Abstract
Polydeoxyribonucleotides (PDRNs) and polynucleotides (PNs) are biologically active DNA-derived polymers with emerging applications in regenerative dentistry. Acting through adenosine A2A receptor activation and modulation of inflammatory responses, these biomolecules promote angiogenesis, enhance fibroblast proliferation, and stimulate extracellular matrix synthesis. In periodontal therapy, their potential to accelerate healing, reduce inflammation, and support the regeneration of gingival connective tissue, periodontal ligament, cementum, and alveolar bone is of increasing clinical interest. This review synthesizes current preclinical and clinical evidence regarding the use of PDRNs and PNs for tissue regeneration in dentistry, including their mechanisms of action, delivery strategies, synergistic effects with biomaterials and growth factors, and safety profile. Furthermore, recent advances in injectable formulations, scaffold integration, and combined therapies are discussed. The review also highlights gaps in evidence, methodological limitations in existing studies, and future research directions needed to establish standardized treatment protocols. A total of 21 studies (10 PDRNs and 11 PNs/ODNs) were analyzed. PDRNs and PNs consistently demonstrated preclinical regenerative efficacy, although robust clinical validation remains limited.
1. Introduction
Periodontal disease is a chronic inflammatory condition characterized by progressive destruction of the gingival connective tissue, periodontal ligament, cementum, and alveolar bone [1]. Left untreated, it can lead to tooth mobility and eventual tooth loss, with significant implications for mastication, esthetics, and overall quality of life. In addition, periodontitis has been linked to systemic diseases such as cardiovascular disease, diabetes mellitus, and adverse pregnancy outcomes, underscoring its importance as a public health concern [2].
Traditional periodontal therapies, including scaling and root planning, flap surgery, and guided tissue regeneration (GTR), aim to control infection and promote healing. However, these approaches often result in repair rather than true regeneration, with limited reformation of functional periodontal structures [3]. Advances in regenerative dentistry have introduced biomaterials, growth factors, and stem cell-based strategies, yet challenges remain in achieving predictable, stable outcomes [4,5].
Therapeutic nucleic acids, including DNA- and RNA-based polymers, have gained increasing attention in regenerative medicine for their ability to modulate cellular behavior and tissue repair. Among them, polydeoxyribonucleotides (PDRNs) and polynucleotides (PNs) represent biologically active DNA-derived macromolecules with demonstrated regenerative potential [6]. A PDRN is a mixture of DNA fragments with molecular weights ranging from 50 to 1500 kDa, typically extracted from salmon or trout sperm [7]. PNs refer to longer-chain DNA polymers, often exceeding the molecular size of a PDRN, which can have distinct physicochemical and pharmacological properties [8]. In addition, oligodeoxynucleotide (ODN)—short, synthetic DNA fragments—has emerged as a related class of nucleic acid therapeutics with promising immunomodulatory and regenerative effects, particularly through toll-like receptor (TLR)-mediated immune modulation and inflammation control [9]. Unlike PDRNs and PNs, which primarily serve as nucleotide sources and act through adenosine A2A receptor pathways, ODNs can be rationally designed to target specific transcriptional regulators or signaling pathways, potentially broadening their applications in periodontal tissue engineering [10]. In addition to biomaterials and nucleic acid-based approaches, stem cell-based regenerative therapies are gaining increasing attention. For instance, mesenchymal stem cells derived from human periapical cysts have shown promising potential in tissue regeneration, offering a minimally invasive and readily available cell source for dental applications [11]. Such cell-based therapies may be combined with bioactive molecules like PDRNs or PNs to achieve synergistic regenerative outcomes.
Tissue regeneration represents a cornerstone of contemporary dental research and clinical innovation, encompassing diverse targets such as periodontal structures, alveolar bone, oral mucosa, dental pulp, and peri-implant tissues. Nucleic acid-based therapeutics offer unique advantages in modulating angiogenesis, osteogenesis, and inflammation, making them highly relevant for multiple regenerative applications in dentistry [12]. While periodontal tissue regeneration has been the most extensively studied area to date, emerging evidence indicates promising potential in other clinical contexts, such as guided bone regeneration, socket preservation, and soft tissue healing [13]. This broader framework highlights the expanding role of nucleic acid-based therapeutics as versatile bioactive agents capable of supporting various dental tissue regeneration strategies.
A primary mechanism of PDRNs involves activation of the adenosine A2A receptor, which exerts potent anti-inflammatory effects and enhances tissue repair [14]. This receptor-mediated pathway upregulates vascular endothelial growth factor (VEGF), promoting angiogenesis and improving blood supply to damaged tissues [15]. In addition, PDRNs and PNs have been reported to stimulate fibroblast proliferation, collagen deposition, and extracellular matrix remodeling, thereby supporting the structural integrity of regenerating tissues [6]. Emerging evidence also suggests potential modulation of bone metabolism through enhanced osteoblast activity and suppression of osteoclast differentiation [16], which may benefit periodontal tissue regeneration in periodontal defects. These multifaceted actions make PDRNs and PNs attractive candidates for adjunctive use in periodontal regenerative therapies.
In dental and maxillofacial applications, PDRNs have been studied in various contexts, including extraction socket preservation [17], sinus floor augmentation [18], and peri-implant bone regeneration [19]. These studies generally report that adjunctive PDRNs can enhance new bone formation and preserve tissue volume in the early healing phase. By contrast, PNs have been less extensively studied in dentistry, but emerging evidence suggests it may improve soft-tissue healing and graft integration. For example, a PN–hyaluronic acid gel has shown clinical benefits when treating periodontal intrabony defects, leading to improved pocket reduction and bone fill in a case series [20]. Preclinical studies further support the pro-regenerative effects of these agents. Experiments in animal models have demonstrated that PDRN administration can accelerate wound healing and increase the quantity of new bone, especially when combined with scaffolds or graft materials [21,22]. Despite these promising findings, the literature on PDRNs/PNs in periodontal regeneration remains fragmentary—published studies vary widely in design, outcome measures, and delivery methods. A consolidated review is therefore warranted to synthesize the current evidence, identify knowledge gaps, and guide future research directions in this field.
This review aims to provide a comprehensive synthesis of the mechanistic basis, preclinical evidence, and translational potential of nucleic acid-based therapeutics—specifically PDRNs, PNs, and ODNs—for tissue regeneration in dentistry. While periodontal tissue regeneration has been the most extensively studied field, this review encompasses a broader range of applications, including alveolar bone, peri-implant tissues, extraction sockets, and oral mucosal regeneration. Both in vitro and in vivo studies are considered, with emphasis on mechanisms of action, delivery strategies, and potential clinical translation. By integrating current evidence across multiple dental tissue targets, this review seeks to guide future research and inform clinical strategies in regenerative dentistry.
2. Literature Search and Study Selection
A comprehensive literature search was conducted in PubMed and Scopus to identify studies investigating the use of PDRNs, PNs, and ODNs in periodontal, peri-implant, and oral tissue regeneration. The search strategy combined relevant keywords and Boolean operators, including
- “oligodeoxynucleotides” AND “dental”;
- “oligodeoxynucleotides” AND “oral”;
- “polydeoxyribonucleotide” AND “dental”;
- “polydeoxyribonucleotide” AND “oral”;
- “polynucleotide” AND “dental”;
- “polynucleotide” AND “oral”.
No restrictions were placed on the publication date. Only articles published in English were included. Grey literature, conference abstracts, and unpublished studies were excluded.
All retrieved records were imported into a reference manager, and duplicate entries were removed. Two reviewers independently screened titles and abstracts to exclude irrelevant studies, followed by full-text assessment against the inclusion criteria. Eligible studies included original in vitro, in vivo, and clinical research directly evaluating PDRNs, PNs, or ODNs in dental or periodontal regenerative applications. Review articles were excluded.
