Acute Effects of Polyphenol-Rich Fruit Juice on Exercise Capacity and Vessels Dilatation in Healthy Humans: A Randomized, Controlled, Crossover Study
Abstract
1. Introduction
2. Methods
2.1. Population
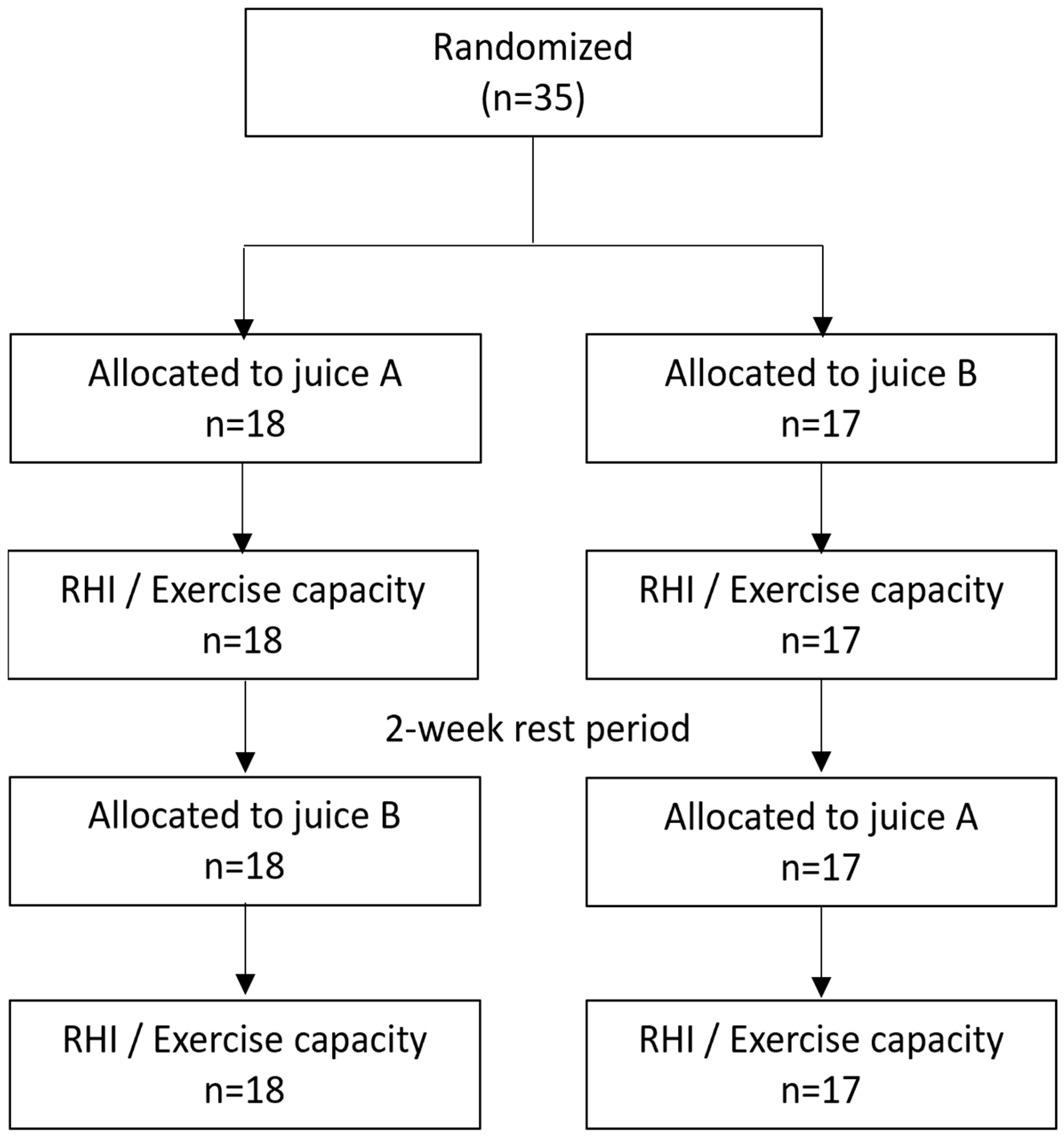
2.2. Experimental Design
2.3. Control (Apple) and Fruit Juices Compositions
2.4. Parameters Determined
2.4.1. Six-Minute Walking Test
2.4.2. Heart Rate and Systemic Blood Pressure
2.4.3. Reactive Hyperemia Index
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Effects of Both Juices on Subject’s Exercise Capacity
3.3. Effects of Juices Intake on Reactive Hyperemia-Index (RHI)
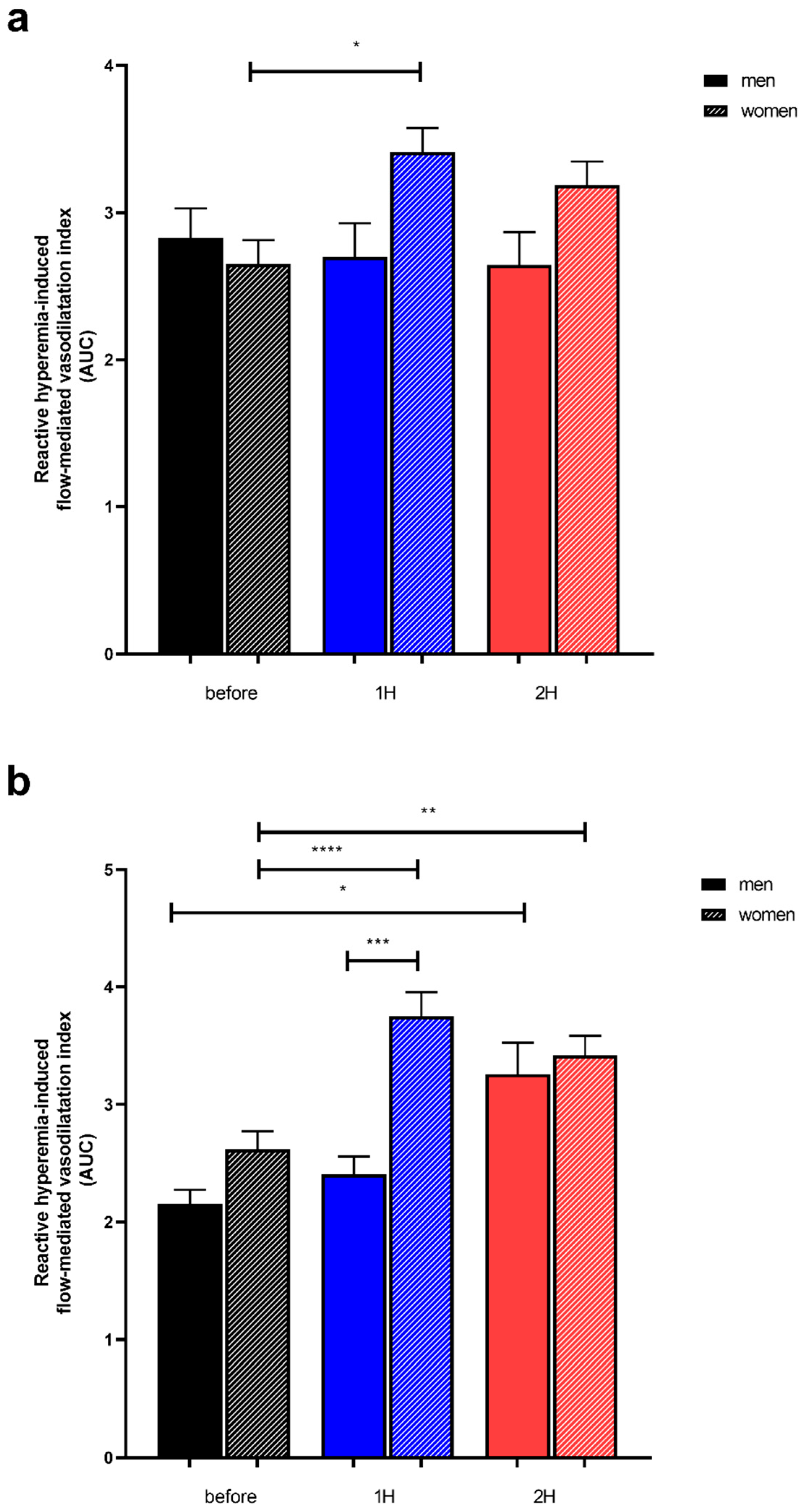
3.3.1. RHI Kinetic After Control Juice Intake, with a Low Polyphenol Content (Apple Juice)
3.3.2. RHI Kinetic After Polyphenol Rich Juice Intake (Fruit Juice)
4. Discussion
4.1. Effect of Increased Reactive Hyperemia on Exercise Capacity
4.2. PP-Rich-Fruit-Juice-Induced Enhancement in Reactive Hyperemia Index Lasted Longer
5. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musial, D.C.; Ajita, M.E.; Bomfim, G.H.S. Benefits of Cilostazol’s Effect on Vascular and Neuropathic Complications Caused by Diabetes. Med. Sci. 2024, 13, 1. [Google Scholar] [CrossRef]
- Damluji, A.A.; Tomczak, C.R.; Hiser, S.; O’Neill, D.E.; Goyal, P.; Pack, Q.R.; Foulkes, S.J.; Brown, T.M.; Haykowsky, M.J.; Needham, D.M.; et al. Benefits of Cardiac Rehabilitation: Mechanisms to Restore Function and Clinical Impact. Circ. Res. 2025, 137, 255–272. [Google Scholar] [CrossRef]
- Keefe, M.S.; Benjamin, C.L.; Casa, D.J.; Sekiguchi, Y. Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review. Appl. Sci. 2024, 14, 10103. [Google Scholar] [CrossRef]
- Whipple, M.O.; Xu, S.; Zhang, D.; Guralnik, J.M.; Spring, B.; Tian, L.; Treat-Jacobson, D.; Zhao, L.; Criqui, M.H.; McDermott, M.M. Home-Based Exercise and Patient-Reported Outcome Measures in Peripheral Artery Disease: The LITE Randomized Clinical Trial. Am. J. Cardiol. 2025, 244, 41–47. [Google Scholar] [CrossRef]
- Schaefer, A.; Piquard, F.; Doutreleau, S.; Mettauer, B.; Epailly, E.; Eisenmann, B.; Lonsdorfer, J.; Geny, B. Reduced Exercise Capacity Is Associated with Reduced Nitric Oxide Production after Heart Transplantation. J. Thorac. Cardiovasc. Surg. 2001, 122, 821–822. [Google Scholar] [CrossRef][Green Version]
