Terbinafine-Loaded PLGA Nanoparticles Applicable to the Treatment of Tinea Fungus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Terbinafine-Loaded PLGA NPs
2.3. Physicochemical Evaluation of Terbinafine-Loaded PLGA NPs
2.3.1. Particle Size and Polydispersity Index
2.3.2. Yield, Content, and Entrapment Efficiency
2.3.3. Release Test
2.4. Animal Experiments
2.5. Determination of Skin Penetration and Intradermal Concentration of Terbinafine
2.6. Data Analysis
3. Results
3.1. Physicochemical Evaluation of Terbinafine-Loaded PLGA NPs
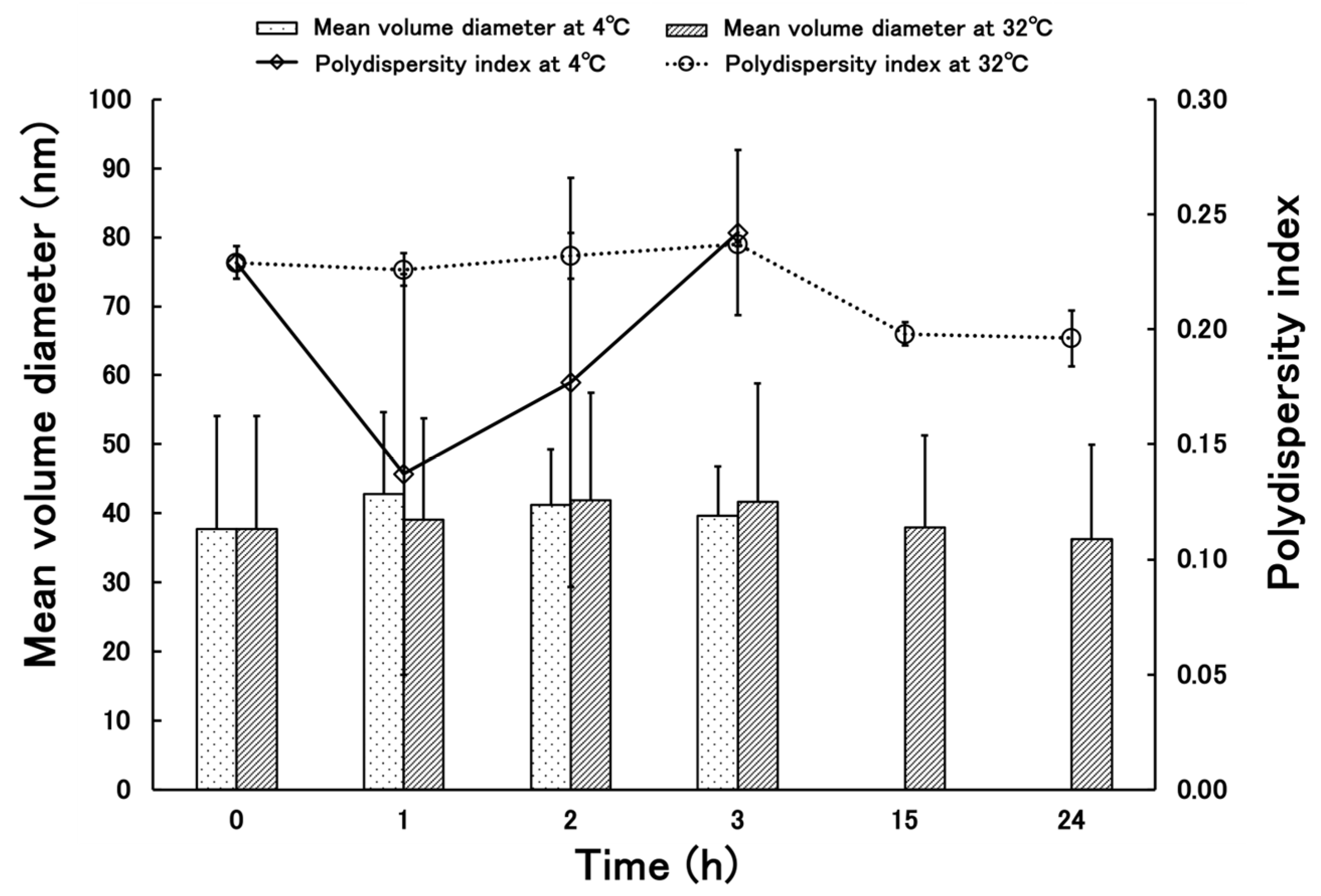
3.2. Stability of Terbinafine-Loaded PLGA NPs
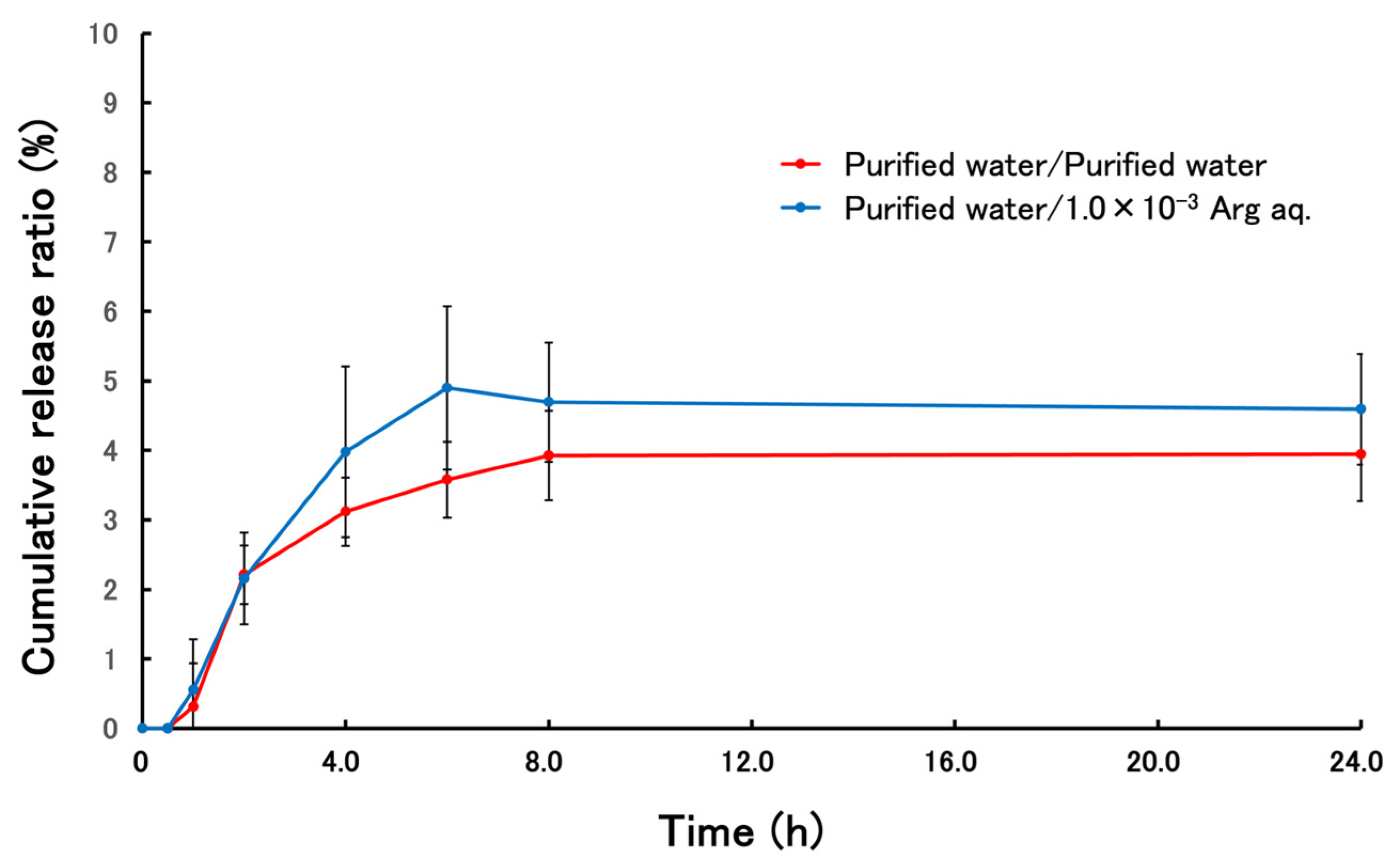
3.3. Release Behavior of Terbinafine-Loaded PLGA NPs
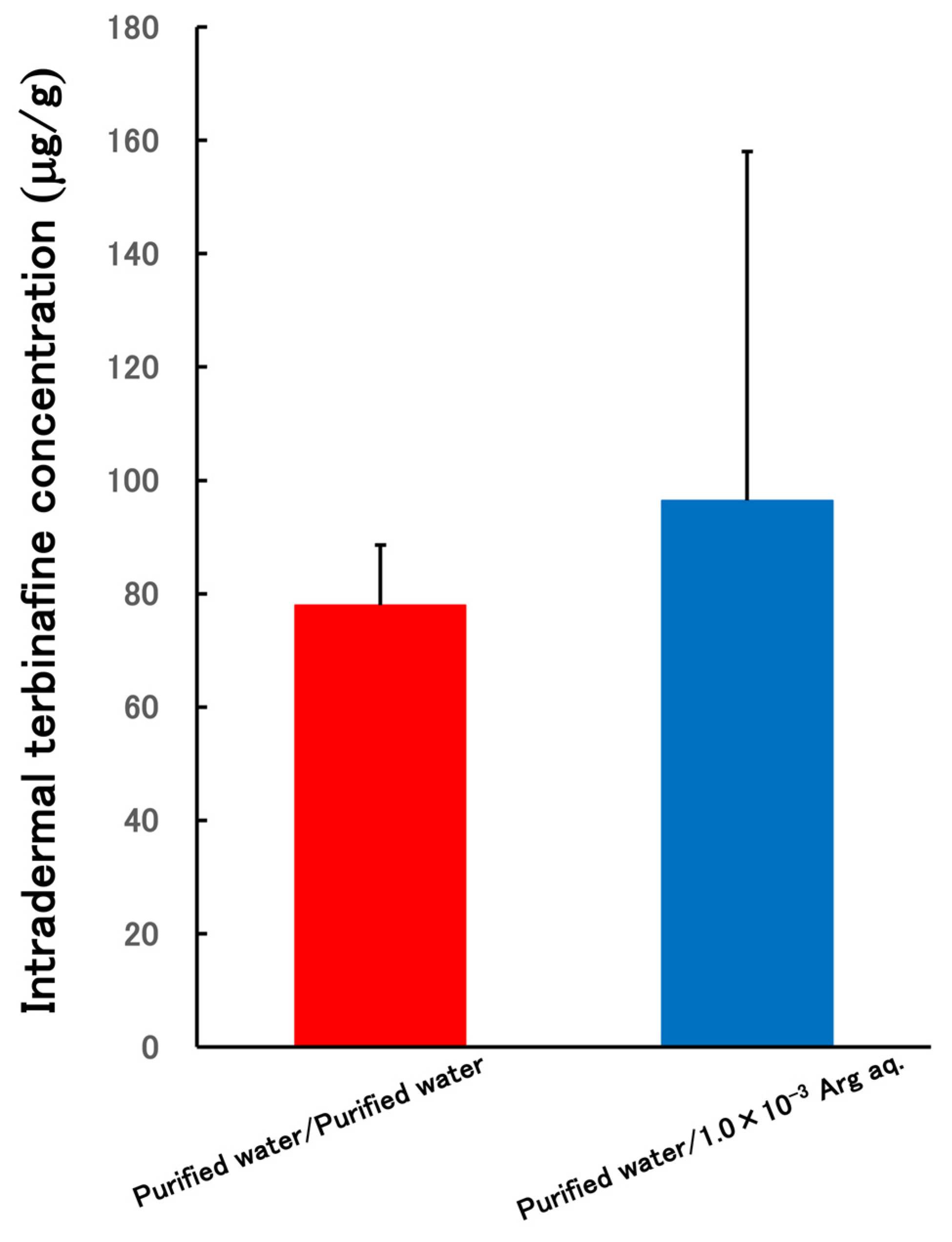
3.4. Skin Permeability Test of PLGA NPs Terbinafine-Loaded
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahoo, A.K.; Mahajan, R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol. Online J. 2016, 7, 77–86. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 165, 165. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335. [Google Scholar] [CrossRef]
