Evaluation of Morphological, Chemical, and Antioxidant Characteristics, and Phenolic Profile of Three Goji Berry Varieties Cultivated in Southwestern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Sample Collection
2.3. Morphological Measurements
2.4. Moisture Content
2.5. Titratable Acidity (TA), pH, and Total Soluble Solids (TSSs)
2.6. Color
2.7. Measurements for the Evaluation of Texture Properties
2.8. Phenolic Compounds Extraction
2.9. Antioxidant Activity
2.9.1. ABTS Assay
2.9.2. DPPH Assay
2.10. Phenolic Compounds Profile
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphological Parameters
3.2. Moisture Content, Total Soluble Solids (TSSs), Titratable Acidity (TA) and pH
3.3. Color
3.4. Texture Analyses
3.5. Antioxidant Capacity
3.6. Total Polyphenols and Phenolic Compound Profile
3.7. ANOVA–Simultaneous Component Analysis (ASCA)
3.8. Pearson Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of Berries and Berry-Based Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji Berry Fruit (Lycium spp.): Antioxidant Compound Fingerprint and Bioactivity Evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality Variation of Goji (Fruits of Lycium spp.) in China: A Comparative Morphological and Metabolomic Analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Vidovic, B.B.; Milincic, D.D.; Kostic, A.Ž.; Pešic, M.B.; Marcetic, M.D.; Djuriš, J.D.; Ilic, T.D. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum Berries (Solanaceae) as Source of Bioactive Compounds for Healthy Purposes: A Review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef]
- Poggioni, L.; Cantini, C.; Binelli, G.; Cai, G.; Conti, V.; Mareri, L.; Romi, M.; Piccini, C. Molecular Analysis by Microsatellite Markers of Goji Plants (Lycium barbarum L.) Grown in Central Italy Reveal Genetic Distinction from Both L. barbarum and L. chinense Species. Plants 2025, 14, 1182. [Google Scholar] [CrossRef]
- Breniere, T.; Fanciullino, A.; Bertin, N.; Borel, P. Effect of Long-Term de Fi Cit Irrigation on Tomato and Goji Berry Quality: From Fruit Composition to in Vitro Bioaccessibility of Carotenoids. Front. Plant Sci. 2024, 15, 1339536. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the Bioactive Compounds, Antioxidant Activity and Chemical Composition of Brazilian Blackberry, Red Raspberry, Strawberry, Blueberry and Sweet Cherry Fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Principal, A.; Ciceoi, R.; Luchian, V.; Tabacu, A.F.; Gutue, M.; Stavrescu-Bedivan, M.M. Goji Berry Gall Mite Expansion in Europe, with Emphasis on Southeastern Part of Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2022, 78, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Leontopoulos, S.; Lampakis, D. Goji Berry: Health Promoting Properties. Nutraceuticals 2022, 2, 32–48. [Google Scholar] [CrossRef]
- Rehman, F.; Zeng, S.; Zhao, Y.; Zhao, J.; Qin, K.; Yang, C.; Huang, H.; Wang, Y. Preservation and Innovation of Goji Berry Germplasm Resources. Med. Plant Biol. 2024, 3, e022. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Amone, F.; Malivindi, R.; Gorgoglione, D.; De Biasio, F.; Scrivano, L.; Pezzi, V.; Puoci, F. Calabrian Goji vs. Chinese Goji: A Comparative Study on Biological Properties. Foods 2017, 6, 30. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic Compounds in Lycium Berry: Composition, Health Benefits and Industrial Applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Povolo, C.; Foschini, A.; Ribaudo, G. Optimization of the Extraction of Bioactive Molecules from Lycium barbarum Fruits and Evaluation of the Antioxidant Activity: A Combined Study. Nat. Prod. Res. 2019, 33, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS Analysis of Bioactive Constituents from Two Romanian Goji (Lycium barbarum L.) Berries Cultivars and Their Antioxidant, Enzyme Inhibitory, and Real-Time Cytotoxicological Evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Dzugalov, H.; Lichev, V.; Yordanov, A.; Kaymakanov, P.; Dimitrova, V.; Kutoranov, G. First Results of Testing Goji Berry (Lycium barbarum L.) in Plovdiv Region, Bulgaria. Sci. Pap. Ser. B Hortic. 2015, LIX, 47–50. [Google Scholar]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical Analysis and Antioxidant Activity of Lycium barbarum (Goji) Cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Tsaniklidis, G.; Papadakis, I.; Ioannou, D.; Tzoutzoukou, C.; Tsantili, E. Nutritional Value and Consumer-Perceived Quality of Fresh Goji Berries (Lycium barbarum L. and L. chinense L.) from Plants Cultivated in Southern Europe. Fruits 2018, 73, 5–12. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the Antioxidant and Antimutagenic Activity of Extracts from Goji Berry of Greek Cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Compounds Profile, Nutritional Compounds and Bioactive Properties of Lycium barbarum L.: A Comparative Study with Stems and Fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Protti, M.; Gualandi, I.; Mandrioli, R.; Zappoli, S.; Tonelli, D.; Mercolini, L. Analytical Profiling of Selected Antioxidants and Total Antioxidant Capacity of Goji (Lycium spp.) Berries. J. Pharm. Biomed. Anal. 2017, 143, 252–260. [Google Scholar] [CrossRef]
- Ilic, T.; Dodevska, M.; Bozic, D.; Kodranov, I.; Marcetic, M.; Vidovic, B. Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef]
- Einpresswire. Available online: https://www.einpresswire.com (accessed on 11 March 2025).
- Einpresswire. The Global Demand for Goji Berries Set to Surge, Market to Grow to $1.89 Billion in 2028. 2024. Available online: https://www.einpresswire.com/article/762854229/the-global-demand-for-goji-berries-set-to-surge-market-to-grow-to-1-89-billion-in-2028 (accessed on 11 March 2025).
