Abstract
The sea buckthorn has proven antioxidant properties. The aim of the study was to determine the effect of the addition of dry Hippophae rhamnoides extract on the functional and oxidative stability of traditional Kazakh molded smoked ham. Sea buckthorn extraction parameters were optimized by factorial experimental design and polynomial regression modelling. The optimal dosages of acid value, peroxide value, TBARS, and sensory-assessed cross-sectional surface were determined. The instrumental color, pH, texture profile, hydrolytic and oxidative changes in lipid and protein fractions, total phenolic content, antioxidant activity, and microbiological status of ham with 3% extract were evaluated over 30 d at 0–4 °C. An increase in total phenols (19.8%), radical scavenging activity against DPPH (33.6%), and FRAP (12.8%) was found. The addition of 3.0% sea buckthorn extract had a minor effect on the oxidative processes. Dry sea buckthorn extract can be successfully added to the production of traditional Kazakh molded smoked ham from horse and camel meat to stabilize the oxidative stability of the product.
1. Introduction
The consumption of horse and camel meat and products made from them is traditional in Kazakhstan [1,2]. Therefore, molded smoked ham made from horse and camel meat is offered on the market, which is very well received by consumers [3]. Unfortunately, both horse and camel meat are rich in both heme iron from myoglobin and pro-oxidant enzyme systems and have a unique fatty acid composition [4]. During salting (by injection) and subsequent vacuum tumbling of the meat, myoglobin interacts with sodium nitrite and initially forms metmyoglobin, which at a later stage interacts with nitric oxide and is transformed into nitrosomyoglobin [5]. After subsequent heat treatment of the salted hams in weakly acidic conditions, the globin protein denatures. Nitrosomyoglobin is converted into nitrosyl-hemochrome [5], a pink pigment responsible for the color of the cut surface of the hams. The attractive pinkish-red color of the cut surface of the hams fades to gray when the product oxidizes in contact with oxygen from the air or is exposed to light [5].
Therefore, the oxidative stability of lipids and pigment resistance of horse and camel meat are considered relatively low. Optimizing the formulation of the meat matrix of such smoked hams is essential to achieving the desired specific quality characteristics of the finished product, such as color, taste, aroma, consistency, and safety. For this reason, the brine recipe contains L-ascorbate and α-tocopherol. Nevertheless, even under these conditions, the color of the cut remains unstable. In order to improve the color and lipid oxidative stability, it is advisable to look for opportunities to add strong antioxidant components, such as polyphenols from some natural plant extracts. For these reasons, oxidative spoilage dominates over microbial spoilage and limits the shelf life of the product [6].
Therefore, following the global trends for the application of natural antioxidants in such meat products, opportunities are being sought to solve these problems by using natural plant extracts with pronounced antioxidant properties [7]. Sea buckthorn is a widespread plant whose fruits have proven antioxidant properties [8].
Bobko et al. [9] propose that sea buckthorn berries (Hippophae rhamnoides Hergo) be applied as a potential natural antioxidant in the meat industry, while Bukarbaev and Abzhanova [10] discuss the use of sea buckthorn extracts as additives for meat products. As a justification for such technologies, Ut’yanov et al. [11] hypothesized that sea buckthorn is a factor that inhibits the processes of formation of heterocyclic aromatic amines in smoked meat products.
Vilas-Franquesa et al. [12] are of a similar opinion. They believe that sea buckthorn-based ingredients have potential for application in the food industry. Vilas Franquesa [13] has developed and characterized products obtained from sea buckthorn berries (Hippophae rhamnoides). Danielski and Shahidi [14] expressed the opinion that due to their specific phenolic composition and biological activity, the fruits and seeds of Hippophae rhamnoides L. can be an unconventional source of natural antioxidants. Papuc et al. [15] also reported high antioxidant activity of sea buckthorn fruits. They believe that the polyphenols contained in sea buckthorn are responsible for this. Kubczak et al. [16] indicated various extracts from leaves and twigs of Hippophae rhamnoides L. as a rich source of nutrients and bioactive compounds with antioxidant activity, and Rop et al. [17] found antioxidant and radical scavenging activity in the fruits of six varieties of sea buckthorn (Hippophae rhamnoides L.). Ko et al. [18] reported similar results. They found that extracts of Hippophae rhamnoides L. possess not only pronounced antioxidant activity but also a whitening effect. Papuc et al. [19] also discussed the antioxidant activity of sea buckthorn (Hippophae rhamnoides) extracts, comparing them with that of common dietary supplements and additives.
According to Fang et al. [20], the antioxidant properties of sea buckthorn (Hippophae rhamnoides) fruits are due to the high concentrations of profiled flavanol glycosides. Tkacz et al. [21] also found increased antioxidant activity in sea buckthorn (Hippophae rhamnoides L.) fruits, accompanied by antienzyme activity, which is modulated by chemical components. Chauhan et al. [22] have proven that aqueous extracts of sea buckthorn (Hippophae rhamnoides) seeds have pronounced antioxidant and antibacterial activity. Negi et al. [23] are of a similar opinion, and they have tested other types of sea buckthorn (Hippophae rhamnoides L.) seed extracts in addition to aqueous extracts. Chawla et al. [24] hypothesized that, in addition to antioxidant activity, fractionated extracts of Hippophae rhamnoides fruits possess pronounced protective properties.
Geetha et al. [25] demonstrated, in vitro, antioxidant and immunomodulatory properties of sea buckthorn (Hippophae rhamnoides). The unanimous opinions of all researchers lead to the conclusion that sea buckthorn can and should be used as a natural additive in meat products. For example, Anchidin et al. [26] increased the antioxidant capacity of functional meat products by infusing them with sea buckthorn oil, thus achieving an effective prophylactic food to combat congenital antioxidant deficiency. However, during ripening, the phytonutrients in sea buckthorn fruits (Hippophae rhamnoides L.) change, and this has a positive correlation effect on their antioxidant properties [27].
Therefore, Mo et al. [28] developed a method for preserving yak meat by applying a sustained release of microencapsulated sea buckthorn essential oil on porous starch and gum arabic. The use of sea buckthorn polyphenols affects the discoloration and lipid peroxidation of ground pork and beef during cooling [29]. Wojtaszeket et al. [30] developed a new type of innovative beef burgers enriched with sea buckthorn fruit juice. These authors studied the physicochemical, antioxidant, and organoleptic properties of ground beef from the burgers and reported another health benefit, namely the antidiabetic properties of sea buckthorn. Bobko et al. [31] also expressed the opinion that the quality parameters of raw cooked meat products can be influenced by treatment with a sea buckthorn fruit preparation (Hippophae rhamnoides L.). Similar data were also reported by Salejda et al. [32], who established the influence of sea buckthorn fruits (Hippophae rhamnoides L.) on the quality characteristics of cooked pork sausages. Mesárošová et al. [33] have shown that sea buckthorn extract (Hippophae rhamnoides var. vitaminnaja) affects the spoilage and shelf life of pork sausages, while Bukarbaev et al. [34] have studied a similar effect on the quality and shelf life of cooked smoked sausages. Finally, Makangali et al. [35] have studied the effect of sea buckthorn seed powder and have shown that this additive can be used in the production of smoked meat products from camel and beef.
The literature review clearly shows the adequacy of the application of sea buckthorn extracts in meat products. Similar studies with molded smoked hams from horse and camel meat have not been conducted.
The main hypothesis of the present study is that by adding of powdered sea buckthorn pomace extract to the brine solution, the total phenolic content and antioxidant activity of the molded smoked ham from horse and camel meat will increase, which will lead to stabilization of color (instrumental color L*, a*, b*), oxidative stability (AV, POV, TBARS, protein oxidation), and other quality indicators (pH, texture profiles, microbiological status) after 30 days of storage at 0–4 °C of the vacuum-packed finished product.
Therefore, the aim of the study was to determine the influence of the addition of the dry sea buckthorn water–ethanol extract (Hippophae rhamnoides) on the functional and oxidative stability of traditional Kazakh molded smoked ham.
2. Materials and Methods
2.1. Materials
The chilled deboned horse and camel meat were delivered from the slaughterhouse of Pervomaiskiye delikatesy Ltd. (Kasyksky village, Kordaysky district, Kazakhstan) (48 h post mortem). The experiment with smoke-molded ham was carried out in the same manufactory by following the technological process for the production of a delicacy product from molded meat (based on horsemeat and camel meat). Raw material was sorted: lean horse meat—muscle cuts with low fat content; horse fat—soft, fast-melting, enhances juiciness and flavor, and coarse-grained; lean camel meat. To prevent dryness, 10–20% fat (ground to pieces with diameter 1–2 cm) was added to the meat. Meat chunks were chopped to a size 5–6 cm thick.
Brine preparation (per 100 L): chilled water 100 L, table salt 6–7 kg, nitrite curing salt (0.6% NaNO2)—1–1.2 kg, additive “Kf Stabinject 101” (manufactured by Koenigshof GmbH, Lebensmitteladditive, Germany) (containing di- and triphosphates (E450 and E451), soy and milk proteins, thickeners (kappa-carrageenan E407 in combination with locust bean gum E410, guar gum E412 and xanthan gum E415), mono- and disaccharides, table salt, and two antioxidants (α-tocopherol and L-ascorbic acid)—0.5–1 kg.
Processing of traditional Kazakh molded smoked ham. The control sample was produced without the addition of sea buckthorn extract but an experimental one—with (3%) sea buckthorn powder extract—3 kg/100 L brine. All ingredients were mixed at 2 °C until fully dissolved. Injecting was carried out using a Günther single-head injector with 16 needles (Günther Maschinenbau GmbH, D-83301 Traunreut, Germany) at pressure 0.2 MPa. In total, 70 L of brine was injected per 100 kg of raw meat. This technology yielded 127.5 ± 1.7 kg of finished product from 100 kg of raw meat materials. Each chunk was evenly injected with deep penetration of the brine. The injected meat with a temperature not higher than +2 °C was loaded into the tumbler at 50% of the drum volume. Tumbling was carried out in the following mode: for the first hour of processing—without rest, under vacuum −8.5 bar (−0.85 MPa) at an angular speed of rotation of the tumbler drum of 5 min−1 (rpm). For the next 5 h—2 min of tumbling at an angular speed of the drum of 3 min−1 (rpm) under a vacuum of −5 bar (−0.5 MPa) and a subsequent rest of 8 min. Tumbling continued until complete distribution of the brine and sea buckthorn powder extract and formation of protein exudate (natural binder). The tumbled pieces of meat and fat were packed tightly into round shapes 30% and were molded by pressing to remove air and residual brine and to form a uniform structure that retained its shape. The molded raw hams were removed from the round shapes and steam-cooked at 80 °C for 90 min until reaching an internal temperature at least 73 °C, proteins coagulated, and meat was fully cooked. Cooked hams were dried at 60 °C for 15 min in order to remove surface moisture to fix the shape, prepare for smoking, and to improve the microbiological stability. The next step was smoking. Alder wood chips are traditionally used in Kazakhstan for smoking because of their balanced lignin composition and low resin content. Alder produces smoke with a high level of phenolic compounds (not determined in this study), which are responsible for the pleasant aroma and have a pronounced antioxidant effect. Thus, it ensures an optimal balance of aroma, flavor, and protective properties, an essential element of the smoking process. The smoke was produced by smoldering alder wood chips, which produce a dense smoke with a rich composition of phenols and other aromatic components. Smoldering ensures uniform exposure of the smoke to the product’s surface, improves sensory characteristics, and extends shelf life due to the antimicrobial and antioxidant effects of phenolic compounds. A smoke generator was used by smoldering a layer of moistened alder wood chips. The smoke had a density of 1.3–1.5 g/cm3 and a speed of movement of 2–3 m/s. The ham was smoked at 60 °C for 20 min and, finally, was rapidly cooled to +4 °C for up to 1 h to prevent bacterial growth and stabilize the product’s structure. The finished product was packed in and vacuum sealing by packed in vacuum shrink film, labelled, and stored at 0–4 °C for 30 days. The packaging material used was vacuum shrink film of the brand Cryovac B620, designed for packaging food products with subsequent heat treatment and storage under vacuum. The main characteristics of the packaging material were as follows. Oxygen transmission rate (OTR): no more than 45 cm3/m2/day at 23 °C, 0% RH; water vapor transmission rate (WVTR): no more than 2.5 g/m2/day at 38 °C, 90% RH; film thickness: 75 µm; shrinkage temperature: 85–90 °C for 3–5 s; film composition: multilayer coextruded structure based on polyamide (PA) and polyethylene (PE), providing barrier properties and heat sealability. This packaging was chosen due to its high barrier properties, which help suppress oxidative processes (due to its low OTR) and reduce moisture loss (low WVTR), which is critical for maintaining the quality of the meat product throughout its shelf life. Furthermore, vacuum sealing combined with shrinkage under the specified temperature conditions ensures a tight adherence of the material to the product surface, preventing the development of aerobic microflora and reducing the risk of cross-contamination.
