Abstract
Soil contamination with petroleum-derived substances, including petrol, is one of the most serious environmental issues of the modern era. These products are characterised by their durability and stability in the environment, their capacity for bioaccumulation and their toxicity to many organisms, including plants. This study aimed to evaluate the impact of petrol contamination on trace element content in the above-ground parts of oats (Avena sativa L.) and to determine the effectiveness of in situ stabilisation methods using compost, bentonite and calcium oxide in reducing bioaccumulation of these elements. Petrol contamination of the soil significantly altered the biomass yield and the concentration of trace elements in the plants. It caused a decrease in the dry matter yield and an increase in the content of most trace elements in the above-ground parts of oats. The most pronounced effects were observed for Cd, Ni, Fe, Co, Cr and Mn, whose concentrations in the plants increased across the entire range of petrol doses. Petrol had a similar effect on Zn and Pb content in the above-ground parts of oats, but only up to a medium level of contamination (5 cm3 kg−1). In contrast to the aforementioned elements, soil contamination with petrol contributed to a decrease in the copper content of the above-ground parts of oats. The materials applied to the soil had a beneficial effect on the biomass and the concentration of certain trace elements in plants. The compost and especially calcium oxide had a positive influence on plant yield. Compared to the series without their application to the soil, all materials reduced the content of Cr, Fe, Cd and, especially, Mn in the above-ground parts of plants. Compost also reduced the content of Pb, while bentonite and calcium oxide reduced the content of Co. Calcium oxide also reduced the content of Cu in the above-ground parts of oats. However, bentonite had a weaker effect than compost and calcium oxide. Changes in the content of other elements in plants after application of the aforementioned materials were often opposite (and dependent on the type of material), with the clearest effect being on nickel content. The materials used in the study produced good results in limiting the impact of minor soil contamination with petrol on the content of certain trace elements in plants.
1. Introduction
Soil contamination with petroleum-derived substances, including petrol, is one of the most serious environmental problems facing the modern world [1]. Petrol is a complex mixture of aliphatic and aromatic hydrocarbons and contains a number of substances with proven toxic, mutagenic and carcinogenic effects [2,3]. These include BTEX compounds (benzene, toluene, ethylbenzene and xylenes), as well as polycyclic aromatic hydrocarbons (PAHs) [4]. These substances enter the soil through leaks in oil refineries, storage facilities, and underground tanks, as well as accidents involving production units and transport pipelines [5]. Oil spills and other activities related to oil extraction and processing result in the release of around 600,000 tonnes of organic pollutants each year [6]. Petroleum products are characterised by their durability and stability in the environment, their bioaccumulation capacity [3] and their toxicity to many soil organisms [7]. According to Gao et al. [8], high doses of petroleum (above 20,000 mg kg−1 of soil) significantly reduce the structural diversity of soil microbial communities and species coexistence patterns, as well as the number of prokaryotic carbon and nitrogen binding genes. Chemical changes in petroleum-contaminated soil primarily include acidification of the soil [9], which results from the hydrolysis of macromolecular substances into smaller molecules, such as CO2 and H2O, and the accumulation of organic acids produced by the microbial degradation of hydrocarbons [10]. The presence of petroleum-derived substances in soil can lead to serious imbalances in ecosystems [11], affecting the availability of macronutrients such as nitrogen [12], as well as the mobility [13] and accumulation of trace elements in crops [14]. Acidification of the soil environment due to petrol contamination increases the solubility of trace elements by causing them to desorb from the soil’s solid phase and become more mobile in the soil solution [15]. Decreased soil pH is accompanied by a reduction in the electronegative charge on colloidal surfaces with variable charges (including organic matter, aluminium and iron oxides, and 1:1 and 2:1 clay minerals), reducing the soil’s sorption capacity for cationic compounds and making them more available to plants [16]. Cheraghi et al. [17] confirmed that the activities of the Tehran Oil Refining Company oil refinery affected the soil’s physicochemical properties, including pH, resulting in a decrease from 7.4 (control) to 5.6. According to Iwegbue’s [18] research, the mobility and accumulation coefficients of Cd, Cu, Pb, Ni, Cr, Zn and Mn increased in oil-contaminated soils compared to control sites.
Petroleum-derived pollutants have a negative impact on plants, including inhibiting germination and seedling growth [19], reducing growth and above-ground biomass [20], and decreasing leaf area and main root system growth [21]. In plant cells, petroleum-derived hydrocarbons induce oxidative stress by increasing the production of reactive oxygen species (ROS) [22], which can lead to membrane lipid peroxidation and nucleic acid damage [23]. Furthermore, petroleum derivatives have a negative impact on fundamental physiological processes in plants, such as photosynthesis, cellular respiration, and water metabolism [24]. Dąbrowski et al. [25] demonstrated that exposure to crude oil impairs the activity of photosystem II (PSII) and photosystem I (PSI) in five herbaceous plant species, reducing the efficiency of electron transport between PSII and PSI. These changes result in disturbances to PSII functioning, leading to reduced photosynthetic efficiency, slower plant growth, and the formation of chlorotic and necrotic changes [22]. Consequently, there is a decrease in plant resistance to abiotic stress factors [26]. The indirect impact of petroleum-derived pollutants on plants occurs mainly through changes in the availability of water, oxygen, and nutrients [25] and disruption to the ionic balance in the root zone [26]. Devatha et al. [27] demonstrated that soil contaminated with 10% petroleum had reduced nitrogen (by 80%), phosphorus (by 33%) and potassium (>99%) content compared to the control. However, in the case of trace elements, a decrease in soil pH due to the presence of hydrocarbons leads to an increase in their solubility and bioavailability [28], as well as increased uptake by plants [29]. According to research by Gospodarek et al. [30], the content of Cd, Pb, Zn, Ni, Fe, Mn, Sr, Ba, As and Al in the roots of field beans increased significantly when petrol was applied at a dose of 6 g kg−1 d.m. of soil, compared to the control object.
Techniques for remediating soil contaminated with petroleum-derived substances can be divided into two strategies: ex situ, which involves physically transferring the contaminated soil to another location for cleaning, and in situ, which involves taking action at the site of contamination [31]. One method of in situ soil remediation is stabilisation, which involves introducing appropriate organic or mineral materials into the soil to reduce the contaminants’ solubility, bioavailability and mobility through processes such as sorption, precipitation or complexation [32]. Shaheen et al. [33] demonstrated that applying bentonite to contaminated floodplain soil reduced the labile fractions of Ni and Zn by 58.7% and 83%, respectively. Undoubtedly, the advantages of in situ stabilisation include high public acceptance, the use of cheap, readily available materials, satisfactory results in a short timeframe, and the absence of secondary waste [34].
Examples of soil additives that can be used successfully in situ stabilisation include compost, bentonite and calcium oxide. As a natural material with a high content of soluble organic matter, compost is highly effective at stabilising petroleum-derived contaminants [35]. Introducing compost into the soil reduces the bioavailability of contaminants, while improving soil quality by balancing pH levels, increasing organic matter and water capacity, and rebuilding microbial populations [36]. Additionally, the presence of humic substances in compost enable trace elements to be immobilised by forming stable organometallic complexes [37]. Bentonite is a clay mineral characterised by a large specific surface area, exceptional sorption capacity and significant ion exchange potential [38], with a high smectite content of 60–75% [39]. As a layered silicate with a 2:1 packet structure, bentonite consists of two tetrahedral silica layers separated by an octahedral aluminium oxide layer and exchangeable cations, most often Ca2+, Mg2+ and Na+ [39,40]. This crystal structure gives the material a high adsorption capacity for trace element cations [41] and organic pollutants, including petroleum derivatives [42]. The mechanism by which trace elements are immobilised using bentonite involves cation exchange, surface adsorption, and intercalation between mineral layers [43]. Conversely, calcium oxide (CaO) primarily neutralises acidity and precipitates trace elements in the form of poorly soluble hydroxides, carbonates, and silicates [44]. Using calcium oxide to remediate petrol-contaminated soils is particularly justified given hydrocarbons’ tendency to reduce soil pH [27]. In the in situ stabilisation technique for soils contaminated with petroleum derivatives, other remediation materials may be used alongside biochar or zeolite. Produced by the pyrolysis of biomass under conditions of limited oxygen access, biochar is particularly effective in immobilising trace elements through electrostatic interactions, complexation, ion exchange, precipitation and chemisorption mechanisms [45] due to its highly porous structure, high content of basic cations and the presence of functional aromatic groups [46]. Gascó et al. [47] demonstrated its usefulness in reducing the accumulation of As, Cu, Co, Cr, Se and Pb in Brassica napus biomass. Mazzurco-Miritana et al. [48] proved that adding biochar to hydrocarbon-contaminated soil (C > 12 = 10.000 mg kg−1) increased degradation by 66.7%, compared to 46% in the control soil. Zeolite is a mineral belonging to the aluminosilicate group with properties similar to bentonite, and has unique adsorption and cation exchange capacities [49]. Xia et al. [50] demonstrated the positive effect of zeolite addition on the degradation of petroleum hydrocarbons in oil-contaminated soil. In an experiment conducted by Radziemska and Mazur [51], zeolite application reduced the content of nickel, chromium and copper in nickel-contaminated soil.