- Yu, J.; Yan, Y.; Zhang, L.; Mi, J.; Yu, L.; Zhang, F.; Lu, L.; Luo, Q.; Li, X.; Zhou, X.; et al. A Comprehensive Review of Goji Berry Processing and Utilization. Food Sci. Nutr. 2023, 11, 7445–7457. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional Constituents and Antioxidant Activities of Eight Chinese Native Goji Genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The Effects of Postharvest Application of Lecithin to Improve Storage Potential and Quality of Fresh Goji (Lycium barbarum L.) Berries. Food Chem. 2017, 230, 241–249. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Zhao, Y.; Chen, S.; Yao, H.; Wang, H.; Wang, C.; Wu, Q. Effects of Short-Term Low Salinity Stress on Non-Volatile Flavor Substances of Muscle and Hepatopancreas in Portunus Trituberculatus. J. Food Compos. Anal. 2022, 109, 104520. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; Colelli, G. Quality of Goji Berry Fruit (Lycium barbarum L.) Stored at Different Temperatures. Foods 2022, 11, 3700. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. Goji Berry (Lycium barbarum): Composition and Health Effects—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects–A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maurer, J.B.B.; Zawadzki-Baggio, S.F.; Ávila, S.; Maciel, G.M.; Haminiuk, C.W.I. Bioactive Compounds of Organic Goji Berry (Lycium barbarum L.) Prevents Oxidative Deterioration of Soybean Oil. Ind. Crops Prod. 2018, 112, 90–97. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D. Health-Promoting Effects of Goji Berries (Lycium barbarum): A Literature Overview. Biol. Life Sci. Forum 2024, 40, 1. [Google Scholar] [CrossRef]
- AOAC International. Methods for the Determination of Moisture, Ash, Protein and Fat. In Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Sánchez-Parra, M.; Ordóñez-Díaz, J.L.; Pérez-Aparicio, J.; Moreno-Rojas, J.M. Physicochemical and Microbiological Changes Associated with Processing in Dry-Cured Tuna. Appl. Sci. 2023, 13, 5900. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture Profiling of Blueberries (Vaccinium spp.) during Fruit Development, Ripening and Storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Silva, J.L.; Marroquin, E.; Matta, F.B.; Garner, J.O.; Stojanovic, J. Physicochemical, Carbohydrate and Sensory Characteristics of Highbush and Rabbiteye Blueberry Cultivars. J. Sci. Food Agric. 2005, 85, 1815–1821. [Google Scholar] [CrossRef]