- Panday, M.; Pandey, D.; Upadhyay, P.; Upadhyay, S. Terbinafine Preferred Antifungal with a Focus on Dermatophytes. Acta Sci. Microbiol. 2020, 3, 65–72. [Google Scholar] [CrossRef]
- Poirier, J.M.; Cheymol, G.J. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 1998, 35, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Glyn, E.; Evans, V.; Sigugeirsson, B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. Br. Med. J. 1999, 318, 1031–1035. [Google Scholar] [CrossRef]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kishida, H.; Okubo, A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study. J. Dermatol. 2017, 44, 753–759. [Google Scholar] [CrossRef]
- Korting, H.C.; Tietz, H.J.; Bräutigam, M.; Mayser, P.; Rapatz, G.; Paul, C. One week terbinafine 1% cream (Lamisil) once daily is effective in the treatment of interdigital tinea pedis: A vehicle controlled study. LAS-INT-06 Study. Med. Mycol. 2001, 39, 335–340. [Google Scholar] [CrossRef]
- Berman, B.; Ellis, C.; Leyden, J.; Lowe, N.; Savin, R.; Shupack, J.; Stiller, M.; Tschen, E.; Zaias, N.; Birnbaum, J.E. Efficacy of a 1-week, twice-daily regimen of terbinafine 1% cream in the treatment of interdigital tinea pedis. Results of placebo-controlled, double-blind, multicenter trials. J. Am. Acad. Dermatol. 1992, 26, 956–960. [Google Scholar] [CrossRef]
- Iverson, S.L.; Uetrecht, J.P. Identification of a reactive metabolite of terbinafine: Insights into terbinafine-induced hepatotoxicity. Chem. Res. Toxicol. 2001, 14, 175–181. [Google Scholar] [CrossRef]
- Ajit, C.; Zaeri, N.; Munoz, S.J.; Suvannasankha, A. Terbinafine-Associated Hepatotoxicity. Am. J. Med. Sci. 2003, 325, 292–295. [Google Scholar] [CrossRef]
- Pyatski, Y.; Flach, C.R.; Mendelsohn, R. FT-IR investigation of Terbinafine interaction with stratum corneum constituents. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183335. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Utreja, P. Vesicular nanocarrier based treatment of skin fungal infections: Potential and emerging trends in nanoscale pharmacotherapy. Asian J. Pharm. Sci. 2019, 14, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Chatur, V.M.; Dhole, S.N. Development and Characterization of Niosomal Gel for Topical Delivery of Luliconazole. Int. J. Pharm. Res. Allied Sci. 2022, 11, 99–107. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.; Guy, R. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef]
- Pearton, M.; Allender, C.; Brain, K.; Anstey, A.; Gateley, C.; Wilke, N.; Morrissey, A.; Birchall, J. Gene Delivery to the Epidermal Cells of Human Skin Explants Using Microfabricated Microneedles and Hydrogel Formulations. Pharm. Res. 2008, 25, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cerc, G.; Vierl, U. Nanotechnology and the transdermal route A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, M.; Saeed, S.; Saleh, E.; Kassem, A.; Arbi, F.; Wanab, A.; Rehman, M.; Rehman, K.; Khan, D.; et al. Nanotherapeutic approaches for transdermal drug delivery systems and their biomedical applications. Eur. Polym. J. 2024, 207, 112819. [Google Scholar] [CrossRef]
- Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm. 1995, 116, 1–9. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.; Yan, R.; Chen, X.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Zhu, Q.; Zhang, M.; Ding, X.; Wang, X.; Hou, X.; Fan, W.; Ding, B.; Wu, X.; et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int. J. Pharm. 2010, 402, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mittal, A.; Jain, A. Enhanced Topical Delivery of Cyclosporin—A Using PLGA Nanoparticles as Carrier. Curr. Nanosci. 2011, 7, 524–530. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125607. [Google Scholar] [CrossRef]
- Tomoda, K.; Yabuki, N.; Terada, H.; Makino, K. Surfactant free preparation of PLGA nanoparticles: The combination of antisolvent diffusion with preferential solvation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 88–93. [Google Scholar] [CrossRef]
- Takeuchi, I.; Makino, K. Biocompatibility and effectiveness of paclitaxel-encapsulated micelle using phosphoester compounds as a carrier fir cancer trearment. Colloids Surf. B Biointerfaces 2019, 177, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, D.; Buono, M.; Kolkhorst, F. Effect of Skin Temperature on the Cholinergic Sensitivity of the Human Eccrine Sweat Gland. Jpn. J. Physiol. 