- Doutreleau, S.; Mettauer, B.; Piquard, F.; Rouyer, O.; Schaefer, A.; Lonsdorfer, J.; Geny, B. Chronic L-Arginine Supplementation Enhances Endurance Exercise Tolerance in Heart Failure Patients. Int. J. Sports Med. 2006, 27, 567–572. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Old-Fashioned, but Still a Superfood—Red Beets as a Rich Source of Bioactive Compounds. Appl. Sci. 2023, 13, 7445. [Google Scholar] [CrossRef]
- Dal-Ros, S.; Zoll, J.; Lang, A.-L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V.B. Chronic Intake of Red Wine Polyphenols by Young Rats Prevents Aging-Induced Endothelial Dysfunction and Decline in Physical Performance: Role of NADPH Oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.-L.; Meyer, A.; Dal-Ros, S.; Auger, C.; Keller, N.; Ramamoorthy, T.G.; Zoll, J.; Metzger, D.; Schini-Kerth, V.; Geny, B. Polyphenols Prevent Ageing-Related Impairment in Skeletal Muscle Mitochondrial Function through Decreased Reactive Oxygen Species Production. Exp. Physiol. 2013, 98, 536–545. [Google Scholar] [CrossRef] [PubMed]
- d’Unienville, N.M.A.; Blake, H.T.; Coates, A.M.; Hill, A.M.; Nelson, M.J.; Buckley, J.D. Effect of Food Sources of Nitrate, Polyphenols, L-Arginine and L-Citrulline on Endurance Exercise Performance: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Int. Soc. Sports Nutr. 2021, 18, 76. [Google Scholar] [CrossRef]
- de Andrade Soares, R.; de Oliveira, B.C.; de Bem, G.F.; de Menezes, M.P.; Romão, M.H.; Santos, I.B.; da Costa, C.A.; de Carvalho, L.C.D.R.M.; Nascimento, A.L.R.; de Carvalho, J.J.; et al. Açaí (Euterpe oleracea Mart.) Seed Extract Improves Aerobic Exercise Performance in Rats. Food Res. Int. 2020, 136, 109549. [Google Scholar] [CrossRef]
- Volpe-Fix, A.R.; de França, E.; Silvestre, J.C.; Thomatieli-Santos, R.V. The Use of Some Polyphenols in the Modulation of Muscle Damage and Inflammation Induced by Physical Exercise: A Review. Foods 2023, 12, 916. [Google Scholar] [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef]
- Hajleh, M.N.A.; Al-Dujaili, E.A.S. Effects of Turmeric Concentrate on Cardiovascular Risk Factors and Exercise-Induced Oxidative Stress in Healthy Volunteers; an Exploratory Study. Adv. Pharm. Bull. 2023, 13, 601–610. [Google Scholar] [CrossRef]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef]