- Christofi, M.; Mourtzinos, I.; Lazaridou, A.; Drogoudi, P.; Tsitlakidou, P.; Biliaderis, C.G.; Manganaris, G.A. Elaboration of Novel and Comprehensive Protocols toward Determination of Textural Properties and Other Sensorial Attributes of Canning Peach Fruit. J. Texture Stud. 2021, 52, 228–239. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of Rootstock and Harvesting Period on the Bioactive Compounds and Antioxidant Activity of Two Orange Cultivars (‘Salustiana’ and ‘Sanguinelli’) Widely Used in Juice Industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Velasco-Ruiz, I.; Velasco-Tejero, C.; Pereira-Caro, G.; Moreno-Rojas, J.M. Seasonal and Morphology Effects on Bioactive Compounds, Antioxidant Capacity, and Sugars Profile of Black Carrot (Daucus carota ssp. sativus Var. atrorubens Alef.). Foods 2024, 13, 1575. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-Check: Validation of Diagnostic Statistics for PLS-DA Models in Metabolomics Studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, M.; Wang, Y.; Zhang, L.; Yao, W.; Feng, M. Integrated Metabolome and Transcriptome Analysis of Differences in Quality of Ripe Lycium barbarum L. Fruits Harvested at Different Periods. BMC Plant Biol. 2024, 24, 82. [Google Scholar] [CrossRef]
- Kruczek, A.; Ochmian, I.; Krupa-Małkiewicz, M.; Lachowicz, S. Comparison of morphological, antidiabetic and antioxidant properties of goji fruits. Acta Univ. Cibiniensis. Ser. E Food Technol. 2020, XXIV, 1–14. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor Quality of Fruits and Vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Viškelis, P.; Šarkinas, A. Determination of the Total Phenolic and Anthocyanin Contents and Antimicrobial Activity of Viburnum opulus Fruit Juice. Plant Foods Hum. Nutr. 2012, 67, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Classification of Textural Characteristics. J. Food Sci. 1963, 28, 385–389. [Google Scholar] [CrossRef]
- Ciacciulli, A.; Chiozzotto, R.; Attanasio, G.; Cirilli, M.; Bassi, D. Identification of a Melting Type Variant among Peach (P. persica L. Batsch) Fruit Textures by a Digital Penetrometer. J. Texture Stud. 2018, 49, 370–377. [Google Scholar] [CrossRef]
- Fuentes-Pérez, M.D.C.; Nogales-Delgado, S.; Ayuso, M.C.; Bohoyo-Gil, D. Different Peach Cultivars and Their Suitability for Minimal Processing. Czech J. Food Sci. 2014, 32, 413–421. [Google Scholar] [CrossRef]
- Río Segade, S.; Soto Vázquez, E.; Díaz Losada, E. Influence of Ripeness Grade on Accumulation and Extractability of Grape Skin Anthocyanins in Different Cultivars. J. Food Compos. Anal. 2008, 21, 599–607. [Google Scholar] [CrossRef]
