Quality Properties of Crackers Enriched with Composite Flours: Effect on Dough and Final Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Dough and Cracker Preparation
2.3. Analysis of Functional Properties of Flours
2.4. Farinograph
2.5. Texture Profile Analysis (TPA) of Dough
2.6. Analysis of Final Product Properties
2.6.1. Water Activity and Moisture Content of Crackers
2.6.2. Physical Parameters of Crackers
2.6.3. Texture Profile Analysis (TPA) of Crackers
2.6.4. Color Analysis of Crackers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Functional Properties of Composite Flours
3.2. Dough Rheology (Farinograph Recordings) of Composite Flours
3.3. The Effect of Composite Flours on Dough Texture
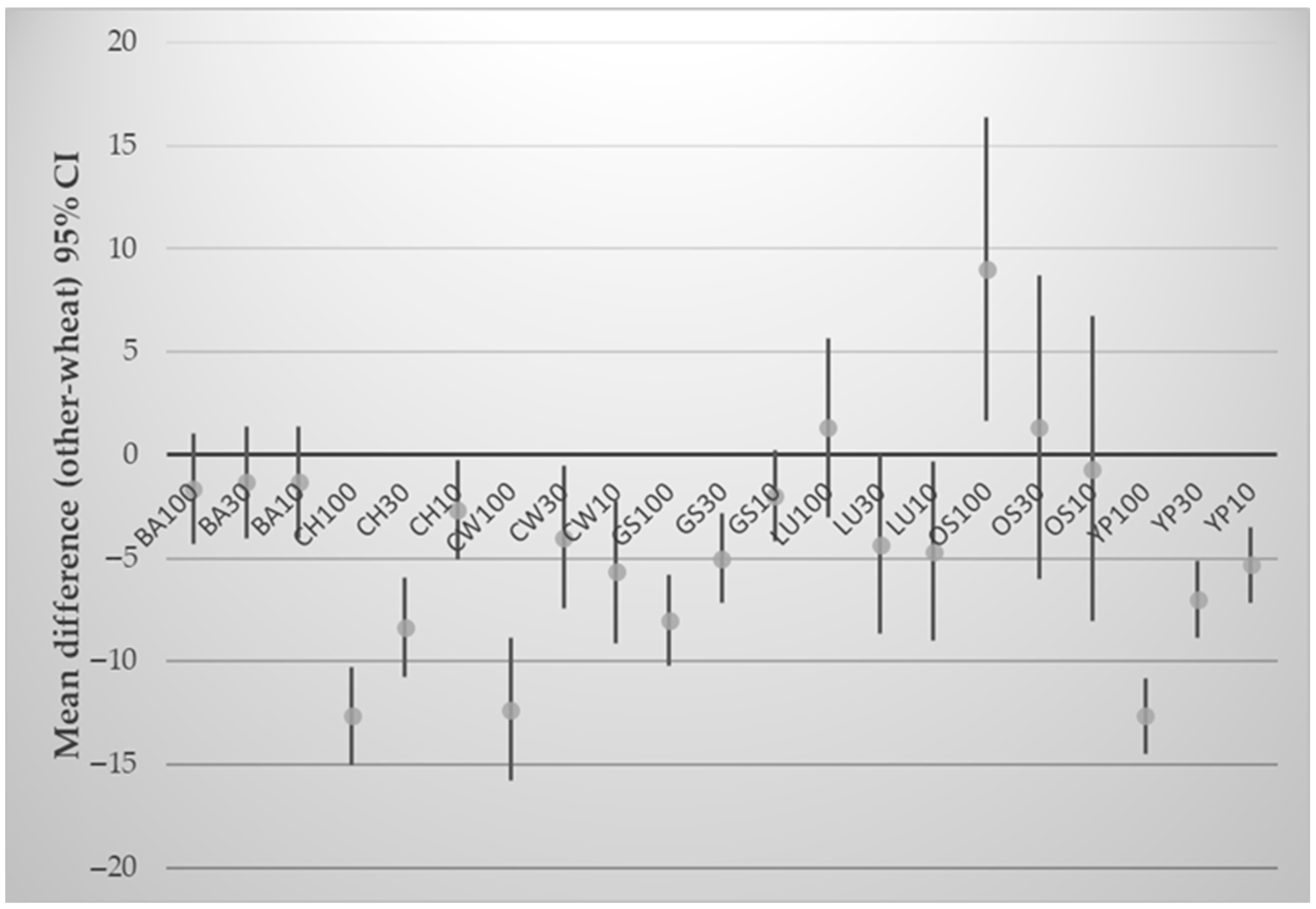
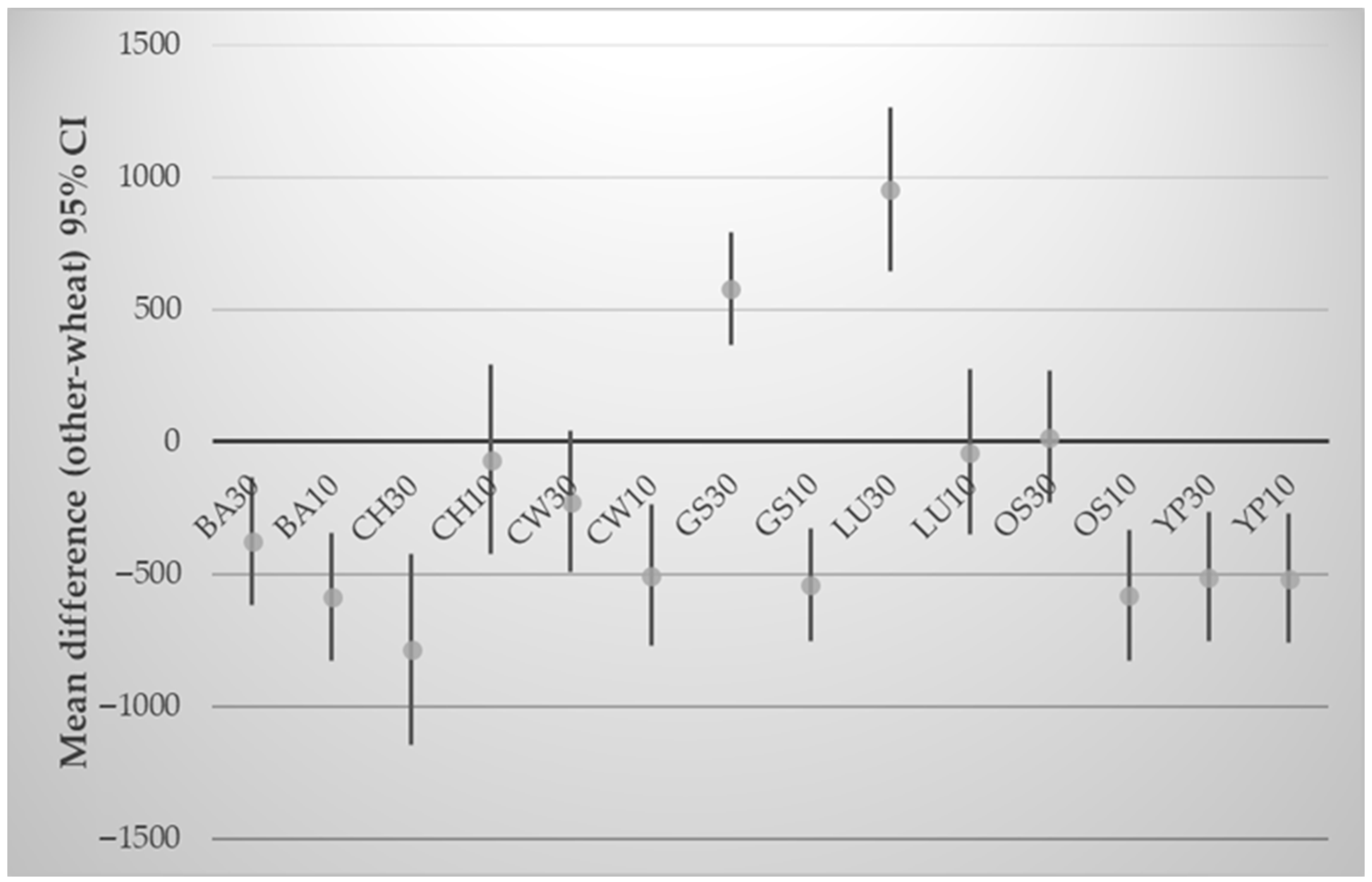
3.4. Effect of Composite Flours on Physicochemical Parameters of Crackers
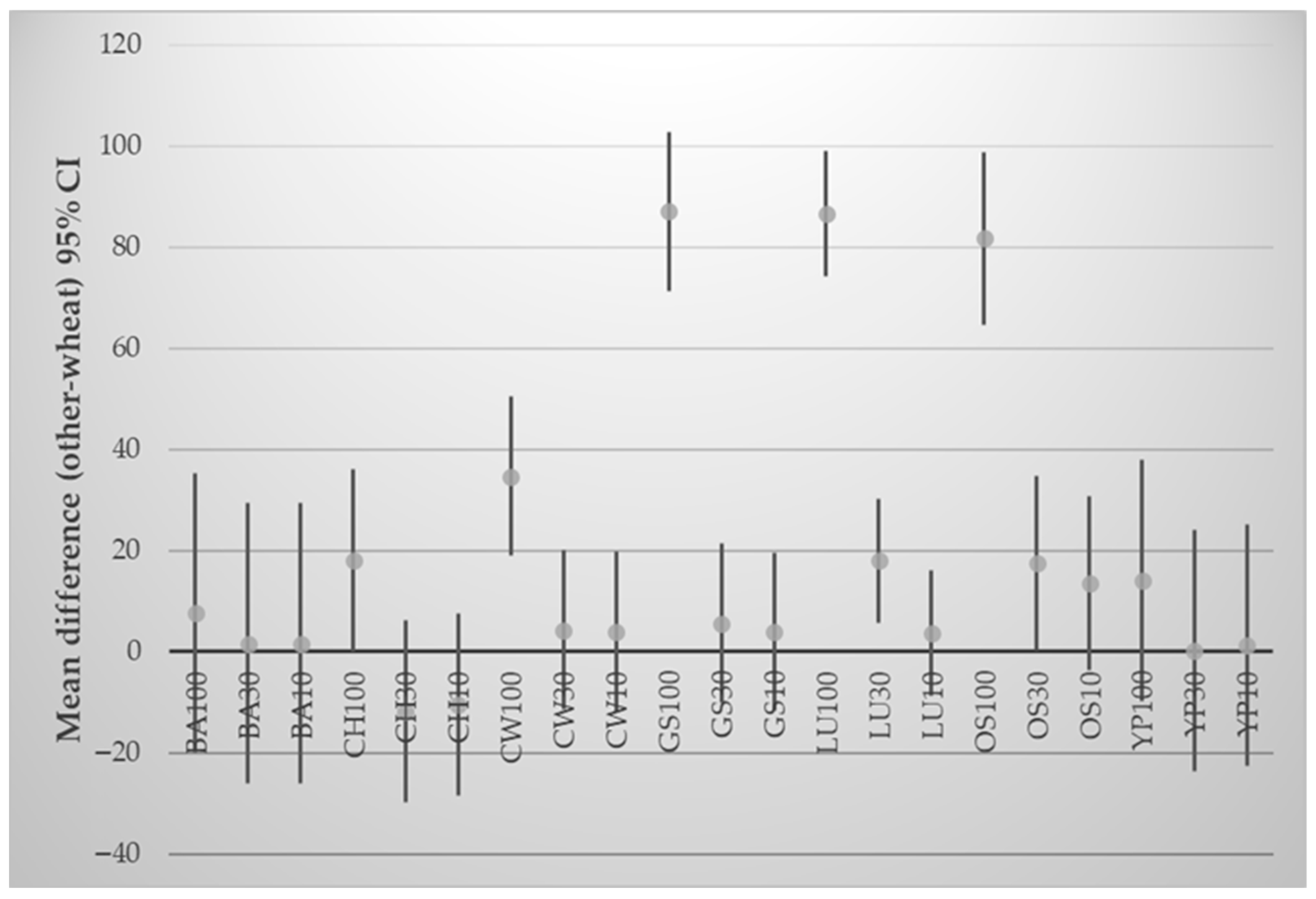
3.5. Effect of Wheat Flour Substitution on the Texture and Color of Crackers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J. Food Sci. Technol. 2015, 52, 5129–5137. [Google Scholar] [CrossRef]
