Aerosol Spraying of Carbon Nanofiber-Based Films for NO2 Detection: The Role of the Spraying Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensing Material
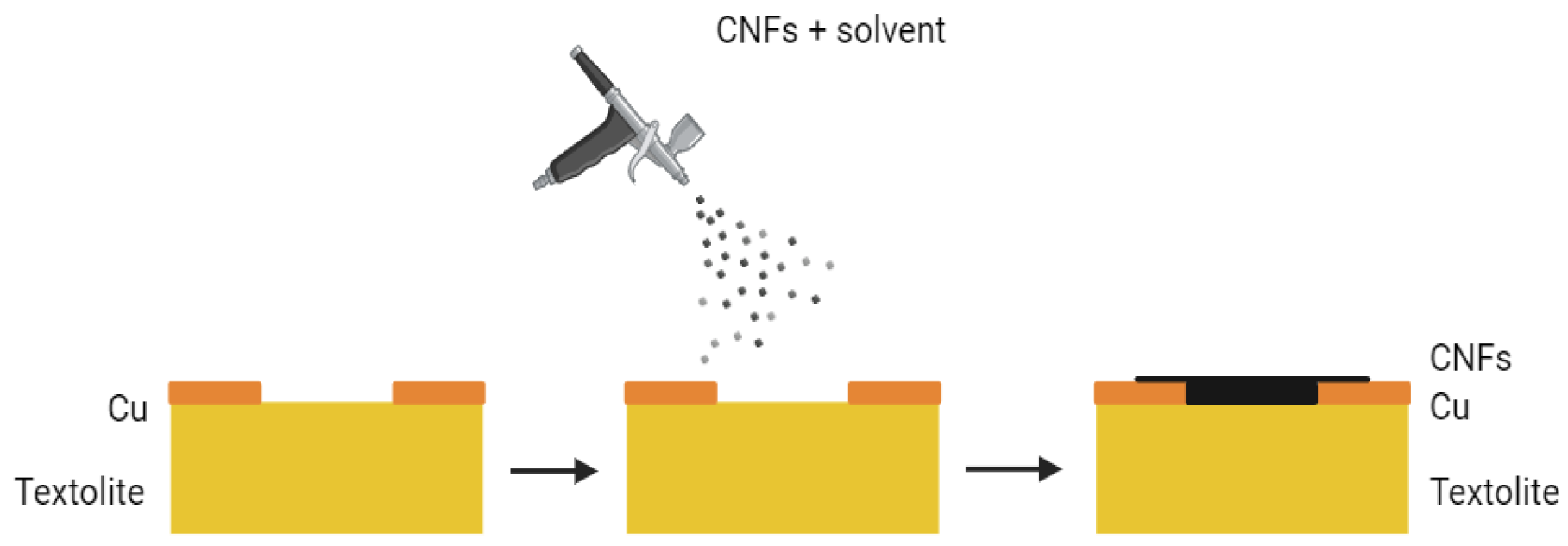
2.2. Aerosol Spraying of CNF-Based Films
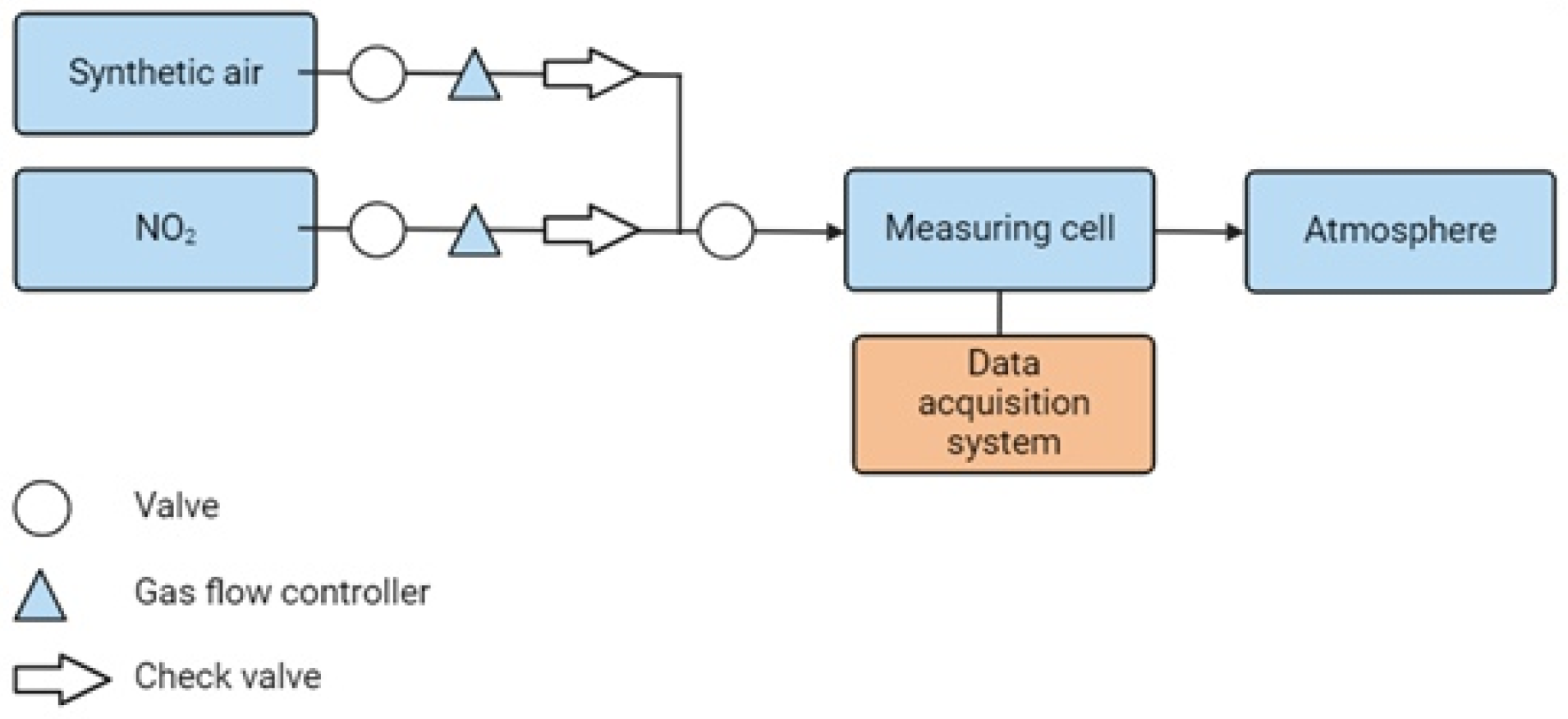
2.3. Testing of Sensors
2.4. Characterization
3. Results and Discussion
3.1. Carbon Nanomaterials
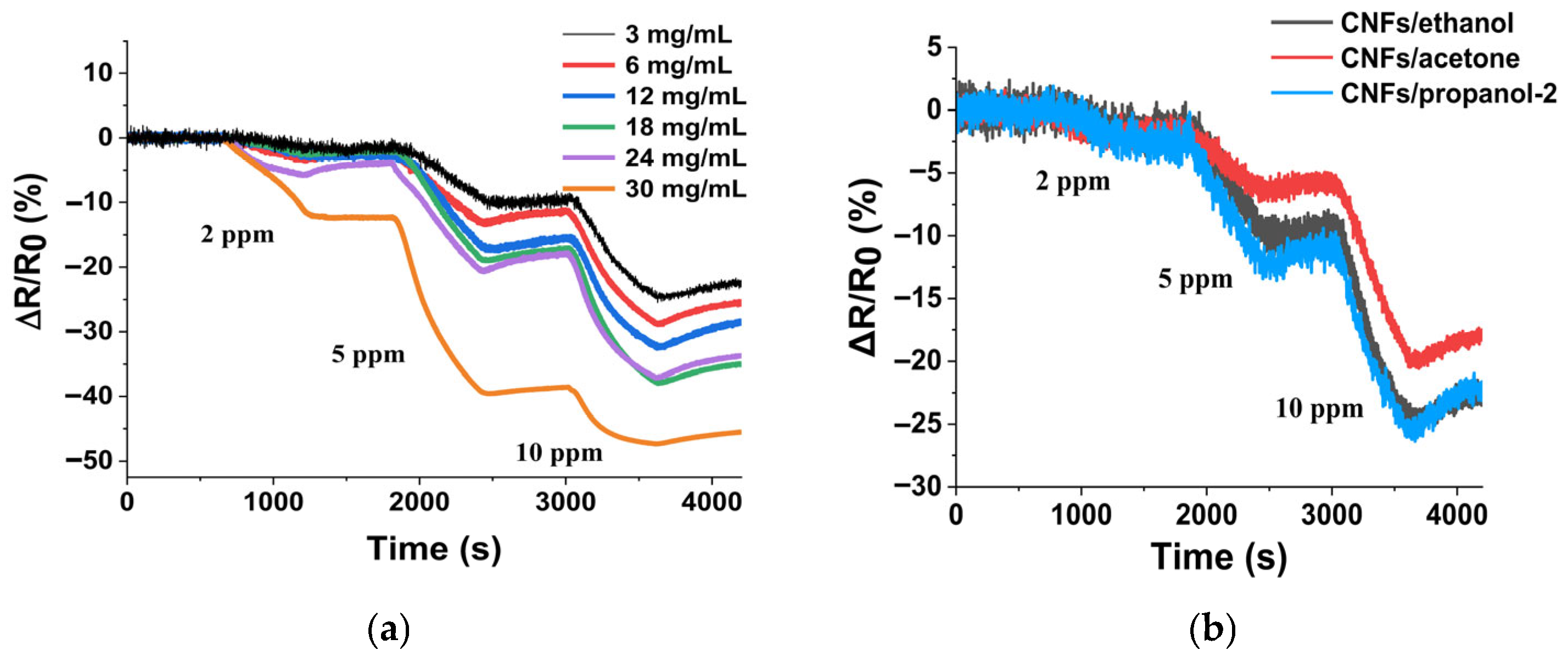
3.2. Effect of Concentration of Sprayed CNF/Ethanol Mixture
3.3. Effect of CNF Film Thickness
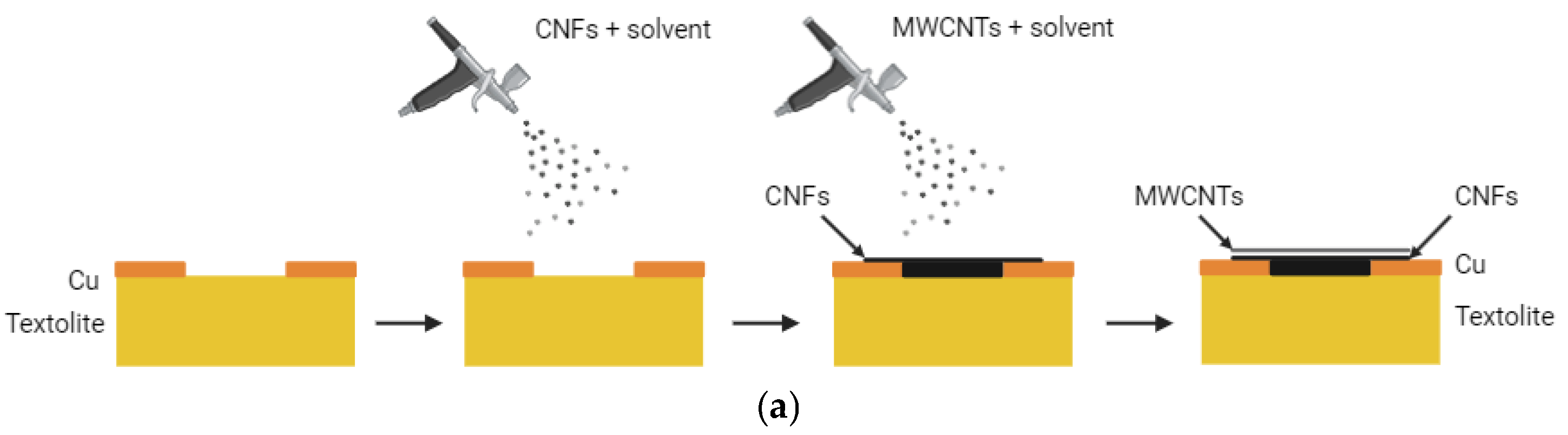
3.4. All-Carbon Hybrid Films Based on CNFs and MWCNTs
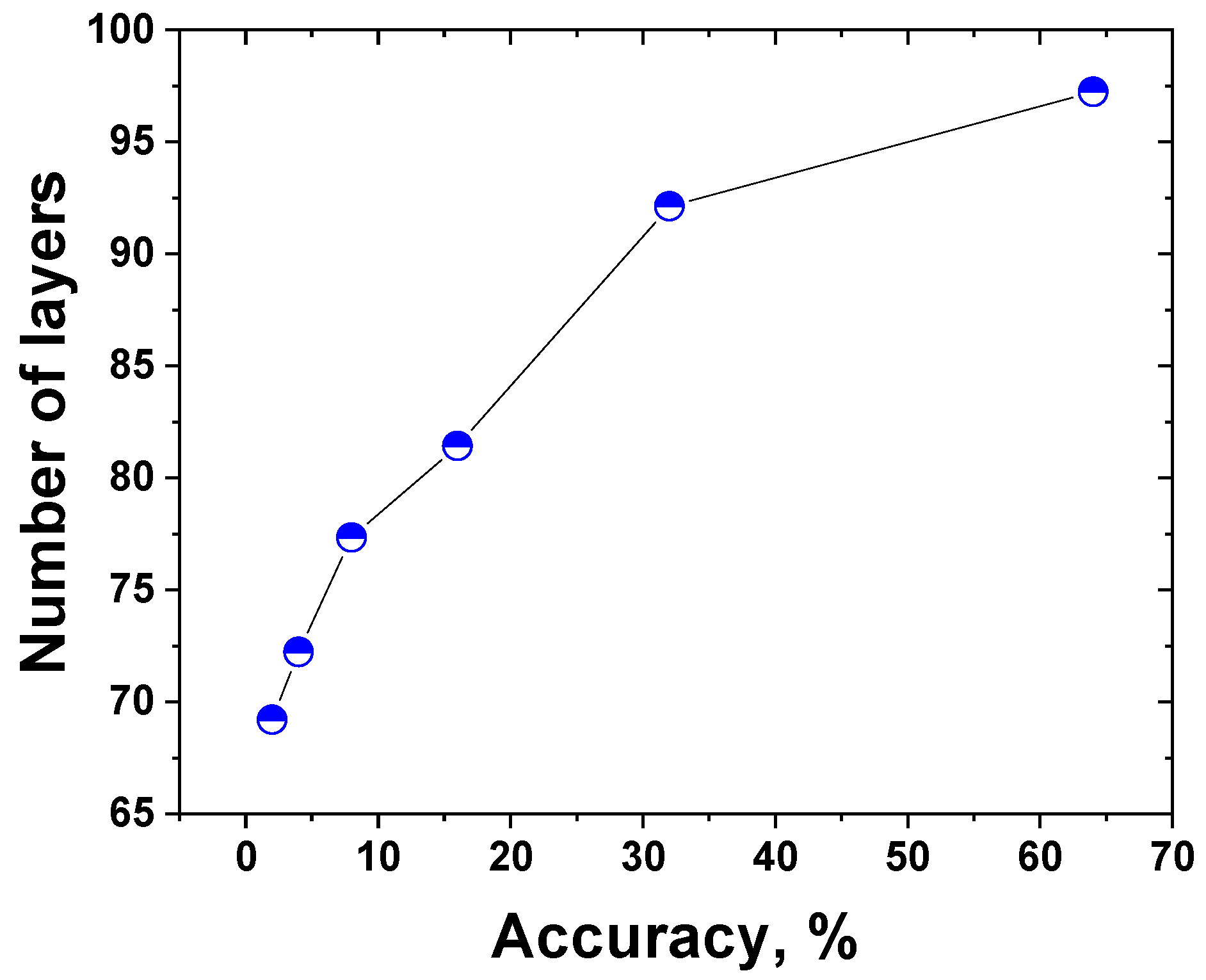
3.5. Machine Learning
- NH3 with a sharply increasing resistance;
- NH3 with a long and strong decrease in resistance before the onset of growth;
- NO2 with an initial rise in resistance followed by a decline;
- NO2 with a continuous decrease in resistance.
- 5.
- Initialization of initial values: i = 0, m—number of shifts, m = 0, width of window is w = 1500 data points. Window will be moved through the range [l0,lmax], where l0 = 0, lmax = 7000 with a step of Δl = 200 datapoints; initial range [a0,b0], where a0 = l0, b0 = w.
- 6.
- A linear regression is used to determine the slope of the time series, which is constructed on the segment [am,bm]. The sign and magnitude of the estimated slope coefficient φm allow us to infer the direction and intensity of data change over time.
- 7.
- m = m + 1, am = am−1 + Δl, bm = bm−1 + Δl.
- 8.
- If bm = lmax, then the algorithm terminates and returns to i = 0.
- 9.
- A linear regression is constructed on the segment [am,bm] and is determined.
- 10.
- If the sign of H has not changed compared to , then proceed to step 3; otherwise, the algorithm terminates and returns i = (am + bm)/2.
- Training set, accounting for 80% of the total size, i.e., 88,566 elements;
- Validation set, accounting for 10% of the total size, i.e., 11,071 elements;