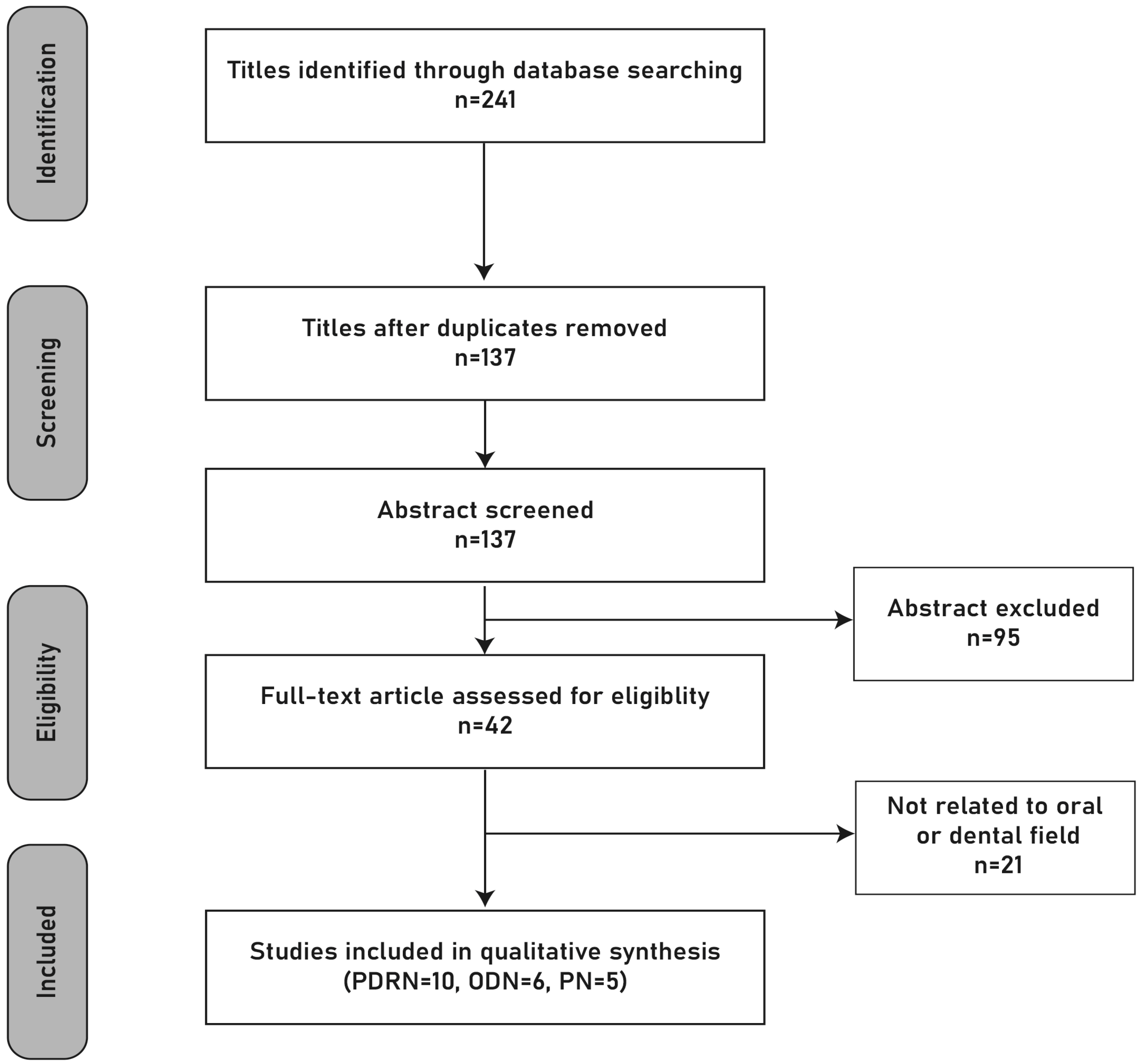
A total of 10 PDRN and 11 PN/ODN studies met the eligibility criteria and were included in the qualitative synthesis. The detailed selection process is illustrated in the PRISMA flow diagram (Figure 1).
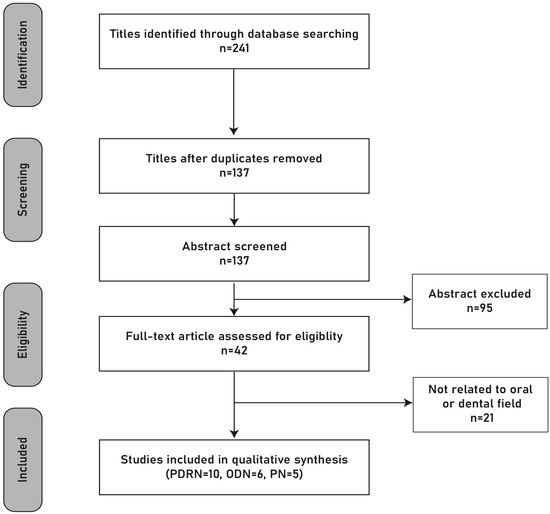
Figure 1.
PRISMA flow diagram. Original schematic illustration prepared by the authors.
In total, 21 studies were included in the review: 10 studies investigated PDRNs, and 11 studies investigated PNs/ODNs. Of these, 13 were preclinical animal or in vitro studies, and 8 were clinical studies.
3. PNs and ODNs in Regenerative Dentistry
3.1. Definition and Characteristics
PNs and ODNs are DNA-based biopolymers with distinct molecular sizes and therapeutic implications. PNs are long chains of nucleotide monomers, typically derived from natural sources such as fish sperm DNA, and are characterized by their viscoelastic, hydrophilic, and biocompatible nature. They can serve both as biological effectors and as structural components in regenerative scaffolds. In contrast, ODN are short, synthetic DNA fragments—usually fewer than 30 bases—designed to interact with specific DNA or transcription factor sequences, modulating gene expression through targeted molecular mechanisms. Both PNs and ODNs are non-immunogenic when appropriately purified, but they differ in their modes of action and pharmacokinetics: PNs often act as extracellular bioactive agents, while ODNs are designed for intracellular gene modulation (Table 1).

Table 1.
Summary of Studies on PNs and ODNs in Dental Regeneration.
3.2. Mechanisms in Regeneration and Wound Healing
PNs exert regenerative effects primarily by stimulating cell proliferation, angiogenesis, and extracellular matrix (ECM) synthesis. They can upregulate fibroblast activity, enhance collagen deposition, and increase VEGF production, thereby accelerating tissue repair. Additionally, their breakdown products serve as nucleoside/nucleotide precursors for DNA repair and replication.
ODNs, particularly decoy ODNs, operate through a different paradigm: they mimic the DNA binding sites of transcription factors and competitively inhibit their interaction with endogenous promoters. For example, nuclear factor-κB (NF-κB) decoy ODNs block pro-inflammatory gene transcription, reducing cytokine expression (IL-1β, IL-6) and osteoclastogenic signaling. This anti-inflammatory modulation can prevent pathological tissue breakdown, as demonstrated in NF-κB decoy ODN-loaded poly(lactic-co-glycolic acid) (PLGA) nanosphere systems, which sustained release and enhanced tissue targeting. By suppressing early inflammatory responses, these ODNs create a microenvironment favorable for periodontal ligament regeneration, cementum repair, and alveolar bone preservation (Figure 2).
Figure 2.
Schematic illustration of the molecular pathways underlying PDRN-induced tissue regeneration. Diagram summarizes representative preclinical models referenced in the text—in vitro (e.g., HUVEC/endothelial assays for angiogenesis; osteoblast/PDL fibroblast cultures for osteogenic/ECM responses) and in vivo (rodent periodontal/orthodontic models, extraction sockets, sinus lift). Original schematic created by the authors [20,23,24,25,26,27,28,29,30,31,32].
