Integration and Innovation in Digital Implantology—Part I: Capabilities and Limitations of Contemporary Workflows: A Narrative Review
Abstract
1. Introduction
- (1)
- summarize the current applications and performance of key digital technologies in implant prosthodontics workflows;
- (2)
- assess their practical benefits and constraints relative to conventional methods; and
- (3)
- identify the critical technical, procedural, and data interfaces that still limit seamless digital integration.
2. Methods
3. Foundations of Digital Implantology
3.1. Current State of Guided Implant Surgery
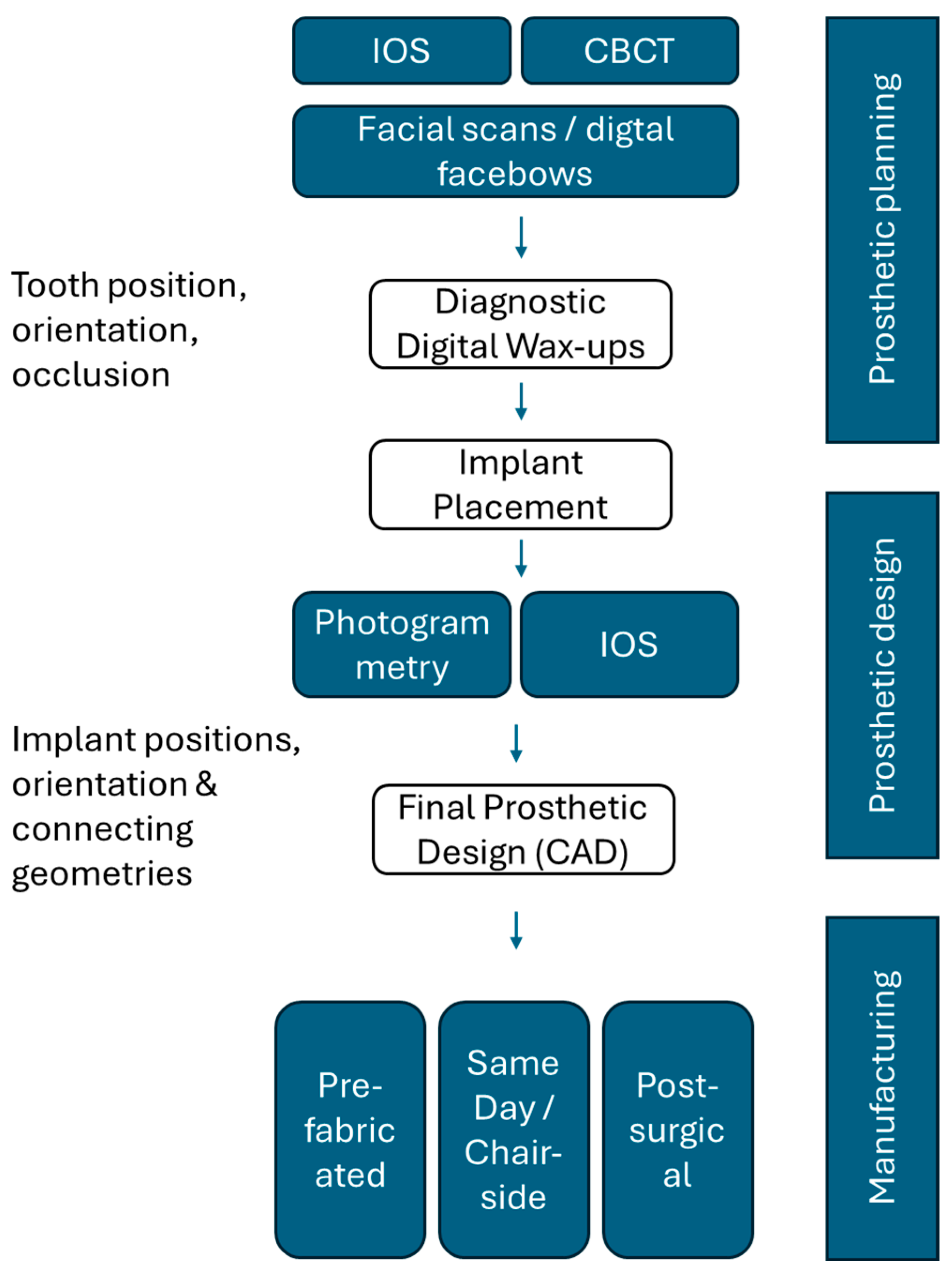
3.2. Current State of Implant-Prosthetic Planning and Manufacturing Workflows
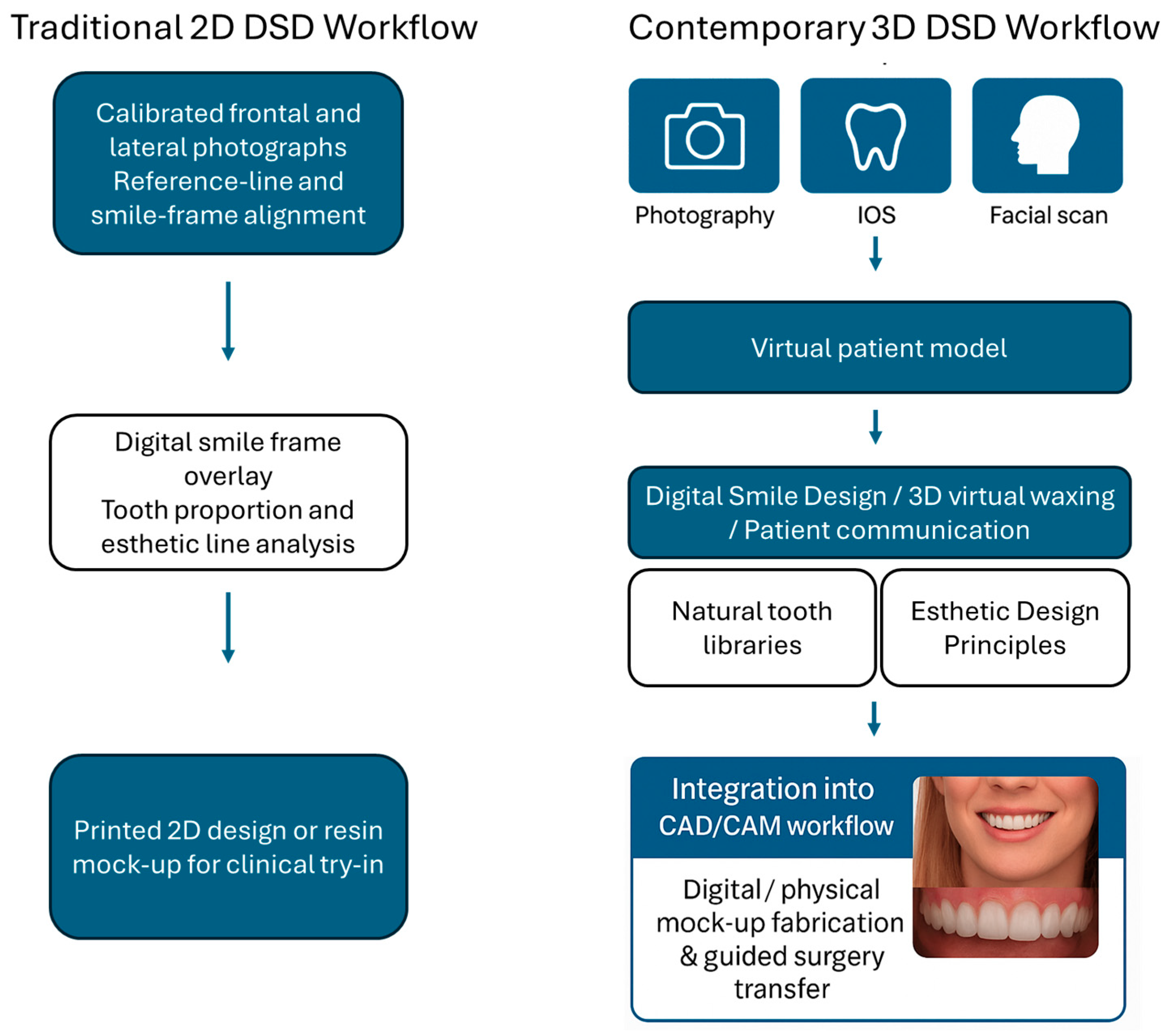
3.3. An Overview of Current Digital Smile Design Workflows
3.4. Virtual Articulators in Functional Digital Prosthodontics
4. Integrated Workflows in Contemporary Digital Implantology
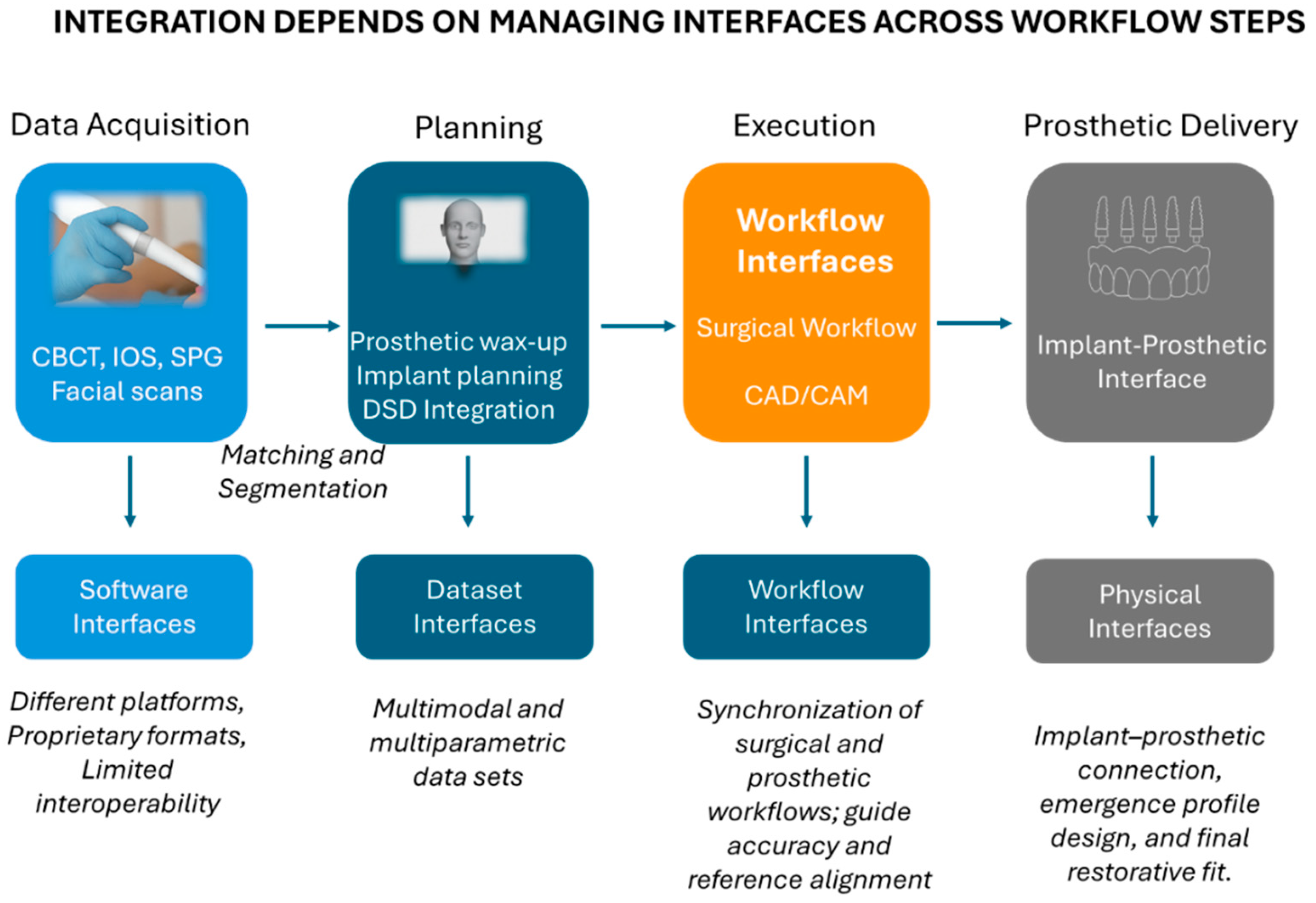
4.1. Conceptual Framework—Workflow Digitization vs. Workflow Integration
4.2. Data Integration and Dataset Interfaces
4.3. Software Interoperability and System Ecosystems
4.4. Human–Machine Interaction and Workflow Feedback Loops
4.5. Future Directions—AI and Robotics as Integrative Enablers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CAIS | Computer-Assisted Implant Surgery |
| CBCT | Cone-Beam Computed Tomography |
| CAD | Computer-Aided Design |
| CAM | Computer-Aided Manufacturing |
| D-CAIS | Dynamic Computer-Assisted Implant Surgery |
| DSD | Digital Smile Design |
| DICOM | Digital Imaging and Communications in Medicine |
| FH | Free-hand |
| FG | Fully Guided |
| IOS | Intraoral Scanner/Intraoral Scanning |
| ML | Machine Learning |
| OBJ | Object File Format |
| PG | Partially Guided |
| PLY | Polygon File Format |
| S-CAIS | Static Computer-Assisted Implant Surgery |
| STL | Standard Tessellation Language |
| VA | Virtual Articulator |
References
- Moörmann, W.H. The Evolution of the CEREC System. J. Am. Dent. Assoc. 2006, 137, 7S–13S. [Google Scholar] [CrossRef]
- Tran, D.; Nesbit, M.; Petridis, H. Survey of UK Dentists Regarding the Use of CAD/CAM Technology. Br. Dent. J. 2016, 221, 639–644. [Google Scholar] [CrossRef]
- Blackwell, E.; Nesbit, M.; Petridis, H. Survey on the Use of CAD-CAM Technology by UK and Irish Dental Technicians. Br. Dent. J. 2017, 222, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassiny, A.; Végh, D.; Bányai, D.; Végh, Á.; Géczi, Z.; Borbély, J.; Hermann, P.; Hegedüs, T. User Experience of Intraoral Scanners in Dentistry: Transnational Questionnaire Study. Int. Dent. J. 2023, 73, 754–759. [Google Scholar] [CrossRef]
- Joda, T.; Zarone, F.; Ferrari, M. The Complete Digital Workflow in Fixed Prosthodontics: A Systematic Review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Yu, J.; Lu, J.; Yan, Z. Comparisons between Digital-Guided and Non-Digital Protocol in Implant Planning, Placement, and Restorations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Evid. Based Dent. Pr. 2023, 23, 101919. [Google Scholar] [CrossRef] [PubMed]
- Scolozzi, P.; Michelini, F.; Crottaz, C.; Perez, A. Computer-Aided Design and Computer-Aided Modeling (CAD/CAM) for Guiding Dental Implant Surgery: Personal Reflection Based on 10 Years of Real-Life Experience. J. Pers. Med. 2023, 13, 129. [Google Scholar] [CrossRef]
- Romandini, M.; Ruales-Carrera, E.; Sadilina, S.; Hämmerle, C.H.F.; Sanz, M. Minimal Invasiveness at Dental Implant Placement: A Systematic Review with Meta-analyses on Flapless Fully Guided Surgery. Periodontol. 2000 2023, 91, 89–112. [Google Scholar] [CrossRef]
- Raico Gallardo, Y.N.; Da Silva-Olivio, I.R.T.; Mukai, E.; Morimoto, S.; Sesma, N.; Cordaro, L. Accuracy Comparison of Guided Surgery for Dental Implants According to the Tissue of Support: A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2017, 28, 602–612. [Google Scholar] [CrossRef]
- Chen, P.; Nikoyan, L. Guided Implant Surgery: A Technique Whose Time Has Come. Dent. Clin. N. Am. 2021, 65, 67–80. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The Accuracy of Static Computer-Aided Implant Surgery: A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A Systematic Review on the Accuracy and the Clinical Outcome of Computer-Guided Template-Based Implant Dentistry. Clin. Oral Implant. Res. 2009, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Laleman, I.; Jacobs, R.; Quirynen, M. Computer-supported Implant Planning and Guided Surgery: A Narrative Review. Clin. Oral Implant. Res. 2015, 26, 69–76. [Google Scholar] [CrossRef]
- Nkenke, E.; Eitner, S.; Radespiel-Tröger, M.; Vairaktaris, E.; Neukam, F.W.; Fenner, M. Patient-centred Outcomes Comparing Transmucosal Implant Placement with an Open Approach in the Maxilla: A Prospective, Non-randomized Pilot Study. Clin. Oral Implant. Res. 2007, 18, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Fortin, T.; Bosson, J.L.; Isidori, M.; Blanchet, E. Effect of Flapless Surgery on Pain Experienced in Implant Placement Using an Image-Guided System. Int. J. Oral Maxillofac. Implant. 2006, 21, 298–304. [Google Scholar]