The finished product (Figure 1) had a weight 800–1100 g with a comparatively round form, composed of whole meat chunks.

Figure 1.
Finished product of traditional Kazakh molded smoked ham from horse and camel meat.
Sea buckthorn pomace (defatted) was obtained as a by-product of juice processing at Yuantai Organic Ltd. (Tiangu, Yanta District, Xi’an, Shaanxi, China) and dried at 50 °C for 18 h to reduce moisture content while maintaining the structure. The dried pomace was ground to powder using laboratory hammer mill LMT-2 (New Technologies Group, Moscow, Russia) at 3000 rpm. The cake was pre-dried to a moisture content of 8%. The particle size after milling was 250–500 µm.
Extraction of sea buckthorn compounds. Ultrasound-assisted extraction was conducted. Sea buckthorn cake was extracted with 70% ethanol at a solvent/solvent ratio of 5:1 (volume/weight). Extraction was performed in a TTC Sapphire 4L ultrasonic bath (JSC Sapphire, Moscow, Russia) (35 kHz, 150 W) at 60 °C for 30 min. The ethanol was removed via rotary evaporation using an IKA RV 5 (IKA–Werke GmbH & Co, Staufen im Breisgau, Germany) at bath temperature 60 °C, absolute pressure 5 kPa, after filtration using a PE-6900 centrifuge (GEO-NDT LLC, Moscow, Russia). The concentrated extracts were dried at 40–50 °C for 24–36 h to yield a powdered form. A dry extract with a yield of 18% (0.18 g of extract per 1 g of dry pomase) was obtained. The moisture content of the finished powder was 6.5%, and the ash content was 3.2%. The total phenolic activity (TPC) by the Folin–Chokaltov method was 42 mg GAE/g of dry extract. The water activity (aw) did not exceed 0.3. This powdered extract had a dark orange color and a characteristic sea buckthorn smell, with good antioxidant activity.
The dry powder extract was dissolved in distilled water and evenly mixed with meat, salt, sugar, and spices. The mixture was molded and air-dried according to traditional practices.
2.2. Experimental Design
Optimization of Formulation Doses of Sea Buckthorn Powder Extract and Additive “Kf Stabinject 101” to Study Their Synergism at Very Low Application Concentrations
A multi-phase approach was undertaken, including optimization of formulation doses of two examined preparations, in vitro antioxidant activity assays, and experimental enrichment of meat samples. Computational modelling using factorial experimental design and polynomial regression was applied to determine optimal dosages for quality retention.
To develop the technology of molded smoked ham, the influence of sea buckthorn powder extract (X1) and additive Kf Stabinject 101 containing α-tocopherol and L ascorbic acid (X2) were optimized for the following quality indicators: acid value (AV)—Y1; peroxide value (POV)—Y2; 2-thiobarbituric acid reactive substances (TBARSs)—Y3; sensory-assessed cross-sectional surface (SACSS)—Y4. To determine the effect of the studied indicators, a full factorial experimental design was used, and a planning matrix was compiled (Table 1). The concept of a “black box” was used to justify the optimal dose of sea buckthorn powder extract added to the formulation of molded smoked ham. The concentration ranges of the sea buckthorn powder extract (0.025–0.10%) and the additive “Kf Stabinject 101” (0–0.10%) were selected based on preliminary tests, including an assessment of their synergistic effect on the quality of the meat product, shelf life, and sensory characteristics of the ham. Our hypothesis is that such low concentrations would provide a good balance between functional efficiency and preservation of the taste and textural properties of the finished product, while minimizing the cost of additives and seeking a strong synergistic effect between the two preparations. The values are given as a percentage of the meat raw material mass. Variable X1—the amount of the extract at 4 levels of variation—was selected in increments of 0.025%. Variable X2—the amount of additive “Kf Stabinject 101” at 3 levels of variation—was selected in increments of 0.05%. The number of levels of determining factors is shown in Table 1.

Table 1.
Planning matrix for a full-factor experiment.
The following indicators, as the target functions Yi, i = 1, 2, 3, 4, were taken, where Y1—AV; Y2—POV; Y3—TBARS; and Y4—the average score of sensory-assessed cross-sectional surface (SACSS). The optimal value of the objective functions was determined by the regression equations below, after general calculation of the ham’s quality parameters, as follows:
Y1 = f1 (x1. x2.) ⇒ max
Y1 = f1 (x1. x2.) ⇒ min
The space factor minx i ≤ x i ≤ maxx i i = 1, 2, 3, 4. Here, Y1, Y2, Y3, and Y4 are dependencies constructed from experimental data; x1. x2.—estimates of the coefficients of the response functions Y1, Y2, Y3, and Y4; minx i, maxx i—two-way restrictions on the main parameters of meat product quality.
2.3. Determination of Quality Parameters of Traditional Kazakh Molded Smoked Ham from Horse and Camel Meat
Formulated ham samples containing 0% (control) and 3% extract were evaluated over 30 d stored at 0–4 °C. Changes in instrumental color (L*, a*, b*), pH value, texture profile (TPA), hydrolytic and oxidative changes in lipid and protein fractions, total phenolic content, antioxidant activity, and microbiological status of hams were examined.
2.4. Methods
Instrumental color. A Konica Minolta colorimeter CR-410 (Konica Minolta Holding, Ramsey, NJ, USA) equipped with a 50 mm aperture, D65 illuminant, and 2◦ standard observer, was used to evaluate the lightness (L*), redness (a*) and yellowness (b*) on the cross-sectional surface of smoked ham [5]. Hams were incubated for 1 h at room temperature, and 1 cm thick ham slices were cut. Color was measured immediately without blooming. Calibration was performed against white reference standard No. 18833116 (Y = 94.3, x = 0.3134, and y = 0.3197). [5]. For each time interval, instrumental color (L*, a*, b*) was measured in seven independent samples (n = 7), each of which was analyzed in duplicate (2 measurements).
pH value.
The pH value of the samples was measured with a Hanna pH meter, model HI99163 (Hanna Instruments, Smithfield, VA, USA), equipped with a meat contact tip type FCO99. The instrument was calibrated before each use with buffer solutions with pH 4.04 and 6.86. The buffer solutions were certified. For each time interval, pH was measured in seven independent samples (n = 7), each of which was analyzed in duplicate (2 measurements). The results are presented as mean ± standard deviation [36].
Texture profile analysis (TPA). Cubic samples (1 × 1 × 1 cm) were excised from each ham sample for analysis. Texture profile analysis was carried out using a TX-700 analyzer equipped with a 25 kg load cell (Lamy Rheology, Champagne au Mont d’Or, France). The measurements were performed at ambient temperature (22–25 °C) employing a double compression protocol with a flat cylindrical probe (60 mm in diameter). Each sample was compressed longitudinally along the muscle fibres to 60% of its original height, at a constant speed of 1 mm/s, with a 5 s interval between the two compression cycles. For each ham, the procedure was repeated seven times (n = 7), each of which was analyzed in duplicate (2 measurements). The results are presented as mean ± standard deviation. Data acquisition and processing were conducted using Rheotex software, version 2.55 (Lamy Rheology, Champagne au Mont d’Or, France). Textural attributes derived from force and area measurements were determined in accordance with the methodology described by Kolev et al. [36].
Extraction of muscle proteins. Protein extraction was performed according to the procedure described by Khan [37], with minor modifications. Meat samples (2.5 g) were homogenised with 48.5 cm3 of phosphate-buffered saline (49 mM Na2HPO4·7H2O, 4.5 mM NaH2PO4·H2O, 23 mM KCl), adjusted to an ionic strength of 0.55. The homogenate was conditioned for 12 h at 0–+4 °C and subsequently centrifuged at 1000× g for 15 min.
Hydrolytic and oxidative changes in lipid fraction.
The acid value (AV) was determined in accordance with ISO 660:2020 [38].
The peroxide value (POV) was measured spectrophotometrically following the method of Shantha and Decker [39].
The 2-thiobarbituric acid reactive substances (TBARSs) were determined according to the procedure of Botsoglou et al. [40] as modified by Moraru Manea et al. [41].
Hydrolysis in the protein fraction. Protein hydrolysis was assessed spectrophotometrically by quantifying the concentration of free amino nitrogen (FAN) according to the method of Vassilev et al. [42].
Protein oxidation. Protein oxidation products, expressed as the concentration of carbonyl groups, were determined using the method described by Mercier et al. [43].
The total phenolic content (TPC) extraction from the phenolic compound. In total, 5 g of sample (ground ham) was homogenised with 20 mL of 50% methanol at 20,000 rpm for 30 s and incubated for 60 min at room temperature (22–25 °C). The mixture was centrifuged (10,000× g) for 10 min, followed by the addition of 20 mL of 70% acetone, mixed, and left to stand for 60 min at room temperature. It was diluted to 50 mL with distilled water and centrifuged again (10,000× g) for 10 min, and the supernatant was collected. The total phenolic content (TPC) was determined based on the reaction between phenolic compounds and the Folin–Ciocalteu reagent, as described by Vardakas et al. [44].
The radical scavenging activity against the 1,1-diphenyl-2-picrylhydrazil (DPPH) radical. The radical scavenging activity against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was assessed according to the method of Brand-Williams et al. [45], with modifications by Dinkova et al. [46].
The transition-metal-chelating activity against ferric (Fe3+) ions—FRAP assay. The ferric ion (Fe3+)-reducing antioxidant power (FRAP) assay was performed according to Benzie and Strain [47], as modified by Dinkova et al. [46].
Sensory analysis. A trained group of 10 participants completed three one-hour training sessions, using reference samples characterizing different levels of color, uniformity, and moisture of the cross-sectional surface, following the guidelines of Meilgaard [48]. Sensory evaluation scores of the cross-sectional surface were assessed by a 5-point hedonic scale, where 1 = strongly dislikes and 5 = strongly likes. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test at a significance level of p < 0.05. The analysis was performed using SPSS version 26 software.
Microbiological evaluation. Microbiological quality was assessed on days 1, 15, and 30 of storage at 0–4 °C using four microbiological indicators:
- Total aerobic plate count (TAPC): an indicator of overall bacterial contamination, representing mesophilic aerobic and facultative anaerobic microorganisms, determined according to GOST R 54354-2011 [49];
- Molds, yeasts, and other spore-forming microorganisms: determined according to GOST 10444.15-94 [50];
- Coliforms: determined in 1.0 g of product according to GOST 31747-2012 [51];
- Salmonella spp.: determined in 25 g of product according to GOST 31659-2012 [52].
