Impact of Three-Fluid Nozzle Emulsification on the Physicochemical and Thermodynamic Properties of Avocado Oil Microcapsules Obtained by Spray Drying
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining the Emulsion with a Three-Fluid Nozzle
2.3. Physicochemical and Rheological Characterization of Emulsions
2.3.1. Droplet Size (DS), Polydispersity Index (PDI), and Zeta Potential (ζ-Potential)
2.3.2. Emulsion Stability Analysis Using the Turbiscan Stability Index (TSI)
2.3.3. Kinetic Modeling of TSI Data
2.3.4. Rheological Characterization
2.4. Preparation of Microcapsules by Spray-Drying
2.4.1. Spray Drying with a Three-Fluid Nozzle (3FN)
2.4.2. Process Yield
2.4.3. Encapsulation Efficiency
2.5. Characterization of Spray-Dried Microcapsules
2.5.1. Moisture Content
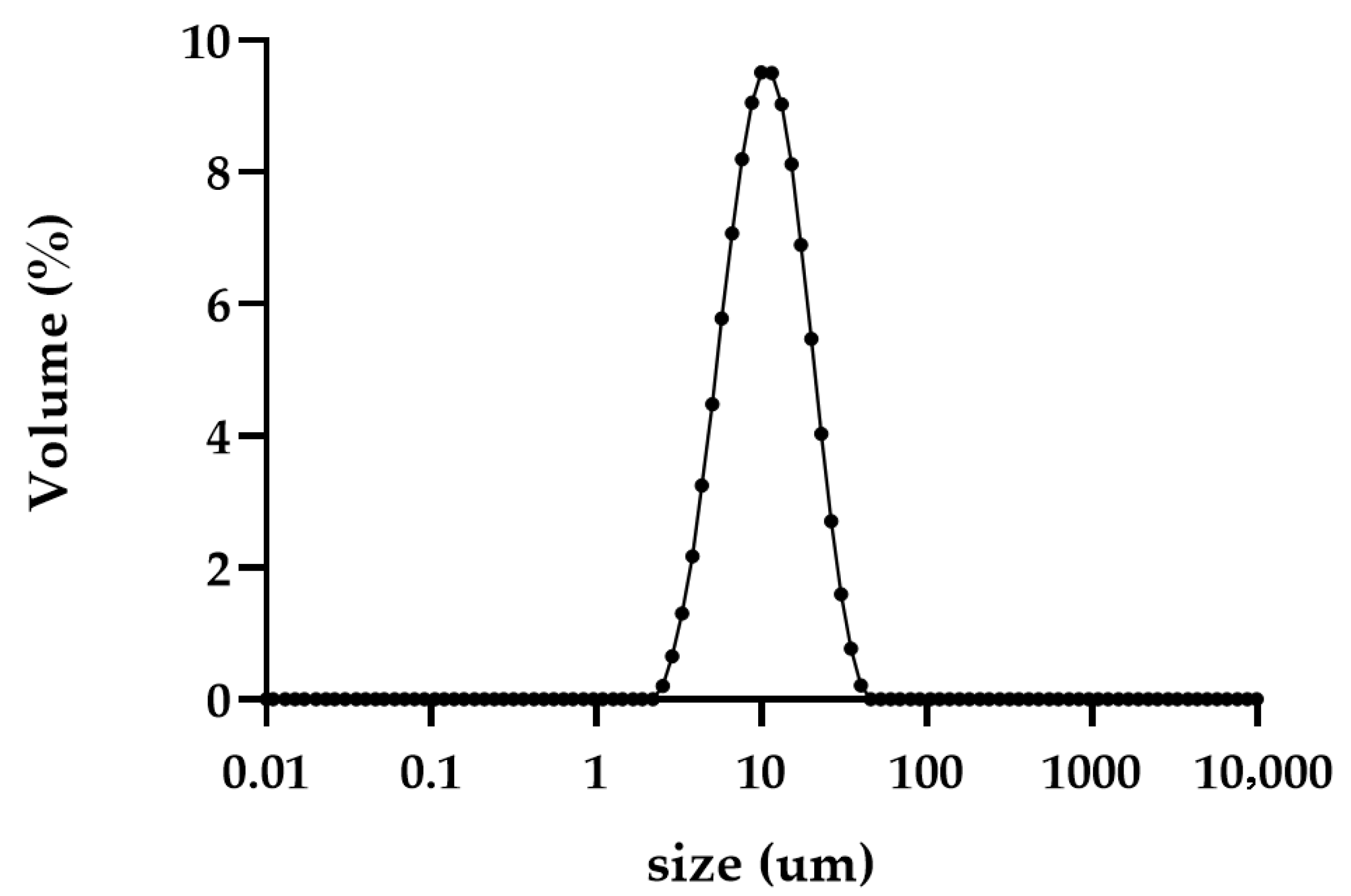
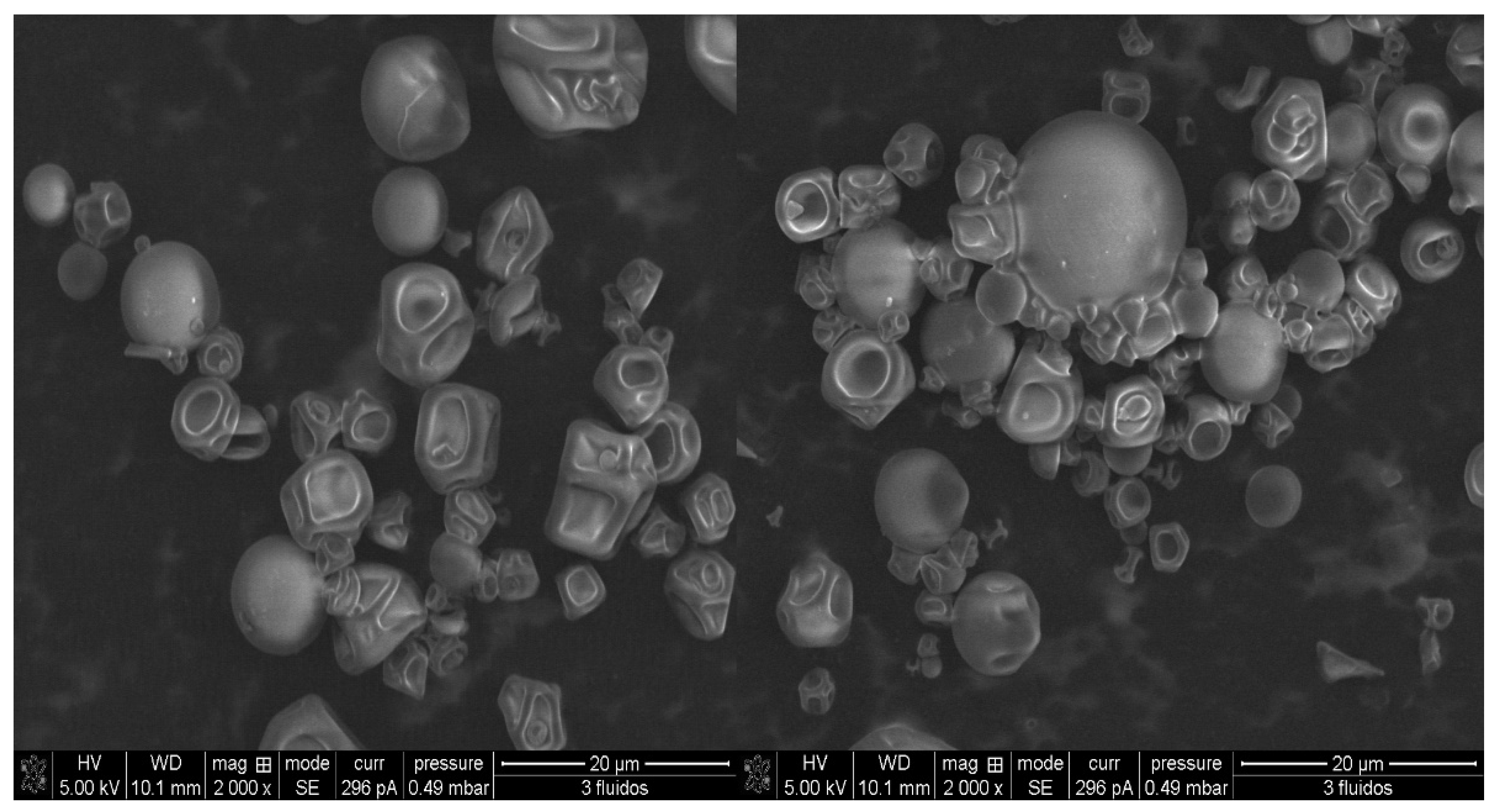
2.5.2. Characterization of Particle Size and Morphology
2.5.3. Evaluation of Powder Flow Function
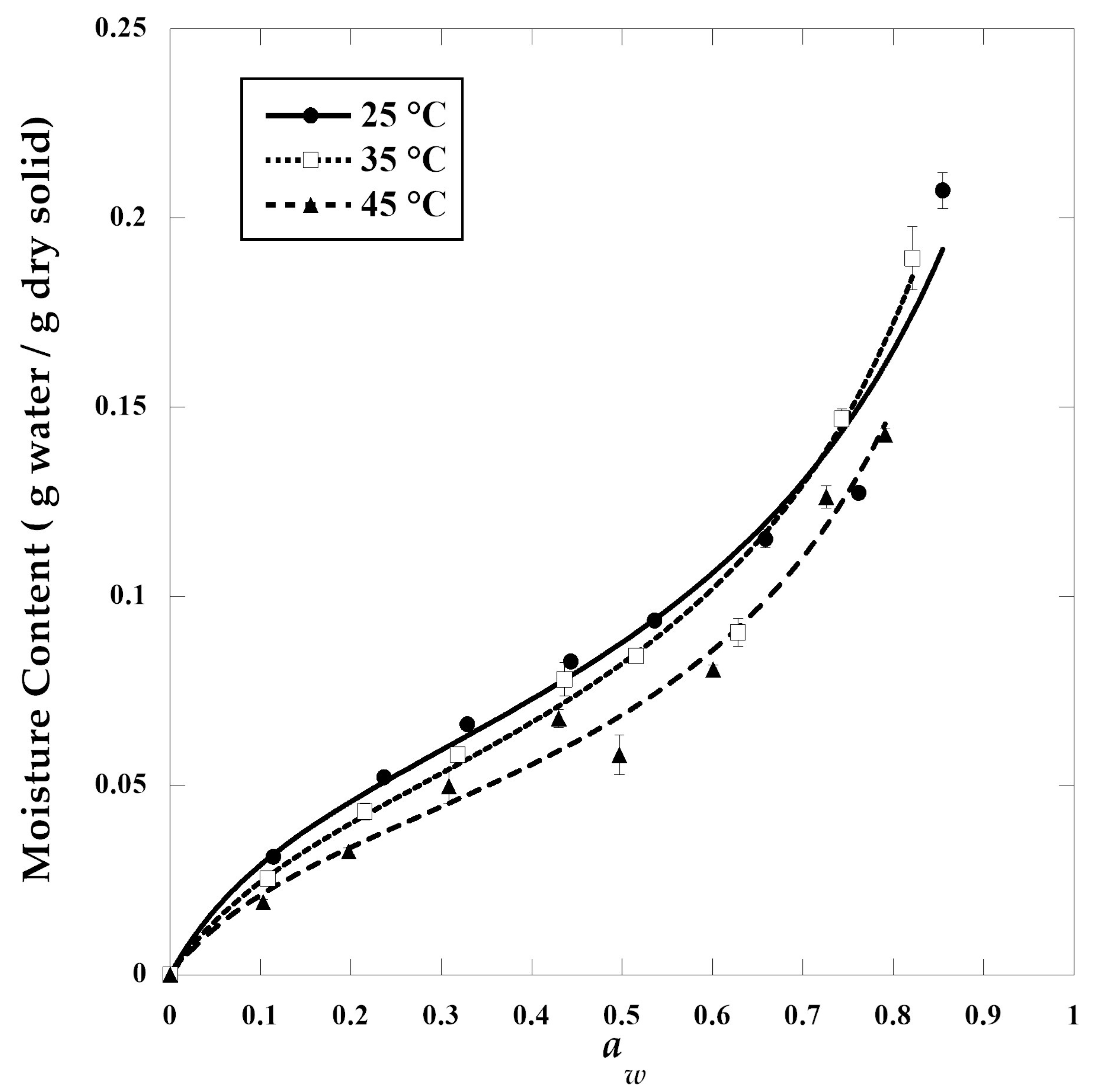
2.5.4. Moisture Sorption Isotherms
2.5.5. Modeling of Water Sorption Isotherms
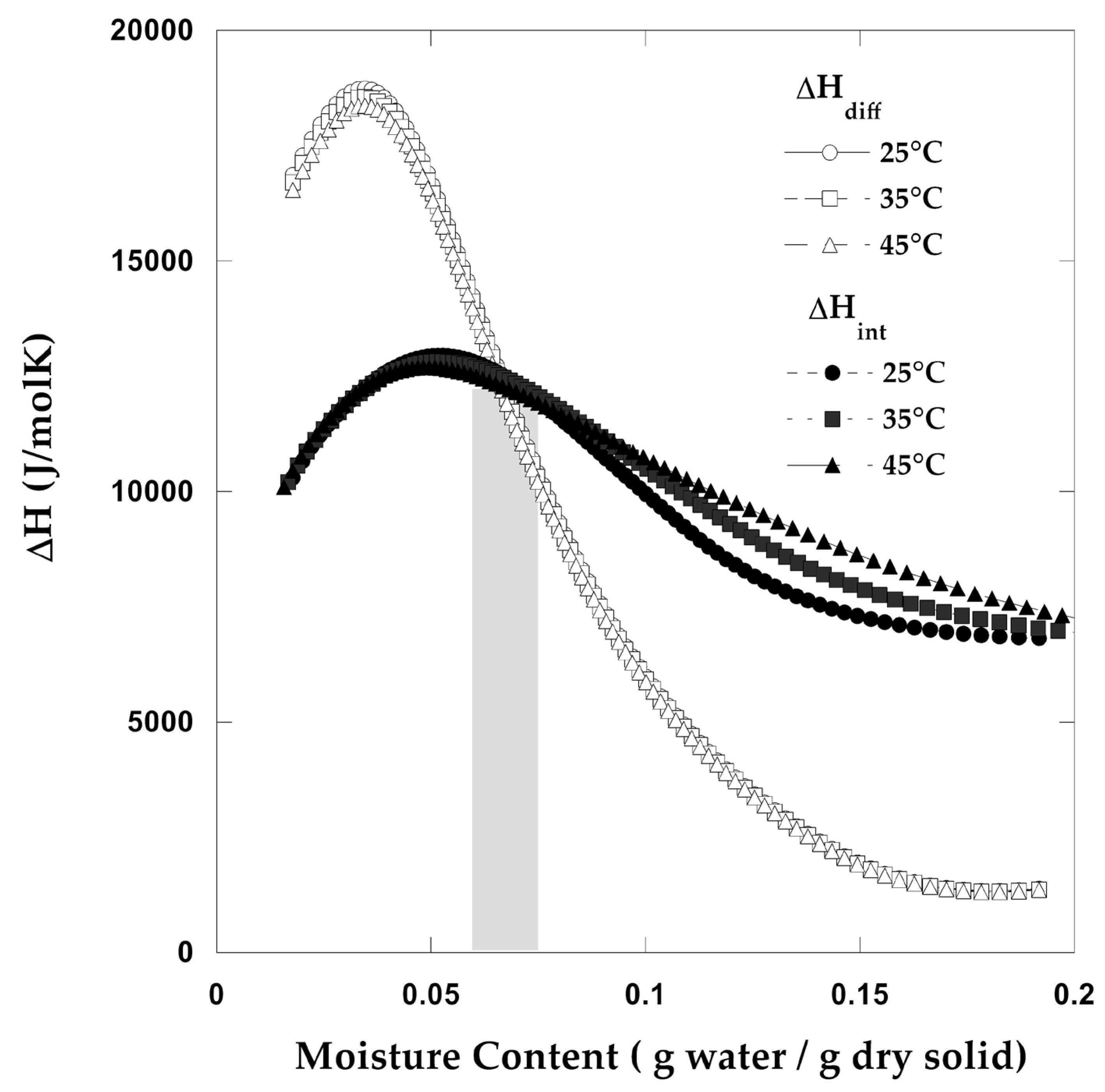
2.5.6. Determination of Thermodynamic Parameters
Differential Properties
Integral Properties
2.5.7. Compensation Theory
2.5.8. Glass Transition Temperature (Tg)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Nozzle Type on Droplet Size (DS), Polydispersity Index (PDI), and ζ-Potential
3.2. Description of Emulsion Stability Using TSI and Its Modeling
3.3. Rheological Characterization of the Emulsions
3.4. Encapsulation Efficiency and Yield
3.5. Powder Rheology
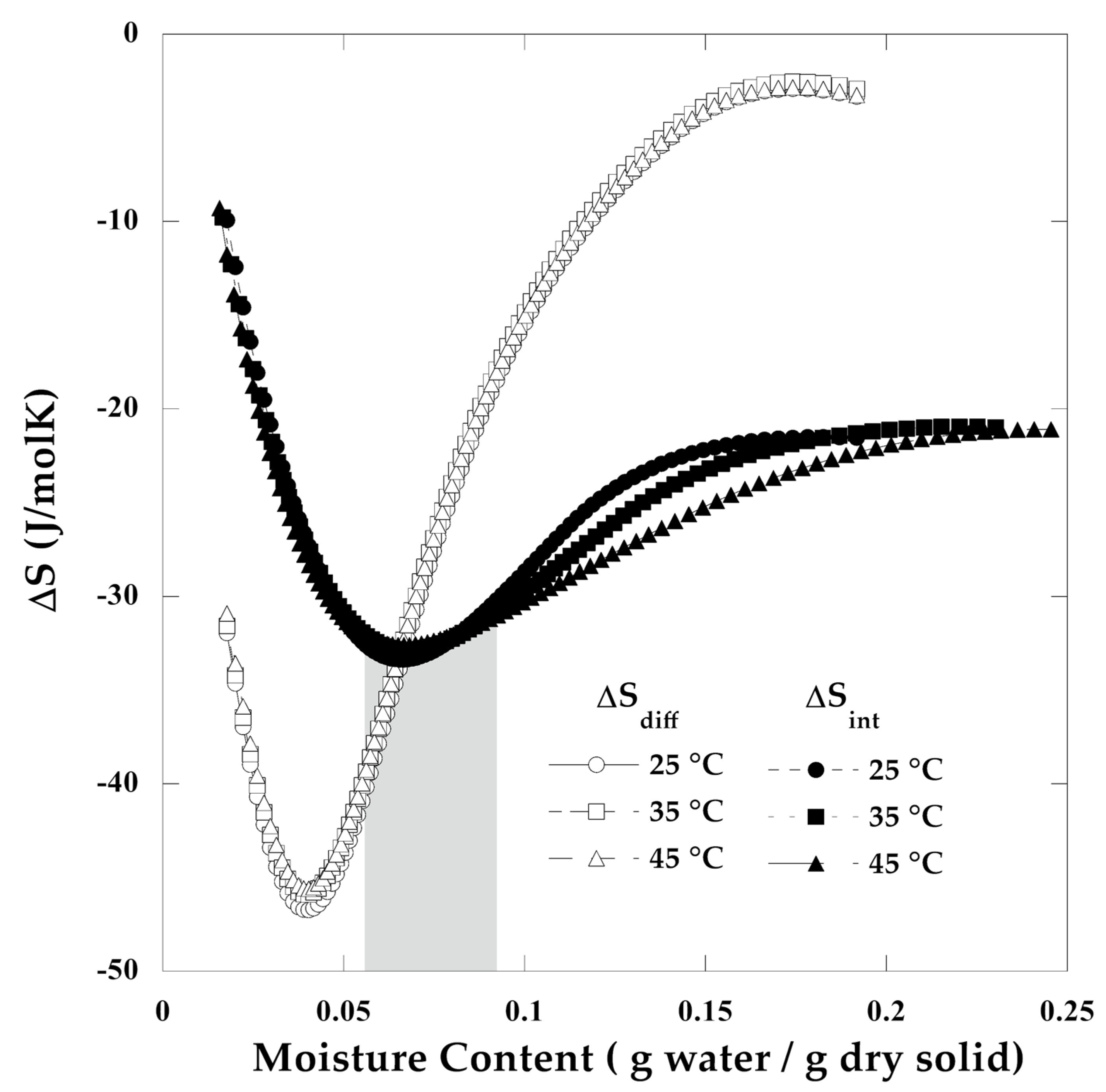
3.6. Adsorption Isotherms and Thermodynamic Properties
3.7. Thermodynamic Properties
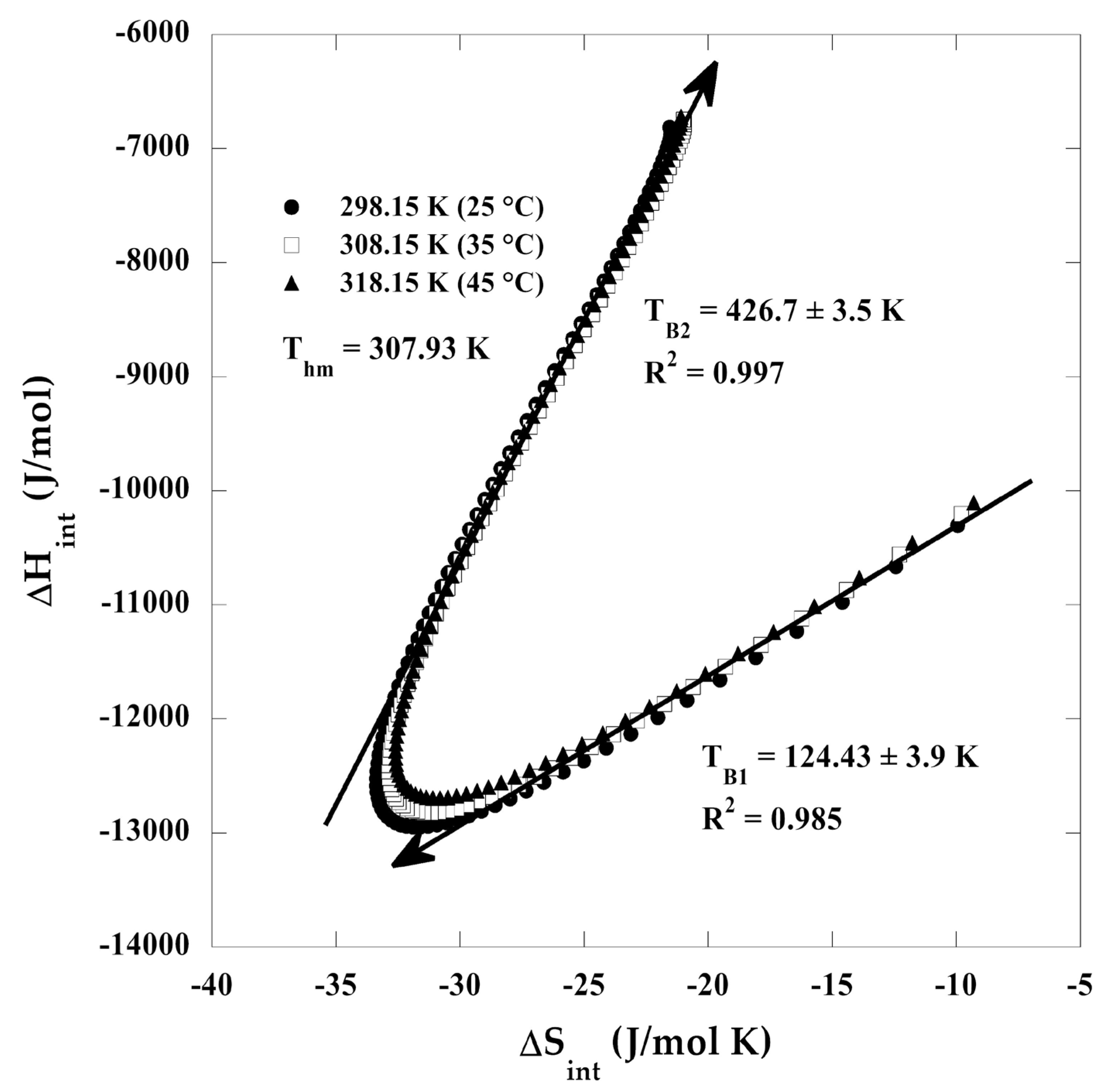
3.8. Enthalpy–Entropy Compensation
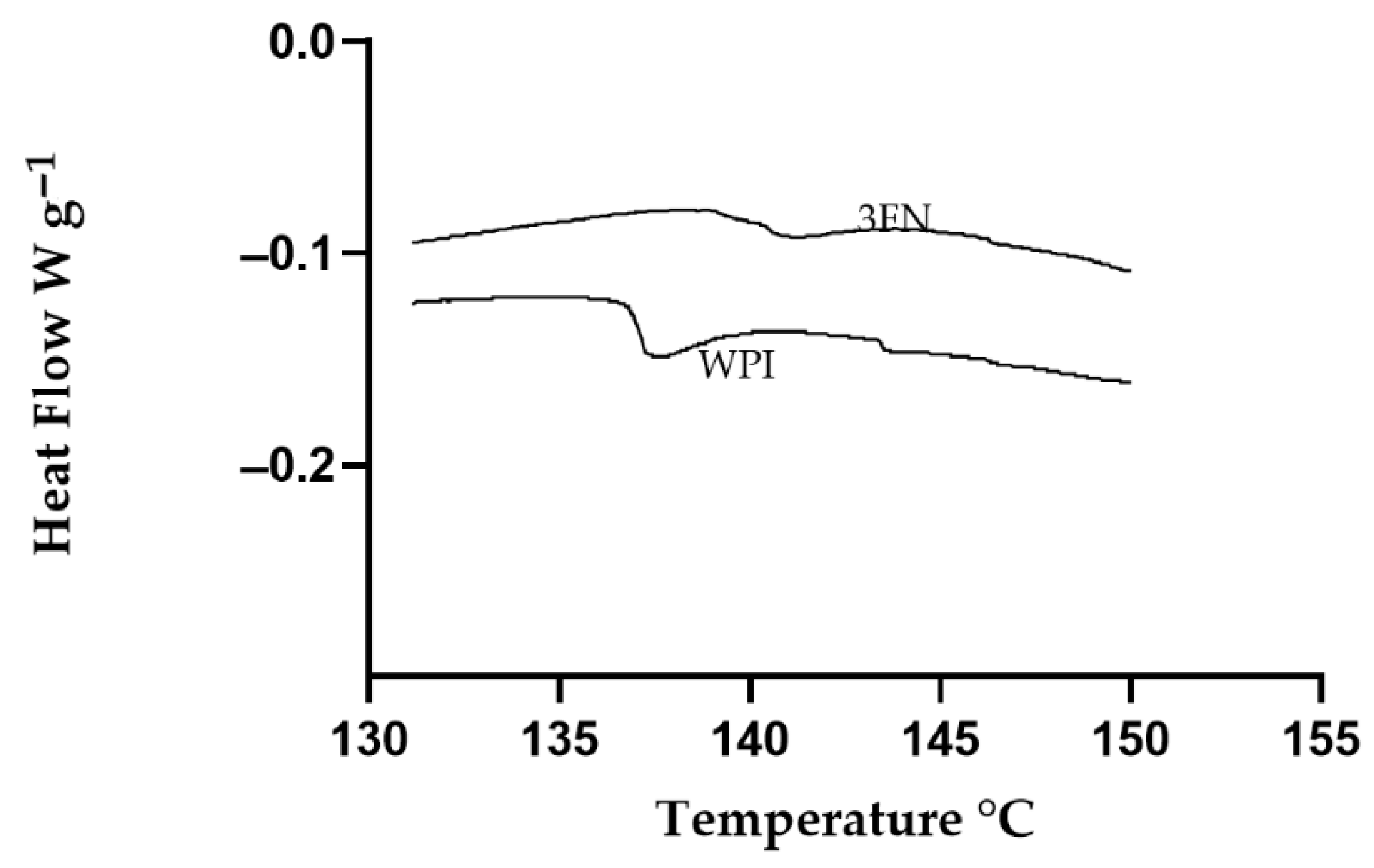
3.9. Glass Transition Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3FN | Three-fluid nozzle |
| 2FN | Two-fluid nozzles |
| WPI | Whey protein isolate |
| DS | Droplet size |
| PDI | Polydispersity Index |
| ζ-Potential | Zeta Potential |
| TSI | Turbiscan Stability Index |
| EE | Encapsulation efficiency |
| GAB | Guggenheim–Anderson–de Boer |
| M0 | Monolayer moisture content |
| Water activity | |
| C | Dimensionless parameter related to heat of sorption of monolayer region |
| K | Dimensionless related to heat of sorption of multilayer region |
| Isosteric heat of water adsorption | |
| Heat of condensation of pure water | |
| Differential enthalpy | |
| Differential entropy | |
| Isokinetic temperature | |
| Gibbs free energy at the isokinetic temperature | |
| Harmonic mean temperature | |
| N | Total number of isotherms used |
| Tg | Glass transition temperature |
| E3FN | Emulsion produced using a three-fluid nozzle |
| RMSE | Root mean square error |
| η | Apparent viscosity |
| Shear rate | |
| O/W | Oil-in-water |
| m1 | Theoretical maximum instability |
| m2 | Time required to reach 50% instability |
| n | Flow index |
| K (Pa·sn) | Consistency coefficient |
| SD | Spray drying |
| db | Dry base |
| D[3,2] | Sauter diameter |
| D[4,3] | Brouckere diameter |
| SEM | Scanning Electron Microscopy |
References