- Gargallo-Albiol, J.; Barootchi, S.; Salomó-Coll, O.; Wang, H. Advantages and Disadvantages of Implant Navigation Surgery. A Systematic Review. Ann. Anat.—Anat. Anz. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Rutkūnas, V.; Auškalnis, L.; Pletkus, J. Intraoral Scanners in Implant Prosthodontics. A Narrative Review. J. Dent. 2024, 148, 105152. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Vazouras, K.; Chen, Y.; Kotina, E.; Natto, Z.; Kang, K.; Chochlidakis, K. Digital vs Conventional Implant Impressions: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 660–678. [Google Scholar] [CrossRef]
- Michelinakis, G.; Apostolakis, D.; Kamposiora, P.; Papavasiliou, G.; Özcan, M. The Direct Digital Workflow in Fixed Implant Prosthodontics: A Narrative Review. BMC Oral Health 2021, 21, 37–61. [Google Scholar] [CrossRef]
- Lee, S.J.; Gallucci, G.O. Digital vs. Conventional Implant Impressions: Efficiency Outcomes. Clin. Oral Implant. Res. 2013, 24, 111–115. [Google Scholar] [CrossRef]
- Delize, V.; Bouhy, A.; Lambert, F.; Lamy, M. Intrasubject Comparison of Digital vs. Conventional Workflow for Screw-retained Single-implant Crowns: Prosthodontic and Patient-centered Outcomes. Clin. Oral Implant. Res. 2019, 30, 892–902. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, P.; Manicone, P.F.; De Angelis, S.; Grippaudo, C.; Gasparini, G.; Liguori, M.G.; Camodeca, F.; Piccirillo, G.B.; Desantis, V.; D’Amato, G.; et al. Patient and Operator Centered Outcomes in Implant Dentistry: Comparison between Fully Digital and Conventional Workflow for Single Crown and Three-Unit Fixed-Bridge. Materials 2020, 13, 2781. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Brägger, U. Time-Efficiency Analysis Comparing Digital and Conventional Workflows for Implant Crowns: A Prospective Clinical Crossover Trial. Int. J. Oral Maxillofac. Implant. 2015, 30, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Arcuri, L.; Moy, P.K. The Smiling Scan Technique: Facially Driven Guided Surgery and Prosthetics. J. Prosthodont. Res. 2018, 62, 514–517. [Google Scholar] [CrossRef]
- Pozzi, A.; Arcuri, L.; Carosi, P.; Laureti, A.; Londono, J.; Wang, H. Photogrammetry Versus Intraoral Scanning in Complete-Arch Digital Implant Impression: A Systematic Review and Meta-Analysis. Clin. Implant Dent. Relat. Res. 2025, 27, e70059. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Chiche, G.; Bahat, O.; Roblee, R.; Coachman, C.; Heymann, H.O. Evolution of Aesthetic Dentistry. J. Dent. Res. 2019, 98, 1294–1304. [Google Scholar] [CrossRef]
- Alves Marlière, D.; Demétrio, M.; Picinini, L.; De Oliveira, R.; Chaves Netto, H.D.M. Accuracy of Computer-Guided Surgery for Dental Implant Placement in Fully Edentulous Patients: A Systematic Review. Eur. J. Dent. 2018, 12, 153. [Google Scholar] [CrossRef]
- Putra, R.H.; Yoda, N.; Astuti, E.R.; Sasaki, K. The Accuracy of Implant Placement with Computer-Guided Surgery in Partially Edentulous Patients and Possible Influencing Factors: A Systematic Review and Meta-Analysis. J. Prosthodont. Res. 2021, 66, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tatakis, D.N.; Chien, H.-H.; Parashis, A.O. Guided Implant Surgery Risks and Their Prevention. Periodontol. 2000 2019, 81, 194–208. [Google Scholar] [CrossRef]
- Fernández-Gil, Á.; Gil, H.; Velasco, M.; Vázquez, J. An In Vitro Model to Evaluate the Accuracy of Guided Implant Placement Based on the Surgeon’s Experience. Int. J. Oral Maxillofac. Implant. 2017, 32, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Kero, T.; Söderberg, R.; Näsström, K. Accuracy of Virtually Planned and CAD/CAM-Guided Implant Surgery on Plastic Models. J. Prosthet. Dent. 2014, 112, 1472–1478. [Google Scholar] [CrossRef]
- Sun, T.-M.; Lee, H.-E.; Lan, T.-H. The Influence of Dental Experience on a Dental Implant Navigation System. BMC Oral Health 2019, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Kirlys, R.; Nedzinskaitė, R.; Rongo, R.; Severino, M.; Puisys, A.; D’Antò, V. Digital Planning Technique for Surgical Guides for Prosthetic Implants before Orthodontic Treatment. Appl. Sci. 2022, 12, 5566. [Google Scholar] [CrossRef]
- Gomes, P.; Karotkin, J.; Alfi, D.; Simmons, J.H.; Scheyer, E.T. Digital Workflow in Interdisciplinary Dentistry with an Airway Analysis: A Case Report with 2-Year Follow-Up. J. Prosthodont. 2025, 34, 80–89. [Google Scholar] [CrossRef]
- Bianchi, J.; Mendonca, G.; Gillot, M.; Oh, H.; Park, J.; Turkestani, N.A.; Gurgel, M.; Cevidanes, L. Three-Dimensional Digital Applications for Implant Space Planning in Orthodontics: A Narrative Review. J. World Fed. Orthod. 2022, 11, 207–215. [Google Scholar] [CrossRef]
- Coachman, C.; Sesma, N.; Blatz, M.B. The Complete Digital Workflow in Interdisciplinary Dentistry. Int. J. Esthet. Dent. 2021, 16, 34–49. [Google Scholar]
- Watanabe, H.; Fellows, C.; An, H. Digital Technologies for Restorative Dentistry. Dent. Clin. N. Am. 2022, 66, 567–590. [Google Scholar] [CrossRef]
- Shirani, M. Trends and Classification of Artificial Intelligence Models Utilized in Dentistry: A Bibliometric Study. Cureus 2025, 17, e81836. [Google Scholar] [CrossRef]
- Samaranayake, L.; Tuygunov, N.; Schwendicke, F.; Osathanon, T.; Khurshid, Z.; Boymuradov, S.A.; Cahyanto, A. The Transformative Role of Artificial Intelligence in Dentistry: A Comprehensive Overview. Part 1: Fundamentals of AI, and Its Contemporary Applications in Dentistry. Int. Dent. J. 2025, 75, 383–396. [Google Scholar] [CrossRef]