Petroleum-contaminated soils are a source of volatile organic compound emissions into the atmosphere, contributing to air pollution and photochemical smog formation [52]. At the same time, the migration of petroleum-derived pollutants into the soil profile can threaten the quality of groundwater [53]. Soil contamination with petroleum derivatives and trace elements is a global environmental and public health issue affecting both developed and developing countries. Montaño-López and Biswas [54] conducted soil testing in 20 community gardens in Guelph, Ontario, Canada, a city with a long history of intensive industrial activity, particularly in the metallurgical sector. Analysis of soil samples taken at two depths (0–15 cm and 15–30 cm) revealed that 17.5% of samples exceeded the Zn standard, 15% exceeded the Pb standard, 45% exceeded the Se standard, and individual samples exceeded the Cd and As standards. Oloruntoba et al. [55] conducted a comprehensive review of the problem of soil, water, and food contamination with trace elements in Nigeria. The study revealed elevated concentrations of Cd, Cu, Ni, Pb, Zn, Co, Cr, Fe and As in soil, water and food, with concentrations exceeding WHO and FAO standards on average. This contamination mainly originates from human activities, including industrial processes, the intensive exploitation of oil in the Niger Delta, vehicle emissions, and the improper use of agrochemicals. Similarly, the problem of soil contamination in European countries requires special attention in the context of historical industrial activity and contemporary sources of contamination. Considering only the loss of ecosystem services, diffuse soil pollution in the European Union generates estimated losses of EUR 3.5–7 billion per year. Conversely, the remediation of contaminated sites costs an average of between EUR 0.3–3 billion per year [56]. Given these figures, research into effective and cost-efficient soil remediation methods is becoming increasingly important in terms of public health, developing sustainable agricultural practices, and ensuring future food security.
While most research on in situ stabilisation methods focuses on soils contaminated exclusively with trace elements, in real field conditions—especially near petrol stations, fuel storage facilities, and transport routes—mixed contamination occurs, with petroleum hydrocarbons co-occurring with trace elements. The interactions between these substances in the presence of various remediation materials are not well understood. For this reason, this study was undertaken to assess the impact of petrol contamination on the trace element content of plants, and to determine the effectiveness of in situ stabilisation methods involving the use of compost, bentonite and calcium oxide in reducing the bioaccumulation of these elements. Furthermore, research on the remediation of petrol-contaminated soils using oats (Avena sativa L.) as a test plant is limited. Oats were chosen as the test plant because they are one of the most important cereal crops cultivated in the temperate climates, ranking sixth globally in terms of cereal production [57]. Oats are primarily used as animal feed and for human consumption (in the form of grain and straw), although the plant is also becoming increasingly important in bioenergy production [58].
2. Materials and Methods
2.1. Experiment Design
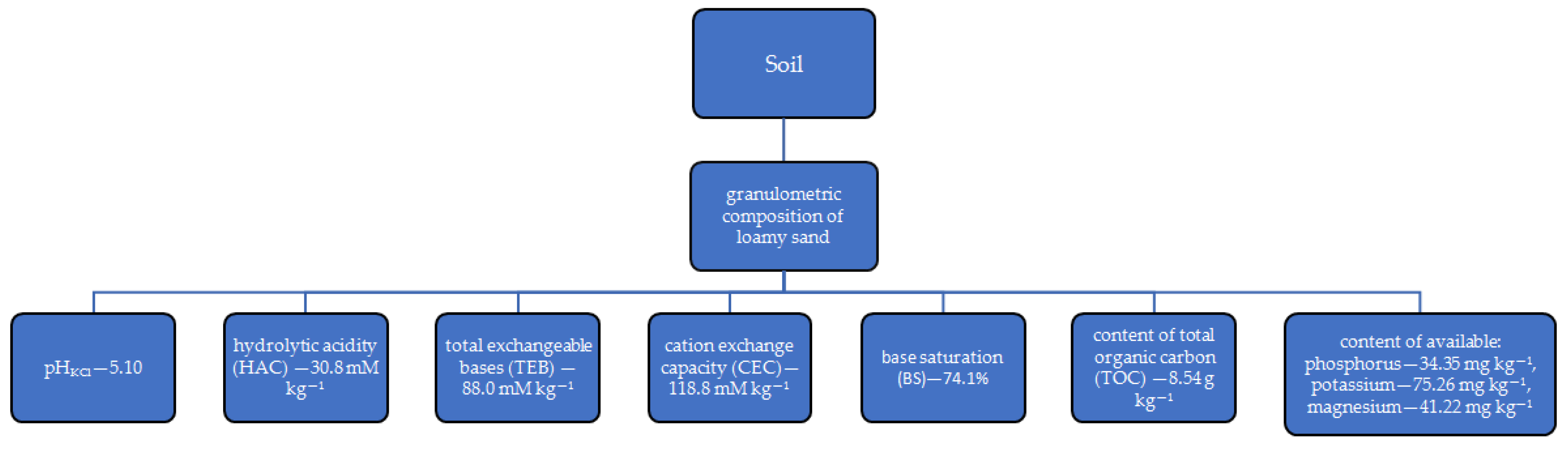
The research was based on a pot experiment conducted in a greenhouse at the University of Warmia and Mazury in Olsztyn, Poland. It was a two-factor vegetation experiment. The top layer of Eutric Cambisol soil [59] was used for the experiment. Its properties are shown in Figure 1.
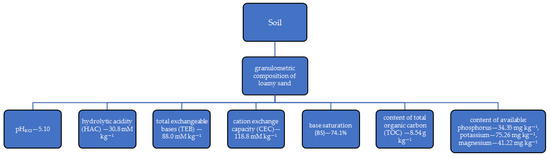
Figure 1.
Soil properties.
The soil used for the experiment was artificially contaminated with the following quantities of petrol: 0, 2.5, 5 and 10 cm3 kg−1 of soil. The characteristics of the petrol were as follows: research octane number (RON)—min. 98.0; motor octane number (MON)—min. 88.0; density at 15 °C—720–775 kg m3; content of Pb—max 5.0 mg dm3; S—max. 10.0 mg dm3; Mn—max. 2.0 mg dm3; hydrocarbons: olefins—max. 18.0% (v/v), aromatics—max. 35.0% (v/v); benzene—max. 1.0% (v/v); oxygen—max. 2.7% (m/m); methanol—max. 3.0% (v/v); ethanol—max 5.0% (v/v); resin (solvent washed)—max. 50 mg dm3 [60]. To limit the impact of the petrol on the soil and plants, compost was added as an organic material, and bentonite (BDC, Niepołomice, Poland) and calcium oxide (50% CaO, Zakład Obrotu Towarami Sp. z o.o., Dwikozy, Poland) were added as mineral materials. These were added to the soil in quantities of 30 g, 20 g and 1.47 g per kg of soil, respectively. The compost was produced from farm waste (23%), cattle manure (33%), garden peat and leaves (44%), mainly from deciduous trees (apple, maple, plum and cherry). The composting process lasted six months. The content of macroelements in the applied materials was as follows: compost—2.32 g P, 1.33 g K, 1.47 g Mg, 15.86 g Ca and 0.12 g Na kg−1 d.m.; bentonite—0.47 g P, 2.43 g K, 5.03 g Mg, 26.72 g Ca and 12.11 g Na kg−1 d.m.; calcium oxide—0.10 g P, 0.77 g K, 2.65 g Mg, 347.99 g Ca and 0.07 g Na kg−1 d.m. Table 1 shows the metal content of the initial soil and the applied materials. The highest concentrations of cadmium, chromium, nickel and cobalt were found in calcium oxide, while the highest concentrations of lead, manganese and iron were found in bentonite. The highest concentrations of zinc and copper were found in compost, while the lowest concentrations of Cd, Pb, Cr, Ni, Mn and Fe were found in compost, and the lowest concentrations of Zn and Cu were found in calcium oxide. To secure the nutritional needs of the plants, basic macro- and micronutrients such as nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), manganese (Mn), molybdenum (Mo) and boron (B) were applied in equal amounts to all experimental combinations. Their doses were as follows: 150 mg of N, 30 mg of P, 75 mg of K, 50 mg of Mg, 5 mg of Mn, 5 mg of Mo and 0.33 mg of B per kg of soil. These elements were applied in the form of CO(NH2)2, KH2PO4, KH2PO4 + KCl, MgSO4·7H2O, MnCl2·4H2O, (NH4)6Mo7O24·4H2O and H3BO3, respectively. When setting up the experiment, the soil was mixed with petrol, the aforementioned macro- and micronutrients, and materials used to neutralise soil contamination by this petroleum-derived substance. The mixture was then poured into 9 kg polyethylene pots. The next step was to sow oats (Avena sativa L.) variety Borowiak (Plant Breeding Station; Borow n/Bielawy, Poland). Once the oats had sprouted, 15 plants were left in each pot. To minimise the risk of errors and ensure the reliability of the experiment results, each experimental object was conducted four times. Constant soil moisture was maintained by watering the plants with tap and redistilled water. The average monthly temperature ranged from 13.6 ± 5.6 °C to 20.1 ± 4.76 °C. The photoperiod ranged from 15 h 22 min to 16 h 56 min, and the insolation ranged from 223.0 h to 368.8 h. Plant material samples were collected for trace element analysis in an analytical laboratory after the oat panicles emerged on the 68th day of the experiment. The results of the analysis of trace elements in the soil were published in a previous paper by Wyszkowski and Kordala [61].

Table 1.
Trace elements content in soil and amendments (mg kg−1 dry matter—d.m.).