- Tavares, L.; Noreña, C.P.Z. Encapsulation of Ginger Essential Oil Using Complex Coacervation Method: Coacervate Formation, Rheological Property, and Physicochemical Characterization. Food Bioprocess Technol. 2020, 13, 1405–1420. [Google Scholar] [CrossRef]
- Alkaltham, M.S.; Uslu, N.; Özcan, M.M.; Salamatullah, A.M.; Mohamed Ahmed, I.A.; Hayat, K. Effect of Drying Process on Oil, Phenolic Composition and Antioxidant Activity of Avocado (cv. Hass) Fruits Harvested at Two Different Maturity Stages. LWT 2021, 148, 111716. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; García-Tejeda, Y.V.; Portilla-Rivera, O.M.; Chávez-Murillo, C.E.; Barrera-Figueroa, V. Effect of Mixed Particulate Emulsifiers on Spray-Dried Avocado Oil-in-Water Pickering Emulsions. Polymers 2022, 14, 3064. [Google Scholar] [CrossRef]
- Ford, N.A.; Spagnuolo, P.; Kraft, J.; Bauer, E. Nutritional Composition of Hass Avocado Pulp. Foods 2023, 12, 2516. [Google Scholar] [CrossRef]
- Romero-Hernandez, H.A.; Sánchez-Rivera, M.M.; Alvarez-Ramirez, J.; Yee-Madeira, H.; Yañez-Fernandez, J.; Bello-Pérez, L.A. Avocado Oil Encapsulation with OSA-Esterified Taro Starch as Wall Material: Physicochemical and Morphology Characteristics. LWT 2021, 138, 110629. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Gayathiri, S.; Preetha, P.; Pandiselvam, R.; Jeevarathinam, G.; Delfiya, D.S.A.; Kothakota, A. Microencapsulation of Bixin Pigment by Spray Drying: Evaluation of Characteristics. LWT 2021, 145, 111343. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent Advances in the Spray Drying Encapsulation of Essential Fatty Acids and Functional Oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, J.; Dai, T.; Ye, J.; Wu, J.; Chen, T.; Liu, C. Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate–Puerarin Composites: Environmental Stability and Interfacial Behavior. Foods 2021, 10, 705. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Cai, J.; Lopez, R.; Lee, Y. Effect of Feed Material Properties on Microencapsulation by Spray Drying with a Three-Fluid Nozzle: Soybean Oil Encapsulated in Maltodextrin and Sugar Beet Pectin. J. Food Process. Preserv. 2023, 2023, 4974631. [Google Scholar] [CrossRef]
- Jauhari, A.K.P.; Wilms, P.F.C.; Corstens, M.N.; Schutyser, M.A.I. Encapsulating Volatiles: The Role of Emulsion Stability on the Retention of d-Limonene during Emulsification and Spray Drying. LWT 2025, 223, 117784. [Google Scholar] [CrossRef]
- Sultana, A.; Adachi, S.; Yoshii, H. Encapsulation of Fish Oil and Essential Fatty Acids by Spray Drying. Sustain. Food Technol. 2023, 1, 827–836. [Google Scholar] [CrossRef]
- Xiao, T.; Ma, X.; Hu, H.; Xiang, F.; Zhang, X.; Zheng, Y.; Dong, H.; Adhikari, B.; Wang, Q.; Shi, A. Advances in Emulsion Stability: A Review on Mechanisms, Role of Emulsifiers, and Applications in Food. Food Chem. X 2025, 29, 102792. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Lie, M.; Antonio-Gutiérrez, O.; López-Díaz, A.S.; López-Malo, A.; Ramírez-Corona, N. Factors Influencing Droplet Size in Pneumatic and Ultrasonic Atomization and Its Application in Food Processing. Discov. Food 2023, 3, 23. [Google Scholar] [CrossRef]
- Höhne, S.; Taboada, M.L.; Schröder, J.; Gomez, C.; Karbstein, H.P.; Gaukel, V. Influence of Nozzle Geometry and Scale-Up on Oil Droplet Breakup in the Atomization Step during Spray Drying of Emulsions. Fluids 2024, 9, 70. [Google Scholar] [CrossRef]