- Salvi, S.; Vu, G.; Gurupur, V.; King, C. Digital Convergence in Dental Informatics: A Structured Narrative Review of Artificial Intelligence, Internet of Things, Digital Twins, and Large Language Models with Security, Privacy, and Ethical Perspectives. Electronics 2025, 14, 3278. [Google Scholar] [CrossRef]
- Najeeb, M.; Islam, S. Artificial Intelligence (AI) in Restorative Dentistry: Current Trends and Future Prospects. BMC Oral Health 2025, 25, 592. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, Y.Y.; Luo, Y.L.; Wu, Y.Y.; Xiang, L.; Yang, X.M.; Qu, Y.L.; Tian, T.R.; Man, Y. Recent Advances in Digital Technology in Implant Dentistry. J. Dent. Res. 2024, 103, 787–799. [Google Scholar] [CrossRef]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 ITI Consensus Report: Digital Technologies. Clin. Oral Implant. Res. 2018, 29, 436–442. [Google Scholar] [CrossRef]
- Joda, T.; Gallucci, G.O. The Virtual Patient in Dental Medicine. Clin. Oral Implant. Res. 2015, 26, 725–726. [Google Scholar] [CrossRef]
- Chen, S.T.; Buser, D. Esthetic Outcomes Following Immediate and Early Implant Placement in the Anterior Maxilla-a Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The Long-Term Efficacy of Currently Used Dental Implants: A Review and Proposed Criteria of Success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant Success, Survival, and Failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.C.; Lang, N.P. Long-Term Evaluation of Non-Submerged ITI Implants. Part 1: 8-Year Life Table Analysis of a Prospective Multi-Center Study with 2359 Implants. Clin Oral Implant. Res. 1997, 8, 161–172. [Google Scholar] [CrossRef]
- Fortin, T.; Coudert, J.L.; Champleboux, G.; Sautot, P.; Lavallée, S. Computer-Assisted Dental Implant Surgery Using Computed Tomography. J. Image Guid. Surg. 1995, 1, 53–58. [Google Scholar] [CrossRef]
- Verstreken, K.; Van Cleynenbreugel, J.; Marchal, G.; Naert, I.; Suetens, P.; van Steenberghe, D. Computer-Assisted Planning of Oral Implant Surgery: A Three-Dimensional Approach. Int. J. Oral Maxillofac. Implant. 1996, 11, 806–810. [Google Scholar]
- Verstreken, K.; Van Cleynenbreugel, J.; Marchal, G.; van Steenberghe, D.; Suetens, P. Computer-Assisted Planning of Oral Implant Surgery. An Approach Using Virtual Reality. Stud. Health Technol. Inf. 1996, 29, 423–434. [Google Scholar]
- van Steenberghe, D.; Naert, I.; Andersson, M.; Brajnovic, I.; Van Cleynenbreugel, J.; Suetens, P. A Custom Template and Definitive Prosthesis Allowing Immediate Implant Loading in the Maxilla: A Clinical Report. Int. J. Oral Maxillofac. Implant. 2002, 17, 663–670. [Google Scholar]
- Vrielinck, L.; Politis, C.; Schepers, S.; Pauwels, M.; Naert, I. Image-Based Planning and Clinical Validation of Zygoma and Pterygoid Implant Placement in Patients with Severe Bone Atrophy Using Customized Drill Guides. Preliminary Results from a Prospective Clinical Follow-up Study. Int. J. Oral Maxillofac. Surg. 2003, 32, 7–14. [Google Scholar] [CrossRef]
- Araujo-Corchado, E.; Pardal-Peláez, B. Computer-Guided Surgery for Dental Implant Placement: A Systematic Review. Prosthesis 2022, 4, 540–553. [Google Scholar] [CrossRef]
- Nomiyama, L.M.; Matumoto, E.K.; Corrêa, M.G.; Cirano, F.R.; Ribeiro, F.V.; Pimentel, S.P.; Casati, M.Z. Comparison between Flapless-Guided and Conventional Surgery for Implant Placement: A 12-Month Randomized Clinical Trial. Clin. Oral Investig. 2023, 27, 1665–1679. [Google Scholar] [CrossRef]
- Oliveira Dos Santos, T.; Lidani, R.; Pauletto, P.; Sabatini, G.; Awais, R.; Dutra, V.; Mezzomo, L.A. Accuracy of Static Computer-Assisted Implant Surgery in Full- Arches: A Systematic Review of Clinical Studies with Meta- Analysis. Int. J. Oral Maxillofac. Implant. 2025, 0, 1–35. [Google Scholar] [CrossRef]
- Laleman, I.; Bernard, L.; Vercruyssen, M.; Jacobs, R.; Bornstein, M.M.; Quirynen, M. Guided Implant Surgery in the Edentulous Maxilla: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, s103–s117. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer Technology Applications in Surgical Implant Dentistry: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef]
- Castro, F.; Pereira, P.; Falcão-Costa, C.; Falcão, A.; Fernandes, J.C.H.; Fernandes, G.V.O.; Rios, J.-V. Comparison of the Accuracy/Precision among Guided (Static), Manual, and Dynamic Navigation in Dental Implant Surgery: A Systematic Review and Meta-Analysis. Oral Maxillofac. Surg. 2025, 29, 170. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Mohanty, A.K.; Raut, A.; Pal, D.; Mahato, M.; Jain, A.; Sarkar, D.F. Comparison of Accuracy of Dental Implant Placement Between Freehand and Guided Implant Surgery-A Systematic Review and Meta-Analysis. J. Maxillofac. Oral Surg. 2025, 24, 865–876. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Barootchi, S.; Marqués-Guasch, J.; Wang, H.-L. Fully Guided Versus Half-Guided and Freehand Implant Placement: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 1159–1169. [Google Scholar] [CrossRef]
- Jorba-García, A.; González-Barnadas, A.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy Assessment of Dynamic Computer–Aided Implant Placement: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 2479–2494. [Google Scholar] [CrossRef] [PubMed]
- Matvijenko, K.; Borusevičius, R. Comparison of Dynamic Navigation Systems in Dental Implantology: A Systematic Literature Review of in Vitro Studies. Int. J. Oral Maxillofac. Surg. 2025, 54, 647–656. [Google Scholar] [CrossRef]