2003, 53, 427–430. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Skin permeability and transdermal delivery route of 50-nm indomethacin-loaded PLGA nanoparticles. Colloids Surf. B Biointerfaces 2017, 159, 312–317. [Google Scholar] [CrossRef]
- Fitch, C.; Platzer, G.; Okon, M.; Garcia-Moreno, B.; McIntosh, L. Arginine: Its pKa value revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef]
- Fujisawa, R.; Sakurai, R.; Oshizaka, T.; Mori, K.; Saitoh, A.; Takeuchi, I.; Sugibayashi, K. Development of PSL-loaded PLGA nanoparticles for the treatment of allergic contact dermatitis. Colloids Interfaces 2024, 8, 39. [Google Scholar] [CrossRef]
- Nakashima, T.; Nozawa, A.; Majima, T. A novel method using micropig stratum corneum in vitro for the evaluation of anti-Trichophyton mentagrophytes activity. Microbiol. Immunol. 2002, 46, 521–525. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp. Dermatol. 2018, 27, 876–883. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Topical Delivery of Drugs for the Effective Treatment of Fungal Infections of Skin. Curr. Pharm. Des. 2015, 21, 2892–2913. [Google Scholar] [CrossRef] [PubMed]
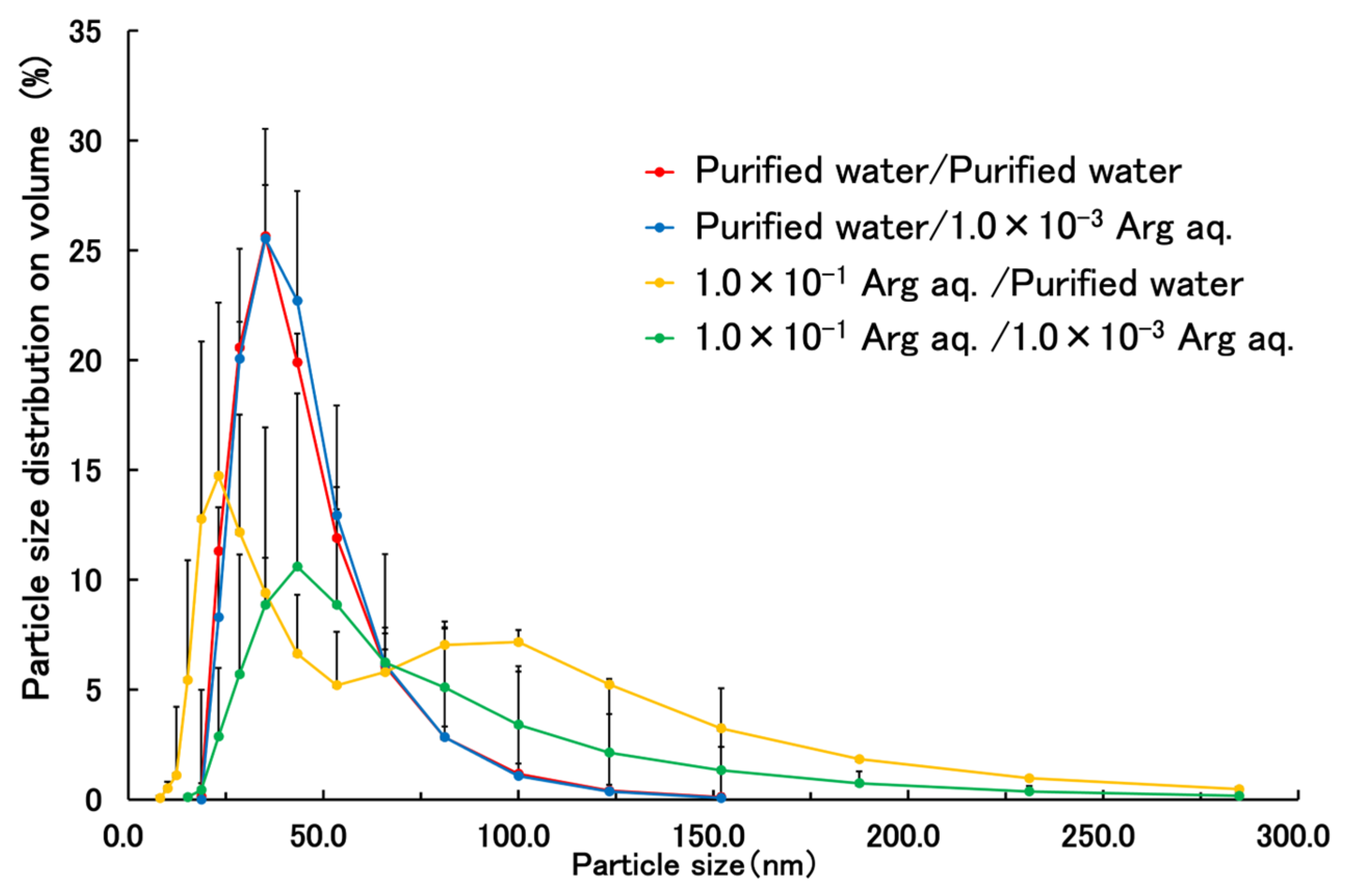
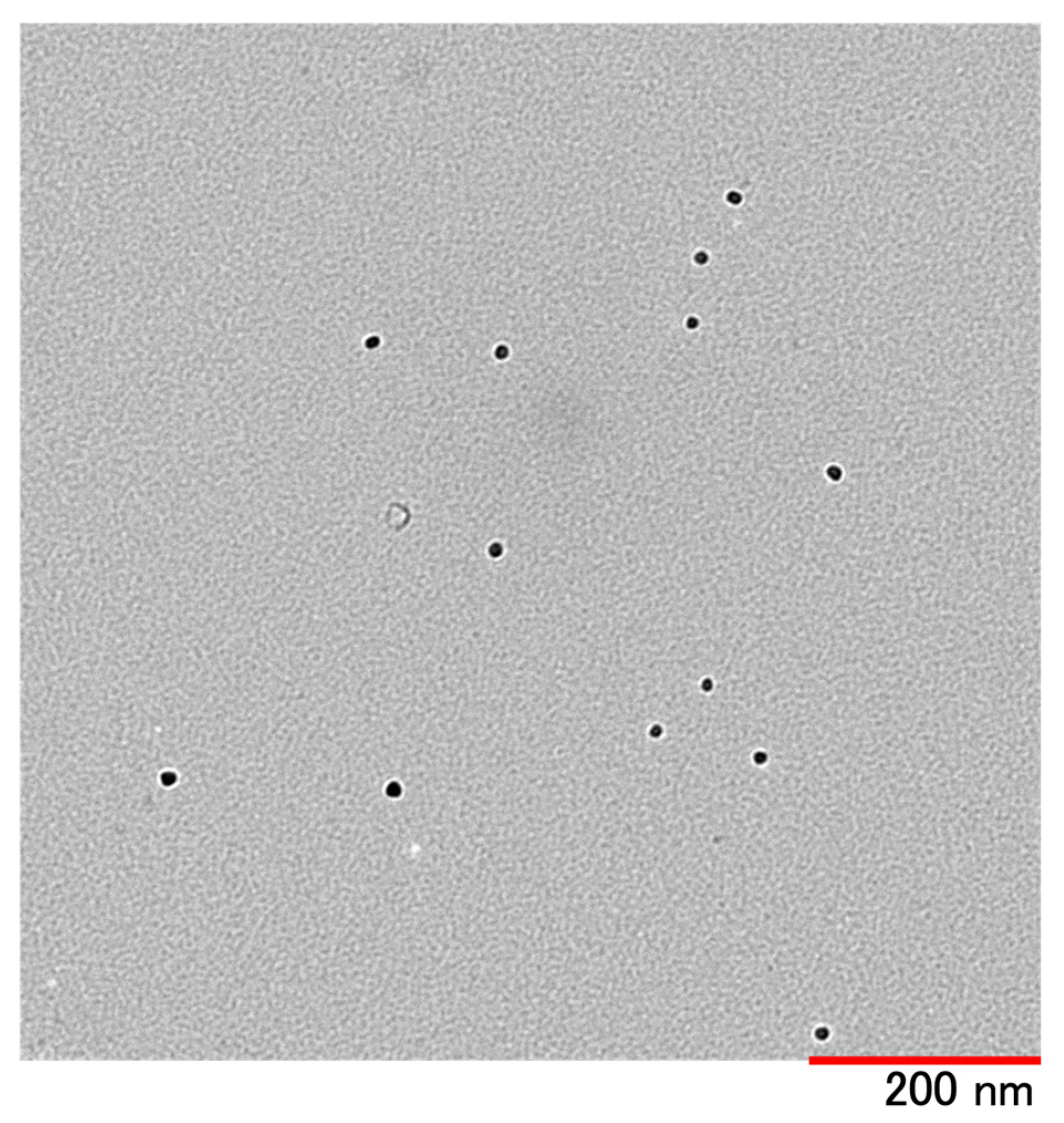
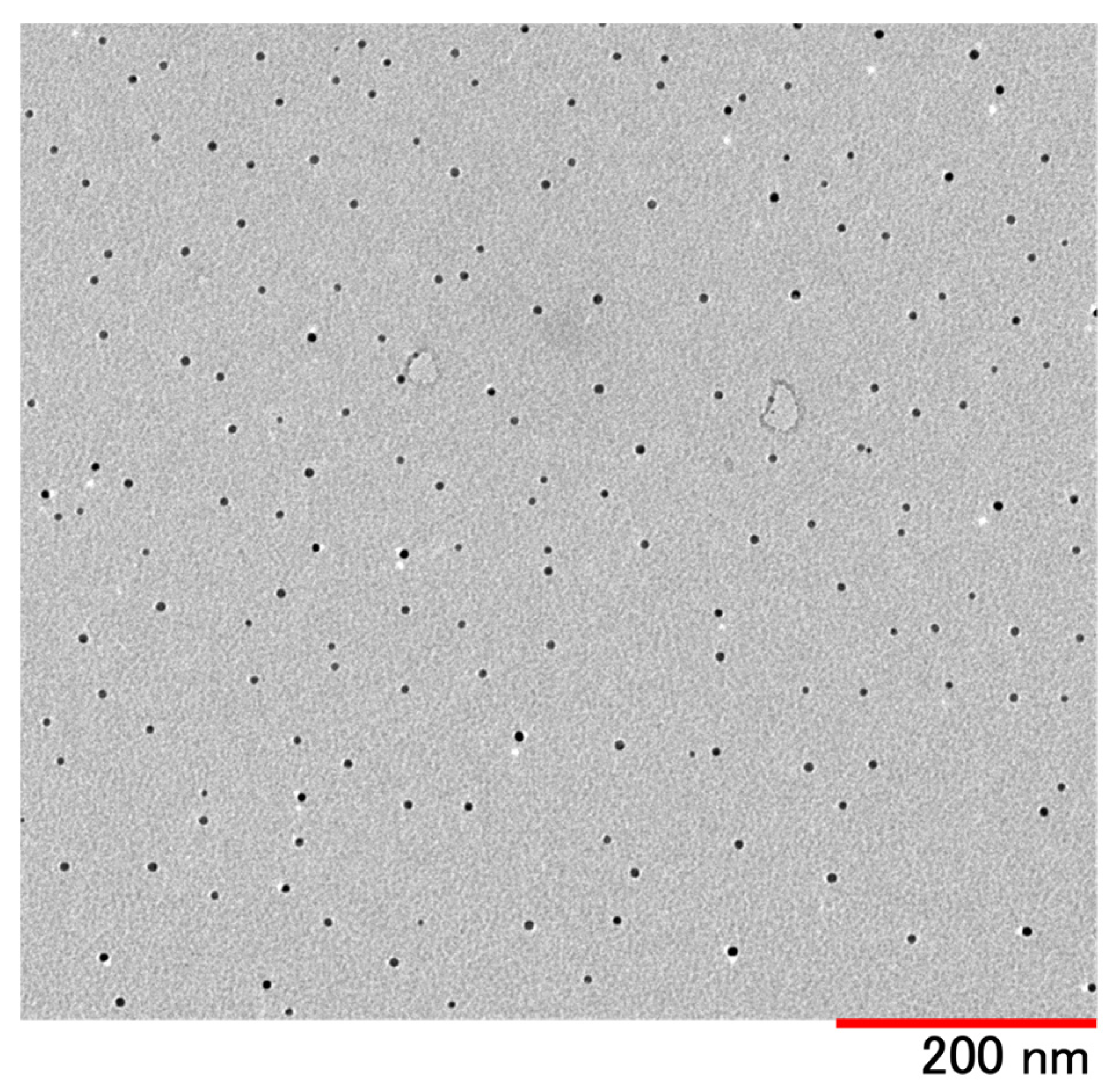

| Good Solvent | Poor Solvent | External Solution | |
|---|---|---|---|
| Condition 1 | Acetone | Purified water | Purified water |
| Condition 2 | Acetone | Purified water | 1.0 × 10−3 w/v% Arg aq. |
| Condition 3 | Acetone | 1.0 × 10−1 w/v% Arg aq. | Purified water |
| Condition 4 | Acetone | 1.0 × 10−1 w/v% Arg aq. | 1.0 × 10−3 w/v% Arg aq. |
| Condition 1 | Condition 2 | Condition 3 | Condition 4 | |
|---|---|---|---|---|
| Mean volume diameter (nm) | 40.6 ± 16.3 | 41.3 ± 15.7 | 55.1 ± 19.5 | 2754.0 ± 3650.3 |
| PDI | 0.168 ± 0.036 | 0.164 ± 0.008 | 0.278 ± 0.047 | 0.294 ± 0.024 |
| Yield (%) | 79.02 ± 5.47 | 83.21 ± 3.19 | 79.29 ± 2.91 | 78.08 ± 6.13 |
| Content (%) | 3.53 ± 0.16 | 5.27 ± 0.43 | 5.24 ± 0.05 | 5.22 ± 0.27 |