- Giongo, L.; Ajelli, M.; Poncetta, P.; Ramos-García, M.; Sambo, P.; Farneti, B. Raspberry Texture Mechanical Profiling during Fruit Ripening and Storage. Postharvest Biol. Technol. 2019, 149, 177–186. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Song, W.O.; Fernandez, M.L.; Bruno, R.S.; Koo, S.I.; Chun, O.K. Development and Validation of an Algorithm to Establish a Total Antioxidant Capacity Database of the US Diet. Int. J. Food Sci. Nutr. 2010, 61, 600–623. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous Determination of Phenolic Acids and Flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A Systematic UHPLC Q-ToF MS Approach for the Characterization of Bioactive Compounds from Freeze-Dried Red Goji Berries (L. barbarum L.) Grown in Serbia: Phenolic Compounds and Phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium barbarum (Goji) as Functional Food: A Review of Its Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10621–10635. [Google Scholar] [CrossRef]
- Zhao, W.H.; Shi, Y.P. Comprehensive Analysis of Phenolic Compounds in Four Varieties of Goji Berries at Different Ripening Stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Mao, Y.; Luo, J.; Cai, Z. Biosynthesis and Regulatory Mechanisms of Plant Flavonoids: A Review. Plants 2025, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [PubMed]
- Gharras, H. El Original Article Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Teissedre, P.; Glories, Y. Effect of PH, Ethanol and Acidity on Astringency and Bitterness of Grape Seed Tannin Oligomers in Model Wine Solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Lopez-Moreno, H.; Phillips, M.; Diaz-Garcia, L.; Torres-Meraz, M.; Jarquin, D.; Loarca, J.; Ikeda, S.; Giongo, L.; Grygleski, E.; Iorizzo, M.; et al. Multiparametric Cranberry (Vaccinium macrocarpon Ait.) Fruit Textural Trait Development for Harvest and Postharvest Evaluation in Representative Cultivars. J. Texture Stud. 2024, 55, e12866. [Google Scholar] [CrossRef] [PubMed]
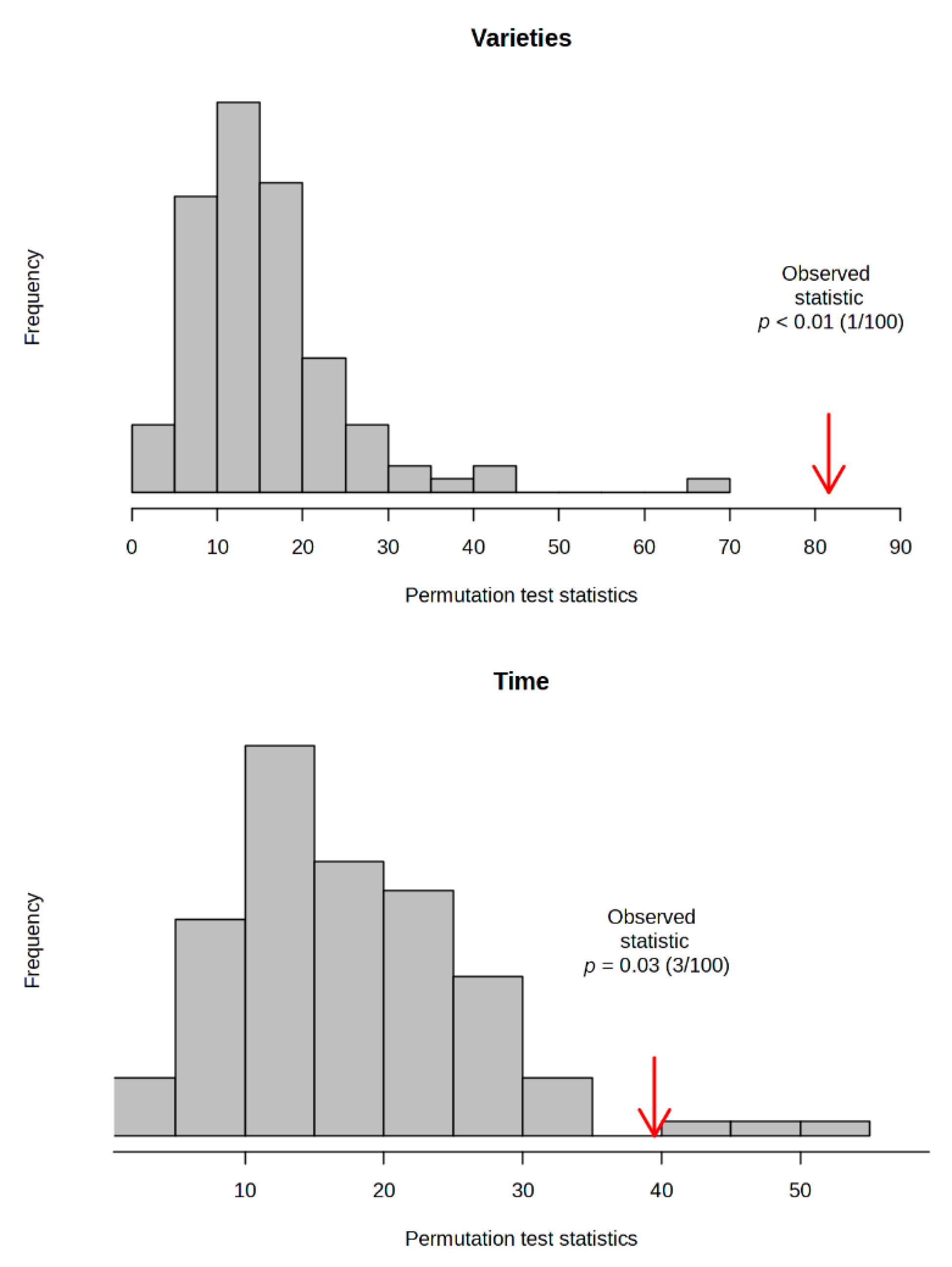

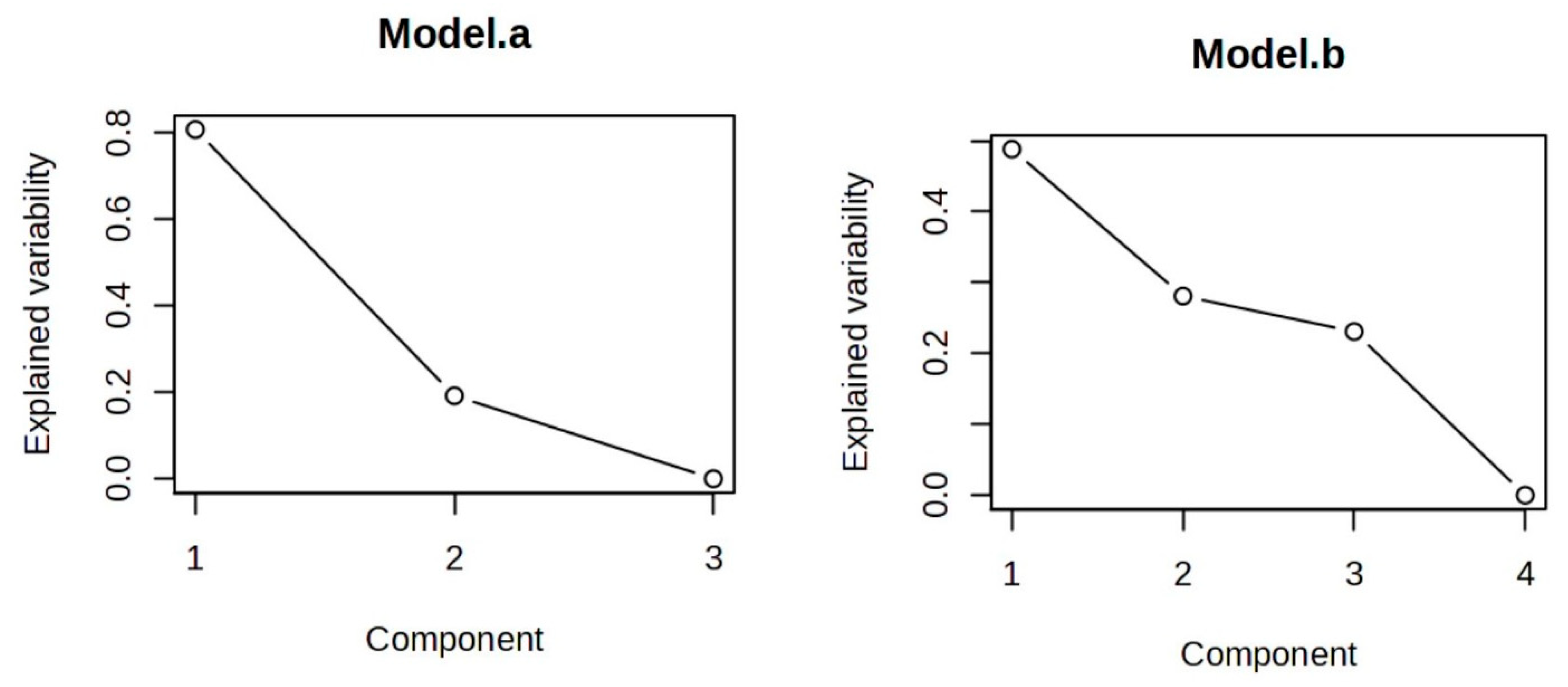
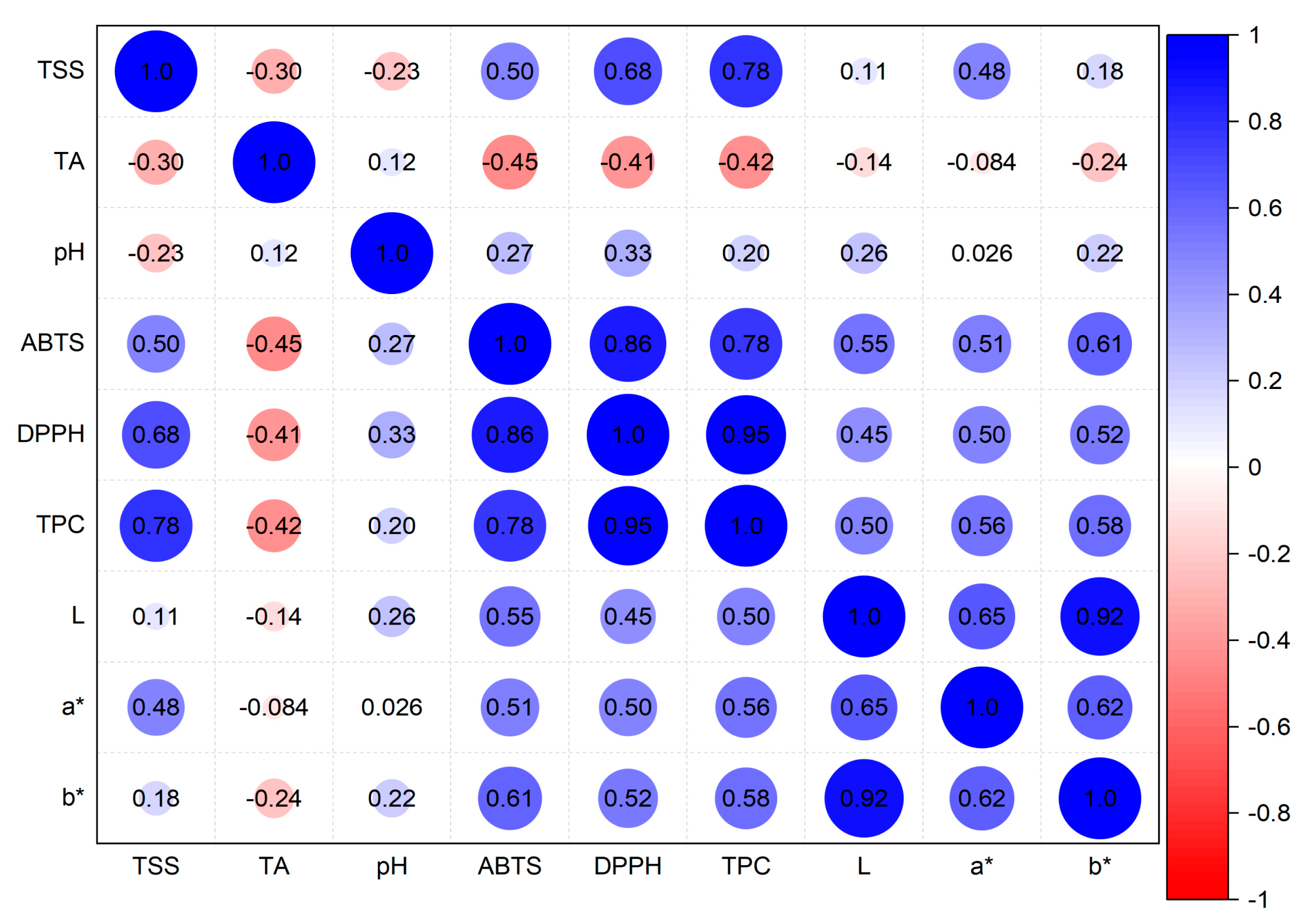
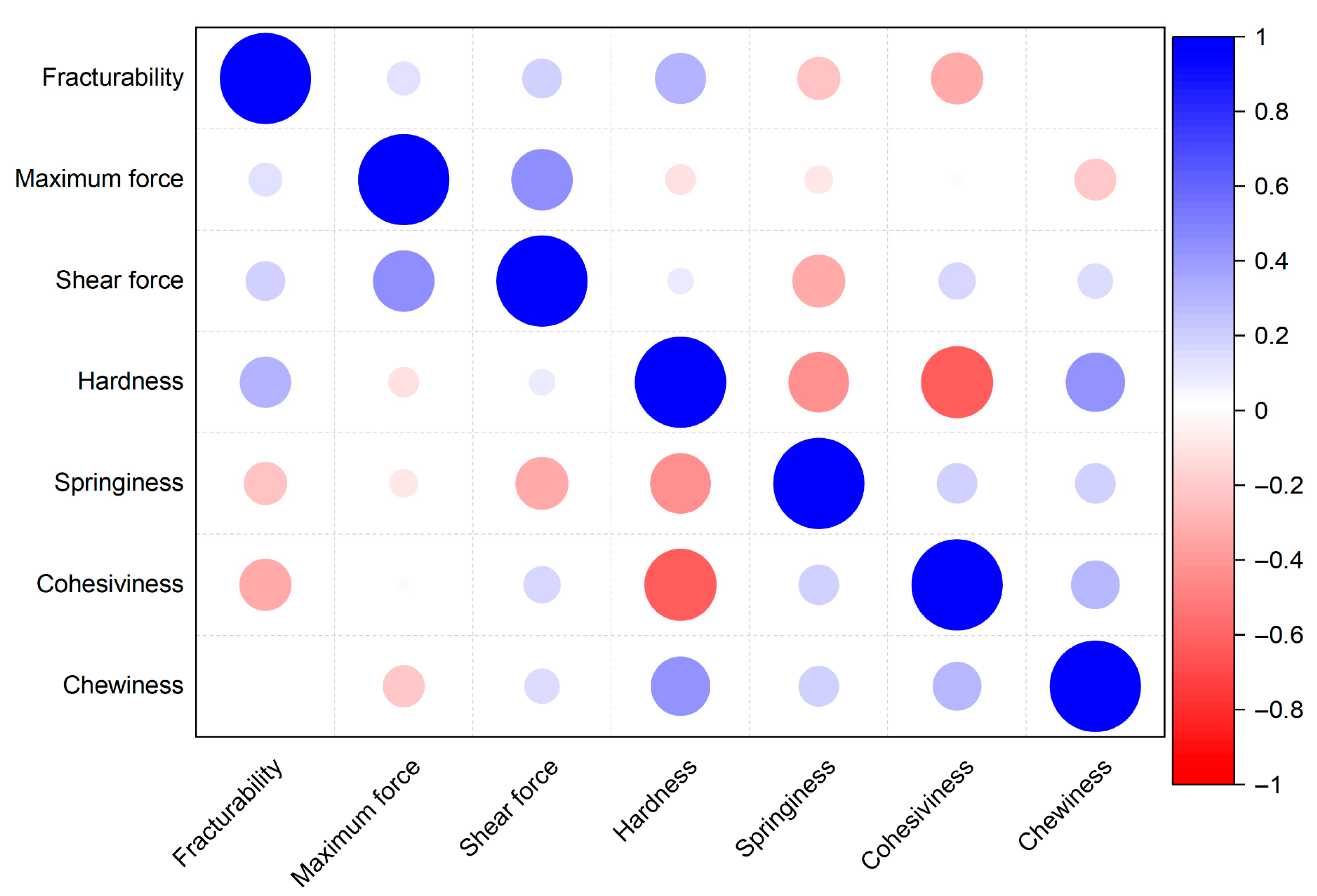

| Varieties | Harvesting Time | ||||||||
| NQ1 | NQ7 | V3 | p-Value | T1 | T2 | T3 | T4 | p-Value | |
| Weight (g/20 berries) | 6.69 ± 0.72 b | 9.79 ± 2.03 a | 9.35 ± 4.92 a | *** | 7.24 ± 1.58 b | 7.98 ± 3.53 b | 8.05 ± 1.22 b | 11.17 ± 4.69 a | *** |
| Width (mm) | 7.59 ± 0.96 b | 9.25 ± 1.15 a | 7.58 ± 1.60 b | *** | 7.82 ± 1.27 b | 7.95 ± 2.06 ab | 8.07 ± 0.97 ab | 8.71 ± 1.36 a | * |
| Length (mm) | 13.32 ± 1.55 b | 15.01 ± 2.64 a | 14.59 ± 3.10 ab | ** | 11.87 ± 1.73 b | 14.73 ± 2.42 a | 15.29 ± 1.88 a | 15.34 ± 2.61 a | *** |