- Chen, S.T.; Buser, D.; Sculean, A.; Belser, U.C. Complications and Treatment Errors in Implant Positioning in the Aesthetic Zone: Diagnosis and Possible Solutions. Periodontol. 2000 2023, 92, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.; Joda, T.; Weber, H.; Brägger, U. Screw Retained vs. Cement Retained Implant-supported Fixed Dental Prosthesis. Periodontol. 2000 2017, 73, 141–151. [Google Scholar] [CrossRef]
- Morton, D.; Gallucci, G.; Lin, W.-S.; Pjetursson, B.; Polido, W.; Roehling, S.; Sailer, I.; Aghaloo, T.; Albera, H.; Bohner, L.; et al. Group 2 ITI Consensus Report: Prosthodontics and Implant Dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 215–223. [Google Scholar] [CrossRef]
- Orentlicher, G.; Horowitz, A.; Kobren, L. Computer-Guided Dental Implant Treatment of Complete Arch Restoration of Edentulous and Terminal Dentition Patients. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 399–426. [Google Scholar] [CrossRef]
- Joda, T.; Derksen, W.; Wittneben, J.G.; Kuehl, S. Static Computer-aided Implant Surgery (s-CAIS) Analysing Patient-reported Outcome Measures (PROMs), Economics and Surgical Complications: A Systematic Review. Clin. Oral Implant. Res. 2018, 29, 359–373. [Google Scholar] [CrossRef]
- Lau, K.; Fung, T.K.C.; Ho, D.K.L.; Pelekos, G.; Fok, M.R. Accuracy of Static and Dynamic Computer-Aided Implant Surgery for Immediate Implant Placement: A Systematic Review and Meta-Analysis. J. Prosthodont. Res. 2025. [Google Scholar] [CrossRef]
- Shirani, M.; Emami, M. Comparison Between Computer-Assisted and Free-Hand Dental Implant Placement: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2025, 0, 1–26. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, C.Z.; Ozdemir, T. Implant Surgery Using Bone- and Mucosa-Supported Stereolithographic Guides in Totally Edentulous Jaws: Surgical and Post-Operative Outcomes of Computer-Aided vs. Standard Techniques. Clin. Oral Implant. Res. 2010, 21, 980–988. [Google Scholar] [CrossRef]
- García-Valdez, D.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; López-López, J.; Núñez-Márquez, E.; Matos-Garrido, N.; Jiménez-Guerra, Á.; Moreno-Muñoz, J.; Rondón-Romero, J.L. Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review. J. Clin. Med. 2025, 14, 6638. [Google Scholar] [CrossRef]
- Cristache, C.M.; Burlibasa, M.; Tudor, I.; Totu, E.E.; Di Francesco, F.; Moraru, L. Accuracy, Labor-Time and Patient-Reported Outcomes with Partially versus Fully Digital Workflow for Flapless Guided Dental Implants Insertion—A Randomized Clinical Trial with One-Year Follow-Up. J. Clin. Med. 2021, 10, 1102. [Google Scholar] [CrossRef]
- Sadilina, S.; Vietor, K.; Doliveux, R.; Siu, A.; Chen, Z.; Al-Nawas, B.; Mattheos, N.; Pozzi, A. Beyond Accuracy: Clinical Outcomes of Computer Assisted Implant Surgery. Clin. Exp. Dent. Res. 2025, 11, e70129. [Google Scholar] [CrossRef]
- Graf, T.; Keul, C.; Wismeijer, D.; Güth, J.F. Time and Costs Related to Computer-assisted versus Non-computer-assisted Implant Planning and Surgery. A Systematic Review. Clin. Oral Implant. Res. 2021, 32, 303–317. [Google Scholar] [CrossRef]
- Younis, H.; Lv, C.; Xu, B.; Zhou, H.; Du, L.; Liao, L.; Zhao, N.; Long, W.; Elayah, S.A.; Chang, X.; et al. Accuracy of Dynamic Navigation Compared to Static Surgical Guides and the Freehand Approach in Implant Placement: A Prospective Clinical Study. Head Face Med. 2024, 20, 30. [Google Scholar] [CrossRef]
- Khaohoen, A.; Powcharoen, W.; Sornsuwan, T.; Chaijareenont, P.; Rungsiyakull, C.; Rungsiyakull, P. Accuracy of Implant Placement with Computer-Aided Static, Dynamic, and Robot-Assisted Surgery: A Systematic Review and Meta-Analysis of Clinical Trials. BMC Oral Health 2024, 24, 359. [Google Scholar] [CrossRef]
- Werny, J.G.; Frank, K.; Fan, S.; Sagheb, K.; Al-Nawas, B.; Narh, C.T.; Schiegnitz, E. Freehand vs. Computer-Aided Implant Surgery: A Systematic Review and Meta-Analysis—Part 1: Accuracy of Planned and Placed Implant Position. Int. J. Implant Dent. 2025, 11, 35. [Google Scholar] [CrossRef]
- Kang, S.; Hou, Y.; Cao, J.; Li, S.; Xue, P.; Jiang, Y. Comparison of Implantation Accuracy Among Different Navigated Approaches: A Systematic Review and Network Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2024, 39, 455–467. [Google Scholar] [CrossRef]
- Marques-Guasch, J.; Bofarull-Ballús, A.; Giralt-Hernando, M.; Hernández-Alfaro, F.; Gargallo-Albiol, J. Dynamic Implant Surgery—An Accurate Alternative to Stereolithographic Guides—Systematic Review and Meta-Analysis. Dent. J. 2023, 11, 150. [Google Scholar] [CrossRef]
- Tang, W.L.; Chao, X.Y.; Ye, Z.; Liu, M.W.; Jiang, H. The Use of Dynamic Navigation Systems as a Component of Digital Dentistry. J. Dent. Res. 2024, 103, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Esposito, M.; Xhanari, E.; Caneva, M.; Meloni, S.M. Computer-Guided vs Freehand Placement of Immediately Loaded Dental Implants: 5-Year Post- Loading Results of a Randomised Controlled Trial. Eur. J. Oral Implant. 2018, 11, 203–213. [Google Scholar]
- Aghaloo, T.; Hadaya, D.; Schoenbaum, T.R.; Pratt, L.; Favagehi, M. Guided and Navigation Implant Surgery: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2023, 38, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Ferri, A.; Del Fabbro, M.; Prati, C.; Gandolfi, M.G.; Marchetti, C. Dynamic Navigation in Implant Dentistry: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2021, 36, e121–e140. [Google Scholar] [CrossRef]