3.3. Current Dental Applications
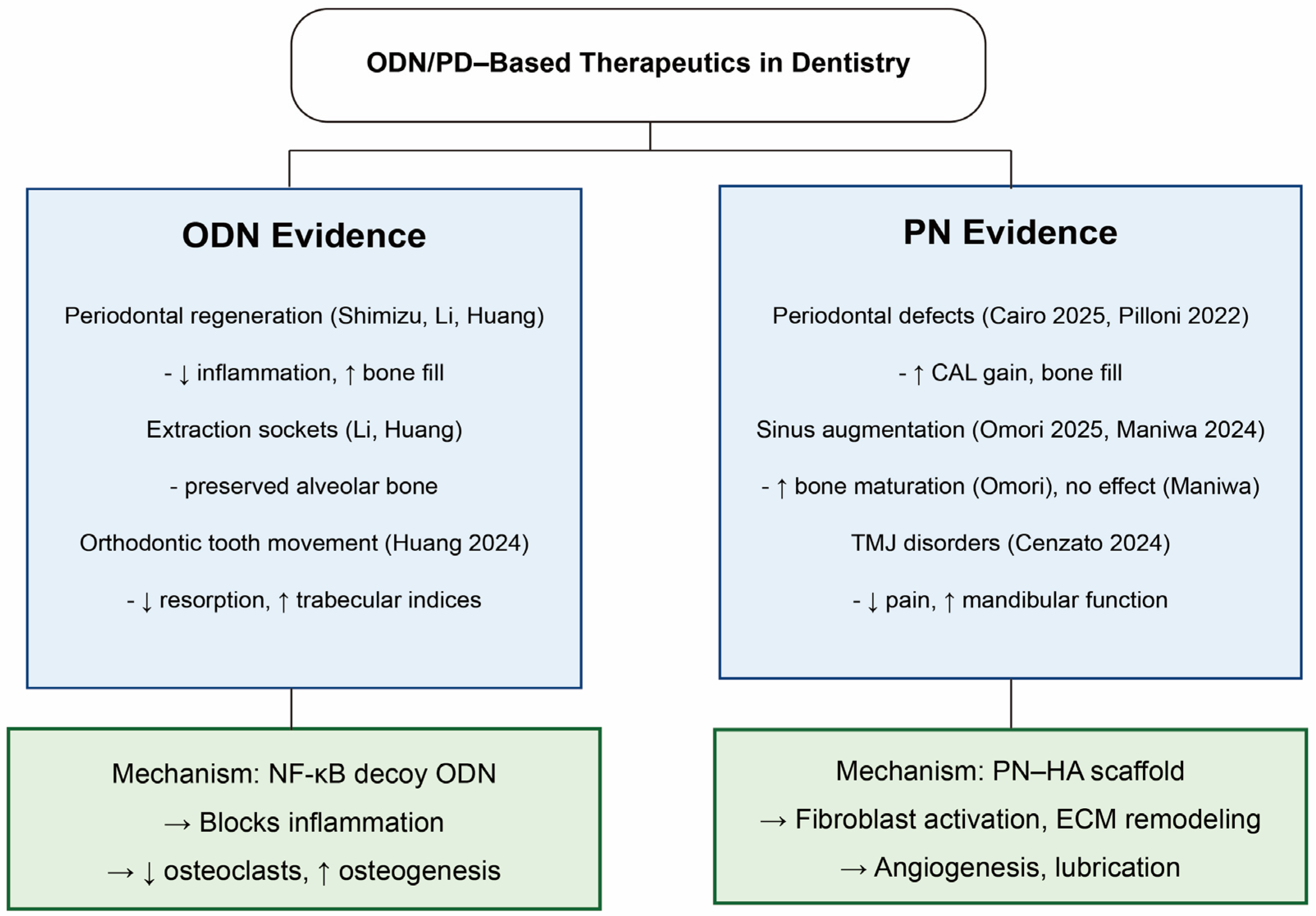
Recent studies have explored PNs and ODNs in various dental contexts (Figure 3):
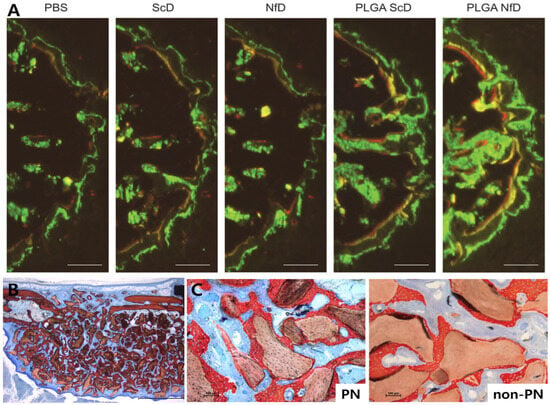
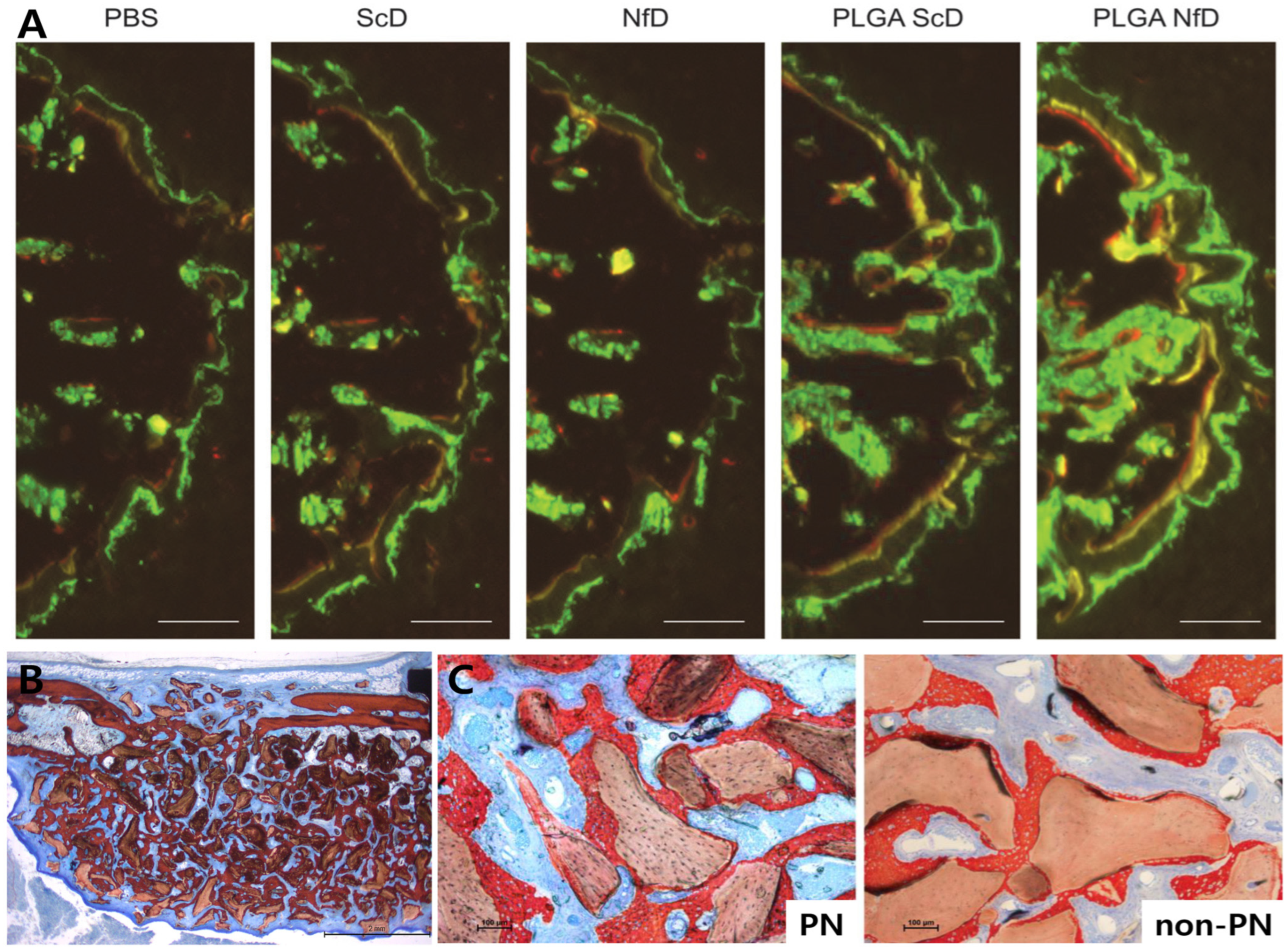
Figure 3.