- Mohandas, A.; Luo, H.; Ramakrishna, S. An Overview on Atomization and Its Drug Delivery and Biomedical Applications. Appl. Sci. 2021, 11, 5173. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Su, Y.; Pathak, V.; Zemlyanov, D.Y.; Cavallaro, A.-A.; Munson, E.J.; Taylor, L.S.; Zhou, Q. Effect of Storage Humidity on Physical Stability of Spray-Dried Naproxen Amorphous Solid Dispersions with Polyvinylpyrrolidone: Two Fluid Nozzle vs. Three Fluid Nozzle. Pharmaceutics 2021, 13, 1074. [Google Scholar] [CrossRef]
- Shi, X.; Lee, Y. Encapsulation of Tributyrin with Whey Protein Isolate (WPI) by Spray-Drying with a Three-Fluid Nozzle. J. Food Eng. 2020, 281, 109992. [Google Scholar] [CrossRef]
- Tatar Turan, F.; Cengiz, A.; Sandıkçı, D.; Dervisoglu, M.; Kahyaoglu, T. Influence of an Ultrasonic Nozzle in Spray-Drying and Storage on the Properties of Blueberry Powder and Microcapsules: Influence of Ultrasonic Nozzle in Spray-Drying and Storage. J. Sci. Food Agric. 2016, 96, 4062–4076. [Google Scholar] [CrossRef]
- Legako, J.; Dunford, N.T. Effect of Spray Nozzle Design on Fish Oil–Whey Protein Microcapsule Properties. J. Food Sci. 2010, 75, E394–E400. [Google Scholar] [CrossRef]
- Taboada, M.L.; Schäfer, A.; Karbstein, H.P.; Gaukel, V. Oil Droplet Breakup during Pressure Swirl Atomization of Food Emulsions: Influence of Atomization Pressure and Initial Oil Droplet Size. J. Food Process Eng. 2020, 44, e13598. [Google Scholar] [CrossRef]
- Villalobos-Espinosa, J.C.; García-Armenta, E.; Alamilla-Beltrán, L.; Quintanilla-Carvajal, M.X.; Azuara-Nieto, E.; Hernández-Sánchez, H.; Perea-Flores, M.D.J.; Gutiérrez-López, G.F. Effect of Pumping and Atomisation on the Stability of Oil/Water Emulsions. J. Food Eng. 2022, 327, 111056. [Google Scholar] [CrossRef]
- Munoz-Ibanez, M.; Azagoh, C.; Dubey, B.N.; Dumoulin, E.; Turchiuli, C. Changes in Oil-in-Water Emulsion Size Distribution during the Atomization Step in Spray-Drying Encapsulation. J. Food Eng. 2015, 167, 122–132. [Google Scholar] [CrossRef]
- Lucas-Aguirre, J.C.; Giraldo-Giraldo, G.A.; Cortés-Rodríguez, M. Thermodynamic Properties of Moisture Sorption and Glass Transition of Coconut (Cocos nucifera L.) Powder Fortified with Physiologically Active Components. In Sorption—New Perspectives and Applications; Margeta, K., Farkaš, A., Eds.; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Fikry, M.; Benjakul, S.; Al-Ghamdi, S.; Mittal, A.; Nilsuwan, K.; Fulleros, R.; Dabbour, M. Sorption Isotherms and Thermodynamic Characteristics of Gelatin Powder Extracted from Whitefish Skin: Mathematical Modeling Approach. Foods 2023, 13, 92. [Google Scholar] [CrossRef]
- Thorpe, G.R. The Significance of the Sorption Isotherm on the Simulated Performance of Grain Driers. Appl. Sci. 2025, 15, 2871. [Google Scholar] [CrossRef]
- Costa, M.F.; Procópio, F.R.; De Figueiredo Furtado, G.; Munhoz Moya, A.M.T.; Cazarin, C.B.B.; Hubinger, M.D. Cinnamon and Paprika Oleoresin Emulsions: A Study of Physicochemical Stability and Antioxidant Synergism. Food Res. Int. 2021, 150, 110777. [Google Scholar] [CrossRef]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. The Effect of Microfluidization Pressure on the Physical Stability of Vitamin A in Oil-in-Water Emulsions. Eur. Food Res. Technol. 2022, 248, 2969–2975. [Google Scholar] [CrossRef]