- Yue, Z.; Luo, Z.; Hou, J.; Zhang, H. Application of 3D Digital Smile Design Based on Virtual Articulation Analysis in Esthetic Dentistry: A Technique. J. Prosthet. Dent. 2025, 133, 24–30. [Google Scholar] [CrossRef]
- Pomares-Puig, C.; Sánchez-Garcés, M.A.; Jorba-García, A. Dynamic and Static Computer-Guided Surgery Using the Double-Factor Technique for Completely Edentulous Patients: A Dental Technique. J. Prosthet. Dent. 2022, 128, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Pomares-Puig, C.; Sánchez-Garcés, M.A.; Jorba-García, A. Dynamic and Static Computer-Assisted Implant Surgery for Completely Edentulous Patients. A Proof of a Concept. J. Dent. 2023, 130, 104443. [Google Scholar] [CrossRef]
- Tattan, M.; Chambrone, L.; González-Martín, O.; Avila-Ortiz, G. Static Computer-aided, Partially Guided, and Free-handed Implant Placement: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Oral Implant. Res. 2020, 31, 889–916. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhu, Y.; Wei, J.; Zhang, C.; Shi, J.; Lai, H. Accuracy of Dynamic Navigation in Implant Surgery: A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2021, 32, 383–393. [Google Scholar] [CrossRef]
- Siqueira, R.; Chen, Z.; Galli, M.; Saleh, I.; Wang, H.; Chan, H. Does a Fully Digital Workflow Improve the Accuracy of Computer-assisted Implant Surgery in Partially Edentulous Patients? A Systematic Review of Clinical Trials. Clin. Implant Dent. Relat. Res. 2020, 22, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Balaguer-Martí, J.C.; Canet-López, Á.; Peñarrocha-Diago, M.; Romeo-Rubio, M.; Peñarrocha-Diago, M.; García-Mira, B. Influence of Splint Support on the Precision of Static Totally Guided Dental Implant Surgery: A Systematic Review and Network Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2023, 38, 157–168. [Google Scholar] [CrossRef]
- Derksen, W.; Wismeijer, D.; Flügge, T.; Hassan, B.; Tahmaseb, A. The Accuracy of Computer-guided Implant Surgery with Tooth-supported, Digitally Designed Drill Guides Based on CBCT and Intraoral Scanning. A Prospective Cohort Study. Clin. Oral Implant. Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef]
- Geng, W.; Liu, C.; Su, Y.; Li, J.; Zhou, Y. Accuracy of Different Types of Computer-Aided Design/Computer-Aided Manufacturing Surgical Guides for Dental Implant Placement. J. Oral Maxillofac. Surg. 2009, 67, 394–401. [Google Scholar]
- Ozan, O.; Turkyilmaz, I.; Ersoy, A.E.; McGlumphy, E.A.; Rosenstiel, S.F. Clinical Accuracy of 3 Different Types of Computed Tomography-Derived Stereolithographic Surgical Guides in Implant Placement. J. Oral Maxillofac. Surg. 2009, 67, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, J.; Ma, C.; Shen, J.; Dong, X.; Lin, D. A Systematic Review of the Accuracy of Digital Surgical Guides for Dental Implantation. Int. J. Implant Dent. 2023, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Z.; Song, L.; Kuo, C.-L.; Shafer, D.M. Clinical Factors Affecting the Accuracy of Guided Implant Surgery-A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pr. 2018, 18, 28–40. [Google Scholar] [CrossRef]
- Mangano, C.; Luongo, F.; Migliario, M.; Mortellaro, C.; Mangano, F.G. Combining Intraoral Scans, Cone Beam Computed Tomography and Face Scans: The Virtual Patient. J. Craniofacial Surg. 2018, 29, 2241–2246. [Google Scholar] [CrossRef]
- Schubert, O.; Schweiger, J.; Stimmelmayr, M.; Nold, E.; Güth, J.-F. Digital Implant Planning and Guided Implant Surgery—Workflow and Reliability. Br. Dent. J. 2019, 226, 101–108. [Google Scholar] [CrossRef]
- Cui, X.; Reason, T.; Pardi, V.; Wu, Q.; Martinez Luna, A.A. CBCT Analysis of Crestal Soft Tissue Thickness before Implant Placement and Its Relationship with Cortical Bone Thickness. BMC Oral Health 2022, 22, 593. [Google Scholar] [CrossRef]
- Carosi, P.; Ferrigno, N.; De Renzi, G.; Laureti, M. Digital Workflow to Merge an Intraoral Scan and CBCT of Edentulous Maxilla: A Technical Report. J. Prosthodont. 2020, 29, 730–732. [Google Scholar] [CrossRef]
- Lanis, A.; Alvarez del Canto, O. The Combination of Digital Surface Scanners and Cone Beam Computed Tomography Technology for Guided Implant Surgery Using 3Shape Implant Studio Software: A Case History Report. Int. J. Prosthodont. 2015, 28, 169–178. [Google Scholar] [CrossRef]
- Yeo, X.H.; Uei, L.J.; Yi, M.; Kungsadalpipob, K.; Subbalehka, K.; Al-Nawas, B.; Mattheos, N. Computer-Assisted Implant Surgery: Patients’ Experience and Perspectives. Clin. Exp. Dent. Res. 2025, 11, e70143. [Google Scholar] [CrossRef] [PubMed]
- Kafedzhieva, A.; Vlahova, A.; Chuchulska, B. Digital Technologies in Implantology: A Narrative Review. Bioengineering 2025, 12, 927. [Google Scholar] [CrossRef]
- Amin, S.A.; Hann, S.; Elsheikh, A.K.; Boltchi, F.; Zandinejad, A. A Complete Digital Approach for Facially Generated Full Arch Diagnostic Wax up, Guided Surgery, and Implant-supported Interim Prosthesis by Integrating 3D Facial Scanning, Intraoral Scan and CBCT. J. Prosthodont. 2023, 32, 90–93. [Google Scholar] [CrossRef]
- Joda, T.; Gallucci, G.O.; Wismeijer, D.; Zitzmann, N.U. Augmented and Virtual Reality in Dental Medicine: A Systematic Review. Comput. Biol. Med. 2019, 108, 93–100. [Google Scholar] [CrossRef]