- Montero, D.; Walther, G.; Diaz-Cañestro, C.; Pyke, K.E.; Padilla, J. Microvascular Dilator Function in Athletes: A Systematic Review and Meta-Analysis. Med. Sci. Sports Exerc. 2015, 47, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Rocha, S.; de Freitas, V. Going “Green” in the Prevention and Management of Atherothrombotic Diseases: The Role of Dietary Polyphenols. J. Clin. Med. 2021, 10, 1490. [Google Scholar] [CrossRef]
- Bapir, M.; Untracht, G.R.; Cooke, D.; McVey, J.H.; Skene, S.S.; Campagnolo, P.; Whyte, M.B.; Dikaios, N.; Rodriguez-Mateos, A.; Sampson, D.D.; et al. Cocoa Flavanol Consumption Improves Lower Extremity Endothelial Function in Healthy Individuals and People with Type 2 Diabetes. Food Funct. 2022, 13, 10439–10448. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Garcia, J.P.; Santana, A.; Baruqui, D.L.; Suraci, N. The Cardiovascular Effects of Chocolate. Rev. Cardiovasc. Med. 2018, 19, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamori, Y. Inhibition of Endothelial Dysfunction by Dietary Flavonoids and Preventive Effects Against Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2020, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, R.M.; Galante, L.A.; Rowland, I.R.; Spencer, J.P.; Commane, D.M. Consumption of a Flavonoid-Rich Açai Meal Is Associated with Acute Improvements in Vascular Function and a Reduction in Total Oxidative Status in Healthy Overweight Men. Am. J. Clin. Nutr. 2016, 104, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kim, S.; Eto, M.; Iijima, K.; Ako, J.; Yoshizumi, M.; Akishita, M.; Kondo, K.; Itakura, H.; Hosoda, K.; et al. Effect of Acute Intake of Red Wine on Flow-Mediated Vasodilatation of the Brachial Artery. Am. J. Cardiol. 2001, 88, A9. [Google Scholar] [CrossRef]
- Agewall, S.; Wright, S.; Doughty, R.N.; Whalley, G.A.; Duxbury, M.; Sharpe, N. Does a Glass of Red Wine Improve Endothelial Function? Eur. Heart J. 2000, 21, 74–78. [Google Scholar] [CrossRef]
