Featured Application
This study demonstrates that pulsed electric field (PEF) technology can be integrated into olive oil production to boost yield and enhance flavor. PEF was particularly effective for varieties with low yields often affected by climatic variability, genetic traits, and/or agronomic factors. As a sustainable, non-thermal complement to conventional extraction methods, PEF improves process efficiency and reduces production losses. By increasing the economic viability of traditional, low-yielding varieties that are otherwise at risk of replacement, the technology also contributes to the preservation of biodiversity. Furthermore, its modulation of volatile organic compounds (VOCs) indicates potential for tailoring the sensory attributes of virgin olive oils (VOOs). Overall, PEF represents a practical approach to improving efficiency, conserving biodiversity, and producing oils with superior sensory quality.
Abstract
This study investigated, for the first time, the effects of pulsed electric field (PEF) pretreatment—applied prior to malaxation at 2–7 kV/cm for 30–90—on oil yield, quality parameters, and volatile profiles of virgin olive oils (VOO) from four representative Croatian autochthonous varieties: Istarska Bjelica and Rosulja (Istria), and Levantinka and Oblica (Dalmatia). Mild PEF conditions significantly increased oil yield (2.5–36%), with the strongest effects observed in varieties with low baseline yields. Basic quality parameters were largely unaffected, but volatile organic compound (VOC) profiles were markedly altered. PEF increased VOCs from the lipoxygenase (LOX) pathway, particularly at lower field strengths, and altered their composition, suggesting changes in the activity of the enzymes involved in their biosynthesis. Among these enzymes, LOX was analyzed, and its activity responded to PEF treatment in a cultivar-dependent manner. Furthermore, PEF generally reduced minor VOCs associated with oxidation and microbial activity. These findings demonstrate the potential of PEF as a non-thermal, sustainable technology for improving oil extraction efficiency while simultaneously enhancing the aroma profile of VOOs.
1. Introduction
Pulsed electric field (PEF) is an emerging, non-thermal food processing technology that has gained significant attention in recent years. It involves subjecting food materials to repeated short pulses of high voltage between two electrodes, typically at room temperature and for a short period of time. This technique significantly reduces energy consumption compared to conventional thermal treatments, while minimizing undesirable changes in the sensory and physical properties of food. As a result, PEF contributes to better preservation of nutritional value and overall product quality. The fundamental mechanism of PEF is to increase the transmembrane potential in cells, leading to electroporation, which increases cell membrane permeability. Due to this effect, PEF has been widely studied for various applications including the extraction of bioactive compounds, microbial inactivation, and as a pretreatment to enhance drying efficiency [1,2]. In addition, the application of PEF can induce molecular-level changes, modifying the structure, and consequently the functional properties, of biomacromolecules such as starches and proteins, as well as changing the enzyme activity [3]. Most published studies have focused on enzyme inactivation by PEF, although some evidence indicates that, under specific conditions, PEF may enhance enzyme activity, depending on the enzyme and the applied parameters [4,5,6].
The specific advantages of PEF technology have driven increased research interest in its application to virgin olive oil (VOO) production. VOO is obtained exclusively through mechanical processes and is renowned for its unique sensory characteristics and high nutritional value. Phenolic compounds are the main compounds linked to high nutritional value of VOO, but they are the primary determinants of its distinctive taste, especially its bitterness and pungency. In contrast, the aroma and overall sensory perception of VOO are largely governed by its volatile organic compound (VOC) profile. VOCs in VOO originate from three main sources: (i) the lipoxygenase (LOX) pathway, (ii) oxidative degradation of fatty acids (OX), and (iii) microbiological activity (MBA) during processing. While OX and MBA are primarily associated with the formation of volatiles that contribute to undesirable sensory attributes, the LOX pathway involves a sequence of enzymatic reactions that generate C5 and C6 VOCs that are key contributors to the pleasant green and fruity aromas of high-quality VOO [7].
One of the main challenges in VOO production is the relatively low efficiency of conventional extraction methods. Typically, only around 80% of the oil contained in the olive fruit is effectively recovered, while the remainder remains trapped within intact cells or emulsified in the aqueous phase [8,9]. Electroporation of cell membranes induced by PEF treatment facilitates the release of oil that is otherwise inaccessible through conventional technology, thereby enhancing extraction efficiency. Increases in oil yield of up to 25%, depending on the specific PEF parameters applied, have been reported in the literature [10,11,12,13]. Importantly, these yield improvements have not been associated with negative effects on oil quality or the development of sensory defects. However, modifications in the VOC profile have been observed. For example, Tamborrino et al. [12] reported a reduction in C5 and C6 alcohols, while Navarro et al. [11] found a significant increase in 2-hexenal, indicating that PEF may influence the activity of enzymes involved in the LOX pathway. Furthermore, Veneziani et al. [14] highlighted the important role of genetic variability among olive varieties in modulating both oil yield and VOC changes under PEF treatment.
Despite the growing global interest in PEF technology as a sustainable approach to improving oil extraction and quality, its effects on Croatian autochthonous olive cultivars remain entirely unexplored at both laboratory and industrial scales. Although Croatia is a relatively small producer of virgin olive oil (VOO) in global terms, its oils are internationally recognized for their exceptional quality [15]. The country’s production is largely carried out in small and medium-sized oil mills, where innovative technologies such as PEF could be more readily implemented, provided their effectiveness is demonstrated [16]. Given that PEF efficiency depends not only on processing parameters but also on olive cultivar and potential interactions between the two [11,13] the objective of this study was to evaluate the effects of PEF pretreatment—applied prior to malaxation at electric field strengths of 2–7 kV/cm and treatment durations of 30–90 s—on oil yield, quality parameters, and volatile organic compound (VOC) profiles of VOO from four major Croatian autochthonous cultivars: Istarska Bjelica and Rosulja (Istria), and Levantinka and Oblica (Dalmatia). This work represents the first comprehensive evaluation of PEF technology in the context of Croatian VOO production.
2. Materials and Methods
2.1. Plant Material
In this study, olive fruits (Olea europaea L.) from two Istrian varieties (Istarska Bjelica and Rosulja) and two Dalmatian varieties (Oblica and Levantinka), each grown in their area of origin, were harvested at their optimal stage of ripeness. The Oblica and Istrian varieties were harvested in mid-October 2022, while Levantinka was harvested in early November that same year. The ripeness of the fruits was assessed using the maturity index (MI), determined based on skin and pulp coloration. The recorded MI values were: 1.32 for Istarska Bjelica, 2.19 for Rosulja, 2.01 for Levantinka, and 1.75 for Oblica. Moisture and oil content were also measured for each variety: Istarska Bjelica had 68.59% moisture and 11.97% oil; Rosulja 66.27% moisture and 10.30% oil; Levantinka 40.42% moisture and 21.87% oil; and Oblica 62.05% moisture and 16.28% oil. All olive fruit characterizations were performed according to the standard methods outlined in the International Olive Council (IOC) guidelines [17].
2.2. Chemicals
4-Methyl-2-pentanol, alkane standard solution (C8–C20), benzamidine, butylated hydroxytoluene (BHT), Coomassie Brilliant Blue G-250, diethyl ether, ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), Triton X-100, and α-linolenic acid (ALA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, anhydrous disodium hydrogen phosphate, isooctane, isopropanol, sodium dihydrogen phosphate dihydrate, sodium hydroxide, and sodium thiosulfate were obtained from Kemika (Zagreb, Croatia). α-Aminocaproic acid (D-norleucine) was purchased from Alfa Aesar (Haverhill, MA, USA), while ethanol and potassium iodide were obtained from Lach-Ner (Neratovice, Czech Republic). o-phosphoric acid was purchased from Fluka (Buchs, Switzerland), hexane from Honeywell (Offenbach, Germany), and bovine serum albumin (BSA) from Santa Cruz Biotechnology (Dallas, TX, USA). 13(S)-Hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid (HPOT) was obtained from Larodan (Solna, Sweden). Hydrochloric acid and starch were purchased from Thermo Fisher Scientific (Waltham, MA, USA). All chemicals were of analytical grade, and HPLC-grade acetonitrile was obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Virgin Olive Oil Production
The olive fruits (1 kg) were crushed using a laboratory-scale metal hammer mill (Enotecnica Pillan, Camisano Vicentino, Italy) operating at 3000 rpm. The resulting olive paste was immediately filled into the PEF treatment chamber (Figure 1), ensuring it was filled to the top to eliminate air gaps and prevent plasma discharge. The PEF treatment was carried out using a high-voltage generator (HVG60/1 PEF; Impel d.o.o., Zagreb, Croatia). The treatment chamber consisted of two stainless steel electrodes with a diameter of 230 mm and were 25 mm apart.
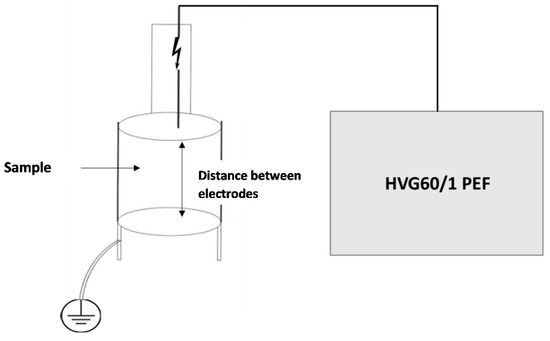
Figure 1.