- Shah, N.; Thakur, M.; Gill, S.; Shetty, O.; Alqahtani, N.M.; Al-Qarni, M.A.; Alqahtani, S.M.; Elagib, M.F.A.; Chaturvedi, S. Validation of Digital Impressions’ Accuracy Obtained Using Intraoral and Extraoral Scanners: A Systematic Review. J. Clin. Med. 2023, 12, 5833. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Arcuri, L.; Block, M.S.; Moy, P.K. Digital Assisted Soft Tissue Sculpturing (DASS) Technique for Immediate Loading Pink Free Complete Arch Implant Prosthesis. J. Prosthodont. Res. 2021, 65, 119–124. [Google Scholar] [CrossRef]
- Sobczak, B.; Majewski, P. An Integrated Fully Digital Prosthetic Workflow for the Immediate Full-Arch Restoration of Edentulous Patients—A Case Report. Int. J. Environ. Res. Public Health 2022, 19, 4126–4137. [Google Scholar] [CrossRef]
- Makarov, N.; Pompa, G.; Papi, P. Computer-Assisted Implant Placement and Full-Arch Immediate Loading with Digitally Prefabricated Provisional Prostheses without Cast: A Prospective Pilot Cohort Study. Int. J. Implant Dent. 2021, 7, 80. [Google Scholar] [CrossRef]
- Gan, N.; Xiong, Y.; Jiao, T. Accuracy of Intraoral Digital Impressions for Whole Upper Jaws, Including Full Dentitions and Palatal Soft Tissues. PLoS ONE 2016, 11, e0158800. [Google Scholar] [CrossRef] [PubMed]
- Wulfman, C.; Naveau, A.; Rignon-Bret, C. Digital Scanning for Complete-Arch Implant-Supported Restorations: A Systematic Review. J. Prosthet. Dent. 2020, 124, 161–167. [Google Scholar] [CrossRef]
- Pozzi, A.; Carosi, P.; Laureti, A.; Mattheos, N.; Pimkhaokham, A.; Chow, J.; Arcuri, L. Accuracy of Navigation Guided Implant Surgery for Immediate Loading Complete Arch Restorations: Prospective Clinical Trial. Clin. Implant Dent. Relat. Res. 2024, 26, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Hansson, L.; Carosi, P.; Arcuri, L. Dynamic Navigation Guided Surgery and Prosthetics for Immediate Loading of Complete-arch Restoration. J. Esthet. Restor. Dent. 2021, 33, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Pariente, L.; Dada, K.; Linder, S.; Dard, M. Immediate Implant Placement in the Esthetic Zone Using a Novel Tapered Implant Design and a Digital Integrated Workflow: A Case Series. Int. J. Periodontics Restor. Dent. 2023, 43, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, B.; Majewski, P.; Egorenkov, E. Survival and Success of 3D-Printed Versus Milled Immediate Provisional Full-Arch Restorations: A Retrospective Analysis. Clin. Implant Dent. Relat. Res. 2025, 27, e13418. [Google Scholar] [CrossRef]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef]
- Wittneben, J.; Wismeijer, D.; Brägger, U.; Joda, T.; Abou-Ayash, S. Patient-reported Outcome Measures Focusing on Aesthetics of Implant- and Tooth-supported Fixed Dental Prostheses: A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 224–240. [Google Scholar] [CrossRef]
- De Bruyn, H.; Raes, S.; Matthys, C.; Cosyn, J. The Current Use of Patient-Centered/Reported Outcomes in Implant Dentistry: A Systematic Review. Clin. Oral Implant. Res. 2015, 26, 45–56. [Google Scholar] [CrossRef]
- Alharkan, H.M. Integrating Digital Smile Design into Restorative Dentistry: A Narrative Review of the Applications and Benefits. Saudi Dent. J. 2024, 36, 561–567. [Google Scholar] [CrossRef]
- Jain, A.; Bhushan, P.; Mahato, M.; Solanki, B.B.; Dutta, D.; Hota, S.; Raut, A.; Mohanty, A.K. The Recent Use, Patient Satisfaction, and Advancement in Digital Smile Designing: A Systematic Review. Cureus 2024, 16, e62459. [Google Scholar] [CrossRef]
- Thomas, P.A.; Krishnamoorthi, D.; Mohan, J.; Raju, R.; Rajajayam, S.; Venkatesan, S. Digital Smile Design. J. Pharm. Bioallied Sci. 2022, 14, S43–S49. [Google Scholar] [CrossRef] [PubMed]
- Luniyal, C.; Shukla, A.K.; Priyadarshi, M.; Ahmed, F.; Kumari, N.; Bankoti, P.; Makkad, R.S. Assessment of Patient Satisfaction and Treatment Outcomes in Digital Smile Design vs. Conventional Smile Design: A Randomized Controlled Trial. J. Pharm. Bioallied Sci. 2024, 16, S669–S671. [Google Scholar] [CrossRef]
- Ackerman, M.B.; Ackerman, J.L. Smile Analysis and Design in the Digital Era. J. Clin. Orthod. 2002, 36, 221–236. [Google Scholar] [PubMed]
- Coachman, C.; Paravina, R.D. Digitally Enhanced Esthetic Dentistry—From Treatment Planning to Quality Control. J. Esthet. Restor. Dent. 2016, 28, S3–S4. [Google Scholar] [CrossRef]
- Coachman, C.; Georg, R.; Bohner, L.; Rigo, L.C.; Sesma, N. Chairside 3D Digital Design and Trial Restoration Workflow. J. Prosthet. Dent. 2020, 124, 514–520. [Google Scholar] [CrossRef]
- Bassett, J.; Kois, J.C. Creating a Hybrid Smile Design Workflow: The Analog Brain Drives the Digital Technology. J. Esthet. Restor. Dent. 2023, 35, 773–786. [Google Scholar] [CrossRef]
- Alikhasi, M.; Yousefi, P.; Afrashtehfar, K.I. Smile Design. Dent. Clin. N. Am. 2022, 66, 477–487. [Google Scholar] [CrossRef]
- Santi, M.R.; Nastri, V.H.T.; Lins, R.B.E. Advanced Digital Planning Approach Using a 3D-Printed Mock-up. A Case Report. Int. J. Esthet. Dent. 2024, 19, 186–194. [Google Scholar]
- Salama, M.A.; Pozzi, A.; Clark, W.A.; Tadros, M.; Hansson, L.; Adar, P. The “Scalloped Guide”: A Proof-of-Concept Technique for a Digitally Streamlined, Pink-Free Full-Arch Implant Protocol. Int. J. Periodontics Restor. Dent. 2018, 38, 791–798. [Google Scholar] [CrossRef]