- Tucci, M.; Del Bo’, C.; Martini, D.; Perna, S.; Marino, M.; Rendine, M.; Gardana, C.; Battezzati, A.; Leone, A.; Bertoli, S.; et al. A Serving of Blueberry (Vaccinium corymbosum) Improves Peripheral Vascular Function but Not Metabolic and Functional Markers in Older Subjects: A Randomized, Controlled, Crossover Study. Food Res. Int. 2024, 197, 115189. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kwon, T.-G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Auger, C.; Kim, J.-H.; Trinh, S.; Chataigneau, T.; Popken, A.M.; Schini-Kerth, V.B. Fruit Juice-Induced Endothelium-Dependent Relaxations in Isolated Porcine Coronary Arteries: Evaluation of Different Fruit Juices and Purees and Optimization of a Red Fruit Juice Blend. Food Funct. 2011, 2, 245–250. [Google Scholar] [CrossRef]
- Cano-Uceda, A.; Pareja-García, P.; Sánchez-Rodríguez, E.; Fraguas-Ramos, D.; Martín-Álvarez, L.; Asencio-Vicente, R.; Rivero-de la Villa, A.; Pérez-Pérez, M.d.M.; Obispo-Portero, B.M.; Morales-Ruiz, L.; et al. Effects of a Short-Term Supervised Exercise Program in Women with Breast Cancer. Appl. Sci. 2024, 14, 6553. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.D.; Regalado-Cabello, P.; Rodríguez-Montes, M.; Cabrera-Martos, I.; Martín-Nuñez, J.; Valenza, M.C. Impact of Core Exercise Training on Gait and Exercise Capacity in People with Multiple Sclerosis: A Systematic Review. Appl. Sci. 2025, 15, 5054. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of Peripheral Vascular Endothelial Function with Finger Arterial Pulse Wave Amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef]
- McCrea, C.E.; Skulas-Ray, A.C.; Chow, M.; West, S.G. Test-Retest Reliability of Pulse Amplitude Tonometry Measures of Vascular Endothelial Function: Implications for Clinical Trial Design. Vasc. Med. Lond. Engl. 2012, 17, 29–36. [Google Scholar] [CrossRef]
- Riou, M.; Oulehri, W.; Momas, C.; Rouyer, O.; Lebourg, F.; Meyer, A.; Enache, I.; Pistea, C.; Charloux, A.; Marcot, C.; et al. Reduced Flow-Mediated Dilatation Is Not Related to COVID-19 Severity Three Months after Hospitalization for SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 1318. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin Mediates Beneficial Effects of Flavanol-Rich Cocoa on Vascular Function in Humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Lejay, A.; Laverny, G.; Paradis, S.; Schlagowski, A.-I.; Charles, A.-L.; Singh, F.; Zoll, J.; Thaveau, F.; Lonsdorfer, E.; Dufour, S.; et al. Moderate Exercise Allows for Shorter Recovery Time in Critical Limb Ischemia. Front. Physiol. 2017, 8, 523. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The 6-Minute Walk: A New Measure of Exercise Capacity in Patients with Chronic Heart Failure. Can. Med. Assoc. J. 1985, 132, 919–923. [Google Scholar] [PubMed]