- López-Hernández, R.E.; García-Solís, S.E.; Monroy-Rodríguez, I.; Cornejo-Mazón, M.; Calderón-Domínguez, G.; Alamilla-Beltrán, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.F. Preparation and Characterization of Canola Oil-in-Water Pickering Emulsions Stabilized by Barley Starch Nanocrystals. J. Food Eng. 2022, 326, 111037. [Google Scholar] [CrossRef]
- Cevik, K.; Yalcin, H.; Konca, Y. Elucidating the Influence of Coating Materials in the Microencapsulation Process of Hempseed Oil Via Spray Drying: A Comprehensive Analysis of Physicochemical Attributes, Oxidation Stability, and Thermal Properties. Food Biophys. 2024, 19, 795–805. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x Paradisi L. Peels. Pharmaceuticals 2024, 17, 596. [Google Scholar] [CrossRef]
- Altuntas, U.; Altin-Yavuzarslan, G.; Ozçelik, B. Enhanced Oxidative Stability and Bioaccessibility of Sour Cherry Kernel Byproducts Encapsulated by Complex Coacervates with Different Wall Matrixes by Spray- and Freeze-Drying. ACS Omega 2023, 8, 23782–23790. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of Juniper Berry Essential Oil (Juniperus communis L.) by Spray Drying: Microcapsule Characterization and Release Kinetics of the Oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Da Silva Anthero, A.G.; Maria Tomazini Munhoz Moya, A.; Souza Torsoni, A.; Baú Betim Cazarin, C.; Dupas Hubinger, M. Characterization of Capsicum Oleoresin Microparticles and in Vivo Evaluation of Short-Term Capsaicin Intake. Food Chem. X 2022, 13, 100179. [Google Scholar] [CrossRef]
- Hashim, N.A.; Mohd Norzi, M.F.A.; Mohd Arshad, Z.I.; Mohd Azman, N.A.; Abdul Mudalip, S.K. Effect of Spray Drying Parameters on the Physicochemical Properties and Oxidative Stability of Oil from Menhaden (Brevoortia spp.) and Asian Swamp Eel (Monopterus albus) Oil Extract Microcapsules. Food Chem. Adv. 2023, 3, 100392. [Google Scholar] [CrossRef]
- Deng, T.; Garg, V.; Pereira Diaz, L.; Markl, D.; Brown, C.; Florence, A.; Bradley, M.S.A. Comparative Studies of Powder Flow Predictions Using Milligrams of Powder for Identifying Powder Flow Issues. Int. J. Pharm. 2022, 628, 122309. [Google Scholar] [CrossRef]
- Lang, K.W.; McCUNE, T.D.; Steinberg, M.P. A Proximity Equilibration Cell for Rapid Determination of Sorption Isotherms. J. Food Sci. 1981, 46, 936–938. [Google Scholar] [CrossRef]
- Labuza, T.P.; Kaanane, A.; Chen, J.Y. Effect of Temperature on the Moisture Sorption Isotherms and Water Activity Shift of Two Dehydrated Foods. J. Food Sci. 1985, 50, 385–392. [Google Scholar] [CrossRef]
- Aviara, N.A. Moisture Sorption Isotherms and Isotherm Model Performance Evaluation for Food and Agricultural Products. In Sorption in 2020s; Kyzas, G., Lazaridis, N., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Othmer, D.F.; Brown, G.G. Correlating Vapor Pressure and Latent Heat Data. Ind. Eng. Chem. 1940, 32, 841–856. [Google Scholar] [CrossRef]
- Wexler, A. Vapor Pressure Formulation for Water in Range 0 to F00 °C. A Revision. J. Res. Natl. Bur. Stand. A Phys. Chem. 1976, 80, 775–785. [Google Scholar] [CrossRef]