Schematic representation of the pulsed electric field (PEF) reactor used for olive paste treatment.
The voltage in the PEF reactor was measured using a Tektronix P6015A high-voltage probe connected to a Tektronix 2 series mixed signal oscilloscope for data recording (Tektronix, Beaverton, OR, USA). Square wave pulses were applied during the treatment and their waveform is presented in Supplementary Material (Figure S1). The energy of each pulse was calculated using Equation (1), described by Leone et al. [10]
where U is the voltage (kV), I is the current (A) and τ is the pulse duration (µs). The PEF treatments were designed according to a central composite experimental plan, with electric field strengths of 2 and 7 kV/cm and treatment durations of 30 and 90 s as factorial points (Table 1). A constant frequency of 150 Hz was applied in all treatments, with each pulse lasting 2 μs. The temperature of the olive paste was measured immediately after each treatment. The observed temperature change ranged from −0.3 °C to 0.4 °C, indicating minimal thermal effects under the applied conditions. Specific energy intensity of the treatment (kJ/kg) was calculated following Equation (2) and obtained values for each experiment are presented in Table 1:
where Wpulse is the energy of pulse (J), fpulse is the pulse frequency (Hz), t is the time of the treatment (s) and m is the mass of the sample (kg).

Table 1.
Experimental plan design with specific energies (kJ/kg) for each treatment.
After the PEF treatment, the olive paste was transferred to a cylindrical stainless-steel vessel placed in a water bath (Stuart SBS40, Cole-Parmer, Vernon Hills, IL, USA). The control samples (prepared in triplicate) were processed identically but without PEF treatment and were malaxed immediately after crushing.
Malaxation was performed using an overhead stirrer (VELP Scientifica, Usmate, Italy) equipped with a modified agitator with paddles on three levels to ensure uniform mixing throughout the paste. Malaxation was carried out at approximately 150 rpm for 40 min at 27 ± 2 °C, without the addition of water. Following malaxation, the olive paste was centrifuged at 5500× g for 10 min using a Rotina 380 centrifuge (Hettich, Tuttlingen, Germany), also without water addition. The resulting liquid phase was decanted into a glass cylinder and the oil fraction was measured to determine the extraction yield. The oil was further clarified by centrifugation (Rotina 380R, Hettich, Tuttlingen, Germany) at 5500 g for 5 min at 18 °C. The obtained VOO was stored in 250 mL dark glass bottles under nitrogen atmosphere at 15–20 °C until further analysis.
2.4. Determination of Oil Yield
The oil yield (Y), defined as the percentage of oil (g) extracted per 100 g of olive paste [18], was calculated using the following Equation (3):
where Voil (mL) is the volume of the extracted oil, 0.915 (g/mL) is the standard density of VOO and Wolives (g) is the mass of the olive paste.
2.5. Determination of Basic Quality Parameters
Basic quality parameters were determined using standard analytical methods. The peroxide value was measured in accordance with ISO 3960 [19]. Free fatty acid content and spectrophotometric measurements in the ultraviolet range (K232, K268, and ΔK) were determined according to the official methods of the International Olive Council (IOC) [20,21].
2.6. Determination of Lipoxygenase Activity
Twenty-five grams of olive paste was sampled after the malaxation phase, flash-frozen in liquid nitrogen, and stored at −20 °C until analysis. This material was used for the determination of lipoxygenase (LOX) activity.
Enzyme extraction was performed using a modified protocol based on the method of Luaces et al. [22]. Briefly, 5 g of frozen olive paste was homogenized in 20 mL of 100 mM phosphate buffer (pH 6.7) containing 0.1% Triton X-100, 1 mM EDTA, 0.1 mM PMSF, 0.1 mM benzamidine, and 5 mM D-norleucine. The homogenization was conducted in two 1-min cycles at 11,000 rpm using a GLH 850 homogenizer (Omni International, Kennesaw, GA, USA). The resulting homogenate was filtered under vacuum through two layers of Miracloth, followed by centrifugation at 27,000× g for 30 min at 4 °C (Rotina 420R, Hettich, Tuttlingen, Germany). The clear supernatant was collected and used as the crude enzyme extract.
LOX activity was determined using a modified version of the method described by Soldo et al. [23], with 25 mM solution of ALA as the substrate, prepared according to the protocol described in our previous work [6]. The reaction mixture consisted of 4 mL of 0.1 M phosphate buffer (pH 6.0), 500 μL of crude enzyme extract, and 500 μL of ALA solution. The mixture was stirred at 25 ± 0.2 °C for 30 min on a magnetic stirrer. The reaction was terminated by adjusting the pH to 2.0 with HCl, followed by the addition of 1 mL of butylated hydroxytoluene (BHT, 0.25 mM) as an internal standard.
The formed ALA hydroperoxides (hydroperoxy octadecatrienoic acids -HPOTs) were extracted three times with 10 mL of a hexane–isopropanol mixture (95:5, v/v). The combined organic extracts were evaporated under reduced pressure at 40 °C using a Hei-VAP rotary evaporator (Heidolph Instruments GmbH & Co., Schwabach, Germany). The dried residue was reconstituted in 1.5 mL of an acetonitrile–water mixture (67:33, v/v).
High-performance liquid chromatography (HPLC) was performed using an Agilent 1200 LC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a C18 column (Luna, 250 mm × 4.6 mm, 5 μm, 100 Å; Phenomenex, Torrance, CA, USA) maintained at 35 °C. The injection volume was 10 μL. Eluent A was 0.25% acetic acid (aqueous), and eluent B was acetonitrile. The flow rate was set to 1 mL/min. The gradient program was as follows: 63% B at 0 min, held until 17 min; ramped to 80% B from 17 to 20 min, held until 32 min; returned to 63% B from 32 to 35 min, followed by equilibration for an additional 10 min. Detection was performed using a diode array detector (DAD) at 234 nm.
HPOTs were identified by comparing their retention times and UV spectra with those of a commercial standard of 13(S)-hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid. Quantification was performed using an internal standard method, taking into account the response factor ratio between HPOT and BHT to express the results as HPOT equivalents. Representative chromatogram of HPOT standard and BHT is presented on Figure S2. LOX activity was defined as the amount of HPOT produced by 1 µmol of enzyme at 25 °C. The method showed good sensitivity, with a limit of detection (LOD) of 0.002 µmol HPOT/mg protein and a limit of quantitation (LOQ) of 0.005 µmol HPOT/mg protein. All enzyme extractions were performed in duplicate, and the enzymatic reactions with ALA were conducted in triplicate.
Protein Determination
The protein content of the enzyme extract was determined using the Bradford method [24]. In brief, 300 μL of the enzyme extract was added to 1.2 mL of Bradford reagent, and the absorbance was measured against a blank after a 5-min reaction at room temperature at 595 nm using UviLine 9400 spectrophotometer (Secomam, Alès, France). Crystalline bovine serum albumin (BSA), at concentrations ranging from 0.005 to 0.5 mg/mL, was used as the standard protein to generate the calibration curve. Protein content of each extract was measured in triplicate.
2.7. Determination of Volatile Components
Volatile compounds were isolated using solid phase microextraction (SPME) and analyzed by gas chromatography coupled with mass spectrometry (GC/MS). A 2 cm DVB/CAR/PDMS fiber with a film thickness of 50/30 μm (Supelco, Bellefonte, PA, USA) was used for extraction. For each sample, 15 mg of internal standard (0.15% solution of 4-methyl-2-pentanol in freshly refined sunflower oil) was added to 10 g of VOO in a 20 mL headspace vial sealed with a silicone septum. The vial was then placed in a heating block at 40 ± 0.5 °C with continuous magnetic stirring (Pierce Reacti-Therm Heating/Stirring Module, Artisan, Champaign, IL, USA). After a 10-min equilibration period, the SPME fiber was exposed to the headspace for 30 min and then immediately desorbed in the GC injector in splitless mode at 260 °C for 1 min.
Analysis was carried out using an Agilent 8890 GC system coupled with an Agilent 7000D Triple Quadrupole mass detector (Agilent Technologies, Santa Clara, CA, USA). Separation was achieved using an HP-5 column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, Santa Clara, CA, USA). The oven temperature program was as follows: initial temperature of 30 °C held for 3 min, ramped to 150 °C at 5 °C/min, then to 250 °C at 20 °C/min, and held at the final temperature for 5 min. The ion source and transfer line temperatures were set at 250 °C and 260 °C, respectively. Helium served as the carrier gas at a constant flow rate of 1.5 mL/min. Mass spectra were recorded over the m/z range 50–550.
To determine retention indices, a homologous series of n-alkanes (C8–C20) was analyzed under identical chromatographic conditions. Volatile compounds were identified by comparing their mass spectra to the NIST mass spectral library and by calculating Kovats retention indices, which were matched with literature values Quantification was achieved using an internal standard, and the method demonstrated good analytical sensitivity (LOD = 0.003 mg/kg; LOQ = 0.010 mg/kg). The isolation and GC–MS analysis of volatile compounds were performed in triplicate to ensure reproducibility.