2.2. Analytical Methodologies
Following drying and grinding, the samples were subjected to wet digestion using the USE-EPA3051A method in a Mars 6 microwave oven (CEM Corporation, Matthews, NC, USA), after which the trace element content was determined by atomic absorption spectrometry (AAS) [62]. Concentrated nitric acid was used as an extractant during the analyses, alongside certified standard and certified reference materials to ensure the reliability of the test results. The digestion process was carried out using the following proportions: 0.3 g of plant material and 10 cm3 of 65% nitric acid. Digestion parameters:—stages: 2 (1st stage—temperature: 140 °C; 2nd stage—temperature: 170 °C), ramp time: 15 min; cooling: 30 min, and a hold time of 10 min for each stage. All spectrophotometric analyses were performed using a SpectrAA 240FS spectrophotometer (Varian Inc., Mulgrave, Australia). Measurements were carried out at the following wavelengths: Cd—228.8 nm, Co—240.7 nm, Cr—357.9 nm, Cu—324.7 nm, Fe—248.3 nm, Mn—279.5 nm, Ni—232.0 nm, Pb—217.0 nm, Zn—213.9 nm. The calibration curves were prepared based on certified reference standards from Fluka (Charlotte, NC, USA): Cd 51994, Pb 16595, Cr 02733, Ni 42242, Zn 188227, Cu 38996, Mn 63534, Fe 16596 and Co 119785.0100. The determination ranges for individual elements were as follows: Cd: 3–1000 µg cm3, Co: 15–30,000 µg cm3, Cr: 15–6000 µg cm3, Cu: 10–2000 µg cm3, Fe: 15–3200 µg cm3, Mn: 5–14,000 µg cm3, Ni: 20–8000 µg cm3, Pb: 30–8000 µg cm3, Zn: 2–14,000 µg cm3. The correlation coefficients of the calibration curves (R2) were ≥0.995 for all elements determined. The limits of detection (LOD) were: Cd: 0.02 µg cm3, Co: 0.05 µg cm3, Cr: 0.06 µg cm3, Cu: 0.03 µg cm3, Fe: 0.06 µg cm3, Mn: 0.02 µg cm3, Ni: 0.1 µg cm3, Pb: 0.1 µg cm3, Zn: 0.01 µg cm3. The limits of quantification (LOQ) are presented above as the lower limits of the calibration ranges. Quality control was ensured by using certified reference material NCS ZC 73,030 (Chinese National Analysis Centre for Iron and Steel, Beijing, China). All samples were analysed in three analytical replicates. In addition, the accuracy of the determinations was verified at regular intervals by re-analysing standard solutions of known concentration, achieving a compliance of 95–105% of the nominal value. Before the experiment began, the soil was dried and sieved, after which its basic properties were determined [63,64,65,66,67,68]. The properties of the soil were determined using classic methods: pH was determined potentiometrically [64]; hydrolytic acidity (HAC) and cation exchange capacity (CEC) were determined using the Kappen method [65]; organic carbon content (TOC) was determined using a TOC analyser (Shimadzu TOC-L CSH/CNS, Shimadzu Corporation, Kyoto, Japan) [66]; the content of available phosphorus and potassium was determined using the Egner-Riehm method [67]; and the content of available magnesium was determined using the Schachtschabel method [68]. In the Kappen method, calcium acetate (0.5 mol dm−3) acts as the hydrolysing agent. This involved shaking the soil sample for 60 min on a rotary mixer. In the Egner-Riehm method, calcium lactate (CH3CHOHCOO)2Ca, acidified with hydrochloric acid to a pH of 3.55 (±0.05), was used for extraction. The soil sample and calcium lactate solution were mixed at a ratio of 1:20 and shaken on a rotary mixer for 90 min.
2.3. Statistical Methods
The results obtained in the studies were analysed statistically using Statistica 13.3 [69]. The Statistica package was used to perform a two-factor analysis of variance (ANOVA), Tukey’s honest significance difference (HSD) test and a principal component analysis (PCA). The percentage of observed variance was calculated using the two-factor ANOVA method’s eta-squared (η2) for each factor studied (petrol contamination, applied materials, and their interaction), which estimates the proportion of total variance attributable to each independent variable. A significance level of p ≤ 0.01 was used in the statistical calculations.
3. Results
3.1. Trace Elements in Plants
Oat yield depended on the level of petrol contamination in the soil and the application of neutralising materials (Table 2). In the series without additives, petrol contamination resulted in a maximum reduction of 44% in the dry matter yield of the oat plants’ above-ground parts, compared to the uncontaminated control. The used materials had a positive effect on oat yield, limiting the negative impact of the petrol contamination. Calcium oxide was the most effective, while bentonite was the least effective. Compared to the series without additives, the average oat yield in the bentonite, compost, and calcium oxide treatments was 4%, 11%, and 19% higher, respectively.

Table 2.
Dry matter yield of above-ground parts of oats—Avena sativa L. (mg kg−1 dry matter—d.m.).
Petrol contamination of the soil had a significant impact on the trace element content of the above-ground parts of oats (Table 3 and Table 4). For most trace elements, an increase in their content in plants was observed with increasing doses of petrol. In the series in which materials were not used to limit the impact of petrol on plants, soil contaminated with the highest dose (10 cm3 kg−1) showed increases in cadmium content of 36%, nickel content of 51%, iron content of 70%, cobalt content of 95%, chromium content of 109%, and manganese content of 175%, compared to the control object (uncontaminated soil). Zinc and lead content also increased by up to 34% and 65%, respectively, but only up to an average level of soil contamination with petrol (5 cm3 kg−1). The highest petrol dose caused a decrease in the content of these elements in the above-ground parts of oats.

Table 3.
Content of cadmium, lead, chromium, nickel and zinc in above-ground parts of oats—Avena sativa L. (mg kg−1 dry matter—d.m.).

Table 4.
Content of copper, manganese, iron and cobalt in above-ground parts of oats—Avena sativa L. (mg kg−1 dry matter—d.m.).
It is likely that at lower doses, petrol acted as an organic solvent, causing partial decomposition of organic matter and releasing previously bound Pb and Zn cations into the soil solution. Additionally, under conditions of lower contamination, petrol could have been used by soil microorganisms as a source of carbon and energy, increasing their metabolic activity and production of organic acids. These metabolites lower the pH of the soil, resulting in reduced adsorption of Pb and Zn on soil particles and their increased availability to oat roots. However, high doses of petrol limited the growth of oat roots, thereby reducing the total uptake of these elements. The opposite relationship was observed for copper, with accumulation in the above-ground parts of oats on petrol-contaminated soil decreasing by up to 55% compared to the control.
The application of compost and mineral materials (bentonite and calcium oxide) to the soil had a significant effect on the trace element content of the plants (Table 3 and Table 4). A reduction in the content of chromium, iron, cadmium and manganese was observed in the above-ground parts of oats when all materials were used, compared to the control series (without materials). All materials had a similar effect on cadmium content, reducing it in plants by an average of 40–43%. The effect on the other aforementioned elements was more varied: the reduction in iron content in the above-ground parts of oats ranged from 22% (bentonite) to 35% (compost); the reduction in chromium content ranged from 17% (bentonite) to 46% (compost); and the reduction in manganese content ranged from 43% (bentonite) to 55% (calcium oxide). Compost had a similar effect on lead content, bentonite on cobalt accumulation and calcium oxide on copper and cobalt content in plants. On average, the reduction in the content of these trace elements in the above-ground parts of oats was 21% (compost), 52% (bentonite), and 47% and 52% (calcium oxide), respectively. In the remaining cases, opposite relationships were observed: all materials caused an increase in nickel and zinc content; compost caused an additional accumulation of copper and cobalt; bentonite caused an increase in lead and copper content; and calcium oxide caused an additional accumulation of lead in the above-ground parts of oats compared to the control series. Bentonite and calcium oxide had a particularly significant impact on the nickel content of the plants. Following their interaction, the nickel content of the above-ground parts of oats increased by 109% and 160%, respectively, compared to the control series (without materials).