Representative dental applications of PNs and ODNs. (A) Rat extraction socket model showing dynamic fluorescent labeling with enhanced mineral apposition following PLGA–NfD treatment [27]. (B) Mouse tooth replantation model showing ground sections at 10 weeks demonstrating increased bone fraction and corticalization, with more pronounced effects in PN-treated sites [26]. (C) Rabbit bilateral sinus lift model showing high-magnification histologic images with bone bridging and osteoconduction, and denser bone formation in PN-treated sites compared with non-PN controls [31]. These findings suggest that PN- and ODN-based approaches can enhance bone healing and hold promise for future clinical applications in dentistry. Abbreviations: PBS, phosphate-buffered saline; ScD, scrambled decoy ODN; NfD, NF-κB decoy ODN; PLGA, poly(lactic-co-glycolic acid). Figure adapted from Omori et al. [26], Huang et al. [27], and Maniwa et al. [31], licensed under CC BY 4.0.
- Periodontal regeneration: ODN-based strategies form the bulk of current evidence. Beginning with the work of Shimizu et al. [23], NF-κB decoy ODNs delivered locally have consistently shown the ability to suppress inflammatory signaling and enhance periodontal tissue repair. Subsequent studies, including those by Li et al. [28], demonstrated that PLGA-encapsulated NF-κB decoys prolong local release and improve bone and periodontal ligament regeneration in rat intrabony defects. More recent reports extended these findings to extraction sockets and orthodontic tooth movement models, where ODN delivery reduced alveolar bone loss, increased bone mineral density, and modulated osteoclast/osteoblast marker expression [27,29]. Collectively, these studies establish ODNs as effective regulators of inflammation and bone remodeling in periodontal contexts.
- PN evidence is more limited. In a retrospective clinical case series, a PN–hyaluronic acid mixture, with or without DBBM, was applied in the treatment of deep intrabony defects [20]. The therapy yielded significant improvements in probing depth reduction, clinical attachment gain, and radiographic bone fill after one year, with smoking identified as a negative predictor of outcomes [20]. Similarly, in a randomized controlled trial of residual periodontal pockets, adjunctive PN–HA gel provided additional reductions in bleeding on probing at deep sites compared with conventional therapy, despite only modest differences in probing depth and attachment outcomes overall [30].
- Implantology: In a rabbit sinus augmentation model, PNs incorporated into graft materials promoted enhanced bone formation and maturation [26]. Another rabbit sinus lift study, however, reported no significant benefit of PN–hyaluronic acid (HA) gel over deproteinized bovine bone mineral (DBBM) alone, indicating variability in outcomes and the need for standardized protocols [31]. ODN delivery systems, through NF-κB inhibition and osteoclast suppression, may also hold value in maintaining peri-implant bone, though this remains preclinical.
- Oral surgery and wound healing: ODN systems have been tested in extraction sockets and graft sites, where they effectively prevented post-extraction bone resorption and promoted osteogenesis [27,28]. These findings highlight their ability to modulate inflammatory signaling while supporting new bone formation, making them promising adjuncts for alveolar ridge preservation and surgical defect repair. PNs’ wound-healing properties have been confirmed in extra-oral tissues, but dental applications remain largely hypothetical.
- Temporomandibular joint (TMJ) disorder: PNs’ applications have also extended to temporomandibular joint pathology. In a randomized clinical trial of TMJ osteoarthritis, PN–HA pericapsular injections significantly reduced pain and improved mandibular kinematics compared with physiotherapy, with no major adverse effects [32].
Overall, PNs currently offer more theoretical translational potential for oral applications, supported mainly by evidence from other tissues [6]. In contrast, ODN has already shown promising results in dental models, particularly in controlling inflammation and supporting alveolar bone and periodontal regeneration. Together, they represent complementary strategies: PNs with trophic and anabolic actions and ODNs as a precise molecular regulator of inflammation and bone remodeling.
4. PDRNs in Regeneration and Dentistry
4.1. Definition and Characteristics
PDRNs are a mixture of deoxyribonucleotide polymers, typically 50–2000 base pairs in length, extracted from natural sources such as salmon sperm DNA. It is highly purified to eliminate immunogenic proteins and lipids. A PDRN is water-soluble, biocompatible, and stable at physiological pH. Its pharmacological effects are primarily mediated through activation of the adenosine A2A receptor, although breakdown products may also serve as nucleotide precursors for DNA repair and replication.
4.2. Mechanistic Evidence from In Vitro Studies
Multiple in vitro investigations have elucidated the cellular and molecular mechanisms underlying the regenerative potential of PDRNs (Table 2). These studies, performed in diverse cell models including fibroblasts, endothelial cells, osteoblasts, and macrophages, collectively demonstrate that PDRNs exert multifaceted effects relevant to periodontal and oral tissue repair.

Table 2.
Summary of In Vitro Studies on PDRN Mechanisms.
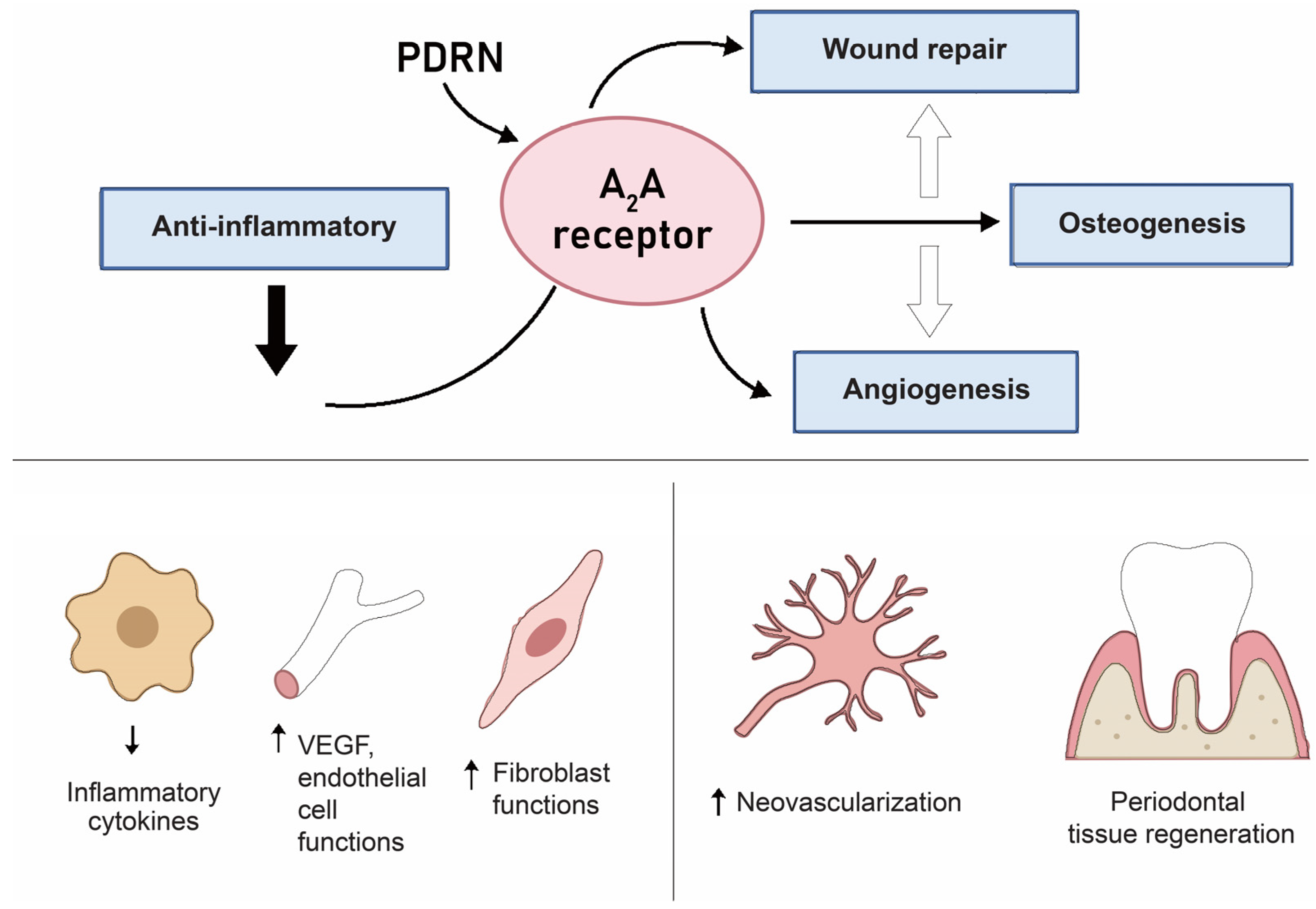
Yun et al. [33] demonstrated that PDRNs at an optimal concentration of 50 µg/mL significantly enhanced proliferation, migration, antioxidant defense, and mitochondrial respiration. These effects were mediated through Akt pathway activation, as pharmacological inhibition of Akt abolished the protective responses. Lee et al. [34] reported a dose-dependent osteogenic effect of PDRNs in 3D gingiva-derived mesenchymal stem cell (GMSC) spheroids. While viability and morphology were unaffected, PDRNs at 75 µg/mL increased calcium deposition, with runt-related transcription factor 2 (RUNX2) expression maximized at 25 µg/mL and collagen type 1 alpha1 (COL1A1) expression at 75 µg/mL. RNA-seq confirmed broad osteogenic gene upregulation, supporting a role for PDRNs in promoting gingival stem cell osteodifferentiation. Picciolo et al. [35] tested PDRNs in human gingival fibroblasts and epithelial cells under inflammatory conditions. At 50 µg/mL, PDRN suppressed NF-κB, tumor necrosis factor-alpha (TNF-α), and IL-6, while increasing IL-10. In parallel, PDRNs restored wingless-related integration site (Wnt)/β-catenin, VEGF, and epidermal growth factor (EGF) expression. These effects were abolished by A2A receptor antagonists, confirming the receptor-dependent mechanism.