- Bessadet, M.; Auduc, C.; Drancourt, N.; Nicolas, E.; El Osta, N. Comparative Analyses of Time Efficiency and Cost in Fabricating Fixed Implant-Supported Prostheses in Digital, Hybrid, and Conventional Workflows: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2025, 133, 689–712. [Google Scholar] [CrossRef]
- Corsalini, M.; Barile, G.; Ranieri, F.; Morea, E.; Corsalini, T.; Capodiferro, S.; Palumbo, R.R. Comparison between Conventional and Digital Workflow in Implant Prosthetic Rehabilitation: A Randomized Controlled Trial. J. Funct. Biomater. 2024, 15, 149. [Google Scholar] [CrossRef]
- Hashemi, A.M.; Hashemi, H.M.; Siadat, H.; Shamshiri, A.; Afrashtehfar, K.I.; Alikhasi, M. Fully Digital versus Conventional Workflows for Fabricating Posterior Three-Unit Implant-Supported Reconstructions: A Prospective Crossover Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 11456. [Google Scholar] [CrossRef]
- Joda, T.; Katsoulis, J.; Brägger, U. Clinical Fitting and Adjustment Time for Implant-Supported Crowns Comparing Digital and Conventional Workflows. Clin. Implant Dent. Relat. Res. 2016, 18, 946–954. [Google Scholar] [CrossRef]
- Lepidi, L.; Galli, M.; Mastrangelo, F.; Venezia, P.; Joda, T.; Wang, H.; Li, J. Virtual Articulators and Virtual Mounting Procedures: Where Do We Stand? J. Prosthodont. 2021, 30, 24–35. [Google Scholar] [CrossRef]
- Chirimar, V.; Trivedi, A.; Saxena, D. Virtual Articulator and Virtual Facebow an Aid in Complete Digital Workflow: A Review Article. IP Ann. Prosthodont. Restor. Dent. 2024, 10, 173–178. [Google Scholar] [CrossRef]
- Hsu, M.R.; Driscoll, C.F.; Romberg, E.; Masri, R. Accuracy of Dynamic Virtual Articulation: Trueness and Precision. J. Prosthodont. 2019, 28, 436–443. [Google Scholar] [CrossRef]
- Al Hamad, K.Q.; Ayyad, J.Q.; Al-Rashdan, B.A.; Al Quran, F.A. Trueness and Precision of Facial Scan and Virtual Patient Representation Workflow. J. Prosthodont. 2025, 34, 703–711. [Google Scholar] [CrossRef]
- Doshi, K.N.; Sathe, S.; Dubey, S.A.; Bhoyar, A.; Dhamande, M.; Jaiswal, T. A Comprehensive Review on Virtual Articulators. Cureus 2024, 16, e52554. [Google Scholar] [CrossRef]
- Lin, H.; Pan, Y.; Wei, X.; Wang, Y.; Yu, H.; Cheng, H. Comparison of the Performance of Various Virtual Articulator Mounting Procedures: A Self-Controlled Clinical Study. Clin. Oral Investig. 2023, 27, 4017–4028. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Scialabba, R.; Lee, J.D.; Sun, J.; Lee, S.J. Assessment of Occlusal Vertical Dimension Change in Mechanical and Virtual Articulation: A Pilot Study. Dent. J. 2022, 10, 212. [Google Scholar] [CrossRef]
- Merli, M.; Aquilanti, L.; Casucci, A.; Nieri, M.; Mariotti, G.; Merli, M.; Rappelli, G. A Personalized 4D Workflow for the Manufacturing of Functional and Removable Esthetic Devices: A Technical Report. J. Esthet. Restor. Dent. 2025, 37, 1645–1654. [Google Scholar] [CrossRef]
- Elgarba, B.M.; Fontenele, R.C.; Tarce, M.; Jacobs, R. Artificial Intelligence Serving Pre-Surgical Digital Implant Planning: A Scoping Review. J. Dent. 2024, 143, 104862. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Krastl, G.; Hecker, H.; Walter, C.; Waltimo, T.; Weiger, R. Strategic Considerations in Treatment Planning: Deciding When to Treat, Extract, or Replace a Questionable Tooth. J. Prosthet. Dent. 2010, 104, 80–91. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Lang, N.P. Prosthetic Treatment Planning on the Basis of Scientific Evidence. J. Oral Rehabil. 2008, 35, 72–79. [Google Scholar] [CrossRef]
- France, K.; Sollecito, T.P. How Evidence-Based Dentistry Has Shaped the Practice of Oral Medicine. Dent. Clin. N. Am. 2019, 63, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Auduc, C.; Douillard, T.; Nicolas, E.; El Osta, N. Fully Digital Workflow in Full-Arch Implant Rehabilitation: A Descriptive Methodological Review. Prosthesis 2025, 7, 85. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, Y.; Gonzalez-Gusmao, I.; Att, W. Complete Digital Workflow in Prosthesis Prototype Fabrication for Complete-Arch Implant Rehabilitation: A Technique. J. Prosthet. Dent. 2019, 122, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Flügge, T.; Derksen, W.; Te Poel, J.; Hassan, B.; Nelson, K.; Wismeijer, D. Registration of Cone Beam Computed Tomography Data and Intraoral Surface Scans—A Prerequisite for Guided Implant Surgery with CAD/CAM Drilling Guides. Clin. Oral Implant. Res. 2017, 28, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Marquez Bautista, N.; Meniz-García, C.; López-Carriches, C.; Sánchez-Labrador, L.; Cortés-Bretón Brinkmann, J.; Madrigal Martínez-Pereda, C. Accuracy of Different Systems of Guided Implant Surgery and Methods for Quantification: A Systematic Review. Appl. Sci. 2024, 14, 11479. [Google Scholar] [CrossRef]
- Biun, J.; Dudhia, R.; Arora, H. The In-vitro Accuracy of Fiducial Marker-based versus Markerless Registration of an Intraoral Scan with a Cone-beam Computed Tomography Scan in the Presence of Restoration Artifact. Clin. Oral Implant. Res. 2023, 34, 1257–1266. [Google Scholar] [CrossRef]
- Preda, F.; Nogueira-Reis, F.; Stanciu, E.M.; Smolders, A.; Jacobs, R.; Shaheen, E. Validation of Automated Registration of Intraoral Scan onto Cone Beam Computed Tomography for an Efficient Digital Dental Workflow. J. Dent. 2024, 149, 105282. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Romero, V.; Jorba-Garcia, A.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy of Dynamic Computer-Assisted Implant Surgery in Fully Edentulous Patients: An in Vitro Study. J. Dent. 2024, 149, 105290. [Google Scholar] [CrossRef]