- Test set, accounting for 10% of the total size, i.e., 11,071 elements.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, Y.-S.; Bhardwaj, A.; Hong, J.-W.; Im, H.-N.; Song, S.-J. Sensing Performance of a YSZ-Based Electrochemical NO2 Sensor Using Nanocomposite Electrodes. J. Electrochem. Soc. 2019, 166, B799–B804. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Y.; Liu, X.; Fu, F.; Gong, Y.; Rao, Y.J.; Chen, Y. Miniature Fiber-Optic NH3 Gas Sensor Based on Pt Nanoparticle-Incorporated Graphene Oxide. Sens. Actuators B Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Chougule, S.S.; Bak, N.-H.; Nam, D.J.; Jung, N.; Cho, H.D.; Kim, S.G.; Kim, M.D. High Performance Langasite Based SAW NO2 Gas Sensor Using 2D g-C3N4@TiO2 Hybrid Nanocomposite. J. Hazard. Mater. 2022, 427, 128174. [Google Scholar] [CrossRef]
- Zamani, C.; Shimanoe, K.; Yamazoe, N. A New Capacitive-Type NO2 Gas Sensor Combining an MIS with a Solid Electrolyte. Sens. Actuators B Chem. 2005, 109, 216–220. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An Overview: Facet-Dependent Metal Oxide Semiconductor Gas Sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas Sensing Based on Organic Composite Materials: Review of Sensor Types, Progresses and Challenges. Mater. Sci. Semicond. Process. 2021, 128, 105744. [Google Scholar] [CrossRef]
- Ma, D.; Su, Y.; Tian, T.; Yin, H.; Huo, T.; Shao, F.; Yang, Z.; Hu, N.; Zhang, Y. Highly Sensitive Room-Temperature NO2Gas Sensors Based on Three-Dimensional Multiwalled Carbon Nanotube Networks on SiO2 Nanospheres. ACS Sustain. Chem. Eng. 2020, 8, 13915–13923. [Google Scholar] [CrossRef]
- Yi, N.; Cheng, Z.; Li, H.; Yang, L.; Zhu, J.; Zheng, X.; Chen, Y.; Liu, Z.; Zhu, H.; Cheng, H. Stretchable, Ultrasensitive, and Low-Temperature NO2 Sensors Based on MoS2@rGO Nanocomposites. Mater. Today Phys. 2020, 15, 100265. [Google Scholar] [CrossRef]