| Moisture (%) | 81.54 ± 2.53 a | 79.06 ± 2.60 b | 80.08 ± 2.60 ab | * | 79.14 ± 1.65 | 79.10 ± 2.76 | 81.75 ± 2.06 | 80.91 ± 3.47 | ns |
| TTA 1 (% citric acid) | 1.10 ± 0.06 b | 0.94 ± 0.12 c | 1.17 ± 0.29 a | *** | 1.21 ± 0.35 a | 0.96 ± 0.12 c | 1.07 ± 0.06 b | 1.04 ± 0.10 b | *** |
| pH | 5.28 ± 0.37 b | 5.19 ± 0.55 c | 5.56 ± 0.83 a | *** | 5.37 ± 0.13 b | 5.22 ± 0.11 d | 5.48 ± 0.62 a | 5.31 ± 0.50 c | *** |
| TSS 2 (°Brix) | 15.41 ± 5.28 ab | 16.65 ± 3.56 a | 13.54 ± 2.74 b | ** | 18.29 ± 4.77 a | 16.21 ± 1.70 ab | 12.82 ± 3.17 c | 13.47 ± 3.99 bc | *** |
| L* | 41.97 ± 3.58 b | 46.30 ± 2.74 a | 42.41 ± 3.36 b | *** | 43.57 ± 2.49 ab | 42.79 ± 3.78 b | 46.02 ± 3.42 a | 41.85 ± 4.10 b | ** |
| a* | 37.37 ± 4.65 b | 41.26 ± 2.57 a | 35.52 ± 3.21 b | *** | 38.52 ± 3.50 | 38.35 ± 3.54 | 38.96 ± 4.16 | 36.37 ± 5.52 | ns |
| b* | 26.89 ± 5.81 b | 35.30 ± 3.29 a | 28.17 ± 4.50 b | *** | 29.70 ± 4.10 b | 28.96 ± 5.07 b | 33.64 ± 5.87 a | 28.18 ± 7.13 b | ** |
| C | 46.11 ± 7.01 b | 54.34 ± 3.64 a | 45.45 ± 4.35 b | *** | 48.68 ± 5.03 ab | 48.14 ± 5.48 ab | 51.63 ± 6.06 a | 46.10 ± 8.48 b | * |
| h° | 35.30 ± 3.44 c | 40.52 ± 2.16 a | 38.26 ± 4.27 b | *** | 37.53 ± 2.31 b | 36.83 ± 3.40 b | 40.59 ± 4.53 a | 37.15 ± 4.42 b | *** |
| ABTS 3 | 1.01 ± 0.10 b | 1.19 ± 0.09 a | 0.94 ± 0.10 c | *** | 1.00 ± 0.12 b | 1.09 ± 0.12 a | 1.16 ± 0.12 a | 0.93 ± 0.12 b | *** |
| DPPH 3 | 0.88 ± 0.05 b | 1.22 ± 0.12 a | 0.82 ± 0.04 c | *** | 0.97 ± 0.20 b | 1.01 ± 0.20 ab | 1.03 ± 0.24 a | 0.88 ± 0.13 c | *** |
| Varieties | Harvesting Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NQ1 | NQ7 | V3 | p-Value | T1 | T2 | T3 | T4 | p-Value | |
| Texture analysis profile (TPA) | |||||||||
| Hardness (N) | 3.05 ± 0.95 a | 2.06 ± 0.83 b | 2.68 ± 0.63 ab | ** | 2.51 ± 0.89 | 2.91 ± 1.08 | 2.22 ± 0.81 | 2.74 ± 0.76 | ns |
| Springiness (N/mm) | 0.72 ± 0.16 b | 0.79 ± 0.05 a | 0.75 ± 0.08 ab | * | 0.78 ± 0.08 ab | 0.70 ± 0.13 b | 0.72 ± 0.07 b | 0.82 ± 0.12 a | ** |
| Cohesiveness (N/mm) | 0.35 ± 0.09 b | 0.42 ± 0.07 ab | 0.44 ± 0.10 a | ** | 0.48 ± 0.12 a | 0.36 ± 0.08 b | 0.41 ± 0.05 ab | 0.36 ± 0.08 b | ** |
| Chewiness (N) | 0.71 ± 0.14 b | 0.66 ± 0.23 b | 0.89 ± 0.37 a | * | 0.92 ± 0.43 | 0.68 ± 0.16 | 0.64 ± 0.14 | 0.77 ± 0.21 | ns |
| 2 mm sensor | |||||||||
| Fracturability (N) | 2.13 ± 0.41 a | 1.62 ± 0.42 b | 2.25 ± 0.91 a | ** | 1.58 ± 0.39 b | 2.65 ± 0.93 a | 1.80 ± 0.23 b | 1.97 ± 0.40 b | *** |
| Mini Kramer/Ottawa sensor | |||||||||
| Maximum force (N) | 19.66 ± 9.05 b | 26.95 ± 5.96 a | 20.42 ± 9.92 ab | * | 18.73 ± 9.40 b | 29.98 ± 6.11 a | 20.39 ± 10.28 b | 20.26 ± 4.89 b | ** |