Statistical analysis
Optimization of processing parameters of the sea buckthorn extraction. The response surface method (RSM) described in detail by Kolev et al. [53] was applied to determine the influence of the independent variables and to optimize the process. The statistical software package Statistica 12.0 version 13.3.721 was used, as well as the “Data Analysis” add-on of the Excel office program version 1803. As a result of the step-by-step forward process, standardized b*-factors, regressive b-coefficients and, Student’s t-criteria were determined to verify the significance and reliability of p. When solving the optimization problem, the set of quality requirements for the finished product was formulated in the form of a set of restrictions that relate to both the chemical composition and percentage the content of individual ingredients. The restrictions on the ratio between the percentages of pairs of ingredients were put. The optimality criterion was the average sensory-assessed score of the finished product. Restrictions imposed on dependent variables were as follows: Y1 (AV) < 3 mg KOH/g; Y2 (POV) < 1 meq O2/kg; Y3 (TBARS) < 0.5 mg MDA/kg; Y4—sensory-assessed cross-sectional surface → max.
Statistical analysis of the data studied the effect of dry sea buckthorn powder extract was analyzed using two-way ANOVA and Tukey’s test (p < 0.05) via GraphPad Prism version is 10.6.1. To assess the influence of the factors “Storage time” and “Sample type” on the studied parameters, a two-way analysis of variance (ANOVA with fixed effects) was used, taking into account the main effects and their interaction. The sample size was n = 7 independent samples in each cell. The assumptions of normal distribution (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) were preliminarily checked. All p-values were two-sided. For multiple comparisons, the Tukey test was used with a significance level of p < 0.05. In cases where assumptions were violated, nonparametric tests were used [54].
3. Results
3.1. Optimization of Formulation Doses of Sea Buckthorn Powder Extract and Additive “Kf Stabinject 101” to Study Their Synergism at Very Low Application Concentrations
For each indicator presented in Table 2, the following were calculated: the mean value M and the mean error value m, the median and mode, the standard deviation S and the variance of the experiment S2 (minimum and maximum values), the range R, the asymmetry A, the kurtosis E, as well as the coefficient of variation V.

Table 2.
Statistical characteristics of the effect of sea buckthorn powder extract addition on the quality of the smoked molded ham.
The statistical characteristics of Table 2 allow for a quantitative interpretation of the empirical data and test hypotheses based on regression analysis of the first approximation.
It was found that the standard errors of the obtained indicators are less than 0.4% of the corresponding mean values. An approximate equality of the mean and the median was observed, with the excess and skewness being negative, and the minimum and maximum values being approximately equal to the mean. It was found that for the obtained values, the coefficient of variation is less than 10%. This is evidence of the proximity of the empirical and normal or generalized-normal distribution.
The results regarding the coordinates of singular points and the corresponding values of the studied response functions Y1, Y2, Y3, and Y4 are presented in Table 3.

Table 3.
Coordinates of stationary points of the dependent experimental parameter’s values.
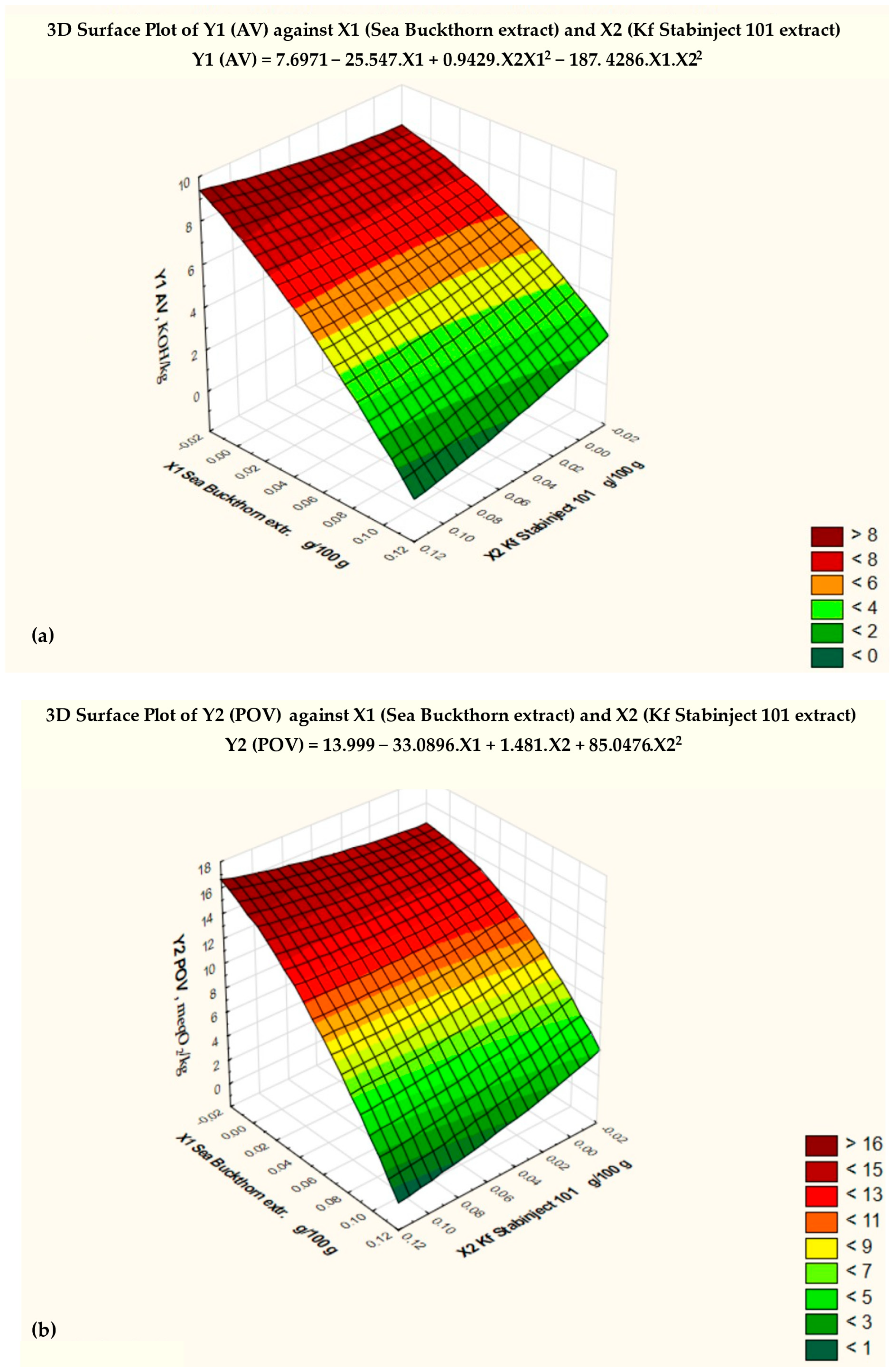
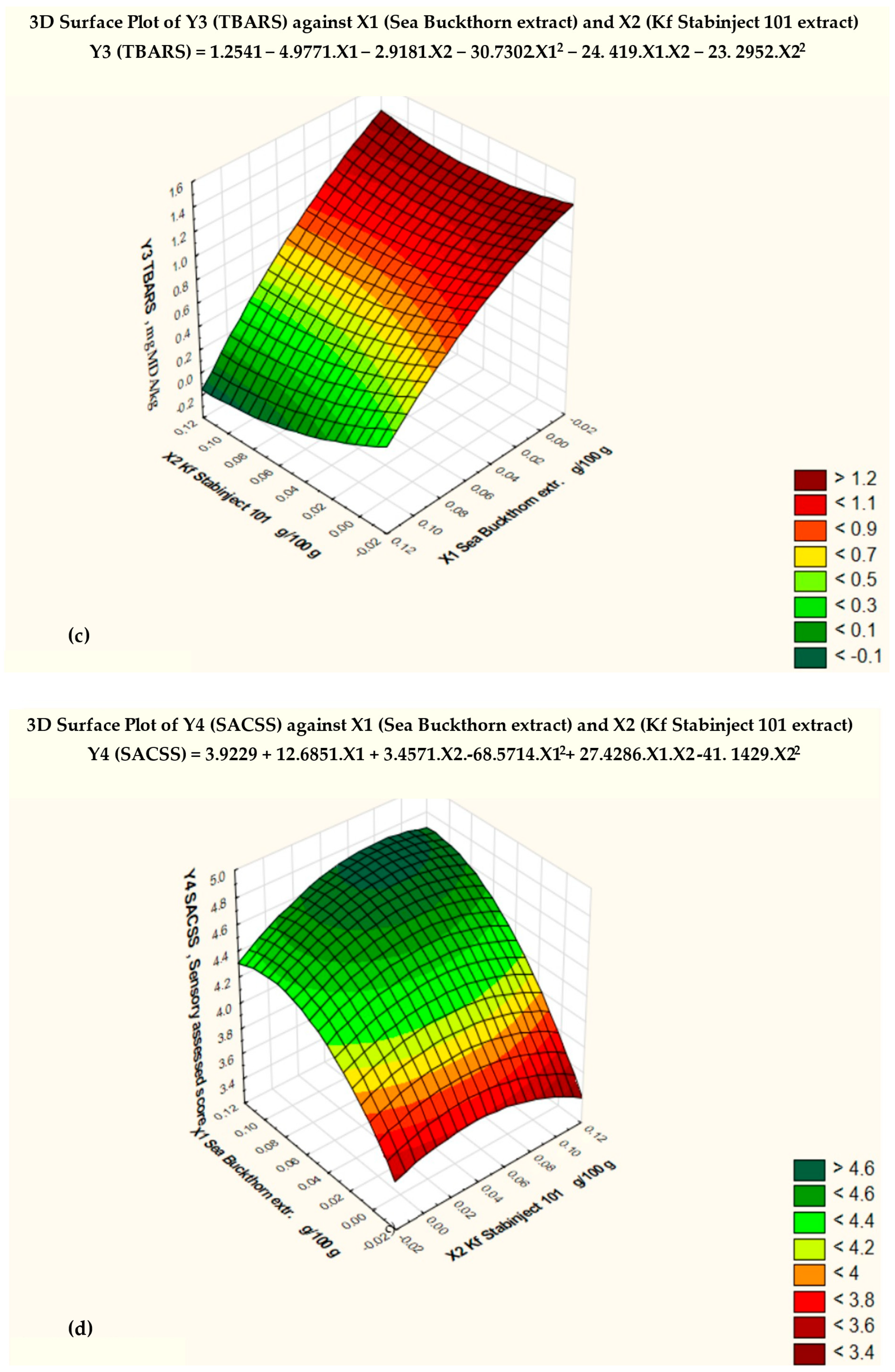
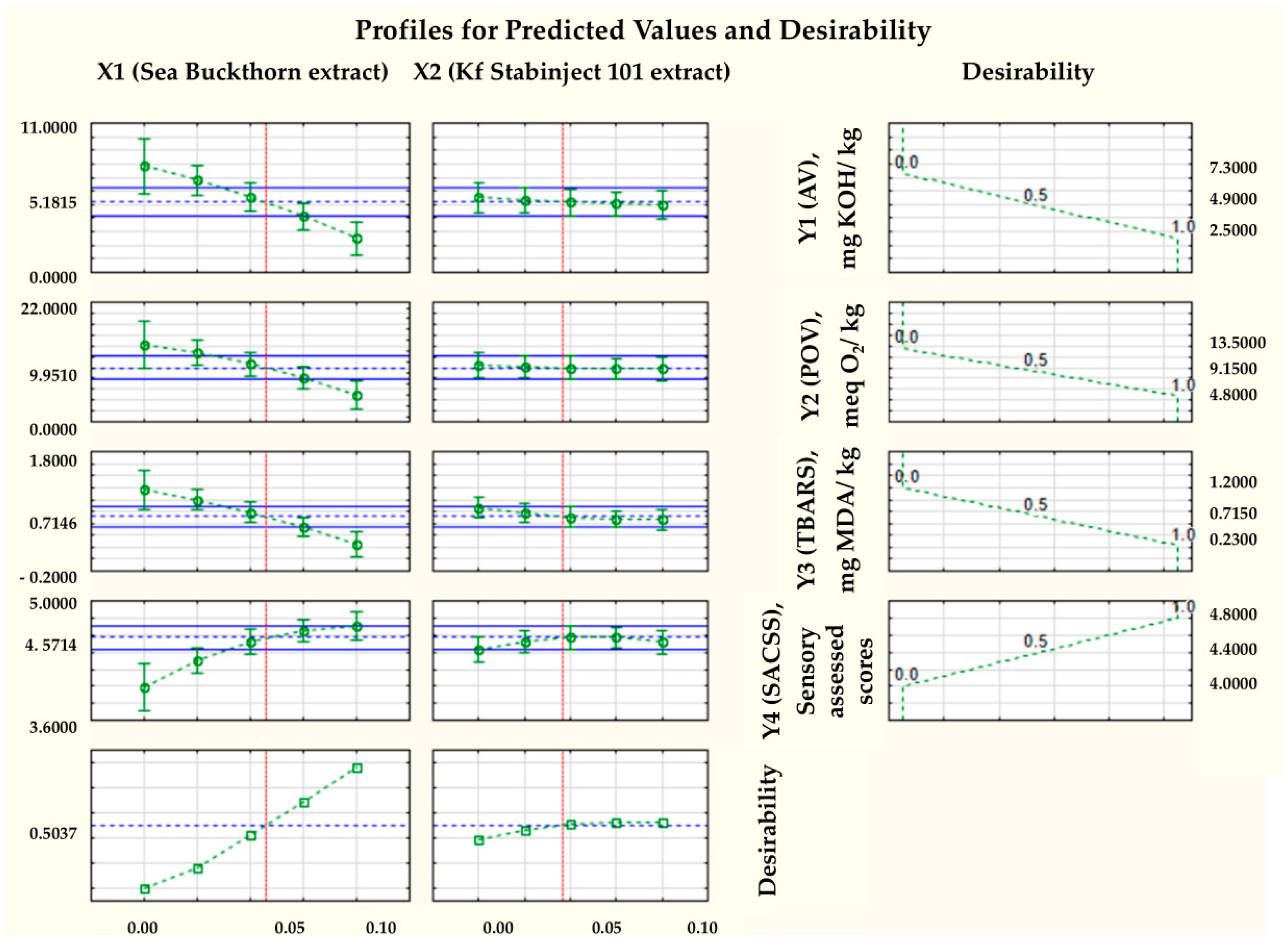
By studying three-dimensional graphs, the regression coefficients of the polynomial model were determined, as well as the dependence of the indicators of the factors Y1, Y2, Y3, and Y4 on the variables X1 and X2 for smoked ham (Figure 2).