- Beristain, C.I.; Garcia, H.S.; Azuara, E. Enthalpy-Entropy Compensation in Food Vapor Adsorption. J. Food Eng. 1996, 30, 405–415. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-Entropy Compensation. 2. Separation of the Chemical from the Statistical Effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- Pérez, A.; De Sousa, A.; López, J.V.; Laredo, E.; Newman, D.; Sandoval, A.J.; Müller, A.J. Thermal and Mechanical Characteristics of a Glassy Food Model Based on Cassava Starch. Appl. Food Res. 2022, 2, 100231. [Google Scholar] [CrossRef]
- Sancho, R.E.; Govindsamy, A.; Pillay, K. Optimization of Growth Conditions for Magnetospirillum Magnetotacticum and Green Synthesis of Metallic Nanoparticles. Appl. Sci. 2023, 13, 8491. [Google Scholar] [CrossRef]
- Shetty, N.; Zhang, Y.; Park, H.; Zemlyanov, D.; Shah, D.; He, A.; Ahn, P.; Mutukuri, T.T.; Chan, H.-K.; Zhou, Q. Surface Composition and Aerosolization Stability of an Inhalable Combinational Powder Formulation Spray Dried Using a Three-Fluid Nozzle. Pharm Res. 2020, 37, 219. [Google Scholar] [CrossRef]
- Kramm, K.; Orth, M.; Teiwes, A.; Kammerhofer, J.C.; Meunier, V.; Pietsch-Braune, S.; Heinrich, S. Influence of Nozzle Parameters on Spray Pattern and Droplet Characteristics for a Two-Fluid Nozzle. Chem. Ing. Tech. 2023, 95, 151–159. [Google Scholar] [CrossRef]
- Orth, M.; Börner, M.; Pietsch-Braune, S.; Heinrich, S. Influence of Spray Parameters on Injected Droplets and Product Properties in Fluidized Bed Spray Granulation. Powder Technol. 2024, 448, 120274. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Domagała, J.; Kasprzak, M.M. Critical Review of Techniques for Food Emulsion Characterization. Appl. Sci. 2024, 14, 1069. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Berski, W.; Krystyjan, M.; Jamróz, E.; Florczuk, A.; Tkaczewska, J.; Zając, M.; Domagała, J.; Lett, A.M.; Ptasznik, S. Effects of Fibre Addition and Processing on the Stability, Rheology and in Vitro Gastric Digestion of Whey Protein-Xanthan Gum Stabilised Emulsions with High Oil Phase. LWT 2023, 178, 114465. [Google Scholar] [CrossRef]
- Ramírez-Brewer, D.; Méndez, D.A.; Garcia-Zapateiro, L.A.; López-Rubio, A.; Fabra, M.J. Rheological Properties, Microstructure and Stability of Oil-in-Water Emulsions Prepared with Mango Kernel Starch (var. Sugar and Tommy). LWT 2024, 194, 115802. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Göksel Saraç, M.; Doğan, M. Investigating the Interfacial and Bulk Rheological Properties of Emulsions Containing Dry Bean Powder. Eur. Food Res. Technol. 2024, 250, 2659–2668. [Google Scholar] [CrossRef]
- Lu, Q.; Pal, R. Steady Shear Rheology and Surface Activity of Polymer-Surfactant Mixtures. Polymers 2025, 17, 364. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of Ultrasound Assisted Emulsification in the Production of Pickering Emulsion Formulated with Chitosan Self-Assembled Particles: Stability, Macro, and Micro Rheological Properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Hou, F.; Yang, S.; Ma, X.; Gong, Z.; Wang, Y.; Wang, W. Characterization of Physicochemical Properties of Oil-in-Water Emulsions Stabilized by Tremella Fuciformis Polysaccharides. Foods 2022, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Bufalini, C.; Campardelli, R. Versatile Emulsion-Based Encapsulation System Production Processes: A Review. Processes 2025, 13, 1409. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of Avocado Oil by Spray Drying Using Whey Protein and Maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Wang, H.; Vehring, R. Mechanistic Formulation Design of Spray-Dried Powders. KONA Powder Part. J. 2023, 40, 149–171. [Google Scholar] [CrossRef]