- Salih, E.; Ayesh, A.I. First Principle Study of Transition Metals Codoped MoS2 as a Gas Sensor for the Detection of NO and NO2 Gases. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 131, 114736. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Kumar, A.; Kashyap, R.; Kumar, D.; Kumar, M. Chemically Functionalized Graphene Oxide Thin Films for Selective Ammonia Gas Sensing. Mater. Res. Express 2020, 7, 015612. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.H.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-Enriched WS2 Nanosheets on Carbon Nanofibers Boosts NO2 Detection at Room Temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Madrigal, M.A.; Montejo-Alvaro, F.; Cernas-Ruiz, A.S.; Rojas-Chávez, H.; Román-Doval, R.; Cruz-Martinez, H.; Medina, D.I. Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors. Int. J. Mol. Sci. 2021, 22, 12968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative Detection of Formaldehyde and Ammonia Gas via Metal Oxide-Modified Graphene-Based Sensor Array Combining with Neural Network Model. Sens. Actuators B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Bielecki, Z.; Stacewicz, T.; Smulko, J.; Wojtas, J. Ammonia Gas Sensors: Comparison of Solid-State and Optical Methods. Appl. Sci. 2020, 10, 5111. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 1–32. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, W.; Zhang, M.; Wang, Y.; Duan, Z.; Tan, C.; Liu, B.; Ouyang, F.; Yuan, Z.; Tai, H.; et al. Edge-Enriched Mo2TiC2Tx/MoS2 Heterostructure with Coupling Interface for Selective NO2 Monitoring. Adv. Funct. Mater. 2022, 32, 2203528. [Google Scholar] [CrossRef]
- Lee, K.; Yoo, Y.K.; Chae, M.S.; Hwang, K.S.; Lee, J.; Kim, H.; Hur, D.; Lee, J.H. Highly Selective Reduced Graphene Oxide (RGO) Sensor Based on a Peptide Aptamer Receptor for Detecting Explosives. Sci. Rep. 2019, 9, 10297. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for Sub-Ppm NO2 Gas Detection Based on Carbon Nanotube Thin Films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Barthwal, S.; Singh, B.; Singh, N.B. ZnO-SWCNT Nanocomposite as NO2 Gas Sensor. Mater. Today Proc. 2018, 5, 15439–15444. [Google Scholar] [CrossRef]