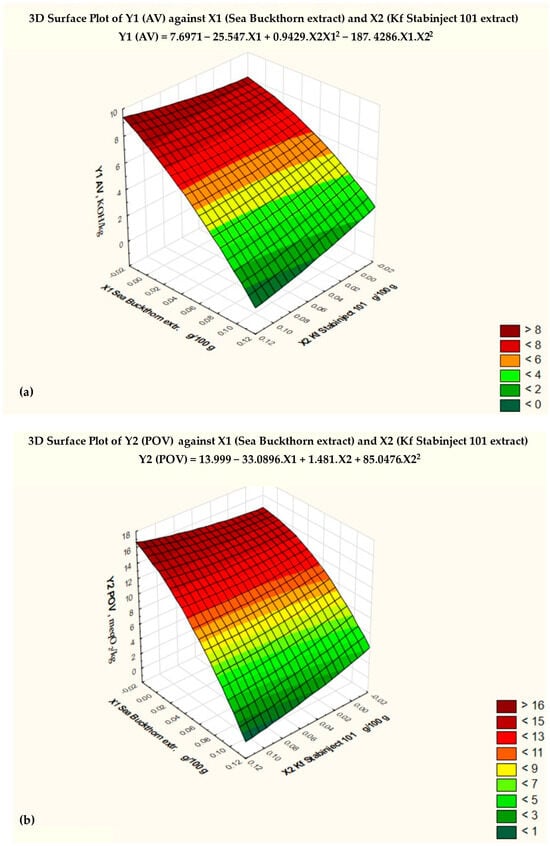
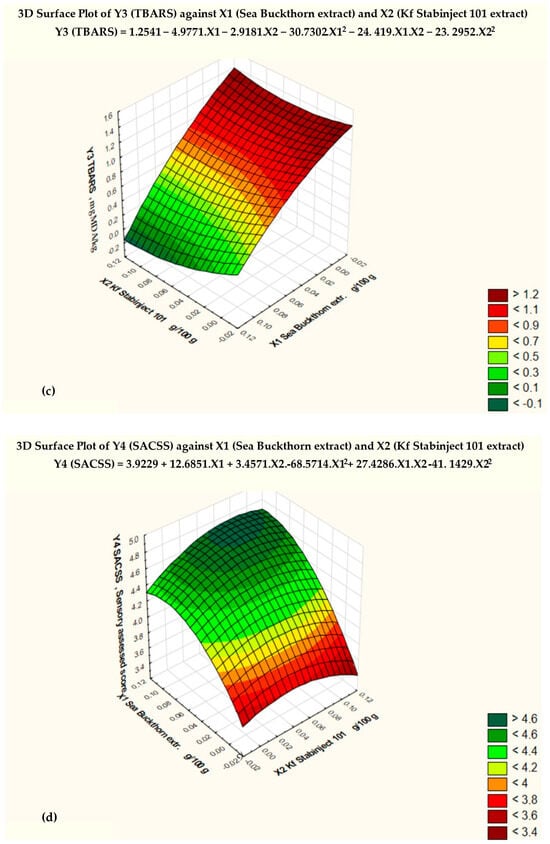
Figure 2.
Response surfaces of the four target functions: (a) acid value = f (x2, x3), x1 = const.; (b) POV = f (x2, x3), x1 = const.; (c) TBARS = f (x2, x3), x1 = const.; (d) sensory-assessed cross-sectional surface = f (x2, x3), x1 = const.
Based on the results of the three-dimensional diagram (Figure 2a) and the regression analysis (Table 4), the conclusion was made that the minimum value of AV (Y1min) = 2.5 mg KOH/kg, with the amount of added sea buckthorn powder extract X1 = 0.1 and the amount of additive “Kf Stabinject 101” X2 = 0.1. For comparison, Y1max of the control sample is 7.3 mg KOH/kg when X1 = 0 and X2 = 0.

Table 4.
Results of regression analysis of the AV (Y1) depending on the amount of sea buckthorn powder extract (X1) and additive “Kf Stabinject 101” (X2).
The regression equation obtained for the factor Y1 (acid value) (Table 4) has the following:
Y1 (AV) = 7.6971 − 27.457 . X1 + 0.9429 . X2 . X12 − 187.4286 . X1 . X22
The regression analysis of the data revealed a strong relationship between the changes in the amounts of the natural antioxidant preparations (X1 and X2) added to the molded smoked ham and the AV. The correlation index (multiple R) is 0.93578, and the coefficient of determination (R-squared) is 0.87556.
Figure 2a shows the critical limit of the AV, based on the Fisher criterion (blue line) and the degree of significance of various factors. The null hypothesis for the factor Y1 (AV) was rejected, and the coefficients of the regression equation were found to be statistically significant (p < 0.05).
The response surface of the mathematical model of the effect of the added antioxidant additives on the peroxide value (POV—Y2) is shown in Figure 2b, and the regression analysis is shown in Table 5. The conclusion was made that the minimum value of POV (Y2min) = 4.8 meq O2/kg with the amount of added sea buckthorn powder extract X1 = 0.1 and the amount of additive “Kf Stabinject 101” X2 = 0.1. For comparison, Y2max of the control sample is 13.5 meq O2/kg when X1 = 0 and X2 = 0.

Table 5.
Results of regression analysis of the POV (Y2) depending on the amount of sea buckthorn powder extract (X1) and additive “Kf Stabinject 101” (X2).
The regression equation obtained for the factor Y2 (peroxide value) (Table 5) has the following:
Y2 (POV) = 13.999 − 33.0286 . X1 + 1.481 . X2 + 85.0476 . X22
The regression analysis of the data revealed a strong relationship between the changes in the amounts of the natural antioxidant preparations (X1 and X2) added to the molded smoked ham and the POV. The correlation index (multiple R) is 0.91693, and the coefficient of determination (R-squared) is 0.84077.
Figure 2b shows the critical limit of the POV based on the Fisher criterion (blue line) and the degree of significance of various factors. The null hypothesis for the factor Y2 (POV) was rejected, and the coefficients of the regression equation were found to be statistically significant (p < 0.05).
Based on the results of the three-dimensional diagram (Figure 2c) and the regression analysis (Table 6), the conclusion was made that the minimum TBARS (Y3min) = 0.23 mg MDA/kg, with the amount of added sea buckthorn powder extract X1 = 0.1 and the amount of additive “Kf Stabinject 101” X2 = 0.1. For comparison, Y3max of the control sample is 1.20 mg MDA/kg when X1 = 0 and X2 = 0.

Table 6.
Results of regression analysis of the TBARS (Y3) depending on the amount of sea buckthorn powder extract (X1) and additive “Kf Stabinject 101” (X2).
The regression equation obtained for the factor Y3 (TBARS) (Table 6) has the following:
Y3 (TBARS) = 1.2541 − 4.9771 . X1 − 2.9181 . X2 − 30.7302 . X12 − 24.419 . X1 . X2 + 23.2952 . X22
The regression analysis of the data revealed a strong relationship between the changes in the amounts of the natural antioxidant preparations (X1 and X2) added to the molded smoked ham and the TBARS. The correlation index (multiple R) is −0.94986, and the coefficient of determination (R-squared) is 0.90223.
Figure 2c shows the critical limit of the TBARS, based on the Fisher criterion (blue line) and the degree of significance of various factors. The null hypothesis for the factor Y3 (TBARS) was rejected, and the coefficients of the regression equation were found to be statistically significant (p < 0.05).
The response surface of the mathematical model of the effect of the added antioxidant additives on the sensory-assessed cross-sectional surface (SACSS − Y4) is presented in Figure 2d. The conclusion was made that the maximum value of sensory-assessed cross-sectional surface (SACSS − Y4min) = 4.0, with the amount of added sea buckthorn powder extract X1 = 0.5 and the amount of additive “Kf Stabinject 101” X2 = 0.075. For comparison, Y4max of the control sample is 4.8 when X1 = 0 and X2 = 0.
The regression equation obtained for the factor Y4 (SACSS) (Table 7) has the following:
Y4 (SACSS) = 3.9229 + 12.6857 . X1 + 3.4571X2 − 68.5714 . X12 + 27.4286 . X1 . X2 − 41.1429 . X22

Table 7.