- Gallardo, M.A.; Martínez-Navarro, M.E.; García Panadero, I.; Pardo, J.E.; Álvarez-Ortí, M. Nutritional enhancement of crackers through the incorporation of by-products from the frozen pumpkin industry. Foods 2025, 14, 42548. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global trends in wheat production, consumption and trade. In Wheat Improvement; Reynolds, M.P., Braun, H.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Czeczotko, M.; Górska-Warsewicz, H.; Zaremba, R. Health and non-health determinants of consumer behavior toward private label products—A systematic literature review. Int. J. Environ. Res. Public Health 2022, 19, 1768. [Google Scholar] [CrossRef]
- Albornoz, R.; García-Salirrosas, E.E.; Millones-Liza, D.Y.; Villar-Guevara, M.; Toyohama-Pocco, G. Using the theory of perceived value to determine the willingness to consume foods from a healthy brand: The role of health consciousness. Nutrients 2024, 16, 13995. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.; Jha, A.; Rasane, P.; Gautam, A.K. Optimization of a process for high fibre and high protein biscuit. J. Food Sci. Technol. 2015, 52, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Drakos, A.; Andrioti-Petropoulou, L.; Evangeliou, V.; Mandala, I. Physical and textural properties of biscuits containing jet milled rye and barley flour. J. Food Sci. Technol. 2019, 56, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, C.; Isik, F. Effects of pomegranate peel supplementation on chemical, physical, and nutritional properties of muffin cakes. J. Food Process. Preserv. 2019, 43, e13868. [Google Scholar] [CrossRef]
- Giannoutsos, K.; Zalidis, A.P.; Koukoumaki, D.I.; Menexes, G.; Mourtzinos, I.; Sarris, D.; Gkatzionis, K. Production of functional crackers based on non-conventional flours: Study of the physicochemical and sensory properties. Food Chem. Adv. 2023, 2, 100194. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Giannoutsos, K.; Devanthi, P.V.P.; Karmiris, P.; Bourni, S.; Monemvasioti, A.; Psimouli, V.; Sarris, D.; Gkatzionis, K. Effect of wheat replacement by pulse flours on the texture, color, and sensorial characteristics of crackers: Flash profile analysis. Int. J. Food Sci. 2022, 2022, 2354045. [Google Scholar] [CrossRef]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Hussey, K.; McCarthy, S.; Gallagher, E. Effect of pulse flours on the physiochemical characteristics and sensory acceptance of baked crackers. Int. J. Food Sci. Technol. 2017, 52, 1155–1163. [Google Scholar] [CrossRef]
- European Commission. Food-Based Dietary Guidelines in Europ. National FBDG Recommendations for Food Groups. Health Prevention Knowledge Gateway. 2025. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/food-based-dietary-guidelines-europe-table-6_en (accessed on 9 November 2025).
- Bolek, S. Olive stone powder: A potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innov. Food Sci. Emerg. Technol. 2020, 64, 102423. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of grape seed flour on the antioxidant profile, textural and sensory properties of waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef]
- Yadav, R.B.; Yadav, B.S.; Dhull, N. Effect of incorporation of plantain and chickpea flours on the quality characteristics of biscuits. J. Food Sci. Technol. 2012, 49, 207–213. [Google Scholar] [CrossRef]
- ICC-Standard No. 115/1; Method for Using the Brabender Farinograph. International Association for Cereal Science and Technology: Vienna, Austria, 1992.