- Oweis, R.J.; Albiss, B.A.; Al-Widyan, M.I.; Al-Akhras, M.A. Hybrid Zinc Oxide Nanorods/Carbon Nanotubes Composite for Nitrogen Dioxide Gas Sensing. J. Electron. Mater. 2014, 43, 3222–3228. [Google Scholar] [CrossRef]
- Huang, M.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Pd-Loaded In2O3 Nanowire-like Network Synthesized Using Carbon Nanotube Templates for Enhancing NO2 Sensing Performance. RSC Adv. 2015, 5, 30038–30045. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Zhang, Y. High-Performance NO2 Gas Sensor Based on Bimetallic Oxide CuWO4 Decorated with Reduced Graphene Oxide. J. Mater. Sci. Mater. Electron. 2020, 31, 6706–6715. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Zheng, Y.; Lee, J.H.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Enhancement of H2S Sensing Performance of P-CuO Nanofibers by Loading p-Reduced Graphene Oxide Nanosheets. Sens. Actuators B Chem. 2019, 281, 453–461. [Google Scholar] [CrossRef]
- Lumbers, B.; Agar, D.W.; Gebel, J.; Platte, F. Mathematical Modelling and Simulation of the Thermo-Catalytic Decomposition of Methane for Economically Improved Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 4265–4283. [Google Scholar] [CrossRef]
- Bannov, A.G.; Lapekin, N.I.; Kurmashov, P.B.; Ukhina, A.V.; Manakhov, A. Room-Temperature NO2 Gas Sensors Based on Granulated Carbon Nanofiber Material. Chemosensors 2022, 10, 525. [Google Scholar] [CrossRef]
- Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors 2022, 22, 5316. [Google Scholar] [CrossRef]
- Orlando, A.; Mushtaq, A.; Gaiardo, A.; Valt, M.; Vanzetti, L.; Costa Angeli, M.A.; Avancini, E.; Shkodra, B.; Petrelli, M.; Tosato, P.; et al. The Influence of Surfactants on the Deposition and Performance of Single-Walled Carbon Nanotube-Based Gas Sensors for NO2 and NH3 Detection. Chemosensors 2023, 11, 127. [Google Scholar] [CrossRef]