Results of regression analysis of the sensory-assessed cross-sectional surface (Y4) depending on the amount of sea buckthorn powder extract (X1) and additive “Kf Stabinject 101” (X2).
The regression analysis of the data revealed a strong relationship between the changes in the amount of the natural antioxidant preparations (X1 and X2, added to the molded smoked ham and the SACSS. The correlation index (multiple R) is 0.88793, and the coefficient of determination (R-squared) is 0.78842.
The null hypothesis for the factor Y4 (SACSS) was rejected, and the coefficients of the regression equation were found to be statistically significant (p < 0.05).
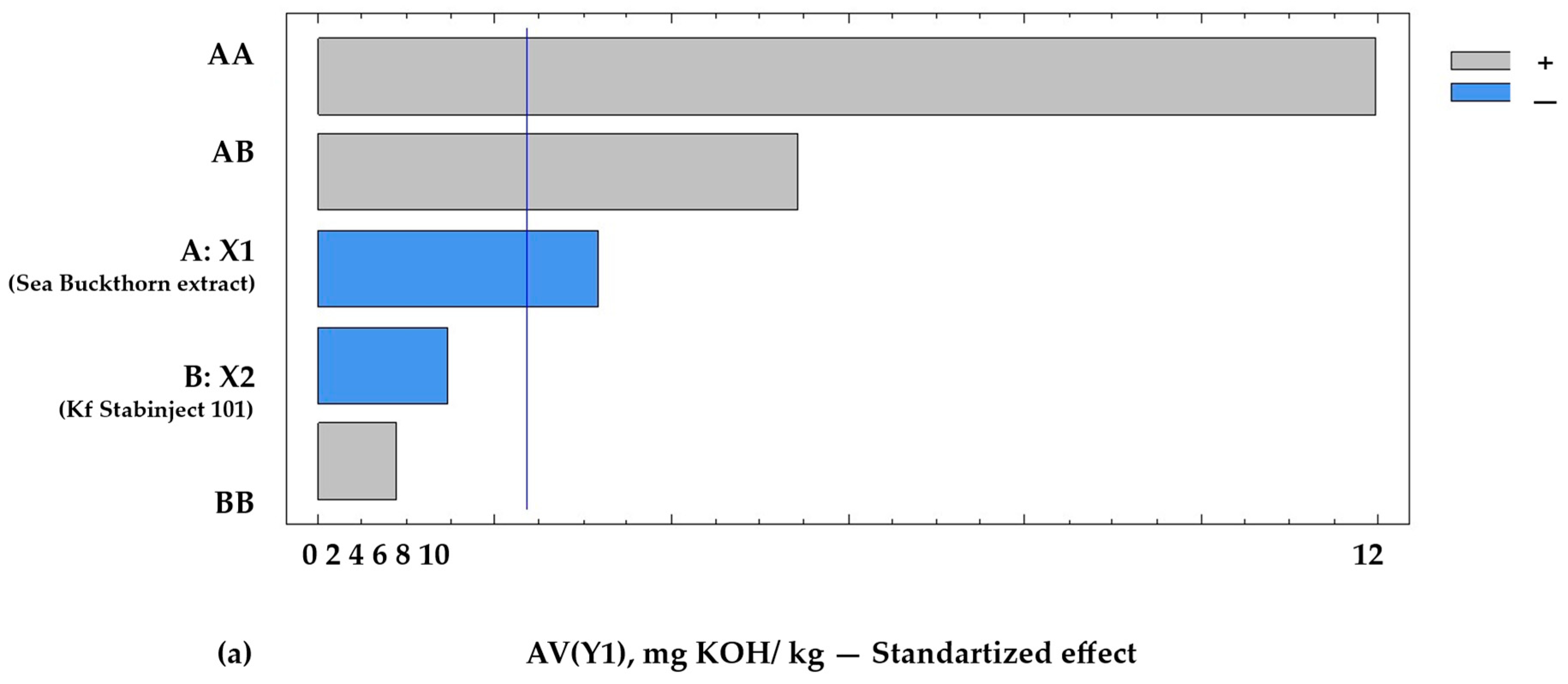
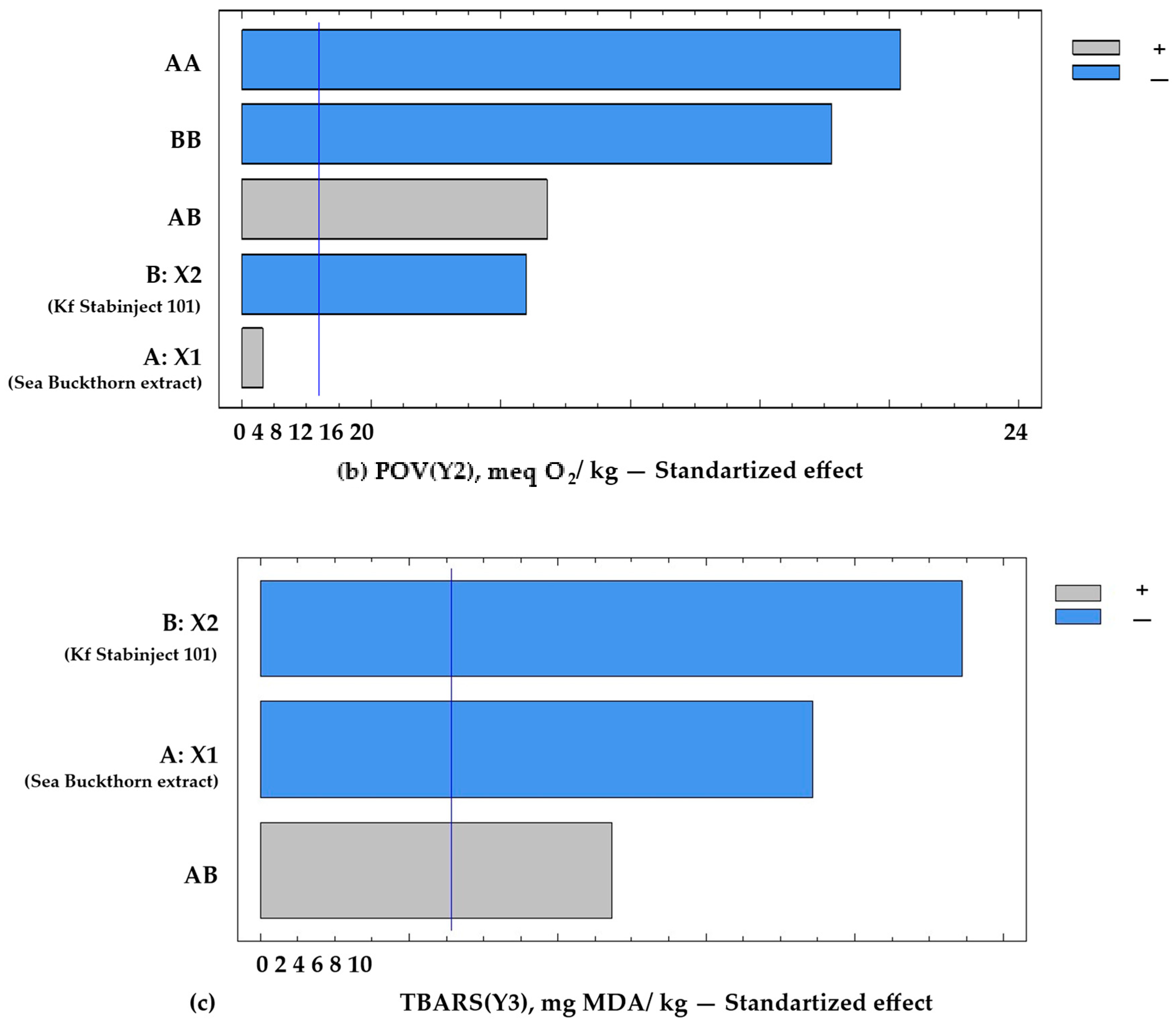
Based on mathematical modelling from the full factorial experiment, it was found that the 13th sample (see Table 1) at levels of the independent variables X1 and X2 (respectively, 0.1% and 0.1%) demonstrated the best quality characteristics of the molded smoked ham. This sample is characterized by minimal acid value (AVmin = 2.5 mg KOH/kg), minimal peroxide value (POVmin = 4.8 meq O2/kg), and minimal 2-thiobarbituric acid reactive compounds (TBARSmin = 0.23 mg MDA/kg), as well as sensory-assessed cross-sectional surface (SACSS = 4.70 closed to max 4.8), which correlates with a high level of product desirability (Figure 3).
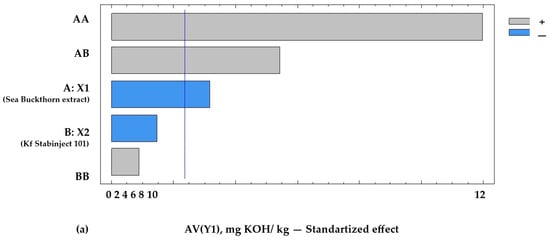
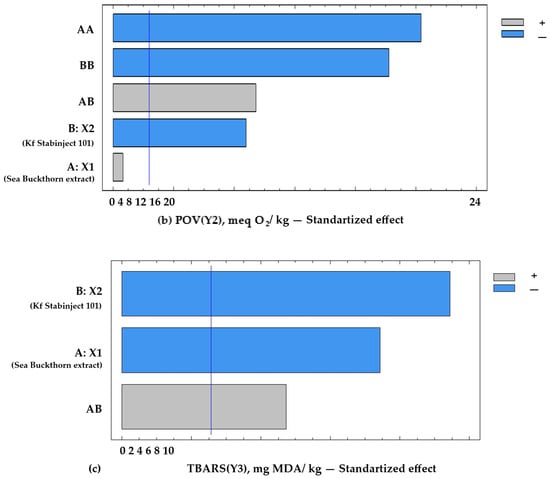
Figure 3.
Pareto chart of standardized effects on the target functions of the experiment: (a) acid value = f (x2, x3), x1 = const.; (b) POV = f (x2, x3), x1 = const.; (c) TBARS = f (x2, x3), x1 = const. Notes: A—the amount of addition of sea buckthorn powder extract X1, %; B—the amount of addition of additive “Kf Stabinject 101” containing α-tocopherol and L-ascorbic acid (X2), %; AB—interfactor interaction between the two factors A and B; AA—the influence of factor A2; BB—influence of factor B2. The reference lines (corrected by Bonferroni alpha) are: AV(Y1min) = 2.5 mg KOH/kg; POV (Y2min) = 4.8 meq O2/kg; and TBARS (Y3min) = 1.2 mg MDA/kg.
The desirability profile of the mathematical model for determining the optimal values of independent variables was shown in Figure 4.
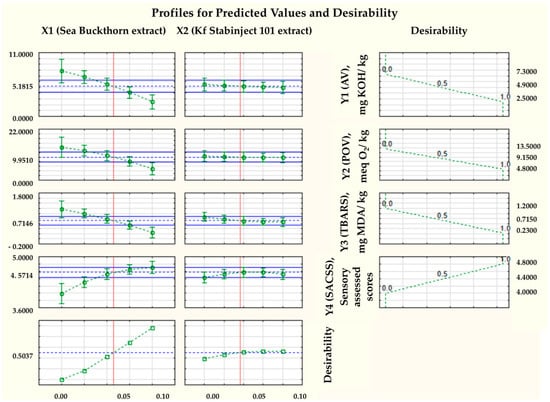
Figure 4.