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Method 935.29; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Awuchi, G.C.; Igwe, S.V.; Echeta, K.C. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019, 5, 139–160. [Google Scholar]
- Zhang, W.; Boateng, I.D.; Xu, J.; Zhang, Y. Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods 2024, 13, 1974. [Google Scholar] [CrossRef]
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the Potential Use of Grape Seed Flour in the Bakery Industry. Foods 2022, 11, 1589. [Google Scholar] [CrossRef]
- Abdel Rahman, M.F.; Elhawary, E.; Hafez, A.M.; Capanoglu, E.; Fang, Y.; Farag, M.A. How Does Olive Seed Chemistry, Health Benefits and Action Mechanisms Compare to Its Fruit Oil? A Comprehensive Review for Valorization Purposes and Maximizing Its Health Benefits. Food Biosci. 2024, 59, 104017. [Google Scholar] [CrossRef]
- Du, S.; Jiang, H.; Yu, X.; Jane, J. Physicochemical and functional properties of whole legume flour. LWT 2014, 55, 308–313. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Magala, M. Rheological and qualitative characteristics of pea flour incorporated cracker biscuits. Croat. J. Food Sci. Technol. 2013, 5, 11–17. [Google Scholar]
- Monnet, A.F.; Eurieult, A.; Berland, S.; Almeida, G.; Jeuffroy, M.H.; Michon, C. Damaged Starch in Pea versus Wheat Flours: Fragmentation Behavior and Contribution of Fine and Coarse Fractions. Cereal Chem. 2019, 96, 465–477. [Google Scholar] [CrossRef]
- Hasmadi, M.; Noorfarahzilah, M.; Noraidah, H.; Zainol, M.K.; Jahurul, M.H.A. Functional properties of composite flour: A review. Food Res. 2020, 4, 1820–1831. [Google Scholar] [CrossRef]
- Labuschagne, M.; Guzmán, C.; Crossa, J.; van Biljon, A. Determining Factors of Durum Wheat Bread Loaf Volume and Alveograph Characteristics Under Optimal, Drought and Heat Stress Conditions. J. Cereal Sci. 2023, 114, 103791. [Google Scholar] [CrossRef]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Boukid, F.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for Bread Fortification: A Necessity or a Choice? Trends Food Sci. Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Chukwuejim, S.; Kadam, D.; Aluko, R.E. Structural, Physicochemical, and Functional Properties of White and Blue Lupin Vicilin and Legumin Fractions. Food Chem. X 2025, 31, 103078. [Google Scholar] [CrossRef]
- Holkovičová, T.; Lauková, M.; Minarovičová, L.; Kohajdová, Z. Wheat-Lupin Flour Blends, Functionally and Nutritionally Valuable Raw Materials for Bakery Applications. ACS Food Sci. Technol. 2025, 5, 3823–3834. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Provatidou, E.; Tsotsiou, D.; Kiosseoglou, V. Dough Rheology and Baking Performance of Wheat Flour–Lupin Protein Isolate Blends. Food Res. Int. 2010, 43, 1009–1016. [Google Scholar] [CrossRef]