- Kuvshinov, G.G.; Mogilnykh, Y.I.; Kuvshinov, D.G.; Yermakov, D.Y.; Yermakova, M.A.; Salanov, A.N.; Rudina, N.A. Mechanism of Porous Filamentous Carbon Granule Formation on Catalytic Hydrocarbon Decomposition. Carbon 1999, 37, 1239–1246. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly Sensitive NO2 Gas Sensor of Ppb-Level Detection Based on In2O3 Nanobricks at Low Temperature. Sens. Actuators B Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.; Byun, Y.T. Highly Sensitive and Selective NO2 Detection by Pt Nanoparticles-Decorated Single-Walled Carbon Nanotubes and the Underlying Sensing Mechanism. Sens. Actuators B Chem. 2017, 238, 1032–1042. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron-Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Sivakumar, R.; Krishnamoorthi, K.; Vadivel, S.; Govindasamy, S. Progress towards a Novel NO2 Gas Sensor Based on SnO2/RGO Hybrid Sensors by a Facial Hydrothermal Approach. Diam. Relat. Mater. 2021, 116, 108418. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Chen, H. MOF-Derived Indium Oxide Hollow Microtubes/MoS2 Nanoparticles for NO2 Gas Sensing. Sens. Actuators B Chem. 2019, 300, 127037. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Wu, M.-J.; Yeh, T.-H.; Lee, C.-T.; Lee, H.-Y. Sensing Mechanism and Characterization of NO2 Gas Sensors Using Gold-Black NP-Decorated Ga2O3 Nanorod Sensing Membranes. ACS Sens. 2024, 9, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Sandler, J.K.W.; Shaffer, M.S.P.; Schwarz, M.K.; Bauhofer, W.; Schulte, K.; Windle, A.H. Formation of Percolating Networks in Multi-Wall Carbon-Nanotube-Epoxy Composites. Compos. Sci. Technol. 2004, 64, 2309–2316. [Google Scholar] [CrossRef]
- Heaney, M. Complex Ac Conductivity of a Carbon Black Composite as a Function of Frequency, Composition, and Temperature. Phys. Rev. B-Condens. Matter Mater. Phys. 1999, 60, 12746–12751. [Google Scholar] [CrossRef]