3.2. Relations Between Variables
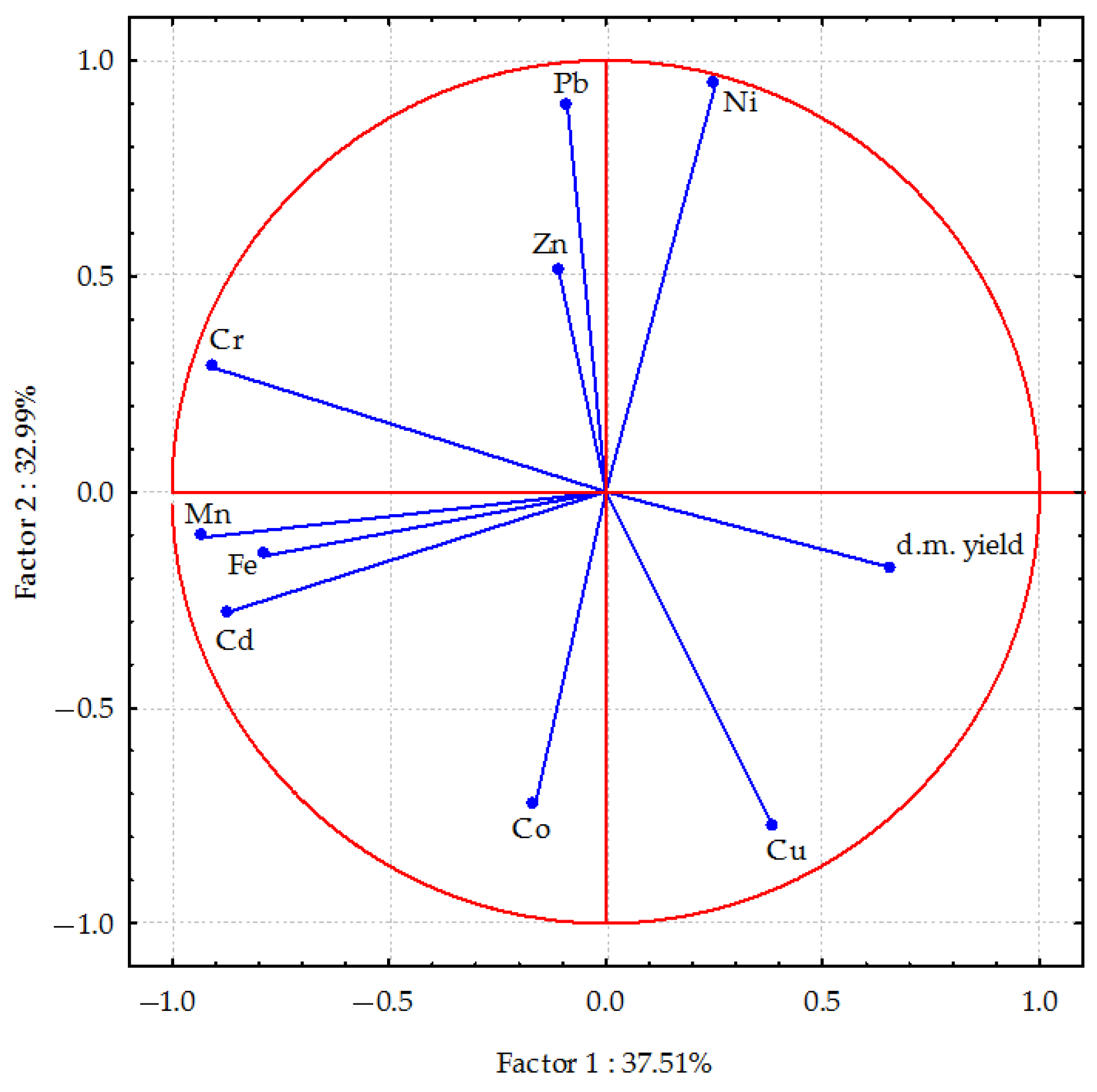
Figure 2 shows the relationships between the trace elements found in oats. Factor 1 explains 37.51% of the total variance in the data, meaning it is the most significant source of variation between the data points. Factor 2 accounts for 32.99% of the variance. The distribution of vectors in Figure 2 indicates a strong positive correlation between nickel and zinc content, as well as a negative correlation between cobalt content and factor 1. Conversely, chromium and lead content in oats were positively correlated, while copper and cobalt content were negatively correlated with factor 2. Longer vectors (e.g., Cu, Cd and Pb) indicate a greater contribution to variability in the data, while shorter vectors (e.g., Zn and Co) indicate a smaller impact. A small angle between vectors indicates a strong correlation. Strong positive correlations were found between elements in plants, particularly cadmium with manganese and iron. Positive correlations were also found between chromium and lead, iron and manganese; between nickel and lead. Partial positive correlations were found between nickel and zinc. The correlations between copper and cobalt were also positive, albeit weaker. Conversely, the copper content in plants was negatively correlated with chromium, lead, nickel and, to a lesser extent, zinc. Similarly, cadmium content in plants was negatively correlated with lead and nickel accumulation, and to a lesser extent, zinc. Dry matter yield of oats was also negatively correlated with manganese, chromium, cadmium and iron.
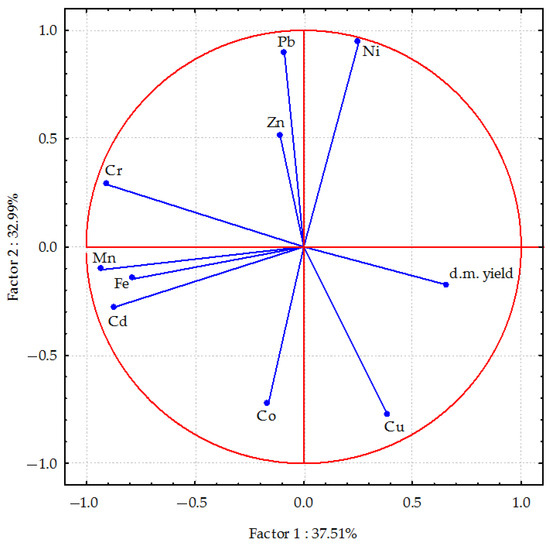
Figure 2.
The relationship between dry matter biomass yield and trace element content in oats (Avena sativa L.) calculated using principal component analysis. d.m. yield—dry matter biomass yield of oats; Cd, Pb, Cr, Ni, Zn, Mn, Fe, Co—content of Cd, Pb, Cr, Ni, Zn, Mn, Fe, Co in above-ground parts of oats.
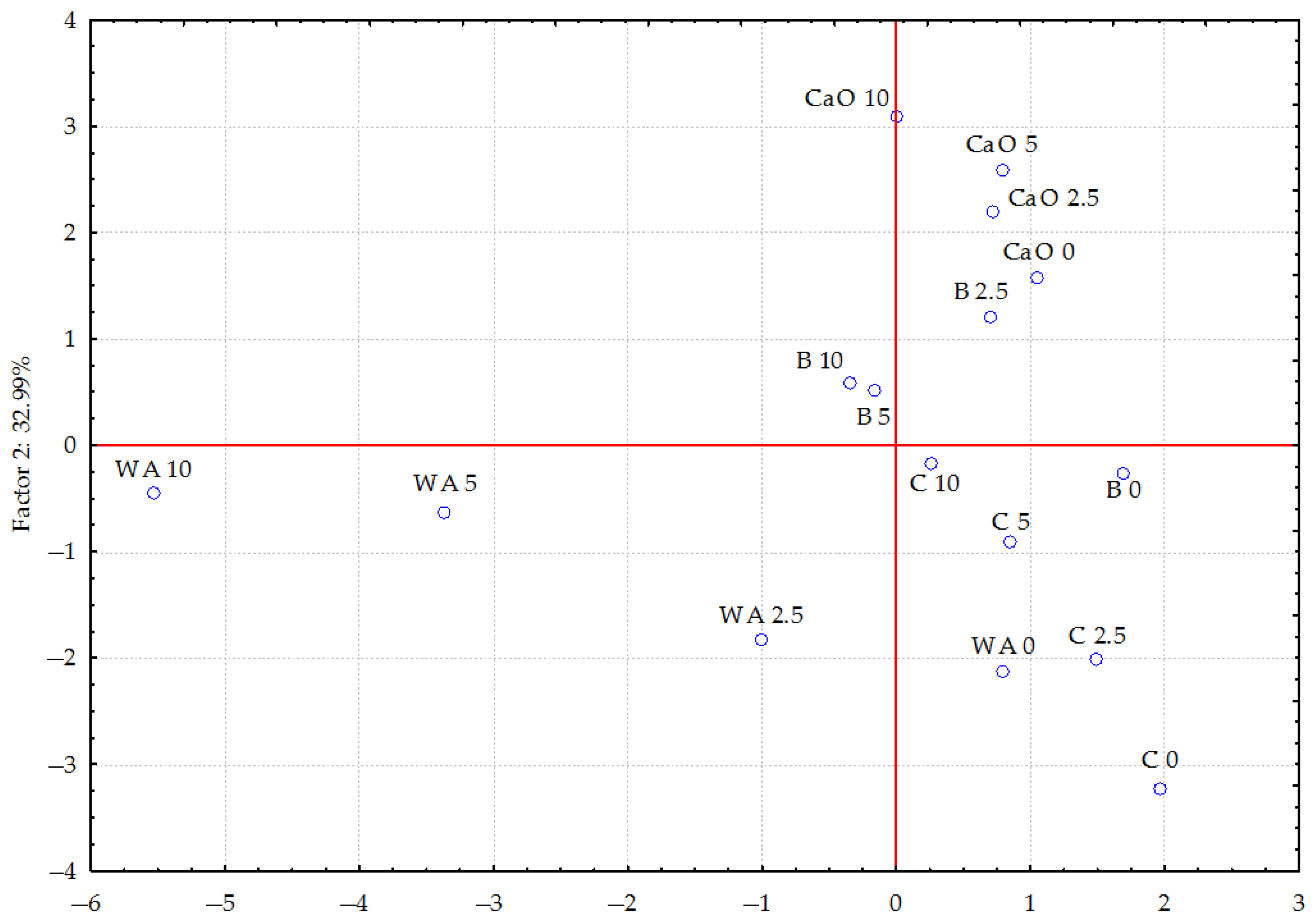
Figure 3 shows that individual doses of petrol without additives have the strongest effect on trace element content in plants compared to other test series in which individual materials were applied. The samples are scattered.
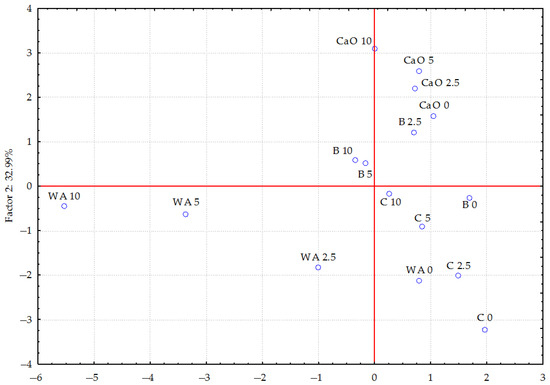
Figure 3.