- Abdullah, M.; Tufail, T.; Hussain, M.; Nadeem, M.; Owais, M.; Zulkiffa, M.; Tanveer, M.H.; Al Jbawi, E. Effect of Sprouted Barley Flour on the Quality of Wheat Bread, Biscuits and Cakes. J. Sci. Food Agric. 2023, 103, 8711–8718. [Google Scholar] [CrossRef]
- Pečivová, P.B.; Kráčmar, S.; Kubáň, V.; Mlček, J.; Juríková, T.; Sochor, J. Effect of Addition of Grape Seed Flour on Chemical, Textural and Sensory Properties of Bread Dough. Potravin. Slovak J. Food Sci. 2016, 10, 368–373. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sharma, G.K.; Khan, M.A.; Govindaraj, T.; Semwal, A.D. Development of multigrain premixes—Its effect on rheological, textural and micro-structural characteristics of dough and quality of biscuits. J. Food Sci. Technol. 2015, 52, 7759–7770. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Dordoni, R.; Garrido, G.D.; Marinoni, L.; Torri, L.; Piochi, M.; Spigno, G. Enrichment of whole wheat cocoa biscuits with encapsulated grape skin extract. Int. J. Food Sci. 2019, 2019, 9161840. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Rodriguez, P.; Gomez, M. Assessing rice flour-starch-protein mixtures to produce gluten-free sugar-snap cookies. LWT 2016, 67, 127–132. [Google Scholar] [CrossRef]
- Melini, V.; Vescovo, D.; Melini, F.; Raffo, A. Bakery Product Enrichment with Phenolic Compounds as an Unexplored Strategy for the Control of the Maillard Reaction. Appl. Sci. 2024, 14, 2647. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Bai, X.; Wang, F. Production of biscuits by substitution with different ratios of yellow pea flour. Grain Oil Sci. Technol. 2019, 2, 91–96. [Google Scholar] [CrossRef]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- Shankar, M.; Simons, C.; Shiv, B.; Mcclure, S.; Levitan, C.; Spence, C. An expectations-based approach to explaining the cross-modal influence of color on orthonasal olfactory identification: The influence of the degree of discrepancy. Atten. Percept. Psychophys. 2010, 72, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
| Rheological Characteristics (Farinograph Testing) | ||||
|---|---|---|---|---|
| Samples | Water Absorption, WA (%) | Dough Development Time, DDT (min) | Stability, STA (min) | Degree of Softening, DS (FU) |
| WF (control) | 61.9 | 2.4 | 6.0 | 78.0 |
| BA30 | 60.0 | 6.2 | 12.6 | 64.0 |
| CH30 | 57.9 | 10.2 | 12.0 | 131.0 |
| LU30 | 68.6 | 6.2 | 5.7 | 50.0 |