Desirability profile of a mathematical model for determining optimal values of independent variables. Notes: The solid blue lines indicate the limits of variation of the independent variables X1 and X2; The green squares and the green segments indicate the average leanness and standard deviation of the dependent variables Y1, Y2, Y3 and Y4; The dashed green lines represent the trend of changes (from 0 to 0.1%) in the mean values of the dependent variables Y1, Y2, Y3 and Y4; The red lines are the reference lines (corrected by Bonferroni alpha), and The dashed blue lines represent the trend of changes in the mean values of the independent variables X1 and X2.
These results confirm our hypothesis that the use of natural antioxidant preparations (the use of natural antioxidants) can effectively improve the functional and oxidative stability of molded smoked ham from horse and camel meat. Using mathematical modelling, the appropriate dose of added sea buckthorn powder extract, and the additive “Kf Stabinject 101” was proven, namely of maximally added −0.1% and 0.1%, respectively. As can be seen from the results obtained, the minimal levels of AV = 2.5 mg KOH/kg and POV = 4.8 meq O2/kg are relatively high. It is concluded that in order to achieve a more pronounced antioxidant effect, the dose of the dry sea buckthorn powder extract should be significantly higher (Figure 4). Therefore, in the next part of this study, we compared molded smoked ham from horse and camel meat without the addition of sea buckthorn powder extract (Control sample) and with the addition of 3% dry sea buckthorn powder extract (Experimental one).
3.2. Effect of the Addition of Dry Sea Buckthorn Powder Extract on the Quality Parameters of Traditional Kazakh Molded Smoked Ham from Horse and Camel Meat
The results of the instrumental recording of the color characteristics of color lightness (L*), red (a*), and yellow color component (b*) of the samples during storage at 0–4 °C are presented in Table 8.

Table 8.
Instrumental color (L*, a*, b*) of samples during storage at 0–4 °C.
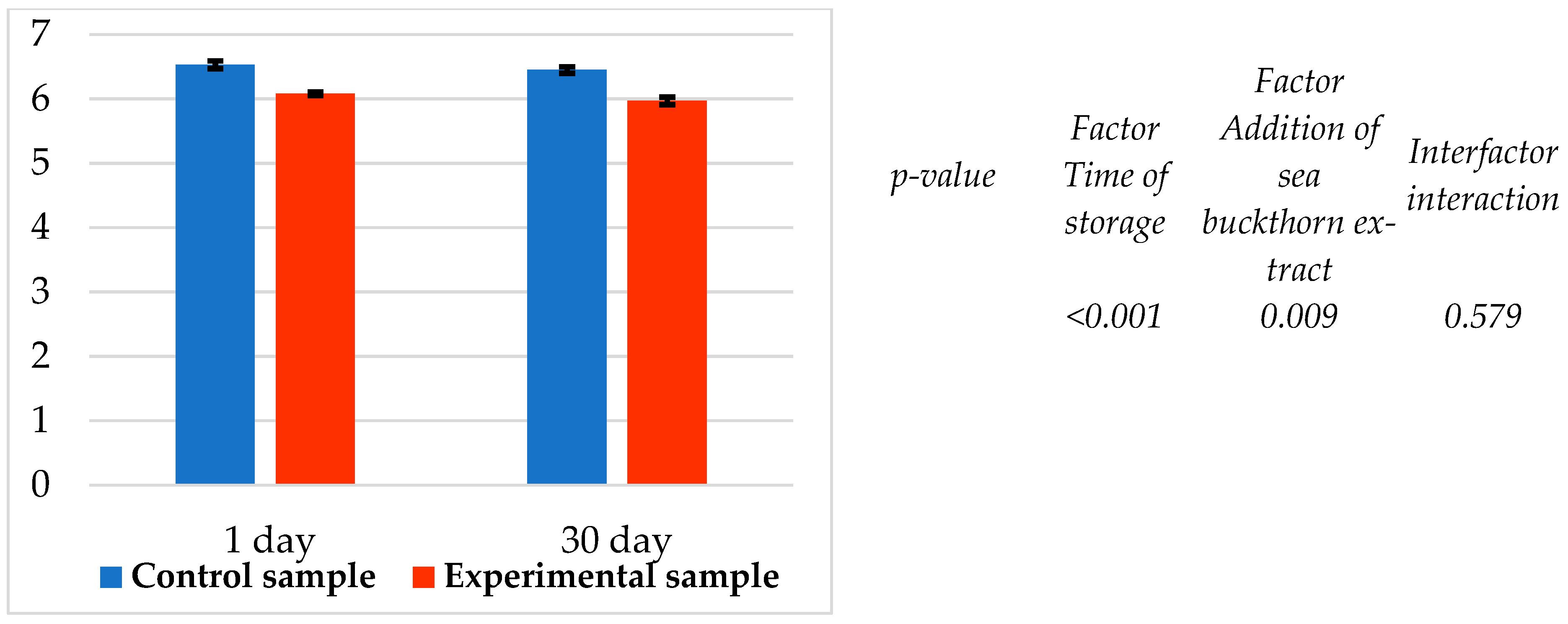
It was found that both factors—time of storage and addition of sea buckthorn extract—significantly (p < 0.05) affected the instrumental color of molded smoked ham (Table 8). It was found that the color lightness (L*) and the yellow color component (b*) significantly (p < 0.05) increased both on the first day of refrigerated storage and after 30 days of storage of the molded smoked ham. On the contrary, the red component of the color (a*) decreased significantly (p < 0.05) (Table 8).
The increase in color lightness is 1.22–1.52 units, and in the yellow component of the color, it is 1.17–1.51 units. Respectively, the red component of the color decreases by 3.7–5.54 units. It is evident that the addition of sea buckthorn powder extract causes significant (p < 0.05) discoloration of the cut surface of the ham, and it appears paler and pinker compared to the control sample.
It was found that both factors—time of storage and addition of sea buckthorn powder extract—significantly (p < 0.05) affected the pH (Figure 5). A similar decreasing trend was also found in the pH value of the experimental sample compared to the control (Figure 5). It was found that the pH value of the experimental sample was lower (p < 0.05; n = 7) than that of the control sample by 0.47 and 0.48 pH units.
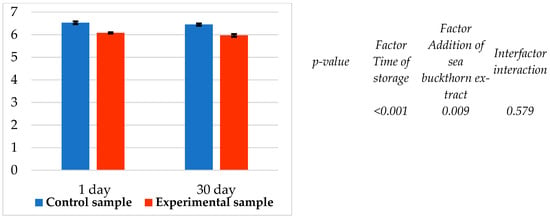
Figure 5.
pH value of samples during storage at 0–4 °C.
The results of the texture profile (TPA)—hardness, cohesiveness, springiness, gumminess, chewiness, and resilience of two samples—of smoked ham during storage at 0–4 °C are presented in Table 9.

Table 9.
Texture profile (TPA) of samples during storage at 0–4 °C.
It was found that the indicators cohesiveness, springiness, and resilience have no significant difference (p > 0.05) in the two studied factors of storage time and addition of sea buckthorn powder extract (Table 9). Regarding the indicators hardness, gumminess, and chewiness, it can be stated that statistically significant (p > 0.05) lower values were found in the experimental sample compared to the control one. The levels of these three parameters showed a trend of significant (p < 0.05) increase during 30 days of storage (Table 9). The thus established trends of changes in hardness, gumminess, and chewiness (Table 9) of the smoked ham samples correspond very well with the data on the lower pH value in the experimental sample compared to the control (Figure 5).
However, more significant is the fact that these three indicators increase significantly (p < 0.05) during refrigerated storage, both in the control and experimental samples (Table 9).
The results of the hydrolytic and oxidative changes in lipid and protein fractions of two samples of molded smoked ham during storage at 0–4 °C are presented in Table 10.

Table 10.
Hydrolytic and oxidative changes in lipid and protein fractions during storage at 0–4 °C.
It was found that both factors (time of storage and addition of sea buckthorn powder extract) significantly influence (p > 0.05) the indicators characterizing lipolytic changes (acid value AV) and primary lipid oxidation products (peroxide value POV). The time of storage only significantly affects (p > 0.05) the secondary lipid oxidation products (TBARSs) and proteolytic processes reflected by changes in free amine nitrogen (FAN) after 1 day of refrigeration (Table 10). An exception to this finding is only the indicator for protein oxidation (protein carbonyl content), in which no levels exceeding the detection limit of the research method were found (Table 10).
The acid value (AV) of the control sample almost doubled—about a 99% increase after 30 days of storage at 0–4 degrees Celsius—while the AV of the experimental sample increased by 1.24 mg KOH/g. At the same time, on day 1 of refrigerated storage, the experimental sample had an AV level of 0.23 mg KOH/kg (by about 20%) higher than in the control sample (Table 10). These data show that the addition of sea buckthorn extract accelerates the lipolysis of smoked ham’s fats. The comparison of the AV data on the 30th day of storage shows that the levels of this indicator in the experimental sample are 0.55 mg KOH/kg higher than in the control sample (Table 10). While the more accelerated course of lipolysis in the control sample is expected and well known from theory and technological practice, the increase in AV in the experimental sample is probably due to the presence of a factor-activating lipolytic activity in the product matrix.
The time of storage significantly affects (p < 0.05) the formation of primary lipid oxidation products, and on the 30th day of storage, POV levels are significantly higher than on the 1st day, both in the control (with 1.08 meq O2/kg) and in the experimental sample (with 0.56 meq O2/kg; Table 10). The addition of dry sea buckthorn powder extract also significantly affects (p < 0.05), but similar to the inversion observed in AV and POV, after 1 day of refrigeration, higher levels were found in the experimental sample (with 0.19 meq O2/kg). After 30 days of storage at 0–4 °C, the levels of primary oxidation products (POV) in the experimental sample are dramatically lower (with 0.33 meq O2/kg) compared to the control sample and do not exceed the acceptable limit of 1.00 meqO2/kg for meat products (Table 10). A very strong interdependence of the data obtained for AV and POV in both samples was established. For both indicators, the trend of changes is the same. This proves the hypothesis that primary lipid oxidation products are formed from released fatty acids during fat hydrolysis.
The time of storage significantly affects (p < 0.05) the formation of secondary lipid oxidation products, and on the 30th day of storage, TBARS levels were significantly higher than on the 1st day, both in the control (by approx. 4.19 times) and in the experimental sample (by about 5.67 times; Table 10). This finding proves that during 30-day refrigerated storage in the molded smoked ham, processes of transformation of primary lipid oxidation products into secondary ones occur. However, it is correct to note that the levels of TBARS on the 1st day of refrigeration are negligible (0.16 and 0.12 mg MDA/kg, respectively), and on the 30th day of storage they do not exceed the limit of 1.00 mg MDA/kg (0.67 and 0.68 mg MDA/kg, respectively), which is considered acceptable for similar types of meat products. The addition of dry sea buckthorn extract did not significantly affect (p > 0.05) the levels of TBARS, and they did not differ statistically significantly comparing the control and experimental samples, both on the 1st and 30th day of storage of the ham at 0–4 °C (Table 10).