| Shear force (N/g of the sample) | 140.20 ± 57.30 c | 170.36 ± 26.77 b | 207.51 ± 40.46 a | *** | 164.03 ± 28.38 b | 205.51 ± 24.86 a | 165.12 ± 53.39 b | 156.09 ± 72.22 b | ** |
| Varieties | Harvesting Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NQ1 | NQ7 | V3 | p-Value | T1 | T2 | T3 | T4 | p-Value | |
| Caffeic acid | 0.014 ± 0.013 b | n.d. | 0.020 ± 0.018 a | *** | 0.006 ± 0.005 c | 0.024 ± 0.018 a | 0.015 ± 0.017 b | 0.001 ± 0.001 d | *** |
| Caffeoylglucoside | 0.410 ± 0.064 c | 0.625 ± 0.060 a | 0.473 ± 0.077 b | *** | 0.486 ± 0.078 bc | 0.555 ± 0.109 a | 0.542 ± 0.127 ab | 0.429 ± 0.109 c | *** |
| Caffeoylquinic acid | 0.269 ± 0.220 a | 0.053 ± 0.022 b | 0.286 ± 0.262 a | *** | 0.178 ± 0.226 bc | 0.398 ± 0.255 a | 0.182 ± 0.169 b | 0.053 ± 0.020 c | *** |
| 4,5-Dicaffeoylquinic acid | 0.002 ± 0.002 b | 0.003 ± 0.003 a | 0.002 ± 0.002 c | *** | 0.002 ± 0.002 bc | 0.005 ± 0.002 a | 0.001 ± 0.001 c | 0.001 ± 0.001 c | *** |
| Chlorogenic acid | 0.455 ± 0.340 b | 0.079 ± 0.047 c | 0.601 ± 0.575 a | *** | 0.257 ± 0.320 bc | 0.811 ± 0.582 a | 0.319 ± 0.302 b | 0.126 ± 0.057 c | *** |
| Ferulic acid | 0.293 ± 0.319 a | 0.335 ± 0.251 a | 0.211 ± 0.222 b | *** | 0.020 ± 0.006 c | 0.081 ± 0.112 c | 0.403 ± 0.086 b | 0.614 ± 0.104 a | *** |
| Feruloylglucose | 5.221 ± 1.220 a | 3.727 ± 0.882 b | 5.147 ± 1.676 a | *** | 6.607 ± 1.335 a | 4.492 ± 0.647 b | 3.820 ± 0.793 b | 3.875 ± 0.619 b | *** |
| Dihydroferulic acid 4-glucuronide | 0.056 ± 0.033 b | 3.069 ± 1.152 a | 0.092 ± 0.044 b | *** | 0.776 ± 1.085 c | 1.047 ± 1.470 b | 0.790 ± 1.168 c | 1.676 ± 2.467 a | *** |
| Dihydroisoferulic acid | 0.005 ± 0.001 a | 0.004 ± 0.001 b | 0.005 ± 0.001 a | *** | 0.004 ± 0.001 bc | 0.006 ± 0.002 a | 0.004 ± 0.001 b | 0.005 ± 0.001 a | *** |
| Dihydroferulic acid | 0.019 ± 0.004 c | 0.040 ± 0.007 a | 0.035 ± 0.008 b | *** | 0.039 ± 0.012 a | 0.029 ± 0.010 bc | 0.031 ± 0.012 b | 0.026 ± 0.009 c | *** |
| p-coumaric acid | 0.045 ± 0.021 b | 2.034 ± 0.660 a | 0.112 ± 0.047 b | *** | 0.636 ± 0.903 bc | 0.454 ± 0.626 c | 0.770 ± 1.062 b | 1.060 ±1.458 a | *** |
| Coumaroylglucoside | 0.150 ± 0.026 b | 3.913 ± 1.477 a | 0.234 ± 0.040 b | *** | 2.146 ± 3.035 a | 0.920 ± 1.075 d | 1.214 ± 1.615 c | 1.450 ± 1.966 b | *** |
| Coumaroyl dihexoside | 0.045 ± 0.015 b | 0.809 ± 0.067 a | 0.070 ± 0.034 b | *** | 0.311 ± 0.388 | 0.287 ± 0.396 | 0.298 ± 0.349 | 0.336 ± 0.424 | ns |
| Sinapic acid | 0.009 ± 0.011 c | 0.015 ± 0.010 b | 0.018 ± 0.016 a | *** | 0.001 ± 0.001 d | 0.032 ± 0.008 a | 0.014 ± 0.006 b | 0.009 ± 0.005 c | *** |
| Sinapoyl hexoside | 1.608 ± 0.324 a | 1.153 ± 0.137 b | 0.869 ± 0.057 c | *** | 1.233 ± 0.533 a | 1.046 ± 0.113 b | 1.284 ± 0.395 ab | 1.278 ± 0.354 a | ** |