| OS30 | 61.3 | 12.9 | 11.0 | 63.0 |
| GS30 | 53.2 | 21.5 | 33.3 | 0.0 |
| Samples | aw | Moisture (%) | Puffiness (%) | Bake Loss (%) | Spread Ratio | Hardness (N) | Fracturability (mm) | Total Energy (J) |
|---|---|---|---|---|---|---|---|---|
| WF | 0.245 ± 0.02 | 4.27 ± 0.05 | 50.00 ± 10.15 | 28.37 ± 1.90 | 23.83 ± 1.82 | 11.65 ± 2.31 | 3.02 ± 1.06 | 20.80 ± 4.83 |
| LU10 | 0.267 ± 0.00 | 4.45 ± 0.17 | 45.00 ± 6.54 | 28.23 ± 0.80 | 24.81 ± 1.23 | 12.31 ± 3.18 | 1.44 ± 0.04 | 14.40 ± 3.18 |
| LU30 | 0.191 * ± 0.00 | 2.94 * ± 0.13 | 28.16 * ± 2.02 | 28.18 ± 1.35 | 27.54 * ± 0.79 | 13.26 ± 2.46 | 1.52 ± 0.45 | 17.24 ± 11.78 |
| CH10 | 0.224 ± 0.02 | 3.25 * ± 0.17 | 43.33 ± 14.65 | 29.50 ± 2.35 | 23.53 ± 2.21 | 13.50 ± 1.03 | 1.70 ± 0.17 | 15.46 ± 1.39 |
| CH30 | 0.182 * ± 0.02 | 2.79 * ± 0.21 | 34.66 ± 5.39 | 30.17 ± 3.63 | 25.63 ± 1.15 | 16.66 ± 5.77 | 1.32 * ± 0.35 | 15.59 ± 2.69 |
| GS10 | 0.136 * ± 0.00 | 2.68 * ± 0.11 | 46.16 ± 8.52 | 29.80 ± 0.40 | 23.75 ± 1.00 | 12.34 ± 0.79 | 2.31 ± 0.32 | 14.88 ± 3.35 |
| GS30 | 0.136 * ± 0.01 | 1.82 * ± 0.06 | 24.33 * ± 3.06 | 29.66 ± 0.95 | 27.70 * ± 0.72 | 6.51 * ± 1.94 | 1.83 ± 0.50 | 8.16 * ± 3.53 |
| OS10 | 0.162 * ± 0.00 | 2.59 * ± 0.02 | 53.83 ± 11.14 | 29.37 ± 0.35 | 22.65 ± 1.68 | 13.03 ± 1.03 | 1.82 ± 0.57 | 14.22 ± 1.74 |
| OS30 | 0.131 * ± 0.01 | 1.84 * ± 0.17 | 22.00 ± 20.42 | 29.83 ± 0.28 | 29.35 ± 4.47 | 9.87 ± 1.50 | 2.70 ± 1.38 | 14.03 ± 3.40 |
| CW10 | 0.143 * ± 0.01 | 2.53 * ± 0.06 | 44.00 ± 16.93 | 27.13 ± 4.37 | 24.49 ± 3.05 | 13.90 ± 3.48 | 2.08 ± 0.10 | 15.60 ± 2.64 |
| CW30 | 0.135 * ± 0.00 | 1.60 * ± 0.02 | 23.00 ± 6.24 | 28.53 ± 2.91 | 28.52 ± 1.43 | 12.63 ± 3.40 | 1.96 ± 0.43 | 15.91 ± 3.71 |
| YP10 | 0.151 * ± 0.01 | 2.81 * ± 0.03 | 41.33 ± 7.11 | 22.13 ± 6.34 | 24.78 ± 1.38 | 14.28 ± 3.58 | 1.88 ± 0.23 | 22.92 ± 11.36 |
| YP30 | 0.137 * ± 0.00 | 1.84 * ± 0.09 | 14.00 * ± 4.92 | 28.91 ± 0.74 | 29.97 * ± 1.06 | 8.09 ± 0.73 | 2.15 ± 0.31 | 11.61 ± 2.04 |
| BA10 | 0.143 * ± 0.01 | 2.47 * ± 0.05 | 48.83 ± 9.57 | 25.21 ± 1.37 | 23.22 ± 1.14 | 18.65 * ± 0.67 | 2.22 ± 0.69 | 19.33 ± 4.51 |
| BA30 | 0.151 * ± 0.01 | 2.69 * ± 0.02 | 54.83 ± 0.58 | 26.86 ± 2.57 | 22.49 ± 0.16 | 14.61 ± 2.40 | 2.11 ± 0.78 | 21.32 ± 7.31 |
| Samples | L* | a* | b* | C* | h° | ΔΕ |
|---|---|---|---|---|---|---|
| WF | 70.60 ± 1.55 | 2.46 ± 1.78 | 28.26 ± 0.98 | 28.43 ± 0.95 | 85.00 ± 3.63 | 0.00 ± 0.00 |
| LU10 | 69.66 ± 3.07 | 5.63 * ± 0.46 | 33.30 * ± 1.35 | 33.76 * ± 1.40 | 80.36 ± 0.47 | 6.68 * ± 0.62 |
| LU30 | 64.76 ± 2.25 | 10.73 * ± 1.04 | 41.03 * ± 0.65 | 42.40 * ± 0.44 | 75.36 * ± 1.55 | 16.50 * ± 0.47 |