- Li, W.; Lefferts, M.J.; Armitage, B.I.; Murugappan, K.; Castell, M.R. Polypyrrole Percolation Network Gas Sensors: Improved Reproducibility through Conductance Monitoring during Polymer Growth. ACS Appl. Polym. Mater. 2022, 4, 2536–2543. [Google Scholar] [CrossRef]
- Armitage, B.I.; Murugappan, K.; Lefferts, M.J.; Cowsik, A.; Castell, M.R. Conducting Polymer Percolation Gas Sensor on a Flexible Substrate. J. Mater. Chem. C 2020, 8, 12669–12676. [Google Scholar] [CrossRef]
- Mukherjee, A.; Jaidev, L.R.; Chatterjee, K.; Misra, A. Nanoscale Heterojunctions of RGO-MoS2 composites for Nitrogen Dioxide Sensing at Room Temperature. Nano Express 2020, 1, 010003. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Konstantinova, E.; Marikutsa, A.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Gaskov, A. Effect of Humidity on Light-Activated No and No2 Gas Sensing by Hybrid Materials. Nanomaterials 2020, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Worsley, M.A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. Effects of Ambient Humidity and Temperature on the NO2 Sensing Characteristics of WS2/Graphene Aerogel. Appl. Surf. Sci. 2018, 450, 372–379. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Q.; Han, F.; Wang, Z.; Tian, B.; Zhao, L.; Dong, T.; Jiang, Z. A Flexible and Wearable NO2 Gas Detection and Early Warning Device Based on a Spraying Process and an Interdigital Electrode at Room Temperature. Microsyst. Nanoeng. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.T.; Navale, S.T.; Patil, V.B. Room Temperature NO2 Gas Sensing Properties of DBSA Doped PPy-WO3 Hybrid Nanocomposite Sensor. Org. Electron. 2015, 19, 15–25. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, D.S.; Choi, H.K.; Lee, D.H.; Kim, J.E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.Y. Flexible Room-Temperature NO2 Gas Sensors Based on Carbon Nanotubes/Reduced Graphene Hybrid Films. Appl. Phys. Lett. 2010, 96, 2–5. [Google Scholar] [CrossRef]