| Total hydroxicinnamic acids | 8.603 ± 1.531 b | 15.858 ± 2.414 a | 8.175 ± 1.336 b | *** | 12.701 ± 3.916 a | 10.185 ± 2.509 bc | 9.687 ± 3.382 c | 10.940 ± 5.853 b | *** |
| Rutin | 0.129 ± 0.037 b | 30.947 ± 2.636 a | 0.147 ± 0.117 b | *** | 11.618 ± 17.882 a | 10.647 ± 16.130 b | 9.812 ± 14.980 c | 9.552 ± 14.654 c | *** |
| Quercetin rutinoside (Rutin isomer) | 0.375 ± 0.050 a | n.d. | 0.365 ± 0.187 a | *** | 0.286 ± 0.222 a | 0.329 ± 0.277 a | 0.219 ± 0.179 b | 0.154 ± 0.138 c | *** |
| Rutin hexoside | 0.060 ± 0.018 b | 0.003 ± 0.001 c | 0.066 ± 0.035 a | *** | 0.034 ± 0.025 c | 0.058 ± 0.052 a | 0.048 ± 0.038 b | 0.031 ± 0.024 c | *** |
| Quercetin 3-glucoside (Isoquercitrin) | 0.006 ± 0.004 b | 0.354 ± 0.065 a | 0.015 ± 0.008 b | *** | 0.109 ± 0.158 ab | 0.108 ± 0.139 b | 0.141 ± 0.204 ab | 0.142 ± 0.212 a | * |
| Kaempferol rutinoside | 0.162 ± 0.099 b | 2.077 ± 0.374 a | 0.066 ± 0.034 b | *** | 0.682 ± 0.975 bc | 0.617 ± 0.811 c | 0.952 ± 1.248 ab | 0.822 ± 1.042 ab | *** |
| Isorhamnetin rutinoside | 0.044 ± 0.036 b | 2.732 ± 0.653 a | 0.024 ± 0.014 b | *** | 0.870 ± 1.312 b | 0.799 ± 1.196 b | 1.252 ± 1.912 ab | 0.812 ± 1.182 b | ** |
| Isorhamnetin glucoside | 0.001 ± 0.001 b | 0.021 ± 0.004 a | 0.002 ± 0.002 b | *** | 0.007 ± 0.009 b | 0.008 ± 0.008 ab | 0.010 ± 0.012 ab | 0.008 ± 0.011 ab | ** |
| Taxifolin | 0.034 ± 0.020 a | 0.004 ± 0.001 c | 0.021 ± 0.018 b | *** | 0.006 ± 0.003 c | 0.021 ± 0.013 b | 0.034 ± 0.024 ab | 0.018 ± 0.022 b | *** |
| Total flavonols | 0.810 ± 0.143 b | 36.152 ± 2.462 a | 0.707 ± 0.383 b | *** | 13.611 ± 20.083 a | 12.595 ± 17.980 ab | 12.473 ± 18.127 bc | 11.546 ± 16.945 c | *** |
| Apigenin | 0.001 ± 0.001 b | 0.002 ± 0.002 a | n.d. | *** | 0.0005 ± 0.0006 c | 0.0019 ± 0.0024 a | 0.0012 ± 0.0011 b | 0.0008 ± 0.0004 c | *** |
| Apigenin rutinoside | n.d. | 0.001 ± 0.001 b | 0.001 ± 0.001 a | *** | 0.0005 ± 0.0004 b | 0.0005 ± 0.0002 b | 0.0011 ± 0.0006 a | 0.0006 ± 0.0002 b | *** |
| Diosmetin | 0.004 ± 0.001 b | 0.002 ± 0.001 c | 0.014 ± 0.007 a | *** | 0.004 ± 0.002 b | 0.005 ± 0.003 b | 0.009 ± 0.009 a | 0.009 ± 0.009 a | *** |
| Total flavones | 0.005 ± 0.001 b | 0.005 ± 0.003 b | 0.015 ± 0.007 a | *** | 0.005 ± 0.003 c | 0.008 ± 0.002 b | 0.011 ± 0.009 a | 0.010 ± 0.009 a | *** |
| Naringenin | 3.007 ± 1.502 b | 10.639 ± 5.123 a | 2.198 ± 1.768 c | *** | 2.979 ± 1.388 c | 7.106 ± 6.214 a | 7.308 ± 6.68 a | 3.733 ± 3.210 b | *** |
| Naringenin 6-glucoside | 1.663 ± 0.554 b | 2.422 ± 1.190 a | 1.243 ± 0.774 c | *** | 0.957 ± 0.159 d | 2.088 ± 0.445 b | 2.766 ± 1.092 a | 1.293 ± 0.776 c | *** |