- Woo, H.-W.; Mai, H.-N.; Lee, D.-H. Comparison of the Accuracy of Image Registration Methods for Merging Optical Scan and Radiographic Data in Edentulous Jaws. J. Prosthodont. 2020, 29, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P. The Passive Fit Concept-A Review of Methods to Achieve and Evaluate in Multiple Unit Implant Supported Screw Retained Prosthesis. J. Dent. Oral Sci. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Pan, Y.; Tsoi, J.K.H.; Lam, W.Y.H.; Zhao, K.; Pow, E.H.N. The Cumulative Effect of Error in the Digital Workflow for Complete-Arch Implant-Supported Frameworks: An in Vitro Study. Clin. Oral Implant. Res. 2022, 33, 886–899. [Google Scholar] [CrossRef]
- Rutkunas, V.; Dirse, J.; Kules, D.; Simonaitis, T. Misfit Simulation on Implant Prostheses with Different Combinations of Engaging and Nonengaging Titanium Bases. Part 1: Stereomicroscopic Assessment of the Active and Passive Fit. J. Prosthet. Dent. 2023, 129, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, J.; Takeichi, T.; Gaviria, A.S.; Peter, L.; Katsoulis, K. Misfit of Implant Prostheses and Its Impact on Clinical Outcomes. Definition, Assessment and a Systematic Review of the Literature. Eur. J. Oral Implantol. 2017, 10, 121–138. [Google Scholar]
- Shely, A.; Nissan, J.; Rosner, O.; Zenziper, E.; Lugassy, D.; Abidulkrem, K.; Ben-Izhack, G. The Impact of Open versus Closed Computer-Aided Design/Computer-Aided Manufacturing Systems on the Marginal Gap of Zirconia-Reinforced Lithium Silicate Single Crowns Evaluated by Scanning Electron Microscopy: A Comparative In Vitro Study. J. Funct. Biomater. 2024, 15, 130. [Google Scholar] [CrossRef]
- Flügge, T.; Kramer, J.; Nelson, K.; Nahles, S.; Kernen, F. Digital Implantology—A Review of Virtual Planning Software for Guided Implant Surgery. Part II: Prosthetic Set-up and Virtual Implant Planning. BMC Oral Health 2022, 22, 23. [Google Scholar] [CrossRef]
- Kernen, F.; Kramer, J.; Wanner, L.; Wismeijer, D.; Nelson, K.; Flügge, T. A Review of Virtual Planning Software for Guided Implant Surgery - Data Import and Visualization, Drill Guide Design and Manufacturing. BMC Oral Health 2020, 20, 251. [Google Scholar] [CrossRef]
- Dada, K.; Pariente, L.; Daas, M. Strategic Extraction Protocol: Use of an Image-Fusion Stereolithographic Guide for Immediate Implant Placement. J. Prosthet. Dent. 2016, 116, 652–656. [Google Scholar] [CrossRef]
- Koch, G.K.; Gallucci, G.O.; Lee, S.J. Accuracy in the Digital Workflow: From Data Acquisition to the Digitally Milled Cast. J. Prosthet. Dent. 2016, 115, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Rutkūnas, V.; Gečiauskaitė, A.; Jegelevičius, D.; Vaitiekūnas, M. Accuracy of Digital Implant Impressions with Intraoral Scanners. A Systematic Review. Eur. J. Oral Implant. 2017, 10 (Suppl. 1), 101–120. [Google Scholar]
- Roberts, M.; Shull, F.; Schiner, B. Maxillary Full-Arch Reconstruction Using a Sequenced Digital Workflow. J. Esthet. Restor. Dent. 2020, 32, 336–356. [Google Scholar] [CrossRef]
- Espona, J.; Roig, E.; Ali, A.; Roig, M. Immediately Loaded Interim Complete-Arch Implant-Supported Fixed Dental Prostheses Fabricated with a Completely Digital Workflow: A Clinical Technique. J. Prosthet. Dent. 2020, 124, 423–427. [Google Scholar] [CrossRef]
- Michelinakis, G.; Nikolidakis, D. Using the Surgical Guide for Impression-Free Digital Bite Registration in the Edentulous Maxilla—A Technical Note. Int. J. Implant Dent. 2019, 5, 19. [Google Scholar] [CrossRef]
- Moura, G.F.; Siqueira, R.; Meirelles, L.; Maska, B.; Wang, H.-L.; Mendonça, G. Denture Scanning Technique for Computer-Guided Implant-Supported Restoration Treatment of Edentulous Patients. J. Prosthet. Dent. 2020, 125, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Lepidi, L.; Galli, M.; Grammatica, A.; Joda, T.; Wang, H.-L.; Li, J. Indirect Digital Workflow for Virtual Cross-Mounting of Fixed Implant-Supported Prostheses to Create a 3D Virtual Patient. J. Prosthodont. 2021, 30, 177–182. [Google Scholar] [CrossRef]
- Chackartchi, T.; Romanos, G.E.; Parkanyi, L.; Schwarz, F.; Sculean, A. Reducing Errors in Guided Implant Surgery to Optimize Treatment Outcomes. Periodontol. 2000 2022, 88, 64–72. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, A.; Lombardi, T. Integration and Innovation in Digital Implantology—Part I: Capabilities and Limitations of Contemporary Workflows: A Narrative Review. Appl. Sci. 2025, 15, 12214. https://doi.org/10.3390/app152212214
Perez A, Lombardi T. Integration and Innovation in Digital Implantology—Part I: Capabilities and Limitations of Contemporary Workflows: A Narrative Review. Applied Sciences. 2025; 15(22):12214. https://doi.org/10.3390/app152212214
Chicago/Turabian StylePerez, Alexandre, and Tommaso Lombardi. 2025. "Integration and Innovation in Digital Implantology—Part I: Capabilities and Limitations of Contemporary Workflows: A Narrative Review" Applied Sciences 15, no. 22: 12214. https://doi.org/10.3390/app152212214
APA StylePerez, A., & Lombardi, T. (2025). Integration and Innovation in Digital Implantology—Part I: Capabilities and Limitations of Contemporary Workflows: A Narrative Review. Applied Sciences, 15(22), 12214. https://doi.org/10.3390/app152212214