4.2.1. Adenosine A2A Receptor—Mediated Anti-Inflammatory Signaling
A consistent finding across mechanistic studies is the activation of the adenosine A2A receptor as the primary pharmacological target of PDRNs [36,37]. This interaction increases intracellular cyclic AMP (cAMP) levels, leading to downstream activation of protein kinase A (PKA) and subsequent inhibition of NF-κB signaling [38,39]. Functionally, this results in reduced expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, and upregulation of anti-inflammatory mediators like IL-10 [39]. Pharmacological blockade of A2A receptors abrogates these effects, confirming the receptor-dependent nature of the pathway [36].
4.2.2. Antioxidant and Anti-Apoptotic Effects
PDRNs reduce oxidative stress markers, including reactive oxygen species (ROS) production and lipid peroxidation, in cells exposed to inflammatory or ischemic insults [40]. These antioxidant effects are associated with increased expression of endogenous antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase [41]. Parallel anti-apoptotic actions have been observed, characterized by reduced caspase-3 activation and preservation of mitochondrial membrane potential [42].
4.2.3. Pro-Angiogenic Signaling
Several studies in human umbilical vein endothelial cells (HUVECs) and microvascular endothelial cultures demonstrate that a PDRN enhances proliferation, migration, and tube formation, processes essential for neovascularization [40]. Mechanistically, a PDRN upregulates VEGF expression through A2A receptor–dependent activation of the ERK1/2 and Akt pathways [40]. These angiogenic effects suggest a pivotal role in early wound healing and granulation tissue formation.
4.2.4. Stimulation of Osteogenic Differentiation
In pre-osteoblast models, PDRN treatment increases alkaline phosphatase (ALP) activity, osteocalcin expression, and mineralized nodule formation [16]. This osteoinductive response is accompanied by activation of ERK and p38 mitogen-activated protein kinase (MAPK) signaling and is partially dependent on A2A receptor stimulation [43]. The ability of PDRNs to promote osteogenesis is particularly relevant for periodontal and peri-implant bone regeneration.
4.2.5. Modulation of Fibroblast Activity and ECM Remodeling
In human dermal and gingival fibroblasts, PDRNs enhance proliferation and increase synthesis of collagen type I and fibronectin [44]. Gene expression analyses reveal upregulation of matrix metalloproteinase inhibitors, suggesting a shift toward ECM preservation and remodeling [45]. These effects underpin improved wound tensile strength and soft tissue stability in preclinical models (Figure 4).
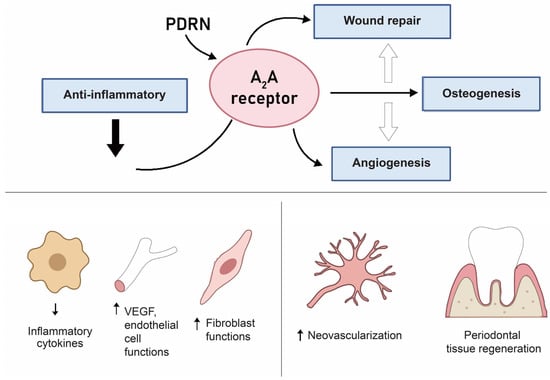
Figure 4.
Schematic drawing for the PDRN mechanism relevant to periodontal regeneration. The figure integrates evidence from in vitro (HUVEC/endothelial tube formation; pre-osteoblast ALP/OCN; dermal/gingival fibroblast ECM synthesis) and in vivo animal models (periodontal and peri-implant defects) highlighting A2A-receptor-mediated VEGF/ERK/Akt signaling, osteogenic pathways, and ECM remodeling. Original schematic created by the authors.
4.3. Preclinical Evidence from Animal Studies
Animal studies provide critical translational evidence supporting the regenerative potential of PDRNs in periodontal and peri-implant applications. Across various species—including rats, rabbits, and beagle dogs—PDRNs have been evaluated in models of extraction sockets, sinus augmentation, ridge preservation, periodontal defects, and peri-implantitis (Table 3 and Figure 5).

Table 3.
Summary of Animal Studies on PDRNs in Dental and Periodontal Regeneration.
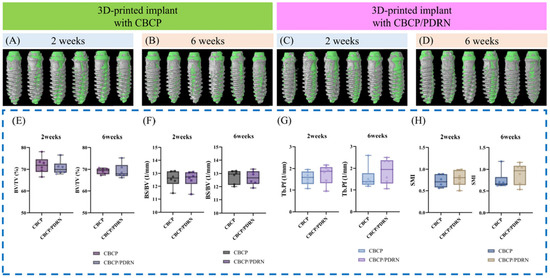
Figure 5.