- Eijkelboom, N.M.; Van Boven, A.P.; Siemons, I.; Wilms, P.F.C.; Boom, R.M.; Kohlus, R.; Schutyser, M.A.I. Particle Structure Development during Spray Drying from a Single Droplet to Pilot-Scale Perspective. J. Food Eng. 2023, 337, 111222. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Factors Determining the Surface Oil Concentration of Encapsulated Lipid Particles: Impact of the Emulsion Oil Droplet Size. Eur. Food Res. Technol. 2020, 246, 1933–1943. [Google Scholar] [CrossRef]
- Lopez, R.; Lee, Y. Advancing Particle Engineering: A Review of Three-Fluid Nozzle Spray Drying for Developing Applications for Food. Food Bioprocess Technol. 2025, 18, 5891–5908. [Google Scholar] [CrossRef]
- Tao, Y.; Tang, Z.; Huang, Q.; Xu, X.; Cheng, X.; Zhang, G.; Jing, X.; Li, X.; Liang, J.; Granato, D.; et al. Effects of Spray Drying Temperature on Physicochemical Properties of Grapeseed Oil Microcapsules and the Encapsulation Efficiency of Pterostilbene. LWT 2024, 193, 115779. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Liu, L.; Li, Y.; Qi, B.; Jiang, L. Preparation of Spray-Dried Soybean Oil Body Microcapsules Using Maltodextrin: Effects of Dextrose Equivalence. LWT 2022, 154, 112874. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of Fingered Citron Extract with Gum Arabic, Modified Starch, Whey Protein, and Maltodextrin Using Spray Drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef]
- Peng, Q.; Meng, Z.; Luo, Z.; Duan, H.; Ramaswamy, H.S.; Wang, C. Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis). Foods 2022, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Kellil, A.; Razem, M. Factors Influencing Food Powder Flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Gouveia Gomes, M.H.G.; Kurozawa, L.E. Improvement of the Functional and Antioxidant Properties of Rice Protein by Enzymatic Hydrolysis for the Microencapsulation of Linseed Oil. J. Food Eng. 2020, 267, 109761. [Google Scholar] [CrossRef]
- Martinović, J.; Ambrus, R.; Planinić, M.; Perković, G.; Šelo, G.; Klarić, A.-M.; Bucić-Kojić, A. Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels 2025, 11, 130. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Microencapsulation by Spray Drying of Omega-3 Lipids Extracted from Oilseeds and Microalgae: Effect on Polyunsaturated Fatty Acid Composition. LWT 2021, 148, 111789. [Google Scholar] [CrossRef]
- Ruan, J.; Li, M.; Liu, Y.; Ye, B.; Ling, C. Adsorption Isotherm and Thermodynamic Properties of Microwave Vacuum Dried Tilapia Fillets. LWT 2022, 166, 113766. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, S. Moisture Sorption Isotherms and Storage Study of Spray-Dried Probiotic Finger Millet Milk Powder. J. Stored Prod. Res. 2023, 102, 102128. [Google Scholar] [CrossRef]