- Xie, T.; Sullivan, N.; Steffens, K.; Wen, B.; Liu, G.; Debnath, R.; Davydov, A.; Gomez, R.; Motayed, A. UV-Assisted Room-Temperature Chemiresistive NO2 Sensor Based on TiO2 Thin Film. J. Alloys Compd. 2015, 653, 255–259. [Google Scholar] [CrossRef]
- Ochieng, P.J.; Maróti, Z.; Dombi, J.; Krész, M.; Békési, J.; Kalmár, T. Adaptive Savitzky–Golay Filters for Analysis of Copy Number Variation Peaks from Whole-Exome Sequencing Data. Information 2023, 14, 128. [Google Scholar] [CrossRef]
- Chow, G.C. Tests of Equality Between Sets of Coefficients in Two Linear Regressions. Econometrica 1960, 28, 591–605. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J.L. Adam: A Method for Stochastic Optimization. In Proceedings of the 3rd International Conference for Learning Representations, San Diego, CA, USA, 7–9 May 2015; pp. 1–15. [Google Scholar]




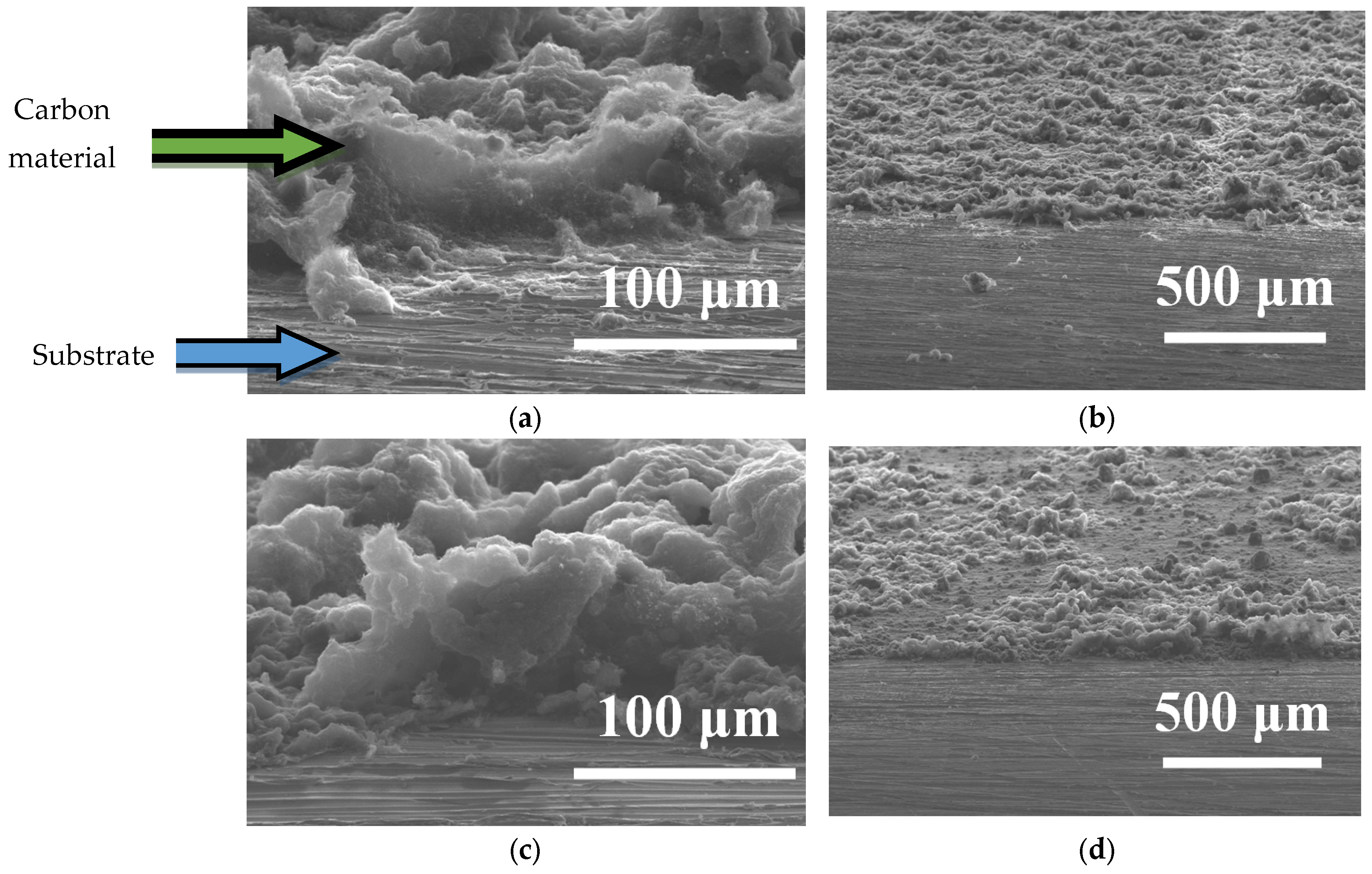
| Properties | CNFs | MWCNTs#1 | MWCNTs#2 |
|---|---|---|---|
| Average diameter 1, nm | 31.2 ± 14.3 | 35.0 ± 3.0 | 26.8 ± 9.0 |
| I(D)/I(G) 2 | 1.0 | 0.56 | 0.64 |
| Specific surface area 3, m2/g | 119 | 68 | 82 |
| Concentration, mg/mL | ΔR/R0, % | Response Time at 2 ppm, s | Recovery Degree at 2 ppm, % | R0, kΩ | SNR | ||
|---|---|---|---|---|---|---|---|
| 2 ppm | 5 ppm | 10 ppm | |||||
| 3 | 1.23 | 9.87 | 24.5 | 387 | 24.5 | 20.4 | 144:1 |
| 6 | 2.7 | 12 | 28.5 | 452 | 25.3 | 7.5 | 782:1 |
| 12 | 2.7 | 16.5 | 32 | 456 | 3.7 | 3.4 | 825:1 |
| 18 | 2.7 | 18.5 | 37.7 | 468 | 20.8 | 3 | 879:1 |
| 24 | 5.6 | 20 | 37 | 471 | 31.2 | 2.47 | 1106:1 |
| 30 | 12 | 39 | 47.3 | 541 | 0 | 0.375 | 1577:1 |
| Solvent | ΔR/R0, % | Response Time at 2 ppm, s | Recovery Degree at 2 ppm, % | R0, kΩ | SNR | ||
|---|---|---|---|---|---|---|---|
| 2 ppm | 5 ppm | 10 ppm | |||||
| Ethanol | 1.23 | 9.87 | 24.5 | 387 | 24.5 | 20.4 | 144:1 |
| Acetone | 1.94 | 6.4 | 19.9 | 438 | 25.7 | 13.4 | 250:1 |
| Propanol-2 | 1.82 | 12 | 25 | 444 | 6 | 42 | 88.5:1 |