2.8. Statistical Analysis
A central composite design (CCD) was employed to investigate the effects of PEF treatment parameters on selected variables. The experimental design included two factorial points: electric field strengths of 2 and 7 kV/cm and treatment durations of 30 and 90 s (Table 1). To assess the influence of olive variety on oil yield, basic quality parameters, lipoxygenase (LOX) activity, and volatile compound composition, a one-way analysis of variance (ANOVA) was conducted on control sample data, followed by Tukey’s Honest Significant Difference (HSD) test for post hoc comparisons. The same approach was applied to evaluate differences in basic quality parameters and volatile profiles between PEF treatments within each variety.
To determine the effects of PEF parameters on yield, LOX activity, and grouped volatile compounds (LOX pathway, OX, and MBA volatiles), response surface methodology (RSM) was applied. All models were fitted using a second-order polynomial equation, and response surface plots were generated to visualize interactions between electric field strength and treatment duration. Model adequacy was assessed using regression coefficients and the determination coefficient (R2).
All statistical analyses were performed using XLSTAT 2023 (Lumivero, Denver, CO, USA), with significance set at p ≤ 0.05.
3. Results and Discussion
3.1. Oil Yield
Oil yields obtained in this study through laboratory-scale extraction varied considerably among the olive varieties studied, ranging from 5% to 16%. The Istrian varieties (Istarska Bjelica 5.31 ± 0.65 and Rosulja 5.74 ± 0.56) had significantly lower oil yields compared to the Dalmatian varieties, with Levantinka having the highest oil yield (16.12 ± 0.19), which was significantly higher than that of Oblica (11.19 ± 0.79) and both Istrian varieties. Previously published data using the Abencor extraction system also indicate considerable variability in oil yield among these varieties. For Istarska Bjelica, reported oil yields range from 10% to 25% [25,26,27,28] while for Rosulja, values between 5% and 18% have been documented [25,29]. According to Žanetić et al. [30], Levantinka typically exhibits an oil content of approximately 20%, with an average yield around 12.5% [28] Oblica displays a broader yield range, varying between 13% and 22%, influenced by cultivation conditions and fruit maturity index [28,31].
According to data from the Croatian Meteorological and Hydrological Service (CMHS), climatic conditions during the oil accumulation period (July–August 2022) differed notably between the regions from which the oil-producing fruits originated (Tables S1 and S2), possibly contributing to the observed differences in oil yield. In Dalmatia, average monthly temperatures during this period were approximately 2 °C higher than those recorded in Istria. However, when compared to the respective five-year regional averages, Dalmatia showed only a marginal increase of +0.3 °C, whereas Istria experienced a more pronounced increase of approximately +1.4 °C. Additionally, both regions received significantly reduced precipitation during the same period. During this period (July–August 2022), precipitation levels were significantly lower than the five-year average—more than eight times lower in Istria and up to twelve times lower in Dalmatia (Tables S1 and S2). Previous research has shown that elevated temperatures during fruit development can adversely affect oil biosynthesis, leading to lower yields [32]. However, the extent of this impact is variety-dependent, as olive genotypes exhibit differing levels of tolerance to heat and drought stress. Oblica, and particularly Levantinka, are recognized for their resilience to water deficit conditions [33,34] which likely contributed to their superior oil yields despite the adverse climatic conditions of the 2021/2022 olive season.
3.2. Lipoxygenase Activity and Composition of Volatile Compounds in Croatian Virgin Olive Oils
Sensory analysis is essential for evaluating VOO quality. Phenolics mainly contribute to bitterness and pungency, while VOCs, particularly those from the LOX pathway, define the green and fruity aroma of high-quality VOO [7]. LOX initiates this pathway by oxidizing polyunsaturated fatty acids, and its activity is considered a limiting step in VOC biosynthesis [35,36]. For this reason, LOX activity was selected in this study as a key parameter for monitoring the formation of desirable VOCs in produced VOOs. LOX activity in Croatian VOOs, presented in Table 2, varied significantly among varieties, with Istrian cultivars, especially Rosulja, showing the highest activity. While varietal genetics play a major role [15,37,38,39,40,41], geographical origin and environmental factors such as temperature also strongly influence LOX activity [42,43]. The elevated activity in Istrian varieties likely reflects the combined effects of these factors (Table 2, Tables S1 and S2).

Table 2.
Lipoxygenase (LOX) activity (µmol HPOT */mg protein) and composition of volatile compounds (VOC) (mg/kg) in control olive oil samples from Croatian varieties. Values are presented as mean ± standard deviation.
The only previous study on LOX activity in Croatian olive varieties, by Soldo et al. [41], focused on Dalmatian cultivars, including Oblica and Levantinka. They reported a maturity-related increase in LOX activity and consistently higher activity in Oblica than in Levantinka. Our results are consistent with this study, as Oblica (MI 1.75) showed higher LOX activity than Levantinka (MI 2.01) (Table 2).
The total mass fraction of LOX-derived VOCs showed only a partial correlation with LOX activity (Table 2). Rosulja had the significantly highest LOX activity but total concentration of its LOX-derived VOCs did not differ significantly from those measured in Istarska Bjelica and Oblica, while Levantinka showed both the lowest activity and VOC content, supporting LOX as a limiting factor in the biosynthesis of these compounds [35]. Overall, Istrian varieties produced more LOX-derived volatiles than Dalmatian varieties (Table 2), consistent with earlier findings linking lower temperatures to higher volatile accumulation [15,44,45].
The composition of VOCs from the LOX pathway also varied by geographical origin. Aldehydes dominated in Istrian oils (68% and 77% for Rosulja and Istarska Bjelica, respectively), while alcohols prevailed in Dalmatian oils (79% and 87% for Levantinka and Oblica, respectively) (Table 2), likely due to higher alcohol dehydrogenase (ADH) activity. In Istrian VOOs, 2-hexenal accounts for more than 50% of LOX-derived volatiles, while in Dalmatian varieties, 2-hexen-1-ol and (Z)-3-hexen-1-ol predominate. In Oblica VOO, (Z)-3-hexen-1-ol accounts for 47.5% and 2-hexen-1-ol for 20%, while in Levantinka, the proportions are nearly reversed (28.6% vs. 47%) (Table 2). These differences indicate variety-specific isomerase activity, which affects the interconversion of LOX-pathway isomers. Enzymatic variation is also observed in alcohol acetyl transferase (AAT), whose activity is higher in Levantinka than in Oblica and Istrian varieties, consistent with the presence of esters (e.g., 3-hexenyl acetate, hexyl acetate) in Levantinka VOO. Lukić et al. [15] reported (Z)-3-hexen-1-ol as the main LOX alcohol in Oblica, while other studies found 2-hexenal to be dominant in both Oblica and Levantinka [41,46]. Ester formation in Dalmatian varieties also varies with harvest season and fruit maturity index [41]. C5 ketones were identified among LOX-derived VOCs, with 1-penten-3-one present in all oils and pentan-3-one found only in Rosulja (Table 2). Most LOX-derived VOCs showed significant inter-variety differences, except for 2-hexen-1-ol, (E)-2-penten-1-ol, and pentan-3-one, which were low and consistent across varieties studied.
In addition to LOX-derived VOCs, OX- and MBA-derived volatiles were detected in all oils (Table 2), and their concentrations differed significantly among varieties. Istarska Bjelica had significantly higher levels, approximately four-fold for OX products and twofold for MBA volatiles, mainly due to 2,4-hexadienal and 4-oxohex-2-enal. Pentanal was significantly higher in Dalmatian varieties, while MBA products varied by origin. Istrian oils, especially Istarska Bjelica, were rich in branched-chain aldehydes (2- and 3-methylbutanal) (Table 2), which are associated with malty and sweet notes [38] and the development of the fusty sensory defect [47,48]. Dalmatian oils had higher concentration of pentan-1-ol (Table 2), linked to winey–vinegary defects [48]. In Istarska Bjelica, these branched aldehydes exceed their odor threshold and likely influence the oil’s sensory profile. In contrast, pentan-1-ol remains below its threshold in Oblica VOO, suggesting no negative impact on sensory quality [7].
3.3. Effect of Pulsed Electric Field on Oil Yield and Quality Parameters
The application of PEF technology in VOO production has been extensively studied over the past decade. When applied after olive crushing and prior to malaxation, PEF can significantly enhance oil extractability and improve oil quality. Electroporation disrupts cell membranes, releasing oil from previously intact cells and facilitating the extraction of intracellular compounds such as phenolics and aroma precursors [1]. During malaxation, these compounds interact with endogenous enzymes, contributing to the formation of desirable non-glyceride components, while coalescing oil droplets promote easier separation [8,9]. Reported increases in oil yield due to PEF pretreatment at this stage, range from 2.3% to 25% [11,12,13,14,49,50,51]. Importantly, these studies consistently show that PEF does not negatively impact the physicochemical quality parameters required for VOO classification, nor does it induce sensory defects. Additionally, PEF may modulate the activity of endogenous enzymes through conformational changes induced by the electric field [3], potentially enhancing the formation of beneficial phenolic and volatile compounds and thereby improving the sensory profile of the oil. In our previous study, we explored the effects of electric field strength and treatment duration on the activity of β-glucosidase and LOX in model systems [6]. Results indicated that electric field strength positively influenced β-glucosidase activity, while treatment duration had a significant effect on LOX activity. Furthermore, Tamborrino et al. [12] reported PEF-induced changes in the concentrations of specific oleuropein and ligstroside derivatives, as well as certain volatile alcohols, likely linked to modulated enzyme activity.