- Jiménez-Regalado, E.J.; Alonso Díaz-Cruz, C.; Velazquez, G.; Yaneli Aguirre-Loredo, R.; Guadarrama-Lezama, A.Y. Thermodynamic Water Adsorption Analysis of Biodegradable Films Based on Corn Starch-Chitosan Added with Triblock Copolymers. J. Food Eng. 2025, 386, 112285. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Krokida, M. Comparative Assessment of Encapsulated Essential Oils through the Innovative Electrohydrodynamic Processing and the Conventional Spray Drying, and Freeze-Drying Techniques. Innov. Food Sci. Emerg. Technol. 2024, 95, 103720. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Jia, H.; Liu, Y. Moisture Sorption Isotherms and Thermodynamic Properties of Tiger Nuts: An Oil-Rich Tuber. LWT 2022, 167, 113866. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Zhang, X.; Zhang, Y.; Zhu, L.; Shi, Q. Sodium Alginate Coating Pretreatment Improved Storage Stability of Heat Pump Dried Scallop Adductors: From Thermal and Thermodynamic Points of View. J. Therm. Anal. Calorim. 2021, 146, 1335–1345. [Google Scholar] [CrossRef]
- Pascual Pineda, L.A.; Contreras, Y.M.; De Lourdes Arévalo Galarza, M.; Morales, M.C.; Marañón, A.H.; Rascón Díaz, M.P.; Andrade, E.F. Clustering Function and Minimum Change in Spreading Pressure as Key Factor to Predict Storage Conditions for Black Pepper Oleoresin Encapsulated by Spray Drying. Food Biosci. 2021, 42, 101215. [Google Scholar] [CrossRef]
- Huerta-Vera, K.; Flores-Andrade, E.; Arévalo-Galarza, M.D.L.C.; Cadena-Iñiguez, J.; Castillo-Morales, M.; Vivar-Vera, G.; Jiménez-Guzmán, J.; Soto-Hernández, R.M. Optimal Storage Conditions for Spray-Dried Chayote Juice (Sechium edule (Jacq.) Sw. cv. Perla Negra) Microencapsulated with Gum Arabic. Food Bioprod. Process. 2025, 150, 296–309. [Google Scholar] [CrossRef]
- Collazos-Escobar, G.A.; Gutiérrez-Guzmán, N.; Váquiro-Herrera, H.A.; Bon, J.; Garcia-Perez, J.V. Thermodynamic Analysis and Modeling of Water Vapor Adsorption Isotherms of Roasted Specialty Coffee (Coffee arabica L. cv. Colombia). LWT 2022, 160, 113335. [Google Scholar] [CrossRef]
- Bonilla, E.; Azuara, E.; Beristain, C.I.; Vernon-Carter, E.J. Predicting Suitable Storage Conditions for Spray-Dried Microcapsules Formed with Different Biopolymer Matrices. Food Hydrocoll. 2010, 24, 633–640. [Google Scholar] [CrossRef]
- Velazquez, G.; Guadarrama-Lezama, A.Y.; Viveros-Contreras, R.; Martin-Polo, M.O.; Diaz-Bandera, D.; Castaño, J. Thermodynamics of Moisture Vapor Sorption and Mechanical Characterization of Methylcellulose and Ethylcellulose Based Films. J. Polym. Environ. 2023, 31, 1498–1509. [Google Scholar] [CrossRef]
- Rascón, M.P.; Huerta-Vera, K.; Pascual-Pineda, L.A.; Contreras-Oliva, A.; Flores-Andrade, E.; Castillo-Morales, M.; Bonilla, E.; González-Morales, I. Osmotic Dehydration Assisted Impregnation of Lactobacillus Rhamnosus in Banana and Effect of Water Activity on the Storage Stability of Probiotic in the Freeze-Dried Product. LWT 2018, 92, 490–496. [Google Scholar] [CrossRef]
- Zhou, P.; Labuza, T.P. Effect of Water Content on Glass Transition and Protein Aggregation of Whey Protein Powders During Short-Term Storage. Food Biophys. 2007, 2, 108–116. [Google Scholar] [CrossRef]
- Opaluwa, C.; Deskovski, S.; Karbstein, H.P.; Emin, M.A. Effect of Oil on the Rheological Properties and Reaction Behavior of Highly Concentrated Wheat Gluten under Conditions Relevant to High Moisture Extrusion. Future Foods 2024, 9, 100307. [Google Scholar] [CrossRef]