The found results for the changes in free amine nitrogen (FAN) after 1 day of refrigeration are similar to those for TBARS (Table 10). The addition of dry sea buckthorn extract did not significantly (p > 0.05) affect the levels of FAN. However, the FAN values in the experimental sample were higher by 0.55 mg Ala/g compared to the control one after 30 days of refrigeration, which is evidence of the more accelerated course of proteolytic processes during storage of hams with the addition of dry sea buckthorn extract (Table 10). It was found that the time of storage has a significant (p < 0.05) effect on the changes in FAN of the two studied samples. After 30 days of refrigeration, the FAN content decreases by 0.91 mg Ala/g in the control sample and by 0.22 mg Ala/g in the experimental one (Table 10). Because the proteolytic enzymes in ham thermally denatured at a temperature of and exceeding 60 °C relatively quickly—within 1–1.5 h after the start of heat treatment—and are practically deactivated during the further 3–4 h of hot smoking and cooking to 73 °C, conditions for the occurrence of proteolytic processes and accumulation of low-molecular-weight amino derivatives of proteins do not exist. It is very likely that the resulting free amines interact with secondary products of lipid oxidation and form products of the Maillard reaction during the smoking and cooking of ham. This is a logical explanation for the reduction in free amine nitrogen levels established by us and for the relatively low levels of TBARS, as well as for the practical absence of protein carbonyls. The low levels of the TBARS (low-molecular-weight oxidized derivatives of the lipids) and protein carbonyls (oxidized derivatives of the muscle proteins) from the ham matrix could be explained by penetration and interaction with phenolic compounds penetrating as a result of the prolonged smoking with goose smoke. As can be seen from the data for FAN in Table 10, the factor of addition of sea buckthorn extract does not have a statistically significant effect on the FAN indicator either (p > 0.05). Therefore, the dispersion of the measurement is large.
It was found that under the experimental conditions, protein oxidation processes are not initiated in the molded smoked ham from horse and camel meat, since the content of protein carbonyls is below the detection limit of the research method used (Table 10). The results obtained thus prove that the technology used for processing of molded smoked ham from horse and camel meat is reliable enough to guarantee prevention of protein oxidation during the storage period of the finished product for up to 30 days at 0–4 °C, regardless of whether or not dry sea buckthorn extract is added.
The results of the total phenolic content and antioxidant activity of two samples of molded smoked ham during storage at 0–4 °C are presented in Table 11.

Table 11.
Total phenolic content and antioxidant activity during storage at 0–4 °C.
The results of the total phenolic content and antioxidant activity of two samples of molded smoked ham during storage at 0–4 °C are presented in Table 11. It was found that the addition of dry sea buckthorn extract to the filling mass of molded smoked ham from horse and camel meat significantly (p < 0.05) increased the TPC by 19.8%, the radical scavenging activity against DPPH radicals by 33.6%, and the transition-metal-chelating activity against ferric (Fe3+) ions by 12.8% (Table 11). The microbiological status of two samples of molded smoked ham during storage at 0–+4 °C is shown in Table 12. The presence of mesophilic aerobic and facultative anaerobic microorganisms, molds, yeasts, and other spore-forming microorganisms, coliforms in 1 g of product, and Salmonella spp. in 25 g of product, was not detected during 30 days of refrigeration, both in the control and experimental samples (Table 12). Thus, the results obtained are evidence that the applied technology for processing molded smoked ham from horse and camel meat guarantees microbial safety of the product, regardless of the addition or not of dry sea buckthorn extract.

Table 12.
Microbiological status of molded smoked ham samples stored at 0–4 °C.
4. Discussion
The obtained results correspond to scientific information from the literature that Hippophae rhamnoides L. plants contain a wide range of phytochemicals and manifest antifungal, antibacterial, antioxidant, antiviral, and anti-inflammatory activities [1,8,12]. These properties are mainly due to the relatively high content of polyphenolic compounds (mainly flavonoids) [12,13,19,55]. The importance of polyphenols in the fruits and extracts of sea buckthorn for their antioxidant activity is especially emphasized [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] and confirmed in our study (Table 11 and Table 12). The recommendation for adding 0.1–3.0% dry sea buckthorn extract to smoked horse and camel meat ham is also confirmed by data presented in other experiments with a wide range of meat products [7,8,9,25,27,28,29,30,31,32,33,34]. Our hypothesis was confirmed by the properties of strong and weak antioxidants, which exhibit more pronounced antioxidant activity at not very high concentrations, but a saturation effect is observed at relatively high concentrations, in which the addition of natural antioxidants becomes ineffective, and in some cases even accelerates the spread of oxidative reactions [53,54].
The findings of Papuk et al. [29] that the addition of 60% ethanol extract of dried sea buckthorn berries causes discoloration of fresh beef and pork minced meat but inhibits lipid peroxidation are similar to our findings of discoloration of the cut surface of horse and camel hams. Our data differ from the reported slight increase in color stability and pH of the filling mass for similar hams when 5, 10, and 15% sea buckthorn seed powder were added by Makangali et al. [35]. The observed changes can be explained by chemical mechanisms associated with cured meat, namely the presence of nitrites, α-tocopherol, and ascorbate in the brine and insufficient concentrations of smoke polyphenolic compounds in the center of the ham. As nitrosyl-hemochrome (the pink pigment responsible for the color of the cut surface of hams) oxidizes, the attractive pink-red color of the cut surface of hams fades to gray. This occurs when the pH decreases or oxidative reactions occur due to the lack of sufficient concentrations of antioxidant reducing agents. The minimal penetration of smoke polyphenolic agents with a highly pronounced antioxidant effect may also explain the observed reduction in redness (a*) of the cut surface color.
On the other hand, the specific chemical composition and antioxidant capacity of chilled sea buckthorn berries [56], sea buckthorn oil [26], different types of fruit extracts, or sea buckthorn pomace may also have an influence. Anchidin et al. [26] found that injection of 3% sea buckthorn oil into pork tenderloin (psoas major) had a positive effect on consumer preferences. The optimality of adding 3% doses of sea buckthorn powder extract obtained from the dried cake of sea buckthorn pomace to the brine for the injection of horse and camel meat ham has been confirmed by us. Kozhkhiyeva et al. [8] reported similar results, finding that the addition of 1% powdered sea buckthorn extract improves the oxidative stability of horse meat delicacies [8] and preserves their sensory and color characteristics for a longer period of time. The data of Anchidin et al. [26] are consistent with ours in concluding that sea buckthorn extract does not have a strong antimicrobial effect, and the reduction in microbial contamination is insignificant.
5. Conclusions
The obtained results and their analysis allow us to conclude that the addition of 3.0% sea buckthorn powder extract increases the antioxidant stability of molded smoked ham from horse and camel meat by increasing the content of total phenols by 19.8%, increases the DPPH radical scavenging activity by 33.6%, and enhances the chelating activity of transition metals against iron (Fe3+) ions by 12.8%. The addition of 3.0% sea buckthorn powder extract stabilizes oxidative stability but causes a decrease in the pH value from 6.08 to 5.97 and discoloration of the cut surface of the ham. On the other hand, concentrations of sea buckthorn extract below 0.2% (w/w) have no effect, and values of 1.0% (w/w) do not lead to a significant improvement in oxidative stability and negatively affect the organoleptic properties of the product. Therefore, it is concluded that the addition of dry powder of sea buckthorn extract can be successfully applied in the production of traditional Kazakh molded smoked ham from horse and camel meat by stabilizing the oxidative stability of the product, but further studies are needed to determine whether concentrations from 0.5 to 3.0% (w/w) will not prove to be more effective in terms of improving the sensory properties of the finished product.
Author Contributions
Conceptualization, M.K.A., S.A.A. and S.G.D.; Methodology, D.B.V.-V. and D.K.B.; Validation, D.K.B.; Formal analysis, N.D.K., A.D.Y.-N. and N.N.N.-D.; Investigation, M.K.A., A.N.K., N.D.K., A.D.Y.-N., N.N.N.-D. and S.G.D.; Resources, S.A.A.; Data curation, D.K.B.; Writing—original draft, S.G.D.; Writing—review & editing, D.K.B. and S.G.D.; Visualization, N.D.K.; Supervision, S.G.D.; Project administration, M.K.A.; Funding acquisition, S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan via Project BR24993234 “Innovative technologies for the production of national products: Intensification and digitalization of meat and dairy products”.
Institutional Review Board Statement
The research was carried out on the basis of letter of assignment No. 1958 of 30 June 2025 of the Vice-Rector for Research and Mobility at the University of Food Technologies–Plovdiv. The sensory evaluation of the samples in the study was carried out using a hedonic scale and did not require ethical consent. It did not involve human experimentation in the same way as clinical and psychological research.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results analyzed or generated during the study will be provided upon request by the corresponding authors to the above-mentioned e-mail addresses: sholpan-ab@mail.ru; n_kolev@uft-plovdiv.bg; s_dragoev@uft-plovdiv.bg; logos2000lt@gmail.com.
Acknowledgments
Authors acknowledge Talgat Kulazhanov—Rector of Almaty Technological University, Almaty, and Galin Ivanov—Rector of the University of Food Technologies, Plovdiv for their assistance and administrative support in conducting research on this project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of variance |
| AV | Acid value |
| DNPH | 2,4-Dinitrophenylhydrazine |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FAN | Free amino nitrogen |
| FRAP | The transition-metal-chelating activity against ferric (Fe3+) ions—FRAP assay |
| GA | Gallic acid |
| GOST R | State Standard of Russia |
| LoD | Limit of detection |
| MDA | Malondialdehyde |
| POV | Peroxide value |
| RSM | Reflection surface method |
| SACSS | Sensory-assessed cross-sectional surface |
| SEM | Standard error of mean |
| TAPC | Total aerobic plate count |
| TBARS | Thiobarbituric acid reactive substances |
| TPA | Texture profile |
| TPC | Total phenolic content |
References
- Batyrbek, A.; Chisbiyah, L.A. Exploring the role of traditional food in developing tourism in Kazakhstan. J. Tour. Culin. Entrep. 2025, 5, 51–71. [Google Scholar] [CrossRef]
- Shoman, A.; Chomanov, U.; Serikbayeva, A.; Mamayeva, L.; Tultabayeva, T.; Kenenbay, G. Evaluation of chemical and nutritional value of camel meat originating from Almaty region. J. Phys. Conf. Ser. 2019, 1362, 012163. [Google Scholar] [CrossRef]
- Uzakov, Y.M.; Kaldarbekova, M.A.; Kuznetsova, O.N. Improved technology for new-generation Kazakh national meat products. Foods Raw Mater. 2020, 8, 76–83. (In Russian) [Google Scholar] [CrossRef]
- Djenane, D.; Aider, M. The one-humped camel: The animal of future, potential alternative red meat, technological suitability and future perspectives. F1000Research 2024, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. American Meat Science Association guidelines for meat color measurement. Meat Mus. Biol. 2023, 6, 12473. [Google Scholar] [CrossRef]
- Mortazavi, S.M.H.; Kaur, M.; Farahnaky, A.; Torley, P.J.; Osborn, A.M. The pathogenic and spoilage bacteria associated with red meat and application of different approaches of high CO2 packaging to extend product shelf life. Crit. Rev. Food Sci. Nutr. 2023, 63, 1733–1754. [Google Scholar] [CrossRef]
- Abilmazhinova, N.; Vlahova-Vangelova, D.; Dragoev, S.; Abzhanova, S.; Balev, D. Optimization of the oxidative stability of horse minced meat enriched with dihydroquercetin and vitamin C as a new functional food. Comp. Ren. Acad. Bulg. Sci. 2020, 73, 1033–1040. [Google Scholar] [CrossRef]
- Kozhkhiyeva, M.; Dragoev, S.; Uzakov, Y.; Nurgazezova, A. Improving of the oxidative stability and quality of new functional horse meat delicacy enriched with sea buckthorn (Hippophae rhamnoides) fruit powder extracts or seed kernel pumpkin (Cucurbita pero L.) flour. Comp. Ren. Acad. Bulg. Sci. 2018, 71, 132–136. [Google Scholar] [CrossRef]
- Bobko, M.; Mesárošová, A.; Mendelová, A.M.; Bobková, A.; Demianová, A.; Poláková, K.; Lidíková, J.; Árvay, J.; Kolesárová, A.; Jurčaga, L. Sea buckthorn (Hippophae rhamnoides Hergo) as potential natural antioxidant for meat industry. Int. Food Res. J. 2024, 31, 1351–1361. [Google Scholar] [CrossRef]
- Bukarbaev, K.M.; Abzhanova, S.A. Use of sea buckthorn extract in meat products. In Proceedings of the XLV International Scientific-Practical Conference “Advances in Science and Technology”, Moscow, Russia, 15 June 2022; Research and Publishing Center “Actualnots.RF”: Moscow, Russia, 2022; pp. 44–46. Available online: https://xn--80aa3afkgvdfe5he.xn--p1ai/AST-45_originalmaket_N.pdf (accessed on 10 October 2025). (In Russian).