Most previous studies on virgin olive oil applied moderate electric field strengths (typically up to 2 kV/cm) with varying treatment durations. However, the cellular and molecular effects induced by PEF are highly dependent on the specific treatment parameters [2]. Additionally, Veneziani et al. [14] highlighted that the response to PEF is cultivar-dependent, further emphasizing the need for variety-specific optimization. Based on these insights, the present study aimed to evaluate the effects of different PEF treatments on four autochthonous Croatian olive cultivars. PEF was applied before malaxation using combinations of electric field strengths (2–7 kV/cm) and treatment durations (30–90 s), based on a CCD. The specific energy input during PEF treatments ranged from 0.018 to 0.708 kJ/kg, aligning well with values commonly reported in the literature for similar applications. These energy levels correspond closely to the applied electric field strengths, number of pulses, and pulse durations used in experiment. Such low to moderate energy inputs are typical for PEF-assisted extraction processes involving plant matrices, particularly under batch-mode conditions, where the preservation of thermolabile compounds is essential [13,52]. The collected data on oil yield, LOX activity, and volatile organic compound (VOC) composition were used to construct two-factor interaction models for each cultivar. Detailed model parameters, including regression coefficients and p-values, are provided in the Supplementary Material (Tables S3 and S4).
Response surface plots derived from the developed models, illustrating the relative change in oil yield for each of the four investigated olive varieties, are presented in Figure 2a–d. The application of PEF treatment led to increased oil extraction efficiency in laboratory-scale experiments across all olive varieties, although the extent of this improvement was dependent on the specific variety. In Istarska Bjelica, the increase ranged from 10.7% to 26.1% (Figure 2a), while in Rosulja, it reached values from 9.7% up to 36% compared to the untreated control (Figure 2b). These enhancements exceed those reported in previous studies at both industrial [12,14,49] and laboratory scales [51]. In contrast, the Dalmatian varieties exhibited a significantly lower increase in oil yield after PEF treatment. Specifically, the Oblica variety showed an increase of up to 4% (Figure 2d), while Levantinka reached a maximum of only 2.5% (Figure 2c). Notably, the Istrian olive varieties, which had lower baseline oil yields for controls (5.5% on average, Table 1), exhibited substantially greater relative improvements upon PEF pretreatment compared to the Dalmatian varieties (Figure 2a–d). This difference can be attributed to differences in the oil-to-water ratio between olive varieties (see Section 2.1. Plant Material). The Istrian varieties, characterized by a lower content, possess a correspondingly higher water content, which enhances the electrical conductivity of the olive paste. Increased conductivity facilitates more effective transmission of PEF, thereby promoting greater cell membrane permeabilization and structural disruption [53], which in turn improves oil release. Ultimately, our results obtained in laboratory conditions indicate that the greatest impact of PEF on oil extraction efficiency can be achieved in olives with lower initial oil yield, potentially due to inherent varietal characteristics or resulting from the increasingly prevalent adverse climatic conditions associated with climate change.
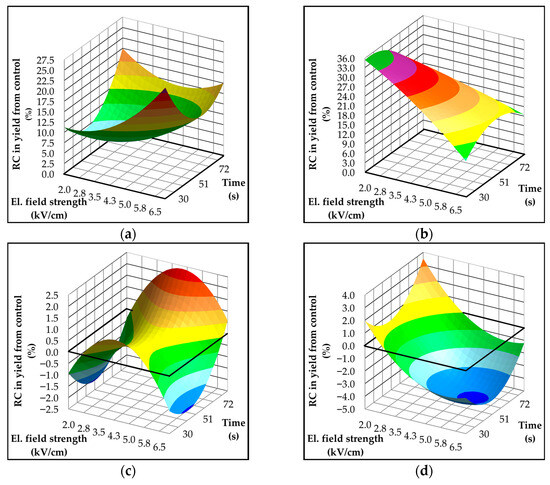
Figure 2.
Three-dimensional response surface plots showing the effects of electric field strength (kV/cm) and treatment time (s) on oil yield (%) in Croatian olive varieties: (a) Istarska Bjelica, (b) Rosulja, (c) Levantinka, and (d) Oblica. Results for each variety are expressed as relative change (RC [%]) compared to the control: RC = 100 × (treatment − control)/control. The solid black line indicates the absolute control yield: 5.31% for Istarska Bjelica (a), 5.74% for Rosulja (b), 16.12% for Levantinka (c), and 11.19% for Oblica (d).
A positive correlation between the intensity of pulsed electric field (PEF) treatment—expressed as electric field strength or specific input energy, which depends on treatment time, pulse width, and frequency—and oil yield in virgin olive oil extraction has been widely reported. Yield generally increases with higher field strengths or longer treatment times, up to an optimal threshold beyond which additional energy input produces diminishing returns or undesired thermal effects [14,50]. However, this relationship is inherently non-linear and strongly variety-dependent. In the present study, clear varietal differences were observed in the response to varying PEF parameters. In Istarska Bjelica (Figure 2a), the highest yield increase was observed at the maximum field strength, whereas treatment time alone had a slightly negative effect. Conversely, at lower field strengths, longer treatment durations were necessary to achieve comparable yield improvements. In Rosulja (Figure 2b), the highest relative increase in yield occurred at lower electric field strengths, indicating an inverse response compared to Istarska Bjelica. A similar pattern was observed in Oblica (Figure 2d), though with an added positive influence of treatment time. Notably, in Oblica, increasing the field strength beyond about 3 kV/cm led to a yield reduction of up to 5% compared to the control. This may be attributed to enhanced emulsion stability resulting from PEF-induced structural modifications of proteins and phospholipids, which are released into the matrix upon cell disruption and contribute to emulsification, thereby reducing extractable oil [54]. Levantinka (Figure 2c), which exhibited the highest initial oil yield among the tested varieties, showed the greatest relative increase at medium field strengths, with an additional positive effect of prolonged treatment time. In contrast, shorter durations at both low and high field strengths resulted in decreased yields relative to the control. It should be noted, however, that, according to the statistical analysis (Table S3), none of the fitted models adequately described the experimental data. Although the R2 values were relatively low (R2 < 0.7), these experimental data remain valuable for identifying overall trends and informing future optimization strategies. The observed variability in the central point replicates illustrates the complexity of the system under investigation.
The basic quality parameters of all VOOs produced—with and without PEF treatment—including acidity, peroxide value (PV), and specific extinction coefficients (K232, K268, and ΔK) were assessed (Table 3). One-way ANOVA revealed a statistically significant effect of PEF treatment on most quality parameters across the tested varieties. However, Tukey’s post hoc test showed that differences from the respective controls were minimal. The largest change in acidity was a reduction of only 0.04% in the Levantinka variety. Additionally, PEF pretreatment had a limited impact on the oxidative deterioration of produced VOOs. A general trend of decreased PV at lower electric field strengths and a slight increase at higher intensities was observed in all varieties except Oblica, where PV consistently increased following application of PEF treatment, with a maximum deviation of 1.7 meq O2/kg compared to the control. In terms of K-values, a slight increase in K232 was recorded for Istarska Bjelica at higher field strengths, while increases in K268 were observed only under extreme treatment conditions. Despite these minor variations, all measured parameters remained well within the regulatory limits for extra VOO established by the EU [55], indicating that PEF pretreatment under laboratory conditions had a minor or negligible impact on oil quality. This outcome supports existing evidence that PEF technology does not significantly affect the quality of VOO, regardless of whether it is applied at industrial [12,14,49] or laboratory scale [51].

Table 3.
Basic quality parameters (acidity, peroxide value—PV and K-values) of virgin olive oil samples extracted with and without pulsed electric field (PEF) pretreatment. Values are reported as mean ± standard deviation.
3.4. Effect of Pulsed Electric Field on Lipoxygenase Activity and Volatile Compounds
Response surface plots illustrating the behavior of LOX activity under PEF treatment for each variety are presented in Figure 3. LOX activity is expressed as a relative difference (%) compared to the untreated control. The results indicate a clear variety-dependent response to PEF. In the Istrian varieties, which exhibited high baseline LOX activity, PEF generally led to a reduction in enzyme activity. Conversely, the Dalmatian varieties, characterized by lower initial LOX activity, showed an increase after the treatment. The reduction in the LOX activity was the most pronounced in Rosulja, which had the highest LOX activity in the control samples and exhibited a reduction of up to 95% after PEF treatment (Figure 3b). In Istarska Bjelica, the decrease was smaller but still notable, with up to a 25% reduction (Figure 3a). The response surface models for the Istrian varieties demonstrated good predictive accuracy (R2 > 0.7), with treatment time identified as the most influential factor (Table S3). Interestingly, the effect of time differed between these two varieties: in Istarska Bjelica, a slight increase in LOX activity was observed at the shortest treatment time, while in Rosulja, the greatest reduction occurred under the same condition. In Istarska Bjelica, all tested factors—except the linear term of electric field strength—had a significant influence on LOX activity (p ≤ 0.05). In Rosulja, treatment time and the quadratic term of electric field strength had significant positive impact on the enzyme’s activity (Table S3).