The influence of petrol and different materials on the trace element content in oats (Avena sativa L.) calculated using principal component analysis. WA—without amendments, C—compost, B—bentonite, and CaO—calcium oxide; 0—0 cm3 (control), 2.5—25 cm3, 5—5 cm3, and 10—10 cm3 petrol per kg of soil.
The samples in the bentonite series are more concentrated. The samples in the compost series are distributed across different quadrants, which could imply that they have different effects on the analysed properties. The position of the samples in the CaO series may indicate specific properties associated with the addition of calcium to the soil. The distribution of samples in the bentonite and CaO series is most similar, which may suggest stabilising properties. The spread of samples in the compost series is greater than in the bentonite and calcium oxide series. The significant distance between the samples in the compost and particularly in the calcium oxide series, compared to the control series (without the addition of materials), suggests that these materials have a greater influence on the trace element content in the above-ground parts of oats more than bentonite does.
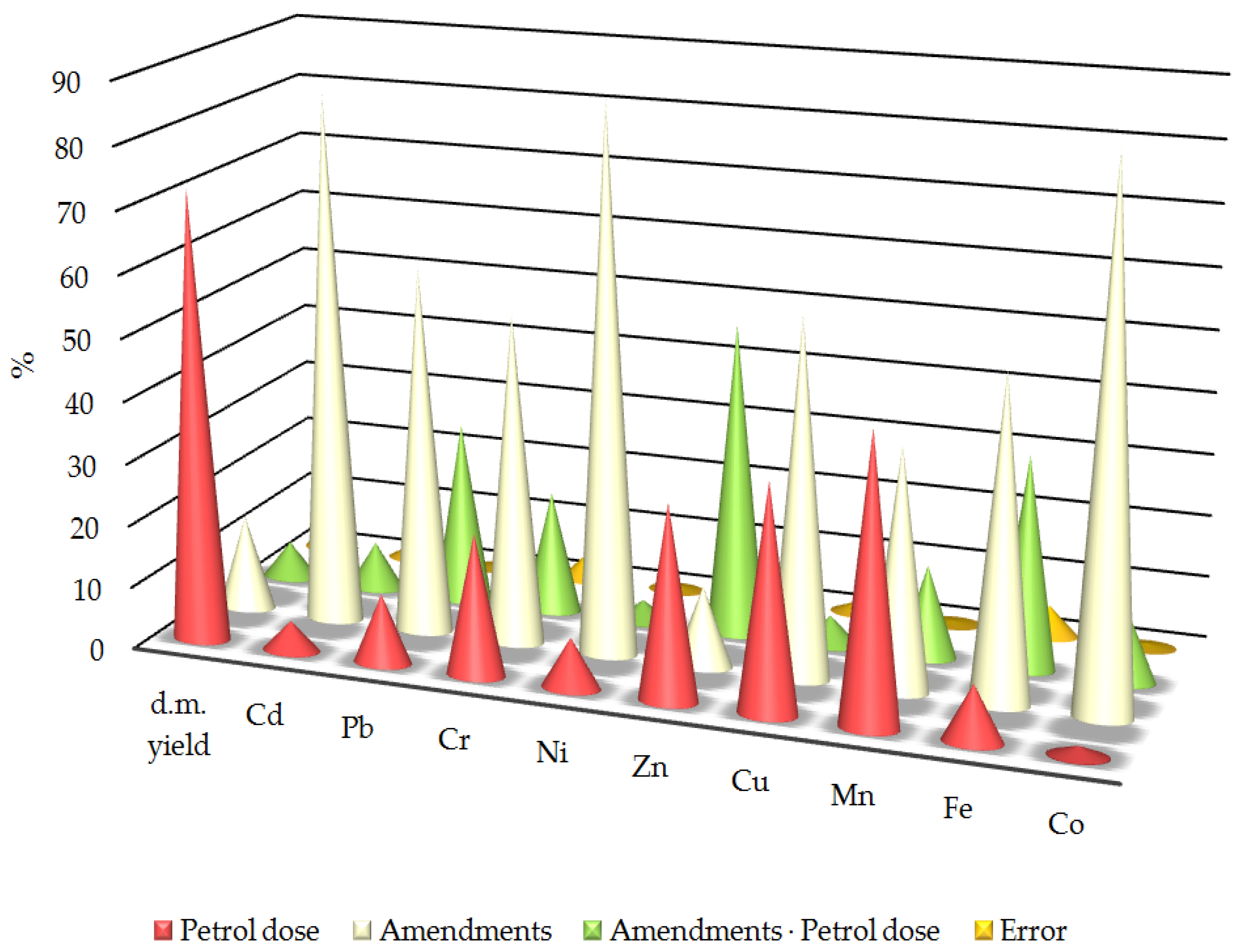
After analysing the observed variability, it was found that the application of materials had a significantly greater impact on the trace element content of oats than soil contamination by petrol (Figure 4). The strongest effects were observed for iron (51.90%), chromium (53.27%), copper (57.31%), lead (58.91%), cadmium (85.21%) and cobalt (86.21%), followed by manganese (38.89%). Conversely, the greatest impact of petrol contamination was only recorded for manganese (45.86%) and dry matter biomass yield (72.61%) with a significant impact also observed for chromium (22.73%), zinc (31.09%) and copper (36.19%). The interaction between the applied materials and the petrol contamination had the greatest effect on the zinc content (50.29%) in the oats. This was also significant for lead (29.38%) and iron (34.25%).
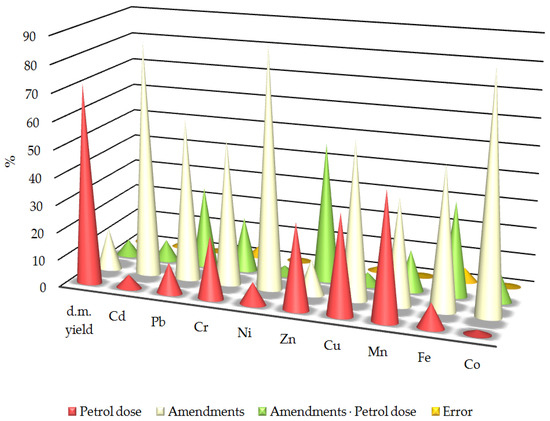
Figure 4.
Relative effect of various factors on trace elements in oats. The figures are shown as percentages. d.m. yield—dry matter yield of oats; Cd, Pb, Cr, Ni, Zn, Mn, Fe, Co—content of Cd, Pb, Cr, Ni, Zn, Mn, Fe, Co in above-ground parts of oats; error—measurement error.
4. Discussion
The obtained research results confirm the complex nature of the interactions within the plant–soil system during the in situ chemical stabilisation of hydrocarbon-contaminated soil. Changes in the content of the analysed elements in the above-ground parts of oats suggest that various mechanisms affect their mobility and availability to plants.
The most significant changes were observed for Cd, Ni, Fe, Co, Cr and Mn. The content of these elements in the above-ground biomass of oats grown in petrol-contaminated soil at a dose of 10 cm3 kg−1 increased considerably compared to the control object. This accumulation can be explained by the increased mobility of these elements under conditions of chemical stress caused by petrol contamination. Literature data [9,70] indicate that petroleum-derived substances can cause soil acidification, leading to increased solubility of trace elements. The activity of microorganisms that decompose hydrocarbons can also lower soil pH, thereby affecting the forms in which trace elements occur, as well as their mobility and availability to plant roots [71]. As soil acidity increases, the sorption of trace element cations decreases due to the dissolution of minerals and hydrated Fe, Mn and Al oxides, to which these cations are bound [72]. Of particular concern from a food safety perspective is the reported 107% increase in Cr content in plants. This element can be toxic at high concentrations, and its accumulation in the edible parts of plants poses a potential threat to human and animal health. The increased uptake of Cr by plants in the presence of petroleum products may be related to changes in soil electrical conductivity [27], redox conditions influenced by petroleum-derived pollutants [73], and the oxidation of Cr(III) to Cr(VI). The latter is characterised by much greater mobility and bioavailability [74], resulting in increased uptake by plant roots. Similar observations were reported by Adesina and Adelasoye [75], who studied the effect of oil contamination on the trace element content of cowpea above-ground parts. Compared to the control (un-contaminated soil), they reported increases in the content of Pb (67.3%), Cd (114.1%), Fe (49.3%), Cr (44.9%), Ni (71.9%), Cu (88.5%) and Zn (440.3%). In this study, the Cu content decreased by 55% in the most contaminated series, while the Pb and Zn contents increased by 34% and 65%, respectively, up to an average level of soil contamination with petrol (5 cm3 kg−1). These differences may be due to variations in the chemical composition of crude oil and petrol, and differences in the absorption mechanisms of cowpeas and oats. In our previous studies [76], increasing doses of petrol resulted in increased accumulation of Pb, Fe, Co, Ni, Cr and Mn in the above-ground biomass of maize. The greatest changes were observed for Ni, Mn and Cr, whose content increased by 62%, 195% and 216%, respectively, compared to the control object. In this study, the accumulation of these elements in the above-ground parts of oats was lower. An increase in Cd content of 36% was observed compared to the uncontaminated control object. These differences between species suggest that there are specific mechanisms for the uptake and transport of trace elements that are characteristic of individual cereals. Differences in the uptake, distribution and accumulation of trace elements between C3 cereal species (such as oats and wheat) and C4 species (such as maize and sorghum) result from variations in root system architecture, root hair density and morphology, transpiration efficiency, and photosynthesis [77]. Rusin et al. [78] confirmed the significant impact of petrol on the chemical composition and trace element content of common wheat. In studies conducted near the Hellenic Petroleum oil refinery in northern Greece, Tsamos et al. [79] showed that long-term exposure of soil to petroleum products leads to significant changes in the trace element profile of vegetables grown in this area. The authors noted particularly high levels of Ni, Mn, and Zn in lettuce leaves. The uptake of trace elements by plants depends on their concentration in the soil and their bioavailability [80]. Oil refinery operations lead to the emission and release of elements such as As, Mn, Ni, Pb, Zn and Cr into the soil, leading to their accumulation and the contamination of ecosystems. According to Tsamos et al. [79], Mn, Ni, Cu and Zn cations are highly bioavailable to plants, while Pb has low bioavailability. This study found that the Ni content in the above-ground biomass of oats increased by 51%, the Cr content by 107%, and the Mn content by 175%. These results confirm the existence of a universal mechanism by which petroleum-derived substances impact the phytoavailability of trace elements. This study investigated the impact of soil contamination with petrol on the Zn and Pb content of oat above-ground biomass. Low and medium doses of petrol (up to 5 cm3 kg−1) resulted in the accumulation of these elements, whereas high doses (10 cm3 kg−1) reduced their content. It is likely that the low and medium doses of petrol had an acidifying effect on the soil, increasing the mobility and availability of Zn and Pb, and thus intensifying their uptake by the plants. Under acidic soil conditions, the concentration of mobile trace elements increases due to the increased solubility of their chemical compounds [72]. In contrast, in the series with the highest level of petrol contamination, the toxic effect of hydrocarbons may have limited root development and root hair density. This constituted a physiological barrier that prevented the effective uptake of trace elements, leading to a reduction in their translocation to shoots. The inhibitory effect of high hydrocarbon concentrations on root system development has been confirmed by Odukoya et al. [81].
