableva, E.S.; Andreeva, Y.V.; Ovchinnikova, T.V.; Kokryakov, V.N. Lactoferrin from Canine Neutrophils: Isolation and Physicochemical and Antimicrobial Properties. Biochemistry 2007, 72, 445–451. [Google Scholar] [CrossRef]
- Tenovuo, J.; Illukka, T.; Vähä-Vahe, T. Non-Immunoglobulin Defense Factors in Canine Saliva and Effects of a Tooth Gel Containing Antibacterial Enzymes. J. Vet. Dent. 2000, 17, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Iacopetti, I.; Perazzi, A.; Badon, T.; Bedin, S.; Contiero, B.; Ricci, R. Salivary pH, Calcium, Phosphorus and Selected Enzymes in Healthy Dogs: A Pilot Study. BMC Vet. Res. 2017, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Özhan, H.K.; Duman, H.; Bechelany, M.; Karav, S. Lactoperoxidase: Properties, Functions, and Potential Applications. Int. J. Mol. Sci. 2025, 26, 5055. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: A Multiple Bioactive Protein—An Overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef] [PubMed]
- Bolesławska, I.; Bolesławska-Król, N.; Jakubowski, K.; Przysławski, J.; Drzymała-Czyż, S. Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer. Molecules 2025, 30, 1507. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F. Characterization of Cytoplasmic Lysozyme Immunoreactivity as a Histiocytic Marker in Normal Canine Tissues. Vet. Pathol. 1986, 23, 763–769. [Google Scholar] [CrossRef]
- Perazzi, A.; Ricci, R.; Contiero, B.; Iacopetti, I. Evaluation of Salivary Biochemistry in Dogs with and without Plaque, Calculus, and Gingivitis: Preliminary Results. Animals 2022, 12, 1091. [Google Scholar] [CrossRef]
- Abreu, V.; Santiago, R.; Carlos, A.; Silva, G.; Da Silva, A.; Sevá, A.; Costa-Dias, R.; Dálety, R.; Cruz, S.; Alzamora, F. Use of antimicrobial photodynamic therapy in the treatment of otitis caused by Malassezia spp. in domestic dogs. Acta Vet. Bras. 2023, 17, 41–47. [Google Scholar] [CrossRef]
- Seeger, M.G.; Ries, A.S.; Gressler, L.T.; Botton, S.A.; Iglesias, B.A.; Cargnelutti, J.F. In vitro antimicrobial photodynamic therapy using tetra-cationic porphyrins against multidrug-resistant bacteria isolated from canine otitis. Photodiagnosis Photodyn. Ther. 2020, 32, 101982. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Kengne, I.C.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Saponins from Melanthera elliptica and Their Synergistic Effects with Antibiotics against Pathogenic Phenotypes. Chem. Cent. J. 2018, 12, 97. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exert Antibacterial Effects against Various Strains of Gram-Positive and Gram-Negative Bacteria: A Comprehensive Study In vitro and In vivo. Evid. Based Complement. Altern. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef]
- Stefanutti, D.; Tonin, G.; Morelli, G.; Zampieri, R.M.; La Rocca, N.; Ricci, R. Oral palatability and owners’ perception of the effect of increasing amounts of spirulina (Arthrospira platensis) in the diet of a cohort of healthy dogs and cats. Animals 2023, 13, 1275. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Sękowski, S.; Kwiatek, A.; Płaczkiewicz, J.; Abdulladjanova, N.; Shlyonsky, V.; Swiecicka, I.; Zamaraeva, M. The Structural Changes in the Membranes of Staphylococcus aureus Caused by Hydrolysable Tannins Witness Their Antibacterial Activity. Membranes 2022, 12, 1124. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as a Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Villanueva, X.; Zhen, L.; Ares, J.N.; Vackier, T.; Lange, H.; Crestini, C.; Steenackers, H.P. Effect of Chemical Modifications of Tannins on Their Antimicrobial and Antibiofilm Effect against Gram-Negative and Gram-Positive Bacteria. Front. Microbiol. 2023, 13, 987164. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Turini, L.; Sarri, G.; Serra, A.; Buccioni, A.; Mele, M. Oral Administration of Chestnut Tannins to Reduce the Duration of Neonatal Calf Diarrhea. BMC Vet. Res. 2018, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Prapaiwong, T.; Srakaew, W.; Wachirapakorn, C.; Jarassaeng, C. Effects of Hydrolyzable Tannin Extract Obtained from Sweet Chestnut Wood (Castanea sativa Mill.) against Bacteria Causing Subclinical Mastitis in Thai Friesian Dairy Cows. Vet. World 2021, 14, 2427–2433. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Cardenia, V.; Rodriguez-Estrada, M.T.; Stefanelli, C.; Grandi, M.; Gatta, P.P.; Biagi, G. In vitro Evaluation of the Effects of a Yucca schidigera Extract and Chestnut Tannins on Composition and Metabolic Profiles of Canine and Feline Faecal Microbiota. Arch. Anim. Nutr. 2017, 71, 395–412. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of Gut Microbiota Biotransformation on Dietary Tannins and Human Health Implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Marrone, G.; Di Lauro, M.; Izzo, F.; Cornali, K.; Masci, C.; Vita, C.; Occhiuto, F.; Di Daniele, N.; De Lorenzo, A.; Noce, A. Possible Beneficial Effects of Hydrolyzable Tannins Deriving from Castanea sativa L. in Internal Medicine. Nutrients 2023, 16, 45. [Google Scholar] [CrossRef]
- Brus, M.; Gradišnik, L.; Trapecar, M.; Škorjanc, D.; Frangež, R. Beneficial Effects of Water-Soluble Chestnut (Castanea sativa Mill.) Tannin Extract on Chicken Small Intestinal Epithelial Cell Culture. Poult. Sci. 2018, 97, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Hoisang, S.; Jitpean, S.; Seesupa, S.; Kamlangchai, P.; Makpunpol, T.; Ngowwatana, P.; Chaimongkol, S.; Khunbutsri, D.; Khlongkhlaeo, J.; Kampa, N. Evaluation of Totarol for Promoting Open Wound Healing in Dogs. Vet. Sci. 2024, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Kaatz, G.W.; Seo, S.M.; Wareham, N.; Williamson, E.M.; Gibbons, S. The Phenolic Diterpene Totarol Inhibits Multidrug Efflux Pump Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 4480–4483. [Google Scholar] [CrossRef]
- Xu, Z.; Krajewski, S.; Weindl, T.; Loeffler, R.; Li, P.; Han, X.; Geis-Gerstorfer, J.; Wendel, H.P.; Scheideler, L.; Rupp, F. Application of Totarol as Natural Antibacterial Coating on Dental Implants for Prevention of Peri-Implantitis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110701. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.J. Studies on a Bactericidal Agent Extracted from a Soil Bacillus: II. Protective Effect of the Bactericidal Agent against Experimental Pneumococcus Infections in Mice. J. Exp. Med. 1939, 70, 11–17. [Google Scholar] [CrossRef]
- Dubos, R.J.; Hotchkiss, R.D. The Production of Bactericidal Substances by Aerobic Sporulating Bacilli. J. Exp. Med. 1941, 73, 629–640. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Torres, M.D.; Mojica, F.J.; Lu, T.K. Next-Generation Precision Antimicrobials: Towards Personalized Treatment of Infectious Diseases. Curr. Opin. Microbiol. 2017, 37, 95–102. [Google Scholar] [CrossRef]
- Egidio, M.; Casalino, L.; De Biasio, F.; Di Paolo, M.; Gómez-García, R.; Pintado, M.; Sardo, A.; Marrone, R. Antimicrobial Properties of Fennel By-Product Extracts and Their Potential Applications in Meat Products. Antibiotics 2024, 13, 932. [Google Scholar] [CrossRef]
- Rühl-Teichner, J.; Jacobmeyer, L.; Leidner, U.; Semmler, T.; Ewers, C. Genomic Diversity, Antimicrobial Susceptibility, and Biofilm Formation of Clinical Acinetobacter baumannii Isolates from Horses. Microorganisms 2023, 11, 556. [Google Scholar] [CrossRef]
- Nakano, M.; Tanaka, M.; Abe, F. The Use of Lactoferrin and Lactoperoxidase for Oral Health. J. Anim. Sci. 2020, 98 (Suppl. S4), 67. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Roles of Lactoferrin and Iron in Immunity. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 11. [Google Scholar] [CrossRef]
- Tresch, M.; Mevissen, M.; Ayrle, H.; Melzig, M.; Roosje, P.; Walkenhorst, M. Medicinal Plants as Therapeutic Options for Topical Treatment in Canine Dermatology? A Systematic Review. BMC Vet. Res. 2019, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; De Martino, L. Methicillin-Resistant Staphylococcus pseudintermedius: Epidemiological Changes, Antibiotic Resistance, and Alternative Therapeutic Strategies. Vet. Res. Commun. 2024, 48, 3505–3515. [Google Scholar] [CrossRef]