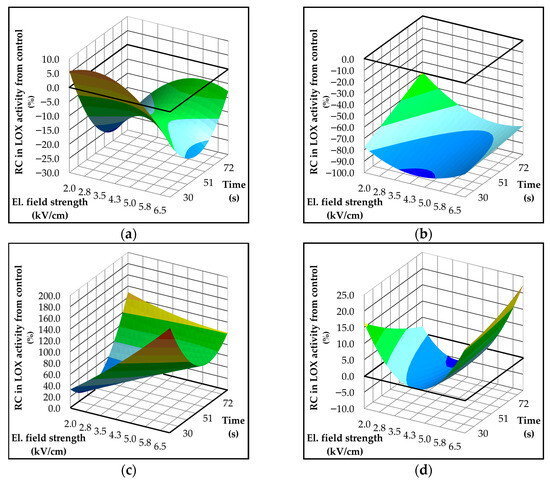
Figure 3.
Three-dimensional response surface plots showing the effect of electric field strength (kV/cm) and treatment time (s) on lipoxygenase (LOX) activity (μmol hydroperoxy-octadecatrienoic acid (HPOT)/mg protein) in Croatian olive varieties (a) Istarska Bjelica; (b) Rosulja; (c) Levantinka; (d) Oblica. Results for each variety are expressed as relative change—RC (%) in LOX activity compared to the control: RC = 100 × (treatment − control)/control. The solid black line indicates the absolute control LOX activity expressed as µmol HPOT/mg protein: 15.48% for Istarska Bjelica (a), 23.46% for Rosulja (b), 2.42% for Levantinka (c), and 9.88% for Oblica (d).
As previously noted, PEF treatment had an opposite effect on LOX activity in the Dalmatian varieties compared to the Istrian varieties. This effect was particularly pronounced in Levantinka, where LOX activity increased by up to 180% relative to the control. Both electric field strength and treatment duration exhibited positive linear effects on enzyme activity; however, their interaction had a negative influence, suggesting that certain parameter combinations may counteract the individual enhancing effects. The greatest increases in LOX activity were observed under two contrasting conditions: high electric field strength with short treatment time, and low field strength with prolonged treatment. In Oblica, a similar trend was observed, with both electric field strength and treatment duration positively influencing LOX activity. The maximum increase occurred under the most intense treatment conditions tested. Despite these observed trends, the response surface models for both Dalmatian varieties showed limited predictive power (R2 < 0.7), indicating that the models do not fully capture the variability or complexity of LOX behavior under PEF in Dalmatian varieties (Table S3).
Possible explanation for the contrasting behavior of LOX activity between the studied variety groups, and the greater sensitivity observed in the Istrian varieties, may lie in differences in fruit water content. As we previously noted, higher moisture levels in the Istrian varieties likely increased the conductivity, enabling more efficient transmission of the electric field during PEF treatment. As a result, the treatment effect may be amplified, potentially causing greater structural disruption of the enzyme and leading to its inactivation. These assumptions are supported by findings by Luo et al. [4], who reported that increasing electric field strength over 5 kV/cm enhances LOX inactivation through conformational changes in the enzyme’s secondary structure. On the other hand, our previous study on LOX behavior in model systems [6] demonstrated that exposure to weak or moderate electric fields can increase LOX activity. This observation supports the results obtained for the Dalmatian varieties, which showed an increase in LOX activity under PEF application.
When comparing the effects of PEF treatment on LOX enzyme activity (Figure 3) and the concentration of volatile compounds generated via the LOX pathway (Figure 4), the results may initially appear inconsistent. In the Istrian varieties, LOX activity was reduced relative to the control, yet an overall increase in LOX-derived volatile compounds was observed. On the other hand, in the Dalmatian varieties, an increase in volatile compounds was also recorded, although not proportional to the observed rise in LOX activity. At first glance, these findings may seem contradictory, particularly considering that LOX activity is generally regarded as the rate-limiting step in the biosynthesis of volatile compounds in VOO [35]. However, upon closer examination, certain patterns and correlations emerge that help better to understand these results.
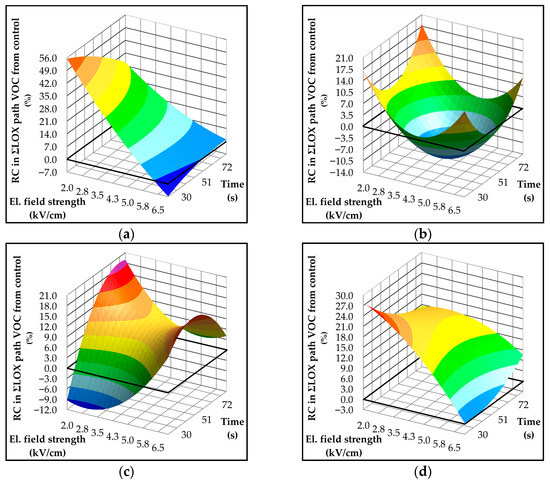
Figure 4.
Three-dimensional response surface plots showing the influence of electric field strength (kV/cm) and treatment time (s) on formation of volatile components (mg/kg) associated with the lipoxygenase (LOX) pathway in Croatian olive varieties (a) Istarska Bjelica; (b) Rosulja; (c) Levantinka; (d) Oblica. Results for each variety are expressed as relative change—RC (%) in LOX pathway compared to the control: RC = 100 × (treatment − control)/control. The solid black line indicates the absolute control value (mg/kg) for each variety: 75.37 for Istarska Bjelica (a), 66.48 for Rosulja (b), 33.54 for Levantinka (c), and 46.80 for Oblica (d).
All of the studied varieties responded better to low electric field strengths. In Istarska Bjelica (Figure 4a), the highest increase in LOX pathway volatiles (~55%) was observed under the mildest PEF treatment conditions, which also coincided with an increase in LOX enzyme activity. Similarly, in Rosulja and Levantinka (Figure 4b,c), the largest increases in LOX-derived volatiles (~18%) were recorded under conditions that produced the highest LOX activity—specifically, low electric field strength combined with longer treatment times. The most notable discrepancy among results was observed in Oblica, where the highest concentration of LOX volatiles occurred at the lowest electric field strength (Figure 4d), despite LOX activity being slightly higher at more intense PEF conditions (Figure 3d).
Several factors may have influenced the observed results. In our previous research using model systems simulating the malaxation process, we found that LOX activity reaches a plateau during malaxation, after which the concentration of hydroperoxides begins to decline [6]. Given the high initial LOX activity in the Istrian varieties (Table 2), partial enzyme inactivation induced by PEF treatment has not been sufficient to prevent the achievement and even an elevation of this activity plateau. Moreover, the achievement of LOX activity plateau could explain the relatively modest increase in LOX-derived volatiles in Levantinka, despite a significant increase in enzyme activity (Figure 3c). It is also important to recognize that LOX is only one of several key enzymes involved in the biosynthesis of volatile compounds. Downstream enzymes such as hydroperoxide HPL, ADH, and AAT may respond differently to PEF treatment, potentially leading to distinct or even competing effects on VOC formation. Therefore, the observed changes in volatile profiles likely reflect the combined influence of multiple enzymatic steps rather than LOX activity alone. In addition, interactions among these enzymes—whether synergistic or competitive—may further contribute to the complexity of PEF-induced responses. The predictive models developed in this study, with the exception of Rosulja, showed limited statistical accuracy (R2 < 0.7; Table S4), indicating that LOX activity alone is insufficient to explain the variability of VOC biosynthesis under PEF treatment. This highlights the need for further investigation, including direct evaluation of the activity of other pathway enzymes and/or proteomic approaches to assess possible PEF-related effects on enzyme expression and conformation. Such studies would provide a more comprehensive understanding of the biochemical mechanisms governing VOC formation under PEF and strengthen the interpretation of pathway-level responses.
The composition of volatile compounds in VOO produced under PEF treatment is presented by variety in Table 4, Table 5, Table 6 and Table 7. Results are expressed as absolute differences in individual compound concentrations (mg/kg) compared to the control. Positive values indicate an increase, while negative values indicate a decrease in compound levels.

Table 4.
Volatile compound composition (mg/kg) in oils extracted from the Istarska Bjelica olive variety after pulsed electric field (PEF) pretreatment. Values are reported as mean ± standard deviation, representing differences in compound content relative to the untreated control.

Table 5.
Volatile compound composition (mg/kg) in oils extracted from the Rosulja olive variety after pulsed electric field (PEF) pretreatment. Values are reported as mean ± standard deviation, representing differences in compound content relative to the untreated control.

Table 6.
Volatile compound composition (mg/kg) in oils extracted from the Levantinka olive variety after pulsed electric field (PEF) pretreatment. Values are reported as mean ± standard deviation, representing differences in compound content relative to the untreated control.

Table 7.
Volatile compound composition (mg/kg) in oils extracted from the Oblica olive variety after pulsed electric field (PEF) pretreatment. Values are reported as mean ± standard deviation, representing differences in compound content relative to the untreated control.