- Utyanov, D.A.; Kulikovsky, A.V.; Knyazeva, A.S.; Kurzova, A.A.; Ivankin, A.N. Methods for reducing the formation of heterocyclic aromatic amines in meat products. All Meat 2021, 2, 58–63. (In Russian) [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Saldo, J.; Juan, B. Potential of sea buckthorn-based ingredients for the food and feed industry—A review. Food Prod. Proc. Nutr. 2020, 2, 17. [Google Scholar] [CrossRef]
- Vilas Franquesa, A. Development and Characterization of Products Derived from Sea Buckthorn (Hippophae rhamnoides) Berries. Ph.D. Thesis, Autonomous University of Barcelona, Bellaterra, Spain, 2021; 493p. Available online: https://hdl.handle.net/10803/673768 (accessed on 10 October 2025).
- Danielski, R.; Shahidi, F. Phenolic composition and bioactivities of sea buckthorn (Hippophae rhamnoides L.) fruit and seeds: An unconventional source of natural antioxidants in North America. J. Sci. Food Agric. 2024, 104, 5553–5564. [Google Scholar] [CrossRef]
- Papuc, C.; Diaconescu, C.; Nicorescu, V.; Criveanu, C. Antioxidant activity of polyphenols from Sea buckthorn fruits (Hippophae rhamnoides). Rev. Chim. Buchar. Origin. Ed. 2008, 59, 392. [Google Scholar] [CrossRef]
- Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Skłodowska, M.; Aralbayeva, A.N.; Nabiyeva, Z.S.; Murzakhmetova, M.K.; Zamaraeva, M.; et al. Hippophae rhamnoides L. leaf and twig extracts as rich sources of nutrients and bioactive compounds with antioxidant activity. Sci. Rep. 2022, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Ercişli, S.; Mlcek, J.; Jurikova, T.; Hoza, I. Antioxidant and radical scavenging activities in fruits of 6 sea buckthorn (Hippophae rhamnoides L.) cultivars. Turk. J. Agric. For. 2014, 38, 224–232. [Google Scholar] [CrossRef]
- Ko, M.S.; Lee, H.J.; Kang, M.J. Antioxidant activities and whitening effects of extracts from Hippophae rhamnoides L. J. East Asian Soc. Diet. Life 2012, 22, 812–817. (In Korean) [Google Scholar]
- Papuc, C.; Diaconescu, C.; Nicorescu, V. Antioxidant activity of sea buckthorn (Hippophae Rhamnoides) extracts compared with common food additives. Rom. Biotech. Lett. 2008, 13, 4049–4053. [Google Scholar]
- Fang, R.; Veitch, N.C.; Kite, G.C.; Porter, E.A.; Simmonds, M.S. Enhanced profiling of flavonol glycosides in the fruits of sea buckthorn (Hippophae rhamnoides). J. Agric. Food Chem. 2013, 61, 3868–3875. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef]
- Negi, P.S.; Chauhan, A.S.; Sadia, G.A.; Rohinishree, Y.S.; Ramteke, R.S. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005, 92, 119–124. [Google Scholar] [CrossRef]
- Raman Chawla, R.C.; Rajesh Arora, R.A.; Shikha Singh, S.S.; Sagar, R.K.; Sharma, R.K.; Raj Kumar, R.K.; Ahuja, P.S. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides. J. Med. Food 2007, 10, 101–109. [Google Scholar] [CrossRef]
- Geetha, S.; Ram, M.S.; Singh, V.; Ilavazhagan, G.; Sawhney, R.C. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)—An in vitro study. J. Ethnopharm. 2002, 79, 373–378. [Google Scholar] [CrossRef]
- Anchidin, B.G.; Lipșa, F.D.; Manoliu, D.R.; Ciobanu, M.M.; Ciobotaru, M.C.; Gucianu, I.; Boișteanu, P.C. Enhancing antioxidant capacity in functional meat products through infusion with sea buckthorn oil to combat inherent antioxidant deficiency. Sci. Pap. Ser. D Anim. Sci. 2024, 67, 366–380. [Google Scholar]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Du, R.; Wang, K.; Puguan, K.; Wang, C.; Liu, Y.; Wang, L.; Wang, L. Sustained-Release Application of Porous Starch/Gum Arabic Microencapsulated Sea Buckthorn Essential Oil in Yak Meat Preservation. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5403113 (accessed on 10 October 2025).
- Papuc, C.; Durdun, N.; Nicorescu, V.; Papuc, C.; Crivineanu, D. The effect of sea buckthorn polyphenols upon discoloration and lipid peroxidation of pork and beef ground meat during refrigeration. Sci. Works Univ. Agronom. Sci. Vet. Med. Buchar. Ser. C Vet. Med. 2010, 56, 136–144. [Google Scholar]
- Wojtaszek, A.; Salejda, A.M.; Nawirska-Olszańska, A.; Zambrowicz, A.; Szmaja, A.; Ambrozik-Haba, J. Physicochemical, antioxidant, organoleptic, and anti-diabetic properties of innovative beef burgers enriched with juices of açaí (Euterpe oleracea Mart.) and sea buckthorn (Hippophae rhamnoides L.) berries. Foods 2024, 13, 3209. [Google Scholar] [CrossRef]
- Bobko, M.; Kročko, M.; Haščík, P.; Tkáčová, J.; Bučko, O.; Bobková, A.; Čuboň, J.; Češkovič, M.; Pavelkova, A. The effect of sea buckthorn (Hippophae rhamnoides L.) berries on parameters of quality raw cooked meat product. J. Microbio. Biotech. Food Sci. 2019, 9, 366–369. [Google Scholar] [CrossRef]
- Salejda, A.M.; Tril, U.; Krasnowska, G. The effect of sea buckthorn (Hippophae rhamnoides L.) berries on some quality characteristics of cooked pork sausages. Int. J. Nutr. Food Eng. 2014, 8, 604–607. [Google Scholar]
- Mesárošová, A.; Lidíková, J.; Bobková, A.; Kročko, M.; Mendelová, A.; Tóth, T.; Nedomová, Š.; Vörösová, D.; Bobko, M. Effect of sea buckthorn (Hippophae rhamnoides var. vitaminnaja) extract on spoilage of pork sausages. J. Microbio. Biotech. Food Sci. 2024, 13, 10420. [Google Scholar] [CrossRef]
- Bukarbayev, K.; Abzhanova, S.; Baibolova, L.; Zhaksylykova, G.; Kulazhanov, T.; Vasilenko, V.; Jetpisbayeva, B.; Katasheva, A.; Sabraly, S.; Yerzhigitov, Y. Effect of hemp protein and sea buckthorn extract on quality and shelf life of cooked-smoked sausages. Foods 2025, 14, 2730. [Google Scholar] [CrossRef]
- Makangali, K.; Konysbaeva, D.; Zhakupova, G.; Gorbulya, V.; Suyundikova, Z. Study of the effect of sea buckthorn seed powder on the production of smoked meat products from camel and beef meat. Period. Tche Quim. 2019, 16, 130–139. [Google Scholar] [CrossRef]
- Kolev, N.; Balev, D.; Dragoev, S.; Popova, T.; Petkov, E.; Dimov, K.; Suman, S.; Salim, A.P.; Vlahova-Vangelova, D. Male layer-type birds (Lohmann Brown Classic Hybrid) as a meat source for chicken pâtés. Appl. Sci. 2025, 15, 6702. [Google Scholar] [CrossRef]
- Khan, A.W. Extraction and fractionation of proteins in fresh chicken musclea. J. Food Sci. 1962, 27, 430–434. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/75594.html (accessed on 10 October 2025).
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 709–712. [Google Scholar] [CrossRef]
- Moraru Manea, A.I.; Cocan, I.; Dumbrava, D.G.; Poiana, M.A. Effect of fruit powders as natural alternatives to sodium nitrite on lipid oxidation in clean-label salami. Foods 2025, 14, 2262. [Google Scholar] [CrossRef]
- Vassilev, K.; Ivanov, G.; Balev, D.; Dobrev, G. Protein changes of chicken light and dark muscles during chilled storage. J. Eco-Agric. Tour. 2012, 8, 263–268. [Google Scholar]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Vardakas, A.; Kechagias, A.; Penov, N.; Giannakas, A.E. Optimization of enzymatic-assisted extraction of bioactive compounds from Olea europaea leaves. Biomass 2024, 4, 647–657. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dinkova, R.; Heffels, P.; Shikov, V.; Weber, F.; Schieber, A.; Mihalev, K. Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vaccinium myrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Food Res. Int. 2014, 65, 35–41. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999; p. 416. ISBN 978-1-0030-4072-9. [Google Scholar] [CrossRef]
- GOST R 54354-2011; Meat and Meat Products. General Requirements and Methods of Microbiological Testing. Federal Agency for Technical Regulation and Metrology (Rosstandart): Moscow, Russia, 2011. Available online: https://www.russiangost.com/p-72038-gost-r-54354-2011.aspx (accessed on 10 October 2025). (In Russian)
- GOST 10444.15-94; Food Products. Methods for Determination Quantity of Mesophilic Aerobes and Facultative Anaerobes. Federal Agency for Technical Regulation and Metrology (Rosstandart): Moscow, Russia, 1994. Available online: https://www.russiangost.com/p-21387-gost-1044415-94.aspx (accessed on 10 October 2025). (In Russian)
- GOST 31747-2012; Food Products. Methods for Detection and Quantity Determination of Coliforms. Federal Agency for Technical Regulation and Metrology (Rosstandart): Moscow, Russia, 2012. Available online: https://www.russiangost.com/p-65997-gost-31747-2012.aspx (accessed on 14 October 2025). (In Russian)
- GOST 10444.15-94; Food Products. Methods for the Detection of Salmonella spp. Federal Agency for Technical Regulation and Metrology (Rosstandart): Moscow, Russia, 1994. Available online: https://www.russiangost.com/p-68638-gost-31659-2012.aspx (accessed on 10 October 2025). (In Russian)
- Kolev, N.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S. Quality changes of cooked sausages influenced by the incorporation of a three-component natural antioxidant blend. BIO Web Conf. 2022, 45, 01006. [Google Scholar] [CrossRef]
- Kolev, N.D.; Vlahova-Vangelova, D.B.; Balev, D.K.; Dragoev, S.G. Stabilization of oxidative processes in cooked sausages by optimization of incorporated biologically active substances. Carp. J. Food Sci. Technol. 2022, 14, 180–188. [Google Scholar] [CrossRef]
- Bayır, H.; Şimşek, B.İ.; Bayır, Y. Hippophae rhamnoides L. botanical, medicinal, traditional, and current use of plant and fruits: A review. New Trend. Med. Sci. 2024, 5, 35–44. [Google Scholar] [CrossRef]
- Nazir, F.; Salim, R.; Bashir, M. Chemical and antioxidant properties of Sea buckthorn (Hippophae rhamnoides). Pharma Innov. J. 2017, 6, 173–176. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).