- Biswas, S.; Kim, I.H. A Thorough Review of Phytogenic Feed Additives in Non-Ruminant Nutrition: Production, Gut Health, and Environmental Concerns. J. Anim. Sci. Technol. 2025, 67, 497–519. [Google Scholar] [CrossRef]
- Chou, H.I.; Chen, K.S.; Wang, H.C.; Lee, W.M. Effects of cranberry extract on prevention of urinary tract infection in dogs and on adhesion of Escherichia coli to Madin–Darby canine kidney cells. Am. J. Vet. Res. 2016, 77, 421–427. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Xiong, H.; Yu, L.X.; Qu, H. Batch-to-batch quality consistency evaluation of botanical drug products using multivariate statistical analysis of the chromatographic fingerprint. AAPS PharmSciTech 2013, 14, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Indrayanto, G.; Rohman, A. Advances in fingerprint analysis for standardization and quality control of herbal medicines. Front. Pharmacol. 2022, 13, 853023. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-phytomedicine: Harnessing plant-derived phytochemicals in nanocarriers for targeted human health applications. Molecules 2025, 30, 3177. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug–microbiota interactions: An emerging priority for precision medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of phytochemicals by liposome cargos: Recent progress, challenges and opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Sharafan, M.; Dziki, A.; Malinowska, M.A.; Sikora, E.; Szopa, A. Targeted delivery strategies for hydrophilic phytochemicals. Appl. Sci. 2025, 15, 7101. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W. Advances in nanotechnology for enhancing the solubility and bioavailability of poorly soluble drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Peira, E.; Chirio, D.; Chindamo, G.; Accomasso, G.; Vercelli, C.; Riganti, C.; Salaroglio, I.C.; Gambino, G.; Re, G.; et al. Human and canine osteosarcoma cell lines: How do they react upon incubation with calcium phosphate-coated lipid nanoparticles carrying doxorubicin and curcumin? Int. J. Pharm. 2025, 668, 124970. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Zhu, Z.; Li, L. The role of plant extracts in enhancing nutrition and health for dogs and cats: Safety, benefits, and applications. Vet. Sci. 2024, 11, 426. [Google Scholar] [CrossRef]
- Klinmalai, P.; Kamonpatana, P.; Sodsai, J.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Kovitvadhi, A.; Areerat, S.; Seubsai, A.; Harnkarnsujarit, N. Modern palatant strategies in dry and wet pet food: Formulation technologies, patent innovations, and market evolution. Foods 2025, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, K.; Guale, F. Australian tea tree (Melaleuca alternifolia) oil poisoning in three purebred cats. J. Vet. Diagn. Investig. 1998, 10, 208–210. [Google Scholar] [CrossRef]
- Kyselová, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Brantley, S.J.; Argikar, A.A.; Lin, Y.S.; Nagar, S.; Paine, M.F. Herb–drug interactions: Challenges and opportunities for safe use. Drug Metab. Dispos. 2014, 42, 301–317. [Google Scholar] [CrossRef]
- Ng, J.Y.; Kim, M.; Suri, A. Exploration of facilitators and barriers to the regulatory toxicology system: A scoping review. Pharm. Policy Pract. 2022, 15, 47. [Google Scholar]
- Quintavalla, F. Phytotherapeutic approaches in canine pediatrics. Vet. Sci. 2024, 11, 133. [Google Scholar] [CrossRef]
- Scahill, K.; Jessen, L.R.; Prior, C.; Singleton, D.; Foroutan, F.; Ferran, A.A.; Arenas, C.; Bjørnvad, C.R.; Lavy, E.; Allerton, F.; et al. Efficacy of antimicrobial and nutraceutical treatment for canine acute diarrhoea: A systematic review and meta-analysis for ENOVAT guidelines. Vet. J. 2024, 303, 106054. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1831/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R1831 (accessed on 14 October 2025).
- Sections 201 and 409 of the Federal Food, Drug and Cosmetic Act. Available online: https://www.govinfo.gov/content/pkg/COMPS-973/pdf/COMPS-973.pdf (accessed on 14 October 2025).