In Istarska Bjelica (Table 4), changes in the total concentration of LOX pathway volatiles were largely driven by variations in the dominant compound, 2-hexenal. Increases were observed after treatments with low electric field strengths (1 and 2 kV/cm) and moderate strength (4.5 kV/cm) applied for 18 sec. In contrast, higher field strengths (7 and 8 kV/cm) led to a substantial reduction in 2-hexenal content. Interestingly, in samples treated with higher electric field intensities, the reduction in 2-hexenal was accompanied by a corresponding increase in its alcohol derivatives which are formed by ADH. This suggests that, while LOX activity may be partially inhibited under higher PEF intensities, ADH activity may be simultaneously promoted, possibly due to different sensitivities of these enzymes to electric field strength.
In Rosulja (Table 5), where 2-hexenal was also the dominant volatile compound, no consistent trend was observed in its concentration following PEF treatment. In contrast to Istarska Bjelica, neither electric field strength nor treatment duration exerted a clear influence on the overall volatile profile, with the exception of esters. Notably, the levels of hexyl acetate and 3-hexenyl acetate increased under the mildest PEF treatments, likely due to enhanced activity of AAT, the enzyme responsible for ester formation.
In Levantinka, PEF treatment significantly affected the majority of volatile compounds derived from the LOX pathway (Table 6). Among all treated samples, the oil obtained following treatment with an electric field strength of 2 kV/cm for 90 s stood out. This sample had a significantly different LOX volatile profile compared to the other produced VOOs. Unlike the other Levantinka samples, where alcohols were typically dominant, the volatile profile of this sample was characterized by significantly higher levels of their corresponding aldehydes accompanied by a reduction in (Z)-3-hexen-1-ol and 2-hexen-1-ol. This shift suggests a possible reduction in ADH activity under these PEF conditions, although the mechanism behind this effect remains unclear. Interestingly, this sample also exhibited the highest concentration of 3-hexenyl acetate, which is synthesized from (Z)-3-hexen-1-ol via the action of AAT. This finding implies that, despite reduced ADH activity, AAT activity may have been maintained or even enhanced. It is noteworthy that the response of ADH to PEF in Levantinka did not follow the same pattern as observed in Istarska Bjelica, suggesting that enzyme sensitivity to PEF may depend on the initial activity levels present in untreated fruit.
The impact of PEF treatment on LOX pathway volatiles was least evident in oils from the Oblica variety (Table 7). Among the compounds analyzed, only (Z)-3-hexen-1-ol, the dominant volatile, and 2-hexenal exhibited sensitivity to PEF. However, no significant differences in (Z)-3-hexen-1-ol concentrations were detected between treated samples, while both the concentration and magnitude of change in 2-hexenal were minimal. These findings suggest that PEF treatment has limited practical relevance for the sensory characteristics of Oblica oils.
The effect of PEF treatment on OX-derived VOCs was generally positive, resulting in a significant reduction of OX products across all studied varieties, as shown in Figure 5. The most pronounced decrease occurred in the Istrian varieties, with Rosulja showing nearly a 70% reduction. This decline in OX compounds, observed in both Istarska Bjelica and Rosulja, was primarily driven by decreased concentrations of secondary oxidation products such as 4-oxohex-2-enal and 2,4-hexadienal (Table 4 and Table 5). In both Istrian varieties (Figure 5a,b), the electric field strength had a significant impact on the reduction of OX products. Furthermore, extending the PEF treatment duration in Rosulja amplified this effect. A less pronounced trend of OX volatile decrease was observed in Dalmatian varieties. For instance, in Levantinka (Figure 5c), shorter treatment times achieved the greatest reduction in OX products, whereas longer durations attenuated this effect. In Oblica (Figure 5d), mild PEF conditions slightly increased OX compounds, but increasing the electric field strength resulted in a significant reduction. This decrease in Oblica oils was primarily associated with reduced levels of 2,4-hexadienal, while 4-oxohex-2-enal was undetectable (Table 7). The reduction of OX products following PEF treatment is likely attributable to enhanced extraction of antioxidants, such as polyphenols and tocopherols [14,49]. According to the results of statistical analysis (Table S4), all predictive models assessing the impact of PEF on OX volatiles exhibited low explanatory power (R2 < 0.5), suggesting that additional factors—such as fatty acid composition, concentrations of antioxidants, presence of prooxidants, and enzymes involved in OX volatile formation—significantly influence the observed outcomes.
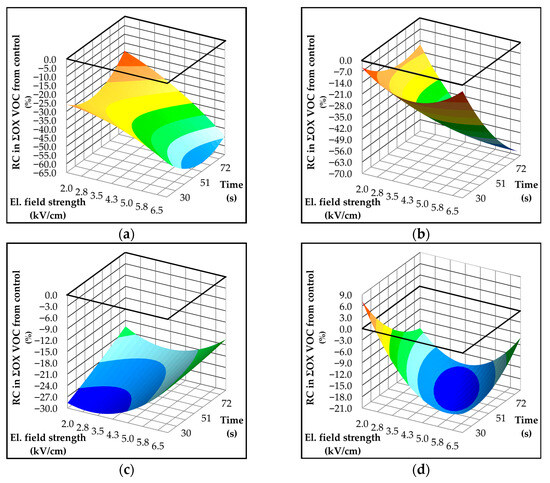
Figure 5.
Three-dimensional response surface plots showing the influence of electric field strength (kV/cm) and treatment time (s) on formation of volatile components (mg/kg) associated with the oxidation (OX) in Croatian olive varieties (a) Istarska Bjelica; (b) Rosulja; (c) Levantinka; (d) Oblica. Results for each variety are expressed as relative change—RC (%) in OX-derived volatiles compared to the control: RC = 100 × (treatment − control)/control. The solid black line indicates the absolute control value (mg/kg) for each variety: 8.53 for Istarska Bjelica (a), 2.16 for Rosulja (b), 1.77 for Levantinka (c), and 1.33 for Oblica (d).
The influence of olive variety, as well as the geographical origin of cultivation, is evident in the response of MBA compounds to PEF treatment (Figure 6). The specific microbiota associated with olives is known to vary depending on the cultivation area, and these microorganisms are responsible for the formation of MBA-related volatiles [56]. The resistance of these microorganisms to inactivation by PEF treatment can differ based on species and cell structure [57]. As we mentioned, in Istrian varieties, the dominant MBA products are branched-chain aldehydes, 2-methylbutanal and 3-methylbutanal (Table 2, Table 4 and Table 5), while in Dalmatian varieties, 1-penten-3-ol is the most abundant (Table 2, Table 6 and Table 7). These differences support the hypothesis that distinct microbial populations are associated with each geographical region and variety. Interestingly, an overall increase in MBA products was observed in Istrian varieties following PEF treatment, with Rosulja showing the most pronounced increase—up to 350% compared to the control. The increase in MBA volatiles was also evident in Istarska Bjelica at moderate electric field strength. However, higher field intensities and longer treatment durations resulted in a significant reduction of approximately 60% (Figure 6a) probably because PEF’s ability to inactivate microorganisms, with the effect enhanced at higher electric field strengths [2]. In contrast, no such reduction was observed in Rosulja (Figure 6b), suggesting that the specific microbial population in this variety may be more resistant to PEF or respond differently to cell permeabilization. Martínez et al. [58] have noted that PEF-induced membrane permeabilization in microbial cells can lead to the release of intracellular metabolites into the surrounding medium. This phenomenon may explain the increase in MBA-related volatiles, as microbial metabolites are released upon membrane disruption.
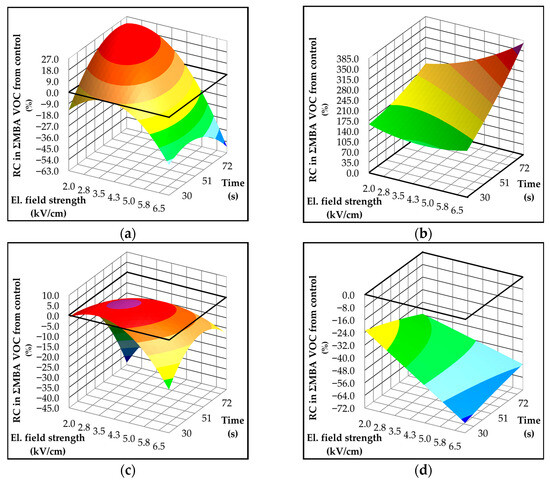
Figure 6.
Three-dimensional response surface plots showing the influence of electric field strength (kV/cm) and treatment time (s) on formation of volatile components (mg/kg) associated with the microbial activity (MBA) in Croatian olive varieties (a) Istarska Bjelica; (b) Rosulja; (c) Levantinka; (d) Oblica. Results for each variety are expressed as relative change—RC (%) in MBA-derived volatiles compared to the control: RC = 100 × (treatment − control)/control. The solid black line indicates the absolute control value (mg/kg) for each variety: 0.89 for Istarska Bjelica (a), 0.12 for Rosulja (b), 0.24 for Levantinka (c), and 0.45 for Oblica (d).
Dalmatian varieties responded to PEF treatment opposite to the Istrian ones. An increase in electric field strength led to the expected reduction in MBA-related volatiles, likely due to the inactivation of microorganisms responsible for off-flavors VOOs. As observed with OX volatiles, the predictive models for MBA compounds (Table S4) showed limited explanatory power and were unable to identify any significant influence of individual treatment parameters on the composition of MBA-derived volatiles.