- Cazzoletti, L.; Zanolin, M.E.; Dorelli, G.; Ferrari, P.; Dalle Carbonare, L.G.; Crisafulli, E.; Alemayohu, M.A.; Olivieri, M.; Verlato, G.; Ferrari, M. Six-Minute Walk Distance in Healthy Subjects: Reference Standards from a General Population Sample. Respir. Res. 2022, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.A.; Theodore, A.P.; Funderburg, B.R.; Waldhelm, A.; McKinley-Barnard, S.K.; Hudson, G.M. Acute (−)-Epicatechin Consumption: Effects on Local Vasodilation Following Resistance Exercise and High-Intensity Exercise Performance. Sports 2020, 8, 22. [Google Scholar] [CrossRef]
- Rouyer, O.; Auger, C.; Charles, A.-L.; Talha, S.; Meyer, A.; Piquard, F.; Andres, E.; Schini-Kerth, V.; Geny, B. Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects. J. Clin. Med. 2019, 8, 859. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Liang, F.; Yang, Y.; Lu, X.; Gu, D. Air Pollution Exposure and Vascular Endothelial Function: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2023, 30, 28525–28549. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Noninvasive Vascular Function Tests for the Future Prediction of Primary Cardiovascular Diseases. Hosp. Pract. 2020, 48, 113–118. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-Rich Apple Improves Endothelial Function in Individuals at Risk for Cardiovascular Disease: A Randomized Controlled Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 1700674. [Google Scholar] [CrossRef]
- Istas, G.; Feliciano, R.P.; Weber, T.; Garcia-Villalba, R.; Tomas-Barberan, F.; Heiss, C.; Rodriguez-Mateos, A. Plasma Urolithin Metabolites Correlate with Improvements in Endothelial Function after Red Raspberry Consumption: A Double-Blind Randomized Controlled Trial. Arch. Biochem. Biophys. 2018, 651, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cheok, A.; Xu, Y.; Zhang, Z.; Caton, P.W.; Rodriguez-Mateos, A. Betalain-Rich Dragon Fruit (Pitaya) Consumption Improves Vascular Function in Men and Women: A Double-Blind, Randomized Controlled Crossover Trial. Am. J. Clin. Nutr. 2022, 115, 1418–1431. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, N.; Raizada, N.; Aslam, M.; Mehrotra, G.; Gambhir, J.K.; Singh, G.; Madhu, S.V. Postprandial Endothelial Dysfunction and CIMT after Oral Fat Challenge in Patients with Type 2 Diabetes Mellitus with and without Macrovascular Disease—A Preliminary Study. Diabetes Metab. Syndr. 2021, 15, 102317. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of Glycemic Variability on Chromatin Remodeling, Oxidative Stress, and Endothelial Dysfunction in Patients With Type 2 Diabetes and With Target HbA1c Levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia Rapidly Suppresses Flow-Mediated Endothelium-Dependent Vasodilation of Brachial Artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Williams, J.S.; Bonafiglia, J.T.; King, T.J.; Gurd, B.J.; Pyke, K.E. No Acute Hyperglycemia Induced Impairment in Brachial Artery Flow-Mediated Dilation before or after Aerobic Exercise Training in Young Recreationally Active Males. Eur. J. Appl. Physiol. 2023, 123, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Basaqr, R.; Skleres, M.; Jayswal, R.; Thomas, D.T. The Effect of Dietary Nitrate and Vitamin C on Endothelial Function, Oxidative Stress and Blood Lipids in Untreated Hypercholesterolemic Subjects: A Randomized Double-Blind Crossover Study. Clin. Nutr. 2021, 40, 1851–1860. [Google Scholar] [CrossRef]
- Green, D.J.; Marsh, C.E.; Thomas, H.J.; Lester, L.; Scurrah, K.J.; Haynes, A.; Naylor, L.H. Exercise and Artery Function in Twins: Sex Differences in a Cross-Over Trial. Hypertension 2023, 80, 1343–1352. [Google Scholar] [CrossRef]
- Alasmari, A.M.; Alsulayyim, A.S.; Alghamdi, S.M.; Philip, K.E.J.; Buttery, S.C.; Banya, W.A.S.; Polkey, M.I.; Armstrong, P.C.; Rickman, M.J.; Warner, T.D.; et al. Oral Nitrate Supplementation Improves Cardiovascular Risk Markers in COPD: ON-BC, a Randomised Controlled Trial. Eur. Respir. J. 2024, 63, 2202353. [Google Scholar] [CrossRef] [PubMed]
- Rossi, I.; Mignogna, C.; Del Rio, D.; Mena, P. Health Effects of 100% Fruit and Vegetable Juices: Evidence from Human Subject Intervention Studies. Nutr. Res. Rev. 2024, 37, 194–238. [Google Scholar] [CrossRef] [PubMed]



| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Fruits and Berries | Total Phenolic Content (g/L GAE) |
| Acerola | 3.12 |
| Apple | 1.70 |
| Aronia | 7.15 |
| Blueberry | 5.94 |