Our own research has shown that soil remediation using compost and mineral materials significantly reduces the average content of Cr, Fe, Cd and Mn in the above-ground biomass of oats. The application of compost also reduced the accumulation of Pb in plants. Compost acts by complexing trace elements with humic compounds, reducing their mobile and exchangeable fractions [82]. Similar results were obtained by Walker et al. [83], who observed limited accumulation of Pb, Zn, Fe and Mn in the tissues of Chenopodium album L. after introducing 27 g kg−1 of compost derived from olive leaves and olive mill waste into the soil. Castaldi et al. [84] confirmed the effectiveness of compost in stabilising soil contaminated with trace elements. They showed that adding 10% of this material to the soil reduced the Pb content by 87% and the Zn content by 31% in the above-ground parts of white lupine (Lupinus albus L.), compared to the control. The effect of compost on soil sorption capacity is particularly important [85]. The organic matter (OM) in compost increases the number of binding sites for metal cations as it contains various functional groups, including -COOH and -OH, which can easily bind trace element ions and form strong anti-desorption complexes [86]. Additionally, organic matter (OM) affects soil pH, which directly impacts element speciation. Under conditions of elevated pH, most trace elements occur in poorly soluble forms, such as hydroxides and carbonate complexes, which limits their uptake by plants [87]. In addition to its alkalising effect, compost introduces organic matter with redox properties into the soil. Humic substances contain various functional groups, including quinones, carboxyl groups, sulphydryl groups, and phenol hydroxyl groups [88], which can participate in electron transfer reactions and create microenvironments with an altered redox potential [89,90]. These oxidation-reduction processes can modify the oxidation state of elements, thereby affecting their solubility and bioavailability [91]. Therefore, the observed changes in the accumulation of trace elements in oat biomass in the individual combinations studied may reflect the combined effects of pH regulation, complexation by organic ligands and redox-induced transformations. The OM supplied with compost stimulates the development of beneficial soil microflora, enhancing the biodegradation of pollutants [92] and improving the supply of nutrients to plants [93]. This affects the overall physiological condition of plants and crop quality.
As a clay mineral with high cation exchange capacity and a negative charge, bentonite is an effective sorbent of trace elements in soils affected by industrial pollution [94]. In our own studies, applying it to soil contaminated with the highest dose of petrol (10 cm3 kg−1) reduced the content of Co, Cr, Fe, Cd and Mn in the above-ground biomass of oats compared to the control series (without material). Studies by Klik et al. [95] found that applying bentonite at 3% of the soil mass resulted in a statistically significant reduction in the bioaccumulation of Cr, Cu, Cd, Zn and Pb in the above-ground parts of Lolium perenne L. and Festuca rubra L., while Zhang et al. [96] reported a reduction in the Cd and Pb content of peppers (Capsicum annuum L.) and cabbage (Brassica pekinensis) grown in crop rotation for five years after a single application of 2.5% bentonite to the soil. The researchers confirmed that bentonite promoted the transformation of available Cd and Pb fractions into highly stable forms, resulting in reduced phytoavailability and uptake by plants. The crystal structure of bentonite, characterised by a high degree of dispersion, a large specific surface area, and a high cation exchange capacity (CEC), enables the effective binding of trace elements [94]. This occurs through ion exchange, surface complexation, and electrostatic interactions [97]. Surface complexation involves trace element ions interacting with bentonite’s functional groups (including hydroxyl (–OH) and silanol (Si–OH)), promoting strong adsorption. During ion exchange, the cations of these elements replace the natural interlayer cations in the mineral structure [43,97]. Electrostatic attraction is also important, whereby the negatively charged surface of bentonite attracts positively charged trace element ions [97]. In addition to immobilising contaminants, the presence of bentonite increases the soil’s sorption capacity, improving its structure and water properties [94]. This ensures the long-term stabilisation of the effects of remediation [96].
Calcium oxide (CaO), also known as quicklime, is one of the most effective alkalising additives used in the remediation of contaminated soils. Schifano et al. [98] demonstrated that adding CaO to soil collected from disused petrol stations significantly reduced petroleum hydrocarbon content and leaching, thereby protecting groundwater. According to these authors, the increase in temperature resulting from the exothermic reaction of quicklime hydration with pore water causes some light petroleum compounds to evaporate, while less volatile aliphatic and aromatic compounds undergo degradation and encapsulation under these conditions. In the case of trace elements, the stabilising effect of liming is primarily based on neutralising soil acidity and increasing pH [99]. Under alkaline pH conditions, trace elements become immobilised through the formation of poorly soluble carbonates, hydroxides, phosphates and oxides. This minimises their mobility and phytoavailability [37]. Additionally, the alkaline nature of calcium compounds decreases the concentration of H+ ions while increasing the number of negatively charged sites on the surface of soil particles. This leads to the increased retention of trace element cations [100]. In this study, applying calcium oxide to areas with the highest levels of petrol contamination reduced the content of Co, Cu, Cr, Fe, Cd and Mn in the above-ground biomass of oats compared to the control object (without addition). Lahori et al. [101] obtained results partially similar results. The authors confirmed the effectiveness of using hydrated lime for in situ soil stabilisation in zinc smelter-contaminated areas. They demonstrated that applying 1% hydrated lime significantly reduced the uptake of trace elements by Chinese cabbage plants. After 45 days, the above-ground parts of the tested plant exhibited reduced accumulation of Pb (20.9%), Cd (67.8%), Cu (9.7%) and Zn (47.4%) compared to the control (no additive). The differences between our results and those of the cited authors may be due to the species of used test plant, the soil’s granulometric composition, and the level and type of contamination. Castaldi et al. [84] reported reductions in the content of Pb (by 87.0%), Cd (by 33.2%) and Zn (by 37.7%) in the biomass of white lupine grown on contaminated soil after the introduction of Ca(OH)2 (0.05% w/w). The researchers also demonstrated that adding Ca(OH)2 increased above-ground lupine biomass by a factor of 3.1 compared to the control. By influencing soil properties, including pH and CEC, calcium oxide creates favourable conditions for the development of microorganisms that degrade anthropogenic pollutants [102] and improves the availability of certain nutrients to plants [103]. This directly affects crop yield quality.
Using the tested sorption materials did not clearly reduce the bioaccumulation of any of the analysed trace elements. An increase in the content of Ni, Zn, Cu and Co (compost), Pb and Cu (bentonite) and Pb (CaO) was observed in the above-ground parts of oats compared to the control. The soil additives introduced have varying affinities for different trace elements [104,105]. This can lead to certain elements being preferentially bound by sorption centres and other ions being displaced from the soil sorption complex. This increases their mobility and availability to the plant root system [16]. This process, known as competitive sorption, is particularly important in soils with complex contamination, where competition for sorption sites determines the fractionation and mobility of trace elements [106]. Changes in the bioaccumulation of the analysed elements may also have resulted from modifications to soil pH induced by the remediation materials used. While an increase in pH generally promotes the immobilisation of trace element cations [107], under alkaline conditions amphoteric elements (e.g., Pb and Cu) [108] can form soluble hydroxyl complexes or oxyanions, thereby increasing their mobility [109]. Furthermore, petroleum-derived hydrocarbons bind with soil organic matter, affecting its structure and disrupting its sorption properties [110], which determines the forms in which trace elements occur. Another possible reason for the increased content of certain trace elements in the above-ground biomass of oats is the formation of soluble complexes with humic substances and petroleum degradation products. This increases the mobility and availability of these elements to the oat root system [106,111,112].