Previous studies have demonstrated that PEF treatment has minimal or negligible effects on the sensory attributes of VOOs, does not induce sensory defects, and may even enhance desirable characteristics [10,11,13,14]. Our results indicate that PEF processing parameters can significantly modulate the concentration and profile of volatile compounds. This modulation is particularly pronounced in volatiles associated with the LOX pathway, although substantial alterations were also observed in volatiles linked to OX processes and in the levels of MBA compounds. Notably, the response to the PEF treatment exhibited significant dependence on the olive variety. To comprehensively evaluate the effects of these compositional changes on oil quality, future studies should integrate sensory analysis along with detailed chemical characterization.
4. Conclusions
This study evaluated the effects of PEF pretreatment on four autochthonous Croatian olive varieties, two Istrian (Istarska Bjelica and Rosulja) and two Dalmatian (Levantinka and Oblica). Different combinations of electric field strengths (2 and 7 kV/cm) and treatment durations (30 and 90 s) were applied prior to malaxation. The results showed that the PEF treatment applied in laboratory conditions improved the efficiency of oil extraction in all varieties tested, with improvements ranging from 2.5% to 36% depending on the variety. In general, the most favorable results were obtained under milder PEF conditions, namely lower field strength and/or shorter treatment duration. The greatest increase in oil yield was observed in varieties with lower initial yield, indicating that PEF is particularly effective in such cases.
The treatment had negligible effects on the basic quality parameters of the VOOs, with all values remaining well below the legal limits for extra VOO classification. In addition, significant changes were observed in the VOC content and composition of the oils produced. All varieties showed an increase in the content of the major fraction of VOCs, the ones originating from the LOX pathway, especially at lower electric field strength. However, the response of LOX enzyme activity itself was variety-dependent—it decreased in varieties with high initial activity (in Rosulja, up to 95%) and increased in varieties with lower activity (in Levantinka, up to 180%). The observed discrepancy between VOC originating from LOX path and LOX activity suggests that other endogenous enzymes involved in the formation of VOCs are also affected by PEF and that their response has a significant impact on the final aroma profile of VOO produced with PEF treatment. In addition, PEF treatment led to a consistent reduction in OX volatiles, indicating improved oxidative stability. The effects on MBA volatiles varied by variety and region, with an increase observed in Istrian varieties and a decrease in Dalmatian varieties, probably due to differences in the indigenous microbiota and their sensitivity to PEF.
Overall, PEF pretreatment is a promising non-thermal technology for enhancing olive oil yield without compromising quality, while influencing the volatile profile in a complex, variety-dependent way. These findings highlight the need to tailor PEF parameters to specific olive cultivars to achieve optimal extraction efficiency and desirable sensory characteristics. Looking ahead, scaling up PEF technology to industrial conditions will be a crucial next step. Future research should focus on process optimization across varieties, integration into existing extraction systems, and comprehensive evaluation of its effects on oil composition, sensory quality, and oxidative stability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app152212139/s1: Figure S1. Voltage of the pulse during the treatment; Figure S2. Chromatographic separation of HPOT (13(S)-Hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid) and BHT (butylated hydroxytoluene). Peaks correspond to the retention times of each compound under the described chromatographic conditions; Table S1: Average monthly air temperature (°C) and total precipitation (mm) for the 2021/2022 olive growing season with the 5-year average (2020–2024) corresponding to the study site in Dalmatia. Data were obtained from the Croatian Meteorological and Hydrological Service (CMHS) for the Split Airport meteorological station; Table S2. Average monthly air temperature (°C) and total precipitation (mm) for the 2021/2022 olive growing season with the 5-year average (2020–2024) corresponding to the study site in Istria. Data were obtained from the Croatian Meteorological and Hydrological Service (CMHS) for the Pula Airport meteorological station; Table S3. Two-factor interaction model parameters for each olive variety (regression coefficient, p-value, determination coefficient (R2), adjusted R2 and contribution of electric field strength to variability of results (%)) for yield and lipoxygenase (LOX) activity. Effects of electric field strength (kV/cm) and pulsed electric field (PEF) treatment time (s); Table S4. Two-factor interaction model parameters for each olive variety (regression coefficient, p-value, determination coefficient (R2), adjusted R2 and contribution of electric field strength to variability of results (%)) for total volatile compound (VOC) derived from LOX pathway from oxidation (OX), and microbiological activity (MBA). Effects of electric field strength (kV/cm) and pulsed electric field (PEF) treatment time (s).
Author Contributions
Conceptualization, K.K., S.B., T.V.P., Z.H. and D.Š.; Data curation, K.K. and D.Š.; Formal analysis, K.K. and D.Š.; Funding acquisition, Z.H. and D.Š.; Investigation, K.F., S.B., M.O., M.I., V.S., M.T. and M.B.; Methodology, K.K. and I.S.; Project administration, K.K. and D.Š.; Resources, K.K., T.V.P., I.S. and D.Š.; Software, K.K.; Supervision, K.K. and D.Š.; Validation, K.K.; Visualization, K.K. and D.Š.; Writing—original draft, K.K. and D.Š.; Writing—review & editing, T.V.P., V.S. and M.J.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under the project number HRZZ-IP-2020-02-7553 and by the Republic of Croatia Ministry of Science and Education through the European Regional Development Fund through the project “Equipping the Semi-Industrial Practice for the Development of New Food Technologies” (KK.01.1.1.02.0001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Acknowledgments
We thank Melisa Trputec (University of Zagreb, Faculty of Food Technology and Biotechnology) for technical assistance in olive oil analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VOO | Virgin olive oil |
| PEF | Pulsed electric field |
| PV | Peroxide value |
| VOC | Volatile organic compounds |
| LOX | Lipoxygenase |
| HPOT | Hydroperoxy-octadecatrienoic acid |
| ADH | Alcohol dehydrogenase |
| AAT | Alcohol acetyl transferase (AAT) |
| OX | Non-enzymatic oxidation of fatty acids |
| MBA | Microbiological activity |
| DAD | Diode array detector |
| ALA | α-linolenic acid |
References
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The Application of PEF Technology in Food Processing and Human Nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Yan, B.; Li, J.; Liang, Q.C.; Huang, Y.; Cao, S.L.; Wang, L.H.; Zeng, X.A. From Laboratory to Industry: The Evolution and Impact of Pulsed Electric Field Technology in Food Processing. Food Rev. Int. 2024, 41, 373–398. [Google Scholar] [CrossRef]
- Ohshima, T.; Tanino, T.; Guionet, A.; Takahashi, K.; Takaki, K. Mechanism of Pulsed Electric Field Enzyme Activity Change and Pulsed Discharge Permeabilization of Agricultural Products. Jpn. J. Appl. Phys. 2021, 60, 060501. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, R.B.; Wang, L.M.; Chen, J.; Guan, Z.C. Conformation Changes of Polyphenol Oxidase and Lipoxygenase Induced by PEF Treatment. J. Appl. Electrochem. 2010, 40, 295–301. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Sobrino-López, Á.; Soliva-Fortuny, R.; Martín-Belloso, O. Influence of High-Intensity Pulsed Electric Field Processing on Lipoxygenase and β-Glucosidase Activities in Strawberry Juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 455–462. [Google Scholar] [CrossRef]
- Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Stuparević, I.; Ivanov, M.; Vukušić Pavičić, T.; Jakoliš, N.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique. Processes 2023, 11, 3283. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Hachicha Hbaieb, R. Beyond the Traditional Virgin Olive Oil Extraction Systems: Searching Innovative and Sustainable Plant Engineering Solutions. Food Res. Int. 2013, 54, 1926–1933. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltran, G.; Sanchez-Villasclaras, S.; Uceda, M.; Jimenez, A. Kneading Olive Paste from Unripe ‘Picual’ Fruits: I. Effect on Oil Process Yield. J. Food Eng. 2010, 97, 533–538. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Ruiz-Méndez, M.V.; Sanz, C.; Martínez, M.; Rego, D.; Pérez, A.G. Application of Pulsed Electric Fields to Pilot and Industrial Scale Virgin Olive Oil Extraction: Impact on Organoleptic and Functional Quality. Foods 2022, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Tamborrino, A.; Urbani, S.; Servili, M.; Romaniello, R.; Perone, C.; Leone, A. Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process. Appl. Sci. 2020, 10, 114. [Google Scholar] [CrossRef]
- Puértolas, E.; de Marañón, I.M. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-Virgin Olive Oil Extracted Using Pulsed Electric Field Technology: Cultivar Impact on Oil Yield and Quality. Front. Nutr. 2019, 6, 134. [Google Scholar] [CrossRef]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Bubola, K.B. Inter-Varietal Diversity of Typical Volatile and Phenolic Profiles of Croatian Extra Virgin Olive Oils as Revealed by GC-IT-MS and UPLC-DAD Analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef]
- Dias, S.; Pino-Hernández, E.; Gonçalves, D.; Rego, D.; Redondo, L.; Alves, M. Challenges and Opportunities for Pilot Scaling-Up Extraction of Olive Oil Assisted by Pulsed Electric Fields: Process, Product, and Economic Evaluation. Appl. Sci. 2024, 14, 3638. [Google Scholar] [CrossRef]
- COI/OH/Doc. No 1 2011; Guide for the Determination of the Characteristics of Oil-Olives; International Olive Council: Madrid, Spain, 2011; Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf (accessed on 5 December 2023).