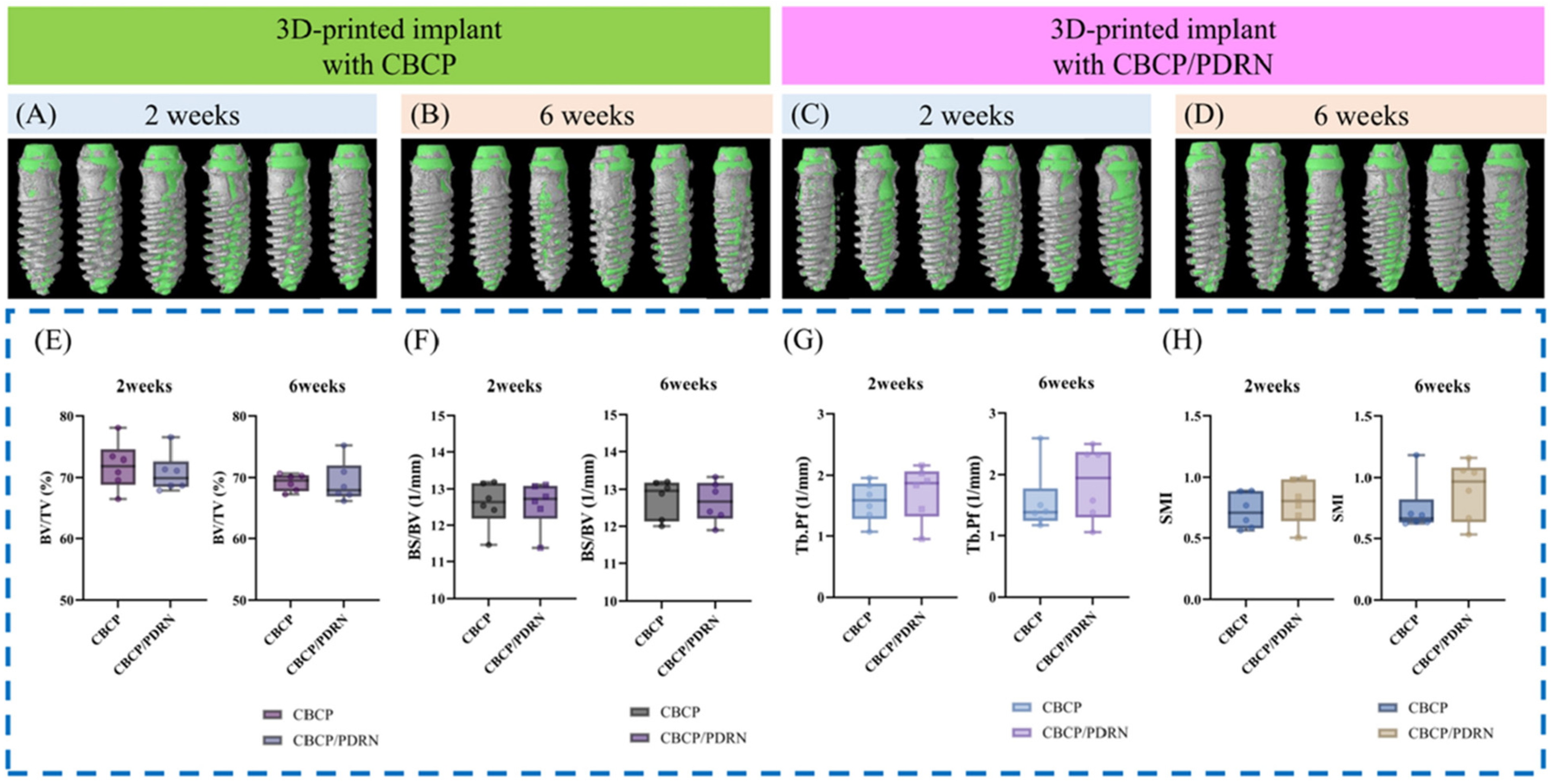
Representative micro-CT outcomes illustrating bone formation within the micro-channeled scaffolds. Beagle dogs underwent buccally positioned 3D-printed implant placement with either CBCP alone (A,B) or CBCP + PDRNs (C,D). Gray and green areas indicate bone volume and implanted regions in the volume of interest. Comparable morphometric parameters, including bone volume/tissue volume (BV/TV) (E), bone surface/bone volume (BS/BV) (F), trabecular bone pattern factor (Tb.Pf) (G), and structure model index (SMI) (H), were observed between the compared groups. Figure adapted from Lee et al. [19], licensed under CC BY 4.0.
4.3.1. Bone Regeneration
Multiple studies demonstrate that PDRNs enhance new bone formation and accelerate maturation when used alone or in combination with graft materials [14,16]. In beagle dog extraction sockets, collagen matrices loaded with PDRNs yielded significantly higher bone volume fraction and trabecular density compared with control scaffolds, with histological evidence of mature lamellar bone [17]. In rabbit sinus augmentation models, PDRNs combined with collagenated biphasic calcium phosphate promoted greater endosinus bone fill and more advanced mineralization [22]. Similar benefits were observed in alveolar ridge augmentation models, where bone-to-implant contact (BIC) ratios and bone fill percentages were markedly increased [19]. These effects are attributed primarily to PDRN-induced angiogenesis and osteoblast activation [17,19].
4.3.2. Peri-Implant Bone Repair
In peri-implant dehiscence models, combining PDRNs with graft materials such as collagenated biphasic calcium phosphate significantly enhanced histomorphometric outcomes—including greater new bone area and more mature lamellar bone formation—compared to graft alone, suggesting both pro-osteogenic and anti-inflammatory actions [19].
4.3.3. Anti-Inflammatory and Angiogenic Mechanisms In Vivo
Preclinical studies consistently show that PDRNs downregulate NF-κB-mediated inflammatory signaling, reducing infiltration of inflammatory cells and cytokine expression in periodontal tissues [45,48]. Concurrently, PDRNs enhance expression of VEGF and promotes neovascularization within regenerative sites, facilitating nutrient delivery and tissue maturation [49].
4.3.4. Translational Implications
The collective animal evidence positions PDRNs as a promising adjunctive agent in periodontal and peri-implant regenerative therapy. Its multi-target activity—spanning inflammation control, angiogenesis, osteogenesis, and fibroblast activation—may offer synergistic benefits when combined with established biomaterials. However, variations in dosing, delivery vehicles, and defect models underscore the need for standardized protocols before clinical translation.
PDRNs and PNs are currently used in other medical fields such as dermatology and ophthalmology, with approved injectable formulations and topical preparations [36,50]. Typical dosage ranges for injectable PDRNs are 2–5 mg/mL, while PNs are often used in hyaluronic acid-based formulations for soft-tissue repair [6]. These clinical experiences provide valuable insight into potential dental applications. Adaptation of these delivery systems to periodontal and peri-implant tissues could facilitate early clinical translation, although standardized dosing and delivery protocols specific to oral tissues remain to be established.
5. Conclusions
PDRNs, PNs, and ODNs are emerging nucleic acid-based therapeutics with demonstrated potential to enhance periodontal and peri-implant tissue regeneration. Acting predominantly through adenosine A2A receptor activation, PDRNs exert anti-inflammatory, angiogenic, and osteogenic effects, while PNs and ODNs provide additional trophic, extracellular matrix-modulating, or gene-regulatory benefits.
Evidence from in vitro and preclinical animal models consistently supports these biological activities, showing improvements in bone fill, connective tissue organization, angiogenesis, and inflammatory control. However, translation into clinical dentistry remains at an early stage. Existing human data are scarce, often limited to small, heterogeneous studies without standardized dosing or delivery protocols.
The overall technology readiness level of these agents for dental applications remains low to moderate, reflecting gaps in robust clinical validation, long-term safety assessments, and regulatory approval pathways. Variability in study designs, biomaterial combinations, and outcome measures further complicates direct comparisons and protocol development.
This review has several limitations: First, while the biological effects of PDRNs, PNs, and ODNs are well supported by preclinical studies, clinical evidence remains limited and heterogeneous. Second, dosing, delivery methods, and outcome measures varied widely across studies, making direct comparisons challenging. Third, most of the available data derive from small-scale animal models and short-term follow-ups, highlighting the need for more robust, standardized clinical research.
Future research should prioritize high-quality randomized controlled trials, head-to-head comparisons with established biologics, and mechanistic studies in human-derived periodontal and peri-implant tissues. Cost-effectiveness analyses and regulatory guidance will also be essential for broader clinical adoption. Until such evidence is available, PDRNs, PNs, and ODNs should be considered promising adjunctive tools, as they are best applied within well-designed research settings rather than routine clinical practice. While most available clinical evidence focuses on periodontal regeneration, other applications such as peri-implant, alveolar ridge preservation, and oral soft tissue regeneration, remain underexplored and warrant further investigation.