| Narirutin | 0.039 ± 0.032 b | 7.047 ± 1.352 a | 0.214 ± 0.071 b | *** | 2.764 ± 4.106 a | 2.319 ±2.542 b | 2.582 ± 3.959 a | 2.495 ± 3.827 a | ** |
| Narirutin glucoside | 4.278 ± 1.227 a | 2.637 ± 0.639 b | 1.691 ± 0.651 c | *** | 3.104 ± 1.285 b | 1.773 ± 0.262 c | 3.693 ±1.495 a | 2.358 ± 1.801 c | *** |
| Eriodyctiol | 0.047 ± 0.026 a | 0.027 ± 0.017 b | 0.030 ± 0.022 b | *** | 0.020 ± 0.013 c | 0.039 ± 0.018 b | 0.046 ± 0.029 a | 0.035 ± 0.026 b | *** |
| Total flavanones | 9.034 ± 2.520 b | 22.772 ± 5.980 a | 5.286 ± 2.781 c | *** | 9.824 ± 5.498 c | 13.324 ± 8.968 b | 16.395 ± 11.595 a | 9.914 ± 7.769 c | *** |
| Phloretin | 0.003 ± 0.001 a | n.d. | 0.002 ± 0.002 b | *** | 0.001 ± 0.001 b | 0.003 ± 0.002 a | 0.003 ± 0.002 a | 0.001 ± 0.001 b | *** |
| Phloridzin | 0.002 ± 0.001 a | n.d. | 0.001 ± 0.001 b | *** | 0.001 ± 0.001 b | 0.001 ± 0.001 a | 0.001 ± 0.001 a | 0.001 ± 0.001 c | *** |
| Total dihydrochalcones | 0.005 ± 0.001 a | n.d. | 0.004 ± 0.003 b | *** | 0.002 ± 0.001 b | 0.004 ± 0.003 a | 0.004 ± 0.003 a | 0.002 ± 0.002 b | *** |
| Total phenolic compounds | 18.458 ± 2.630 b | 74.787 ± 4.339 a | 14.186 ± 4.120 c | *** | 36.143 ± 29.382 a | 36.115 ± 29.366 a | 38.570 ± 32.885 a | 32.412 ± 29.804 b | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Garrido, M.E.; Sánchez-Parra, M.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M. Evaluation of Morphological, Chemical, and Antioxidant Characteristics, and Phenolic Profile of Three Goji Berry Varieties Cultivated in Southwestern Spain. Appl. Sci. 2025, 15, 11999. https://doi.org/10.3390/app152211999
García-Garrido ME, Sánchez-Parra M, Ordóñez-Díaz JL, Moreno-Rojas JM. Evaluation of Morphological, Chemical, and Antioxidant Characteristics, and Phenolic Profile of Three Goji Berry Varieties Cultivated in Southwestern Spain. Applied Sciences. 2025; 15(22):11999. https://doi.org/10.3390/app152211999
Chicago/Turabian StyleGarcía-Garrido, María Elena, Mónica Sánchez-Parra, José Luis Ordóñez-Díaz, and José Manuel Moreno-Rojas. 2025. "Evaluation of Morphological, Chemical, and Antioxidant Characteristics, and Phenolic Profile of Three Goji Berry Varieties Cultivated in Southwestern Spain" Applied Sciences 15, no. 22: 11999. https://doi.org/10.3390/app152211999
APA StyleGarcía-Garrido, M. E., Sánchez-Parra, M., Ordóñez-Díaz, J. L., & Moreno-Rojas, J. M. (2025). Evaluation of Morphological, Chemical, and Antioxidant Characteristics, and Phenolic Profile of Three Goji Berry Varieties Cultivated in Southwestern Spain. Applied Sciences, 15(22), 11999. https://doi.org/10.3390/app152211999