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-scale Optimization of Olive Oil Extraction: Simultaneous Addition of Enzymes and Microtalc Improves the Yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/71268.html (accessed on 4 December 2023).
- COI/T.20/Doc. No 34/Rev. 1 2017; Method Determination of Free Fatty Acids, Cold Method; International Olive Council: Madrid, Spain, 2017; Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-34-Rev.-1-2017.pdf (accessed on 5 December 2023).
- COI/T.20/Doc. No 19/Rev. 5 2019; Method of Analysis Spectrophotometric Investigation in the Ultraviolet; International Olive Council: Madrid, Spain, 2019; Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/Method-COI-T.20-Doc.-No-19-Rev.-5-2019-2.pdf (accessed on 4 December 2023).
- Luaces, P.; Sanz, C.; Pérez, A.G. Thermal Stability of Lipoxygenase and Hydroperoxide Lyase from Olive Fruit and Repercussion on Olive Oil Aroma Biosynthesis. J. Agric. Food Chem. 2007, 55, 6309–6313. [Google Scholar] [CrossRef]
- Soldo, B.; Šprung, M.; Mušac, G.; Pavela-Vrančić, M.; Ljubenkov, I. Evaluation of Olive Fruit Lipoxygenase Extraction Protocols on 9- and 13-Z,E-HPODE Formation. Molecules 2016, 21, 506. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Novoselić, A.; Krapac, M.; Lukić, I. Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom. Foods 2020, 9, 1445. [Google Scholar] [CrossRef]
- Marcelić, Š.; Vidović, N.; Pasković, I.; Lukić, M.; Špika, M.J.; Palčić, I.; Lukić, I.; Petek, M.; Pecina, M.; Herak Ćustić, M.; et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae 2022, 8, 203. [Google Scholar] [CrossRef]
- Majetić Germek, V.; Butinar, B.; Pizzale, L.; Bučar-Miklavčič, M.; Conte, L.S.; Koprivnjak, O. Phenols and Volatiles of Istarska Bjelica and Leccino Virgin Olive Oils Produced with Talc, NaCl and KCl as Processing Aids. J. Am. Oil Chem. Soc. 2016, 93, 1365–1372. [Google Scholar] [CrossRef]
- Škevin, D.; Balbino, S.; Žanetić, M.; Jukić Špika, M.; Koprivnjak, O.; Filipan, K.; Obranović, M.; Žanetić, K.; Smajić, E.; Radić, M.; et al. Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process. Foods 2025, 14, 2564. [Google Scholar] [CrossRef] [PubMed]
- Product-Specification-EXTRA-VIRGIN-OLIVE-OIL-OF-HERZEGOVINA. Available online: https://fsa.gov.ba/wp-content/uploads/2025/06/Product-specification-EXTRA-VIRGIN-OLIVE-OIL-OF-HERZEGOVINA.pdf (accessed on 20 May 2025).
- Žanetić, M.; Jukić Špika, M.; Ožić, M.M.; Brkić Bubola, K. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Žanetić, M.; Kraljić, K.; Pasković, I.; Škevin, D. Changes in Olive Fruit Characteristics and Oil Accumulation in ‘Oblica’ and ‘Leccino’ during Ripening. Acta Hortic. 2018, 1199, 543–548. [Google Scholar] [CrossRef]
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High Temperature Environment Reduces Olive Oil Yield and Quality. PLoS ONE 2020, 15, e0231956. [Google Scholar] [CrossRef]
- Strikic, F.; Bandelj Mavsar, D.; Perica, S.; Cmelik, Z.; Satovic, Z.; Javornik, B. The Main Croatian Olive Cultivar, ‘Oblica’, Shows High Morphological but Low Molecular Diversity. J. Hortic. Sci. Biotechnol. 2009, 84, 345–349. [Google Scholar] [CrossRef]
- Žanetić, M.; Cerretani, L.; Del Carlo, M. Preliminary Characterisation of Monovarietal Extra-Virgin Olive Oils Obtained from Different Cultivars in Croatia. J. Commod. Sci. Technol. Qual. 2007, 46, 79–94. [Google Scholar]
- Sánchez-Ortiz, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Synthesis of Volatile Compounds of Virgin Olive Oil Is Limited by the Lipoxygenase Activity Load during the Oil Extraction Process. J. Agric. Food Chem. 2012, 60, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Salas, J.J.; Williams, M.; Harwood, J.L.; Sánchez, J. Lipoxygenase Activity in Olive (Olea europaea) Fruit. J. Am. Oil Chem. Soc. 1999, 76, 1163–1168. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar Influence on the Volatile Components of Olive Oil Formed in the Lipoxygenase Pathway. LWT-Food Sci. Technol. 2021, 147, 111485. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 Varietal Virgin Olive Oils by Their Volatile Compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- García-Vico, L.; Belaj, A.; Sánchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile Compound Profiling by HS-SPME/GC-MS-FID of a Core Olive Cultivar Collection as a Tool for Aroma Improvement of Virgin Olive Oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the Key Aroma Compounds in Turkish Olive Oils from Different Geographic Origins by Application of Aroma Extract Dilution Analysis (AEDA). J. Agric. Food Chem. 2014, 62, 391–401. [Google Scholar] [CrossRef]
- Soldo, B.; Jukić Špika, M.; Pasković, I.; Vuko, E.; Polić Pasković, M.; Ljubenkov, I. The Composition of Volatiles and the Role of Non-Traditional LOX on Target Metabolites in Virgin Olive Oil from Autochthonous Dalmatian Cultivars. Molecules 2024, 29, 1696. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Attilio, C.; Serraiocco, A. Regional and Cultivar Comparison of Italian Single Cultivar Olive Oils According to Flavor Profiling. Eur. J. Lipid Sci. Technol. 2013, 115, 196–210. [Google Scholar] [CrossRef]
- Padilla, M.N.; Hernández, M.L.; Sanz, C.; Martínez-Rivas, J.M. Stress-Dependent Regulation of 13-Lipoxygenases and 13-Hydroperoxide Lyase in Olive Fruit Mesocarp. Phytochemistry 2014, 102, 80–88. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of Agroclimatic Parameters on Phenolic and Volatile Compounds of Chilean Virgin Olive Oils and Characterization Based on Geographical Origin, Cultivar and Ripening Stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 38, pp. 83–147. [Google Scholar]
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C.I.G.; Jerković, I. Phytochemicals and Other Characteristics of Croatian Monovarietal Extra Virgin Olive Oils from Oblica, Lastovka and Levantinka Varieties. Molecules 2015, 20, 4395–4409. [Google Scholar] [CrossRef]
- Angerosa, F.; Lanza, B.; Marsilio, V. Biogenesis of “fusty” defect in virgin olive oil. Grasas Aceites 1996, 47, 142–150. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Li, G.; Li, C.; Li, W.; Bi, Y.; Wei, J. Pulsed Electric Field Treatment Improves the Oil Yield, Quality, and Antioxidant Activity of Virgin Olive Oil. Food Chem. X 2024, 22, 101372. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Ripalda, M.; Herrero-Continente, T.; Barranquero, C.; Dávalos, A.; de las Hazas, M.C.L.; Álvarez-Lanzarote, I.; Sánchez-Gimeno, A.C.; Raso, J.; Arnal, C.; et al. Pulsed Electric Field Increases the Extraction Yield of Extra Virgin Olive Oil without Loss of Its Biological Properties. Front. Nutr. 2022, 9, 1065543. [Google Scholar] [CrossRef] [PubMed]
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, A.C. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioproc. Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Grgić, T.; Bleha, R.; Smrčkova, P.; Stulić, V.; Pavičić, T.V.; Synytsya, A.; Iveković, D.; Novotni, D. Pulsed Electric Field Treatment of Oat and Barley Flour: Influence on Enzymes, Non-Starch Polysaccharides, Dough Rheological Properties, and Application in Flat Bread. Food Bioproc. Technol. 2024, 17, 4303–4324. [Google Scholar] [CrossRef]
- Töpfl, S. Pulsed Electric Fields (PEF) for Permeabilization of Cell Membranes in Food-and Bioprocessing: Applications, Process and Equipment Design and Cost Analysis. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 22 September 2006. [Google Scholar]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Commission Delegated Regulation Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. 2022. Official Journal of the European Union 2022, L 282, 1–52.
- Fakas, S.; Kefalogianni, I.; Makri, A.; Tsoumpeli, G.; Rouni, G.; Gardeli, C.; Papanikolaou, S.; Aggelis, G. Characterization of Olive Fruit Microflora and Its Effect on Olive Oil Volatile Compounds Biogenesis. Eur. J. Lipid Sci. Technol. 2010, 112, 1024–1032. [Google Scholar] [CrossRef]
- Saldaña, G.; Álvarez, I.; Condón, S.; Raso, J. Microbiological Aspects Related to the Feasibility of PEF Technology for Food Pasteurization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1415–1426. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food. Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).