Areas contaminated with petroleum-derived substances have a lower self-cleaning capacity, mainly due to the toxic effect of hydrocarbons on native microorganisms that can degrade pollutants [113]. The in situ stabilisation methods tested under pot experiment conditions in this study, using natural and inexpensive sorption materials, have shown potential as effective technological solutions for areas contaminated with petroleum products while maintaining soil fertility. Our research results contribute to a better understanding of the impact of petroleum-derived substances on soil chemistry changes and trace element bioaccumulation in vegetation in anthropogenically contaminated areas. A clear advantage of the pot experiment is that it allows for the homogenisation of the soil and the control of environmental parameters. However, the small soil volume (9 kg) in the pot may lead to faster petroleum volatilisation than in field conditions, as well as limiting the space available for root growth. This could result in an overestimation of the remediation effectiveness of the materials used. For this reason, before making final recommendations on agricultural practices, field studies must be conducted. These studies should include: verification of the effectiveness of methods using other crop species and species that spontaneously colonise degraded areas; assessment of their effectiveness at higher levels of petrol contamination and in the presence of other petroleum-derived substances; tests on different soil types; long-term field experiments that take into account variability in environmental conditions and the full plant development cycle.
This study expands our knowledge of the impact of petrol contamination on the chemical composition of crops by analysing oat-specific responses. The different pattern of trace element uptake observed in oats in our study, under conditions of soil contamination and the application of organic and inorganic sorbents, differs from that observed in other crops such as maize due to oats’ different physiological characteristics as a C3 cereal. Furthermore, this study directly compares the effectiveness of organic and inorganic sorbents characterised by different trace element immobilisation mechanisms under identical soil and controlled environmental conditions.
5. Conclusions
The yield and concentration of trace elements in the above-ground parts of oats were influenced by both petrol contamination and the application of compost and mineral materials (such as bentonite and calcium oxide) to the soil. Petrol contamination significantly impacted the biomass and the trace element content of the plants. It decreased the dry matter yield and an increased the content of most trace elements in the above-ground parts of oats. The most striking effects were observed for cadmium, nickel, iron, cobalt, chromium and manganese, with levels increasing across the entire range of petrol doses. Petrol had a similar effect on zinc and lead levels in oat above-ground parts, but only up to a medium level of soil contamination (5 cm3 kg−1). In contrast to the aforementioned elements, petrol contamination of the soil contributed to a decrease in copper content in the above-ground parts of oats.
The materials applied to the soil had a beneficial effect on the biomass and the concentration of certain trace elements in plants. Compost and calcium oxide in particular had a positive influence on plant yield. Compared to the series without their application to the soil, all materials reduced the content of chromium, iron, cadmium and, in particular, manganese in the above-ground parts of plants. Compost also reduced the levels of lead, bentonite and calcium oxide—cobalt, while calcium oxide reduced the levels of copper in the above-ground parts of oats. However, bentonite had a weaker effect than that of compost and calcium oxide. Changes in the content of other elements in plants after the application of these materials were often opposite (and dependent on the type of material), with the clearest effect being on nickel content. These results suggest that the tested in situ stabilisation methods have potential, but stabilisation techniques must be selected on a case-by-case basis and comprehensive multi-element monitoring must be conducted in field conditions.
In pot experiments, the used sorption materials (compost, bentonite and calcium oxide) had a beneficial effect on reducing the bioaccumulation of certain trace elements in the above-ground parts of oats grown on soil contaminated with low levels of petrol (2.5, 5 and 10 cm3 kg−1 of soil; harvested on day 68). These results provide a basis for comprehensive field studies to fully verify the effectiveness of the tested stabilisation methods in remediating soils contaminated with petroleum products. Further research is required to investigate the long-term effectiveness of the stabilisation methods used, as well as the dynamics of changes in bioaccumulation of trace elements over time. This should include measurements at various stages of the test plant’s development and monitoring of changes in soil properties and element mobility over time. Testing should also be conducted at higher levels of petrol contamination.
Author Contributions
Conceptualisation, M.W.; methodology, M.W.; validation, M.W.; formal analysis, M.W. and N.K.; investigation, N.K.; resources, M.W.; data curation, M.W.; writing—original draft preparation, M.W. and N.K.; writing—review and editing, M.W. and N.K.; visualisation, M.W. and N.K.; supervision, M.W.; project administration, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the Department of Agricultural and Environmental Chemistry, Faculty of Agriculture and Forestry, University of Warmia and Mazury in Olsztyn (grant No. 30.610.004-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Adipah, S. Introduction of petroleum hydrocarbons contaminants and its human effects. J. Environ. Sci. Public Health 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 62813. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef] [PubMed]
- Ghahri, A.; Seydi, P.; Ranjbar, A.; Hatami, H.; Beheshti, T.; Seydi, E. Evaluation of exposure to volatile organic compounds (BTEX) and Polycyclic Aromatic Hydrocarbons (PAHs) in gas station workers and oxidative stress assessment in Karaj City. Toxicol. Rep. 2024, 13, 101767. [Google Scholar] [CrossRef] [PubMed]
- Haghollahi, A.; Fazaelipoor, M.H.; Schaffie, M. The effect of soil type on the bioremediation of petroleum contaminated soils. J. Environ. Manag. 2016, 180, 197–201. [Google Scholar] [CrossRef]
- Das, N.; Bhuyan, B.; Pandey, P. Correlation of soil microbiome with crude oil contamination drives detection of hydrocarbon degrading genes which are independent to quantity and type of contaminants. Environ. Res. 2022, 215, 114185. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Gao, H.; Wu, M.; Liu, H.; Xu, Y.; Liu, Z. Effect of petroleum hydrocarbon pollution levels on the soil microecosystem and ecological function. Environ. Pollut. 2022, 293, 118511. [Google Scholar] [CrossRef]
- Zahermand, S.; Vafaeian, M.; Bazyar, M.H. Analysis of the physical and chemical properties of soil contaminated with oil (petroleum) hydrocarbons. Earth Sci. Res. J. 2020, 24, 163–168. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar. RSC Adv. 2019, 9, 35304–35311. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Regulation of the microbiome in soil contaminated with diesel oil and gasoline. Int. J. Mol. Sci. 2025, 26, 6491. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of soil from petroleum contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Trace element contents in petrol-contaminated soil following the application of compost and mineral materials. Materials 2022, 15, 5233. [Google Scholar] [CrossRef]
- Gospodarek, J.; Rusin, M.; Kandziora-Ciupa, M.; Nadgórska-Socha, A. The subsequent effects of soil pollution by petroleum products and its bioremediation on the antioxidant response and content of elements in Vicia faba plants. Energies 2021, 14, 7748. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Cheraghi, M.; Sobhan, A.S.; Lorestani, B.; Merrikhpour, H.; Parvizimosaed, H. Biochemical and physical characterization of petroleum hydrocarbon contaminated soils in Tehran. J. Chem. Health Risks 2015, 5, 199–208. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A. Assessment of heavy metal speciation in soils impacted with crude oil in the Niger Delta, Nigeria. Chem. Speciat. Bioavailab. 2011, 23, 7–15. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, Y.; Li, D. Germination of grass species in soil affected by crude oil contamination. Int. J. Phytorem. 2018, 20, 567–573. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Revitalization of soil contaminated by petroleum products using materials that improve the physicochemical and biochemical properties of the soil. Molecules 2024, 29, 5838. [Google Scholar] [CrossRef]
- Ekundayo, E.; Emede, T.; Osayande, D. Effects of crude oil spillage on growth and yield of maize (Zea mays L.) in soils of midwestern Nigeria. Plant Foods Hum. Nutr. 2001, 56, 313–324. [Google Scholar] [CrossRef]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczak, A.; Dybowski, M.; Jośko, I.; Kusiak, M.; Sikora, M.; Czech, B. The antioxidant defense responses of Hordeum vulgare L. to polycyclic aromatic hydrocarbons and their derivatives in biochar-amended soil. Environ. Pollut. 2022, 294, 118664. [Google Scholar] [CrossRef] [PubMed]
- Olaranont, Y.; Stewart, A.B.; Traiperm, P. Physiological and anatomical responses of a common beach grass to crude oil pollution. Environ. Sci. Pollut. Res. 2018, 25, 28075–28085. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Małuszyńska, I.; Małuszyński, M.J.; Pawluśkiewicz, B.; Gnatowski, T.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M. Photosynthetic efficiency of plants as an indicator of tolerance to petroleum-contaminated soils. Sustainability 2024, 16, 10811. [Google Scholar] [CrossRef]
- da Silva Correa, H.; Blum, C.T.; Galvão, F.; Maranho, L.T. Effects of oil contamination on plant growth and development: A review. Environ. Sci. Pollut. Res. 2022, 29, 43501–43515. [Google Scholar] [CrossRef]