Author Contributions
Conceptualization, J.-Y.K. and S.-G.K.; methodology, M.-H.H.; software, U.G.; validation, U.G., J.-Y.K. and S.-G.K.; formal analysis, M.-H.H.; investigation, M.-H.H.; resources, M.-H.H.; data curation, S.-G.K.; writing—original draft preparation, J.-Y.K.; writing—review and editing, S.-G.K.; visualization, M.-H.H.; supervision, U.G.; project administration, S.-G.K.; funding acquisition, M.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Regional Innovation System & Education (RISE) program through the Gangwon RISE Center, funded by the Ministry of Education (MOE) and the Gangwon State (G.S.), Republic of Korea (2025-RISE-10-004). (P0028969, Regional Anchor company-Academia Partnership Innovation Development).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALP | Alkaline phosphatase |
| A2A | Adenosine A2A receptor |
| BIC | Bone-to-implant contact |
| BMD | Bone mineral density |
| BS/BV | Bone surface/bone volume |
| BV/TV | Bone volume/tissue volume |
| CBCP | Collagenated biphasic calcium phosphate |
| DBBM | Deproteinized bovine bone mineral |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ERK | Extracellular signal-regulated kinase |
| FGF-2 | Fibroblast growth factor 2 |
| HA | Hyaluronic acid |
| HUVEC | Human umbilical vein endothelial cell |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa B |
| NfD | NF-κB decoy oligodeoxynucleotide |
| OCN | Osteocalcin |
| ODN | Oligodeoxynucleotide |
| PBS | Phosphate-buffered saline |
| PDL | Periodontal ligament |
| PDRN | Polydeoxyribonucleotide |
| PLGA | Poly(lactic-co-glycolic acid) |
| PN | Polynucleotide |
| RUNX2 | Runt-related transcription factor 2 |
| ScD | Scrambled decoy oligodeoxynucleotide |
| SMI | Structure model index |
| Tb.Pf | Trabecular bone pattern factor |
| TGF-β1 | Transforming growth factor beta 1 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
References
- Szmirnova, I.; Szmirnov, G.; Gellérd, E.; Németh, Z.; Kivovics, M.; Szabó, G. Challenges and strategies in dental care for patients with intellectual disabilities in Hungary. Maxillofac. Plast. Reconstr. Surg. 2025, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, A.C.; Wong, M.L.; Allen, F.; Gao, X. Periodontal disease and systemic health: An update for medical practitioners. Ann. Acad. Med. Singap. 2022, 51, 567–574. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Seo, J.I.; Lim, J.H.; Jo, W.M.; Lee, J.K.; Song, S.I. Effects of rhBMP-2 with various carriers on maxillofacial bone regeneration through computed tomography evaluation. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 40. [Google Scholar] [CrossRef]
- Solidum, J.G.N.; Ceriales, J.A.; Ong, E.P.; Ornos, E.D.B.; Relador, R.J.L.; Quebral, E.P.B.; Lapeña, J.F.F., Jr.; Tantengco, O.A.G.; Lee, K.Y. Nanomedicine and nanoparticle-based delivery systems in plastic and reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 15. [Google Scholar] [CrossRef]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From polydeoxyribonucleotides (PDRNs) to polynucleotides (PNs): Bridging the gap between scientific definitions, molecular insights, and clinical applications of multifunctional biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef]
- Chae, D.; Oh, S.W.; Choi, Y.S.; Kang, D.J.; Park, C.W.; Lee, J.; Seo, W.S. First report on microbial-derived polydeoxyribonucleotide: A sustainable and enhanced alternative to salmon-based polydeoxyribonucleotide. Curr. Issues Mol. Biol. 2025, 47, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, H.J.; Jung, R.J.; Won, C.Y.; Ohk, S.O.; Kim, H.T.; Roh, N.K.; Yi, K.H. High-resolution 3-D scanning electron microscopy (SEM) images of DOTTM polynucleotides (PN): Unique scaffold characteristics and potential applications in biomedicine. Skin Res. Technol. 2024, 30, e13667. [Google Scholar] [CrossRef] [PubMed]
- Nigar, S.; Shimosato, T. Cooperation of oligodeoxynucleotides and synthetic molecules as enhanced immune modulators. Front. Nutr. 2019, 6, 140. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y.; Chen, D.; Li, Q.; Shi, S.; Huang, X.; Fang, M.; Yang, M. Advances in the mechanisms and applications of inhibitory oligodeoxynucleotides against immune-mediated inflammatory diseases. Front. Pharmacol. 2023, 14, 1119431. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.; Negruțiu, M.L.; Rusu, L.C.; Riviș, M. Mesenchymal stem cells derived from human periapical cysts and their implications in regenerative medicine. Biomedicines 2023, 11, 2436. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The effects of polydeoxyribonucleotide on wound healing and tissue regeneration: A systematic review of the literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chen, J.-Y.; Suo, W.-H.; Shao, W.-R.; Huang, C.-Y.; Li, M.-T.; Li, Y.-Y.; Li, Y.-H.; Liang, E.-L.; Chen, Y.-H.; et al. Unlocking the future of periodontal regeneration: An interdisciplinary approach to tissue engineering and advanced therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef]
- Lee, W.; Son, A.; Ji, J.; Han, E.; Cho, J.Y.; Kim, J.W.; Kim, Y.O.; Kong, H.J.; Kim, H. Elucidating interactome dynamics of the A2A adenosine receptor in the presence of polydeoxyribonucleotide. J. Proteome Res. 2025, 24, 3741–3750. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Ko, Y.C.; Lee, J.; Urban, I.; Seol, Y.J.; Lee, Y.M.; Koo, K.T. The adjunctive effect of polydeoxyribonucleotide on bone formation in alveolar ridge preservation: A pre-clinical in vivo study. J. Clin. Periodontol. 2024, 51, 1034–1043. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Koo, K.T.; Seol, Y.J.; Lee, Y.M. The impact of polydeoxyribonucleotide on early bone formation in lateral-window sinus floor elevation with simultaneous implant placement. J. Periodontal Implant Sci. 2023, 53, 157–169. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Seol, Y.J.; Lee, Y.M.; Koo, K.T. Effect of polydeoxyribonucleotide on early bone formation in lateral bone augmentation with immediate implant placement: An experimental in vivo study. Sci. Rep. 2023, 13, 16853. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Cavalcanti, R.; Barbato, L.; Nieri, M.; Castelluzzo, W.; di Martino, M.; Pilloni, A. Polynucleotides and hyaluronic acid (PN-HA) mixture with or without deproteinized bovine bone mineral as a novel approach for the treatment of deep in-frabony defects: A retrospective case series. Int. J. Periodontics Restor. Dent. 2025, 45, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Huh, C.K.; Lee, J.H.; Kim, K.W.; Kim, M.Y. Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 7. [Google Scholar] [CrossRef]
- Lim, H.; Hong, J.Y.; Shin, S.I.; Chung, J.H.; Thoma, D.S.; Jung, R.E.; Lim, H.C. Effects of polydeoxyribonucleotide (PDRN) on endosinus bone regeneration following sinus floor elevation: An experimental in vivo pilot study. Clin. Oral Implants Res. 2025, 36, 239–249. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakagami, H.; Morita, S.; Tsukamoto, I.; Osako, M.K.; Nakagami, F.; Shimosato, T.; Minobe, N.; Morishita, R. New treatment of periodontal diseases by using NF-kappaB decoy oligodeoxynucleotides via prevention of bone resorption and promotion of wound healing. Antioxid. Redox Signal 2009, 11, 2065–2075. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, Y.; Huang, S.; Ruiz, S.; Kawai, T.; Bai, Y.; Han, X. CpG ODN/mangiferin dual delivery through calcium alginate hydrogels inhibits immune-mediated osteoclastogenesis and promotes alveolar bone regeneration in mice. Biology 2023, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Salcedo, A.; Yamazaki, T.; Ohshima, H. Effects of synthetic toll-like receptor 9 ligand molecules on pulpal immuno-modulatory response and repair after injuries. Biomolecules 2024, 14, 931. [Google Scholar] [CrossRef]