- Foster, K.D.; Bronlund, J.E.; Paterson, A.H.J. The Prediction of Moisture Sorption Isotherms for Dairy Powders. Int. Dairy J. 2005, 15, 411–418. [Google Scholar] [CrossRef]
- Paramita, V.D.; Panyoyai, N.; Kasapis, S. The Mechanical Glass Transition Temperature Affords a Fundamental Quality Control in Condensed Gels for Innovative Application in Functional Foods and Nutraceuticals. Foods 2025, 14, 2098. [Google Scholar] [CrossRef]
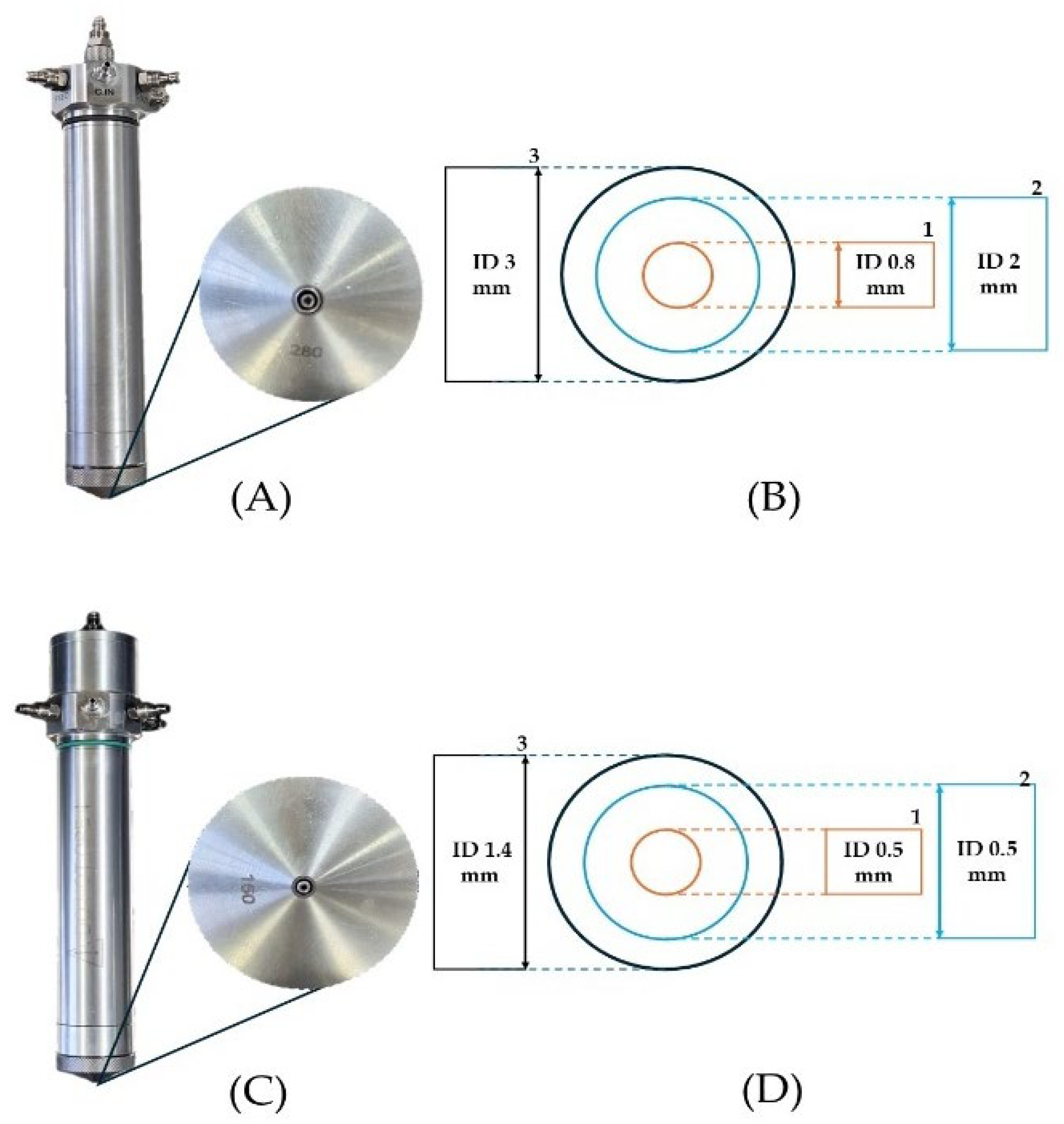
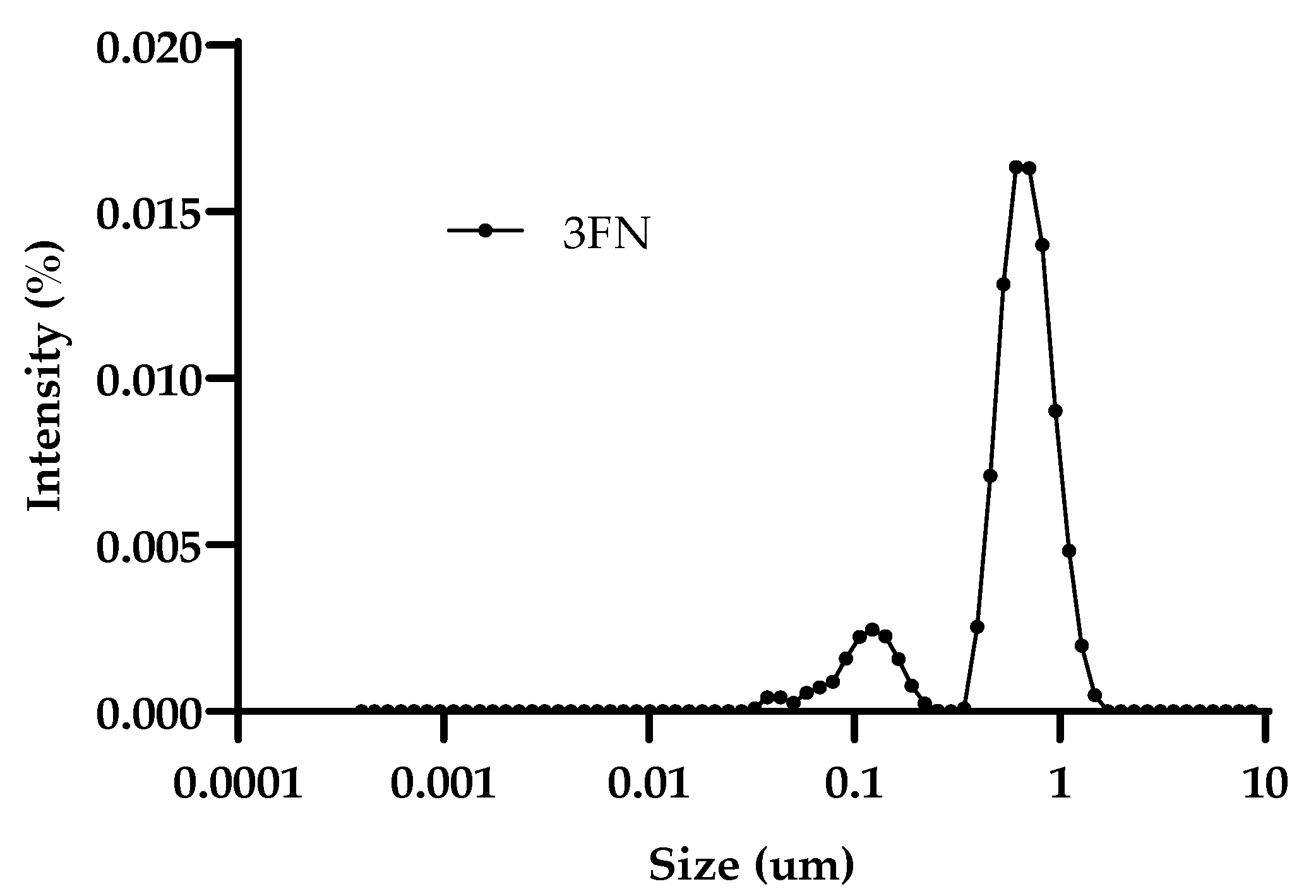
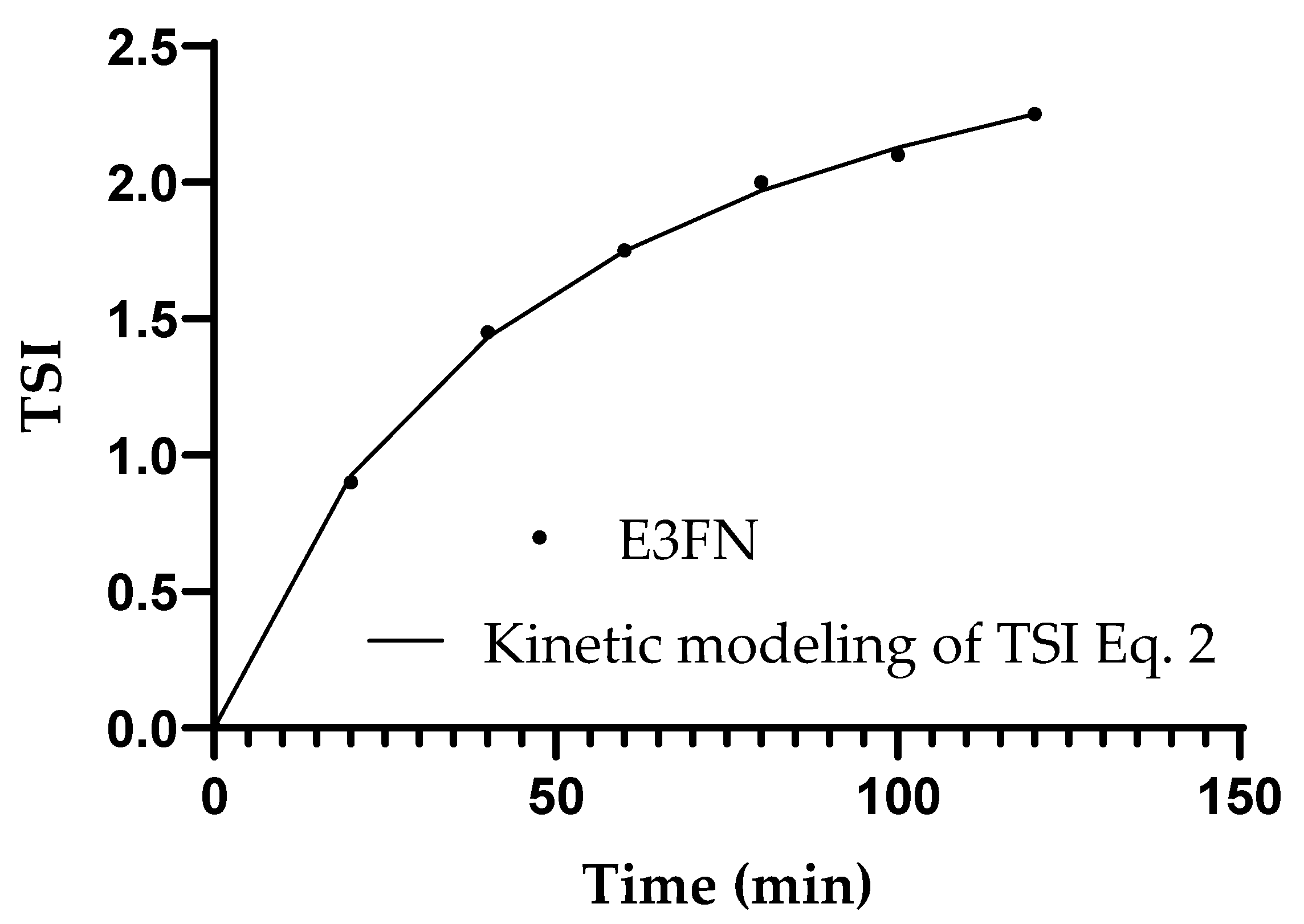
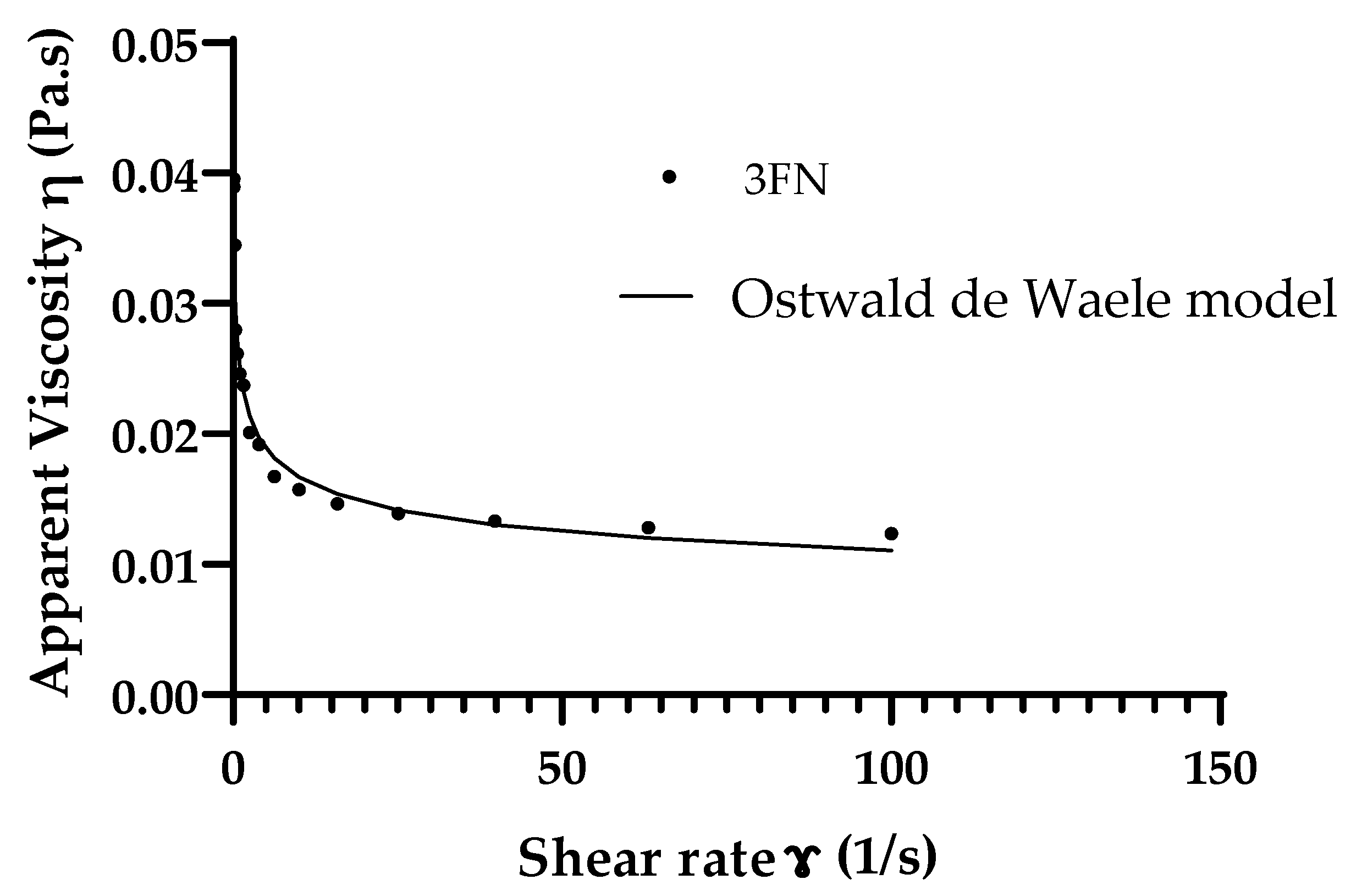
| Droplet Size (µm) | |||||
|---|---|---|---|---|---|
| Sample | Average Size (µm) | Peak 1 | Peak 2 | ζ-Potential (mV) | PDI |
| E3FN | 0.89 ± 24.03 | 0.89 (78.2) | 0.15 (21.8) | −23.90 ± 1.69 | 0.63 ± 0.04 |
| Emulsions | Parameters of the Model | |||
|---|---|---|---|---|
| m1 | m2 (min) | R2 | RMSE | |
| E3FN | 3.15 | 48.14 | 0.999 | 0.002 |
| Samples | K (Pa·sn) | n | R2 |
|---|---|---|---|
| E3FN | 0.02 ± 0.003 | 0.82 ± 0.03 | 0.997 |
| Properties | 3FN |
|---|---|
| Yield (%) | 71.73 ± 1.59 |
| Encapsulation efficiency (%) | 57.83 ± 0.07 |
| Moisture (% db) | 3.63 ± 0.41 |
| Particle Diameter (um) | Volume Diameter (um) | ||||
|---|---|---|---|---|---|
| D[3,2] | D[4,3] | d10 | d50 | d90 | |
| 3FN | 8.38 ± 0.17 | 11.15 ± 0.18 | 4.73 ± 0.10 | 9.76 ± 0.21 | 19.55 ± 0.27 |
| GAB | Temperature (°C) | |||
|---|---|---|---|---|
| 25 | 35 | 45 | ||
| 3FN | M0 | 0.06 | 0.05 | 0.04 |
| C | 8.87 | 7.291 | 7.965 | |
| K | 0.81 | 0.87 | 0.90 | |
| R | 0.997 | 0.998 | 0.991 | |
| E (%) | 0.444 | 0.435 | 0.007 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Marañón, A.; Flores-Andrade, E.; Yáñez-Fernández, J.; Carvajal, M.T.; Pascual-Pineda, L.A.; Alamilla-Beltrán, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.F. Impact of Three-Fluid Nozzle Emulsification on the Physicochemical and Thermodynamic Properties of Avocado Oil Microcapsules Obtained by Spray Drying. Appl. Sci. 2025, 15, 11798. https://doi.org/10.3390/app152111798
Hernández-Marañón A, Flores-Andrade E, Yáñez-Fernández J, Carvajal MT, Pascual-Pineda LA, Alamilla-Beltrán L, Hernández-Sánchez H, Gutiérrez-López GF. Impact of Three-Fluid Nozzle Emulsification on the Physicochemical and Thermodynamic Properties of Avocado Oil Microcapsules Obtained by Spray Drying. Applied Sciences. 2025; 15(21):11798. https://doi.org/10.3390/app152111798
Chicago/Turabian StyleHernández-Marañón, Anahí, Enrique Flores-Andrade, Jorge Yáñez-Fernández, M. Teresa Carvajal, Luz Alicia Pascual-Pineda, Liliana Alamilla-Beltrán, Humberto Hernández-Sánchez, and Gustavo F. Gutiérrez-López. 2025. "Impact of Three-Fluid Nozzle Emulsification on the Physicochemical and Thermodynamic Properties of Avocado Oil Microcapsules Obtained by Spray Drying" Applied Sciences 15, no. 21: 11798. https://doi.org/10.3390/app152111798
APA StyleHernández-Marañón, A., Flores-Andrade, E., Yáñez-Fernández, J., Carvajal, M. T., Pascual-Pineda, L. A., Alamilla-Beltrán, L., Hernández-Sánchez, H., & Gutiérrez-López, G. F. (2025). Impact of Three-Fluid Nozzle Emulsification on the Physicochemical and Thermodynamic Properties of Avocado Oil Microcapsules Obtained by Spray Drying. Applied Sciences, 15(21), 11798. https://doi.org/10.3390/app152111798