| CH10 | 71.20 ± 1.66 | 6.10 ± 1.91 | 29.56 ± 2.06 | 30.23 ± 2.32 | 78.50 ± 2.86 | 4.37 * ± 2.39 |
| CH30 | 64.46 * ± 1.89 | 12.56 * ± 1.21 | 31.86 ± 1.26 | 34.26 * ± 0.70 | 68.50 * ± 2.66 | 12.54 * ± 1.53 |
| GS10 | 53.53 * ± 1.42 | 7.23 * ± 2.67 | 22.03 * ± 0.60 | 23.26 * ± 1.00 | 71.96 * ± 6.36 | 18.93 * ± 0.71 |
| GS30 | 39.06 * ± 0.40 | 11.00 * ± 0.10 | 15.56 * ± 0.40 | 19.10 * ± 0.26 | 54.83 * ± 1.01 | 35.03 * ± 0.44 |
| OS10 | 46.00 * ± 0.26 | 5.63 ± 0.38 | 17.50 * ± 0.79 | 18.36 * ± 0.86 | 72.16 * ± 0.65 | 27.02 * ± 0.48 |
| OS30 | 30.83 * ± 2.40 | 5.06 ± 1.47 | 10.66 * ± 0.23 | 11.83 * ± 0.55 | 64.83 * ± 6.86 | 43.56 * ± 2.09 |
| CW10 | 62.63 * ± 2.57 | 12.23 * ± 1.29 | 33.10 * ± 0.53 | 35.30 * ± 0.26 | 69.73 * ± 2.28 | 13.66 * ± 2.20 |
| CW30 | 49.23 * ± 2.83 | 17.20 * ± 0.20 | 28.30 ± 2.17 | 33.10 * ± 1.73 | 58.66 * ± 2.03 | 26.08 * ± 2.33 |
| YP10 | 61.20 * ± 1.54 | 12.10 * ± 0.72 | 32.63 * ± 0.40 | 34.83 * ± 0.32 | 69.63 * ± 1.21 | 14.24 * ± 1.41 |
| YP30 | 46.66 * ± 1.25 | 16.80 * ± 0.62 | 25.03 * ± 0.84 | 30.13 * ± 0.35 | 56.20 * ± 1.82 | 28.16 * ± 1.45 |
| BA10 | 66.80 ± 2.26 | 8.73 * ± 1.25 | 33.33 * ± 1.20 | 34.53 * ± 1.46 | 75.33 * ± 1.62 | 9.15 * ± 1.91 |
| BA30 | 67.86 ± 1.65 | 7.36 * ± 1.18 | 31.30 * ± 0.44 | 32.13 * ± 0.72 | 76.76 * ± 1.85 | 6.48 * ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoutsos, K.; Koukoumaki, D.I.; Bountziouka, V.; Poriazi, T.; Papageorgiou, M.; Sarris, D.; Gkatzionis, K.; Naziri, E. Quality Properties of Crackers Enriched with Composite Flours: Effect on Dough and Final Product. Appl. Sci. 2025, 15, 12361. https://doi.org/10.3390/app152312361
Giannoutsos K, Koukoumaki DI, Bountziouka V, Poriazi T, Papageorgiou M, Sarris D, Gkatzionis K, Naziri E. Quality Properties of Crackers Enriched with Composite Flours: Effect on Dough and Final Product. Applied Sciences. 2025; 15(23):12361. https://doi.org/10.3390/app152312361
Chicago/Turabian StyleGiannoutsos, Konstantinos, Danai Ioanna Koukoumaki, Vasiliki Bountziouka, Tonia Poriazi, Maria Papageorgiou, Dimitris Sarris, Konstantinos Gkatzionis, and Eleni Naziri. 2025. "Quality Properties of Crackers Enriched with Composite Flours: Effect on Dough and Final Product" Applied Sciences 15, no. 23: 12361. https://doi.org/10.3390/app152312361
APA StyleGiannoutsos, K., Koukoumaki, D. I., Bountziouka, V., Poriazi, T., Papageorgiou, M., Sarris, D., Gkatzionis, K., & Naziri, E. (2025). Quality Properties of Crackers Enriched with Composite Flours: Effect on Dough and Final Product. Applied Sciences, 15(23), 12361. https://doi.org/10.3390/app152312361