- Komala, M.G.; Ong, S.G.; Qadri, M.U.; Elshafie, L.M.; Pollock, C.A.; Saad, S. Investigating the regulatory process, safety, efficacy and product transparency for nutraceuticals in the USA, Europe and Australia. Foods 2023, 12, 427. [Google Scholar] [CrossRef]
- Kelidari, M.; Abdi-Moghadam, Z.; Sabzevar, T.E.; Khajenouri, M.; Shakeri, A. Preparation and therapeutic implications of essential oil-based nanoemulsions: A review. Colloids Surf. B Biointerfaces 2025, 255, 114939. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hong, T.; Cui, P.; Wang, J.; Xia, J. Antimicrobial peptides towards clinical application: Delivery and formulation. Adv. Drug Deliv. Rev. 2021, 175, 113818. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, T.; McDonough, C.M.; Gold, S.E.; Chen, C.; Glenn, A.E.; Pokoo-Aikins, A. Synergistic effects of essential oils and organic acids against Aspergillus flavus contamination in poultry feed. Toxins 2023, 15, 635. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jung, I.G.; Yum, S.H.; Hwang, Y.J. In vitro synergistic inhibitory effects of plant extract combinations on bacterial growth of methicillin-resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Frame, L.A. Fiber, microbiomes, and SCFAs: Insights from companion animal models to inform personalized nutrition. mSystems 2025, 10, e0145424. [Google Scholar] [CrossRef]
- Hamadou, M.; Mune Mune, M.A. AI-driven bioactive peptide discovery of next-generation metabolic biotherapeutics. Appl. Food Res. 2025, 5, 101291. [Google Scholar] [CrossRef]
- Bilal, H.; Khan, M.N.; Khan, S.; Shafiq, M.; Fang, W.; Khan, R.U.; Rahman, M.U.; Li, X.; Lv, Q.L.; Xu, B. The role of artificial intelligence and machine learning in predicting and combating antimicrobial resistance. Comput. Struct. Biotechnol. J. 2025, 27, 423–439. [Google Scholar] [CrossRef]
- Marco-Fuertes, A.; Marin, C.; Lorenzo-Rebenaque, L.; Vega, S.; Montoro-Dasi, L. Antimicrobial resistance in companion animals: A new challenge for the One Health approach in the European Union. Vet. Sci. 2022, 9, 208. [Google Scholar] [CrossRef]
- Pollock, J.; Low, A.S.; McHugh, R.E.; Muwonge, A.; Stevens, M.P.; Corbishley, A.; Gally, D.L. Alternatives to antibiotics in a One Health context and the role genomics can play in reducing antimicrobial use. Clin. Microbiol. Infect. 2020, 26, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial usage and resistance in companion animals: A cross-sectional study in three European countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Slana, M.; Dolenc, M.S. Environmental risk assessment of antimicrobials applied in veterinary medicine: A field study and laboratory approach. Environ. Toxicol. Pharmacol. 2013, 35, 131–141. [Google Scholar] [CrossRef] [PubMed]
| Compound | Reference Number |
|---|---|
| Alkaloids | [33,34,35] |
| Antimicrobial peptides | [27,29,31,36,37,38,39,40,41,42,43,44,45,46,47,48,49] |
| Biotics | [30,50,51,52,53,54,55] |
| Catechins | [56] |
| Cranberry | [50,57] |
| Curcumin | [58,59,60] |
| Ensolisin | [61,62] |
| Essential oils | [26,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] |
| Flavonoids | [81,82,83,84] |
| Lactoferrin | [46,62,85,86,87,88,89,90,91,92,93,94,95] |
| Lactoperoxidase | [90] |
| Lysozime | [88,94,96,97] |
| Photodynamic compounds | [98,99] |
| Saponins | [100,101] |
| Spirulin | [102] |
| Tannins | [103,104,105,106,107,108,109,110,111,112] |
| Totarol | [113,114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercelli, C.; Amadori, M.; Gambino, G.; Danieli, D.; Crimi, S.; Re, G. Natural Antimicrobial Compounds in Veterinary Medicine: Focus on Companion Animals. Appl. Sci. 2025, 15, 12388. https://doi.org/10.3390/app152312388
Vercelli C, Amadori M, Gambino G, Danieli D, Crimi S, Re G. Natural Antimicrobial Compounds in Veterinary Medicine: Focus on Companion Animals. Applied Sciences. 2025; 15(23):12388. https://doi.org/10.3390/app152312388
Chicago/Turabian StyleVercelli, Cristina, Michela Amadori, Graziana Gambino, Davide Danieli, Sara Crimi, and Giovanni Re. 2025. "Natural Antimicrobial Compounds in Veterinary Medicine: Focus on Companion Animals" Applied Sciences 15, no. 23: 12388. https://doi.org/10.3390/app152312388
APA StyleVercelli, C., Amadori, M., Gambino, G., Danieli, D., Crimi, S., & Re, G. (2025). Natural Antimicrobial Compounds in Veterinary Medicine: Focus on Companion Animals. Applied Sciences, 15(23), 12388. https://doi.org/10.3390/app152312388