| Grape | 1.99 |
| Strawberry | 2.03 |
| Flavanols Content (mg/L) | |
| Flavan-3-ols | 13.1 |
| Procyanidin B1 | 11.8 |
| Procyanidin B2 | 15.3 |
| Epicatechin | 12.8 |
| Gallocatechin-3-O-gallate | 0.8 |
| Apple Juice (g/L) | Fruit Juice (g/L) | |
|---|---|---|
| Fat | 0 | 0 |
| Sugar | 98 | 122.4 |
| Protein | 0 | 0 |
| Salt | 0 | 0 |
| Vitamin C | 0 | 0.40 |
| n | Total 35 | Women 24 | Men 11 |
|---|---|---|---|
| Age (years) | 48.9 ± 1.50 | 49.1 [45.36; 52.83] | 48.48 [42.23; 54.74] |
| BMI (kg/m2) | 25.96 ± 0.94 | 25.83 [23.39; 28.26] | 26.25 [22.69; 29.82] |
| Systolic BP (mm Hg) | 129.1 ± 3.57 | 121.2 [113.2; 129.1] | 146.3 [136.3; 156.3] |
| Diastolic BP (mm Hg) | 81.03 ± 3.84 | 78.08 [67.01; 89.16] | 87.45 [80.41; 94.50] |
| Heart rate (bpm) | 68.34 ± 1.66 | 69.83 [66.26; 73.40] | 65.09 [56.96; 73.22] |
| Baecke index | 7.45 ± 0.21 | 7.27 [6.68; 7.86] | 7.85 [7.46; 8.24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouyer, O.; Charles, A.-L.; Auger, C.; Talha, S.; Andres, E.; Charloux, A.; Schini-Kerth, V.; Geny, B. Acute Effects of Polyphenol-Rich Fruit Juice on Exercise Capacity and Vessels Dilatation in Healthy Humans: A Randomized, Controlled, Crossover Study. Appl. Sci. 2025, 15, 11553. https://doi.org/10.3390/app152111553
Rouyer O, Charles A-L, Auger C, Talha S, Andres E, Charloux A, Schini-Kerth V, Geny B. Acute Effects of Polyphenol-Rich Fruit Juice on Exercise Capacity and Vessels Dilatation in Healthy Humans: A Randomized, Controlled, Crossover Study. Applied Sciences. 2025; 15(21):11553. https://doi.org/10.3390/app152111553
Chicago/Turabian StyleRouyer, Olivier, Anne-Laure Charles, Cyril Auger, Samy Talha, Emmanuel Andres, Anne Charloux, Valerie Schini-Kerth, and Bernard Geny. 2025. "Acute Effects of Polyphenol-Rich Fruit Juice on Exercise Capacity and Vessels Dilatation in Healthy Humans: A Randomized, Controlled, Crossover Study" Applied Sciences 15, no. 21: 11553. https://doi.org/10.3390/app152111553
APA StyleRouyer, O., Charles, A.-L., Auger, C., Talha, S., Andres, E., Charloux, A., Schini-Kerth, V., & Geny, B. (2025). Acute Effects of Polyphenol-Rich Fruit Juice on Exercise Capacity and Vessels Dilatation in Healthy Humans: A Randomized, Controlled, Crossover Study. Applied Sciences, 15(21), 11553. https://doi.org/10.3390/app152111553