- Omori, H.; Botticelli, D.; Silva, E.R.; Xavier, S.P.; Souza, S.L.S.; Kusano, K.; Baba, S. Analysis of implant osseointegration, bone repair, and sinus mucosa integrity using Bio-Oss® and hyaluronic acid-polynucleotide gel (Regenfast®) in maxillary sinus augmentation in rabbits. Dent. J. 2025, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Ishida, Y.; Li, K.; Rintanalert, D.; Hatano-Sato, K.; Oishi, S.; Hosomichi, J.; Usumi-Fujita, R.; Yamaguchi, H.; Tsujimoto, H.; et al. NF-κB decoy ODN-loaded Poly(Lactic-co-glycolic Acid) nanospheres inhibit alveolar ridge resorption. Int. J. Mol. Sci. 2023, 24, 3699. [Google Scholar] [CrossRef]
- Li, K.; Ishida, Y.; Hatano-Sato, K.; Ongprakobkul, N.; Hosomichi, J.; Usumi-Fujita, R.; Kaneko, S.; Yamaguchi, H.; Ono, T. Nuclear factor-kappa B decoy oligodeoxynucleotide-loaded poly lactic-co-glycolic acid nanospheres promote periodontal tissue healing after tooth replantation in rats. J. Periodontol. 2022, 93, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Ishida, Y.; Hatano-Sato, K.; Oishi, S.; Hosomichi, J.; Usumi-Fujita, R.; Yamaguchi, H.; Tsujimoto, H.; Sasai, A.; Ochi, A.; et al. NF-κB decoy oligodeoxynucleotide-loaded poly lactic-co-glycolic acid nanospheres facilitate socket healing in orthodontic tooth movement. Int. J. Mol. Sci. 2024, 25, 5223. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Trezza, C.; Carere, M.; De Filippis, A.; Marsala, R.L.; Marini, L. Clinical effects of the adjunctive use of polynucleotide and hyaluronic acid-based gel in the subgingival re-instrumentation of residual periodontal pockets: A randomized, split-mouth clinical trial. J. Periodontol. 2023, 94, 354–363. [Google Scholar] [CrossRef]
- Maniwa, N.; Xavier, S.P.; Scombatti de Souza, S.; Silva, E.R.; Botticelli, D.; Morinaga, K.; Baba, S. Sequential bone repair in rabbit sinus lifts using Bio-Oss and hyaluronic acid-polynucleotide gel (Regenfast). J. Funct. Biomater. 2024, 15, 361. [Google Scholar] [CrossRef]
- Cenzato, N.; Crispino, R.; Russillo, A.; Del Fabbro, M.; Tartaglia, G.M. Clinical effectiveness of polynucleotide TMJ injection com-pared with physiotherapy: A 3-month randomised clinical trial. Br. J. Oral Maxillofac. Surg. 2024, 62, 807–812. [Google Scholar] [CrossRef]
- Yun, Y.G.; Yeo, D.; Shin, S.J.; Shin, J.S.; Lee, J.H.; Kim, H.W. Polydeoxyribonucleotide enhances the bioactivities of stem cells from human exfoliated deciduous teeth through Akt activation. Biochem. Biophys. Res. Commun. 2024, 739, 150947. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwa, S.; Cho, S.; Kim, J.H.; Song, H.J.; Ko, Y.; Park, J.B. Impact of polydeoxyribonucleotides on the morphology, viability, and osteogenic differentiation of gingiva-derived stem cell spheroids. Medicina 2024, 60, 1610. [Google Scholar] [CrossRef]
- Picciolo, G.; Mannino, F.; Irrera, N.; Altavilla, D.; Minutoli, L.; Vaccaro, M.; Arcoraci, V.; Squadrito, V.; Picciolo, G.; Squadrito, F.; et al. PDRN, a natural bioactive compound, blunts inflammation and positively reprograms healing genes in an “in vitro” model of oral mucositis. Biomed. Pharmacother. 2021, 138, 111538. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Baek, A.; Cho, Y.; Kim, S.H.; Baek, D.; Hwang, J.; Cho, S.R.; Kim, H.J. Therapeutic effects of polydeoxyribonucleotide in an in vitro neuronal model of ischemia/reperfusion injury. Sci. Rep. 2023, 13, 6004. [Google Scholar] [CrossRef]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Jeon, J.W.; Chung, J.Y.; Han, J.H. Adenosine A2A receptor agonist polydeoxyribonucleotide ameliorates short-term memory impairment by suppressing cerebral ischemia-induced inflammation via MAPK pathway. PLoS ONE 2021, 16, e0248689. [Google Scholar] [CrossRef]
- Jang, S.K.; Choi, J.; Lim, H.W.; Kim, H.G.; Yoo, Y.M. Comparative analysis of melatonin and polydeoxyribonucleotide: Possible benefits of co-treatment effects and potential synergistic applicability. Int. J. Mol. Sci. 2025, 26, 5703. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, I.; Mannino, F.; Irrera, N.; Minutoli, L.; Arcoraci, V.; Altavilla, D.; Cavallini, G.M.; Guarini, S.; Squadrito, F.; Pallio, G. Beneficial effects of polydeoxyribonucleotide (PDRN) in an in vitro model of fuchs endothelial corneal dystrophy. Pharmaceuticals 2022, 15, 447. [Google Scholar] [CrossRef]
- Shin, S.M.; Baek, E.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Polydeoxyribonucleotide exerts opposing effects on ERK activity in human skin keratinocytes and fibroblasts. Mol. Med. Rep. 2023, 28, 148. [Google Scholar] [CrossRef]
- An, J.; Park, S.H.; Ko, I.G.; Jin, J.J.; Hwang, L.; Ji, E.S.; Kim, S.H.; Kim, C.J.; Park, S.Y.; Hwang, J.J.; et al. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced lung injury by inhibiting apoptotic cell death in rats. Int. J. Mol. Sci. 2017, 18, 1847. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Galfo, F.; Oteri, G.; Atteritano, M.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Aliquò, F.; et al. Adenosine receptor stimulation improves glucocorticoid-induced osteoporosis in a rat model. Front. Pharmacol. 2017, 8, 558. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.; Wang, H.; Lee, G.; Kim, S.; Ryu, Y.H.; Chang, N.H.; Kang, Y.W. Analysis of skin regeneration and barrier-improvement efficacy of polydeoxyribonucleotide isolated from panax ginseng (C.A. Mey.) adventitious root. Molecules 2023, 28, 7240. [Google Scholar] [CrossRef]
- Oh, N.; Hwang, J.; Kang, M.S.; Yoo, C.Y.; Kwak, M.; Han, D.W. Versatile and marvelous potentials of polydeoxyribonucleotide for tissue engineering and regeneration. Biomater. Res. 2025, 29, 0183. [Google Scholar] [CrossRef]
- Lee, H.K.; Hong, J.Y.; Shin, S.I.; Herr, Y.; Lim, H.C.; Chung, J.H. Soft-tissue volume augmentation using a connective tissue graft and a volume-stable collagen matrix with polydeoxyribonucleotide for immediate implant placement: A pilot study in a dog model. J. Periodontal Implant Sci. 2024, 54, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Hong, J.Y.; Shin, S.; Shin, S.Y.; Chung, J.H.; Thoma, D.S.; Lim, H.C. Preclinical investigation on the effect of collagen matrix with polydeoxyribonucleotide at buccally positioned implants. Clin. Implant Dent. Relat. Res. 2025, 27, e13411. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a bioactive natural compound, ameliorates imiquimod-induced psoriasis through NF-κB pathway inhibition and Wnt/β-catenin signaling modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef]
- Mari, R.; Ramamurthy, J.; Rudhra, K.; Krishnaswamy, N. Efficacy of polydeoxyribonucleic acid (PDRN) in periodontal regeneration: A systematic review of clinical outcomes. J. Oral Biol. Craniofac. Res. 2025, 15, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, M.; Tomasello, E.M.; Caporossi, A. Clinical Evaluation of Corneal Epithelialization after Photorefractive Keratectomy in Patients Treated with Polydeoxyribonucleotide (PDRN) Eye Drops: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Ophthalmol. 2004, 14, 284–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).