| Entrapment efficiency (%) | 58.83 ± 2.78 | 87.76 ± 7.16 | 87.35 ± 0.84 | 86.52 ± 4.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, R.; Sakurai, R.; Oshizaka, T.; Mori, K.; Saitoh, A.; Takeuchi, I.; Sugibayashi, K. Terbinafine-Loaded PLGA Nanoparticles Applicable to the Treatment of Tinea Fungus. Appl. Sci. 2025, 15, 12357. https://doi.org/10.3390/app152312357
Fujisawa R, Sakurai R, Oshizaka T, Mori K, Saitoh A, Takeuchi I, Sugibayashi K. Terbinafine-Loaded PLGA Nanoparticles Applicable to the Treatment of Tinea Fungus. Applied Sciences. 2025; 15(23):12357. https://doi.org/10.3390/app152312357
Chicago/Turabian StyleFujisawa, Ryo, Ryuse Sakurai, Takeshi Oshizaka, Kenji Mori, Akiyoshi Saitoh, Issei Takeuchi, and Kenji Sugibayashi. 2025. "Terbinafine-Loaded PLGA Nanoparticles Applicable to the Treatment of Tinea Fungus" Applied Sciences 15, no. 23: 12357. https://doi.org/10.3390/app152312357
APA StyleFujisawa, R., Sakurai, R., Oshizaka, T., Mori, K., Saitoh, A., Takeuchi, I., & Sugibayashi, K. (2025). Terbinafine-Loaded PLGA Nanoparticles Applicable to the Treatment of Tinea Fungus. Applied Sciences, 15(23), 12357. https://doi.org/10.3390/app152312357