| Average Thickness of the Film, μm | ΔR/R0, % | Response Time at 2 ppm, s | Recovery Degree at 2 ppm, % | R0, kΩ | SNR | ||
|---|---|---|---|---|---|---|---|
| 2 ppm | 5 ppm | 10 ppm | |||||
| 15 ± 5 | 7 | 32.9 | 46.2 | 731 | 19.7 | 2.76 | 735:1 |
| 40 ± 7 | 9.4 | 35 | 45.7 | 762 | 11.6 | 1.62 | 1583:1 |
| 55 ± 15 | 10.6 | 35.5 | 46.3 | 754 | 10.5 | 1.14 | 1892:1 |
| Sample | Operating Temperature, °C | NO2 Concentration, ppm | Sensor Response ΔR/R0, % | Ref. |
|---|---|---|---|---|
| 11.1 wt% rGO/SnO2 | 25 | 100 | 26 | [43] |
| PPy-WO3 | 38 | 5 | 15 | [44] |
| CNTs/rGO | 20 | 10 | 20 | [45] |
| CNFs (drop casting) | 25 | 10 | 5.1 | [25] |
| TiO2 | n/a (room temperature) | 100 | 2.4 | [46] |
| CNFs (aerosol spraying; 30 mg/mL) | 25 | 2 | 12 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishin, A.; Golovakhin, V.; Maksimovskiy, E.; Vostretsova, E.; Timofeev, V.; Bannov, A. Aerosol Spraying of Carbon Nanofiber-Based Films for NO2 Detection: The Role of the Spraying Technique. Appl. Sci. 2025, 15, 12110. https://doi.org/10.3390/app152212110
Shishin A, Golovakhin V, Maksimovskiy E, Vostretsova E, Timofeev V, Bannov A. Aerosol Spraying of Carbon Nanofiber-Based Films for NO2 Detection: The Role of the Spraying Technique. Applied Sciences. 2025; 15(22):12110. https://doi.org/10.3390/app152212110
Chicago/Turabian StyleShishin, Artyom, Valeriy Golovakhin, Eugene Maksimovskiy, Ekaterina Vostretsova, Vladimir Timofeev, and Alexander Bannov. 2025. "Aerosol Spraying of Carbon Nanofiber-Based Films for NO2 Detection: The Role of the Spraying Technique" Applied Sciences 15, no. 22: 12110. https://doi.org/10.3390/app152212110
APA StyleShishin, A., Golovakhin, V., Maksimovskiy, E., Vostretsova, E., Timofeev, V., & Bannov, A. (2025). Aerosol Spraying of Carbon Nanofiber-Based Films for NO2 Detection: The Role of the Spraying Technique. Applied Sciences, 15(22), 12110. https://doi.org/10.3390/app152212110