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The impact of soil pH on heavy metals uptake and photosynthesis efficiency in Melissa officinalis, Taraxacum officinalis, Ocimum basilicum. Molecules 2022, 27, 4671. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Importance of compost, bentonite, and calcium oxide in reducing trace element content in maize on agricultural soil contaminated with diesel oil. Agriculture 2023, 13, 1948. [Google Scholar] [CrossRef]
- Gospodarek, J.; Rusin, M.; Nadgórska-Socha, A. The long-term effect of petroleum-derived substances and their bioremediation on the host plant (Vicia faba L.) and a herbivore (Sitona spp.). Agronomy 2020, 10, 1066. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment. Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, F.; Xing, X.; Peng, J.; Wang, J.; Ji, M.; Li, B.L. A review on remediation technology and the remediation evaluation of heavy metal-contaminated soils. Toxics 2024, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Rinklebe, J.; Selim, M. Impact of various amendments on immobilization and phytoavailability of nickel and zinc in a contaminated floodplain soil. Int. J. Environ. Sci. Technol. 2015, 12, 2765–2776. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef]
- Vouillamoz, J.; Milke, M.W. Effect of compost in phytoremediation of diesel-contaminated soils. Water Sci. Technol. 2001, 43, 291–295. [Google Scholar] [CrossRef]
- Henderson, S.C.; Dhar, A.; Naeth, M.A. Reclamation of hydrocarbon contaminated soils using soil amendments and native plant species. Resources 2023, 12, 130. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Muslim, W.A.; Al-Nasri, S.K.; Albayati, T.M.; Salih, I.K. Investigation of bentonite clay minerals as a natural adsorbents for Cs-137 real radioactive wastewater treatment. Desalin. Water Treat. 2024, 317, 100121. [Google Scholar] [CrossRef]
- Krupskaya, V.; Zakusin, S.; Zakusina, O.; Belousov, P.; Pokidko, B.; Morozov, I.; Zaitseva, T.; Tyupina, E.; Koroleva, T. On the question of finding relationship between structural features of smectites and adsorption and surface properties of bentonites. Minerals 2025, 15, 30. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Kumararaja, P.; Shabeer, T.A.; Manjaiah, K.M. Effect of bentonite on heavy metal uptake by amaranth (Amaranthus blitum cv. Pusa Kirti) grown on metal contaminated soil. Horti. Soc. Ind. 2016, 73, 224–228. [Google Scholar] [CrossRef]
- Qv, M.; Bao, J.; Wang, W.; Dai, D.; Wu, Q.; Li, S.; Zhu, L. Bentonite addition enhances the biodegradation of petroleum pollutants and bacterial community succession during the aerobic co-composting of waste heavy oil with agricultural wastes. J. Hazard. Mater. 2024, 462, 132655. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Xie, H. Preparation, characterization and intercalation mechanism of bentonite modified with different organic ammonium. Chem. Eng. Sci. 2025, 301, 120758. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Gan, X.; Zhang, M.; Xue, M.; Li, J. Comparative experimental study on calcium oxide, calcium hydroxide, and calcium carbonate solidified zinc-contaminated Red Clay. Geofluids 2022, 2022, 8428982. [Google Scholar] [CrossRef]
- Becerra-Agudelo, E.; López, J.E.; Betancur-García, H.; Carbal-Guerra, J.; Torres-Hernández, M.; Saldarriaga, J.F. Assessment of the application of two amendments (lime and biochar) on the acidification and bioavailability of Ni in a Ni-contaminated agricultural soils of northern Colombia. Heliyon 2022, 8, e10221. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar remediation of soil: Linking biochar production with function in heavy metal contaminated soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Gascó, G.; Álvarez, M.L.; Paz-Ferreiro, J.; Méndez, A. Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Riotinto (Spain). Chemosphere 2019, 231, 562–570. [Google Scholar] [CrossRef]
- Mazzurco-Miritana, V.; Passatore, L.; Zacchini, M.; Pietrini, F.; Peruzzi, E.; Carloni, S.; Rolando, L.; Garbini, G.L.; Caracciolo, A.B.; Silvani, V.; et al. Promoting the remediation of contaminated soils using biochar in combination with bioaugmentation and phytoremediation techniques. Sci. Rep. 2025, 15, 11231. [Google Scholar] [CrossRef]
- Viana, R.S.R.; Chagas, J.K.M.; Paz-Ferreiro, J.; de Figueiredo, C.C. Enhanced remediation of heavy metal-contaminated soils using biochar and zeolite combinations with additives: A meta-analysis. Environ. Pollut. 2024, 367, 125617. [Google Scholar] [CrossRef]
- Xia, M.; Xu, K.; Zhang, S.; Zhang, C.; Wang, X.; Li, J. Insights into the low-temperature rapid catalytic pyrolysis and remediation mechanism of weathered petroleum-contaminated saline-alkali soil using Beta zeolite. Environ. Res. 2024, 263, 120266. [Google Scholar] [CrossRef]
- Radziemska, M.; Mazur, Z. Content of selected heavy metals in Ni-contaminated soil following the application of halloysite and zeolite. J. Ecol. Eng. 2016, 17, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Volatile Organic Compounds (VOCs) emitted from petroleum and their influence on photochemical smog formation in the atmosphere. J. Pet. Environ. Biotechnol. 2012, 3, e104. [Google Scholar] [CrossRef]
- Yanxun, S.; Yani, W.; Hui, Q.; Yuan, F. Analysis of the groundwater and soil pollution by oil leakage. Procedia Environ. Sci. 2011, 11, 939–944. [Google Scholar] [CrossRef]
- Montaño-López, F.; Biswas, A. Are heavy metals in urban garden soils linked to vulnerable populations? A case study from Guelph, Canada. Sci. Rep. 2021, 11, 11286. [Google Scholar] [CrossRef]
- Oloruntoba, A.; Omoniyi, A.O.; Shittu, Z.A.; Ajala, R.O.; Kolawole, S.A. Heavy metal contamination in soils, water, and food in Nigeria from 2000–2019: A systematic review on methods, pollution level and policy implications. Water Air Soil Poll. 2024, 235, 586. [Google Scholar] [CrossRef]
- Panagos, P.; Jones, A.; Lugato, E.; Ballabio, C. A Soil monitoring law for Europe. Glob. Chall. 2025, 9, 2400336. [Google Scholar] [CrossRef]
- Akasa, L.U. Trends of oat production area, productivity and utilization status in Ethiopia. Am. J. Biosci. Bioinforma. 2023, 2, 14–19. [Google Scholar] [CrossRef]
- Tobiasz-Salach, R.; Stadnik, B.; Bajcar, M. Oat as a potential source of energy. Energies 2023, 16, 6019. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; p. 236. Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 10 June 2025).
- Characteristics of 95 Unleaded Gasoline. 2025. Available online: https://www.orlen.pl/en/for-business/products/fuels/petrol/unleaded-95 (accessed on 22 October 2025).
- Wyszkowski, M.; Kordala, N. Mineral and organic materials as factors reducing the effect of petrol on heavy metal content in soil. Materials 2024, 17, 3528. [Google Scholar] [CrossRef]
- US-EPA Method 3051A. Microwave Assisted Acid Digestion of Sediment, Sludges, Soils, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–30. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 10 June 2025).
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 1224. [Google Scholar]
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Jaremko, D.; Kalembasa, D. A comparison of methods for the determination of cation exchange capacity of soils. Ecol. Chem. Eng. 2014, 21, 487–498. [Google Scholar] [CrossRef]
- Shimadzu Corporation. Shimadzu Analytical and Measuring Instruments. In User’s Manual; Shimadzu Corporation: Kyoto, Japan, 2016. [Google Scholar]
- Korzeniowska, J.; Stanisławska-Glubiak, E. Evaluation of the Egner–Riehm DL and Mehlich 3 tests for the determination of phosphorus: The influence of soil properties on extraction efficiency and test conversion. Agronomy 2024, 14, 2921. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Oliveira, V.S.; Marchiori, J.J.P.; Ferreira, T.C.; Bernabé, A.C.B.; Boone, G.T.F.; Pereira, L.L.S.; Carriço, E. The nutrient magnesium in soil and plant: A review. Int. J. Plant Soil Sci. 2023, 35, 136–144. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), version 13.3; TIBCO Software: Palo Alto, CA, USA, 2017. [Google Scholar]
- Elgazali, A.; Althalb, H.; Elmusrati, I.; Ahmed, H.M.; Banat, I.M. Remediation approaches to reduce hydrocarbon contamination in petroleum-polluted soil. Microorganisms 2023, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse soil pollution from agriculture: Impacts and remediation. Sci. Total Environ. 2025, 962, 178398. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Arroyo, S.; Rosano-Ortega, G.; Martínez-Gallegos, S.; Pérez-Armendariz, B.; Vega-Lebrún, C.A. Reduction of hydrocarbons in contaminated soil through paired sorption and advanced oxidation processes. Soil Secur. 2021, 4, 100013. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Adesina, G.; Adelasoye, K. Effect of crude oil pollution on heavy metal contents, microbial population in soil, and maize and cowpea growth. Agric. Sci. 2014, 5, 43–50. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Role of different material amendments in shaping the content of heavy metals in maize (Zea mays L.) on soil polluted with petrol. Materials 2022, 15, 2623. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z. Systematic comparison of C3 and C4 plants based on metabolic network analysis. BMC Syst. Biol. 2012, 6 (Suppl. S2), S9. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Barczyk, G.; Nadgórska-Socha, A. Antioxidant responses of Triticum aestivum plants to petroleum-derived substances. Ecotoxicology 2018, 27, 1353–1367. [Google Scholar] [CrossRef]
- Tsamos, P.; Stefanou, S.; Noli, F. Assessment of distribution of heavy metals and radionuclides in soil and plants nearby an oil refinery in northern Greece. Case Stud. Chem. Environ. Eng. 2024, 9, 100593. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Odukoya, J.; Lambert, R.; Sakrabani, R. Impact of crude oil on yield and phytochemical composition of selected green leafy vegetables. Int. J. Veg. Sci. 2019, 25, 554–570. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D. Behaviors of heavy metals (Cd, Cu, Ni, Pb and Zn) in soil amended with composts. Environ. Technol. 2016, 37, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Melis, P. Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 2005, 60, 365–371. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the influence of compost and biochar amendments on the mobility and uptake of heavy metals by green leafy vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Shrestha, P.; Bellitürk, K.; Görres, J.H. Phytoremediation of heavy metal-contaminated soil by switchgrass: A comparative study utilizing different composts and coir fiber on pollution remediation, plant productivity, and nutrient leaching. Int. J. Environ. Res. Public Health 2019, 16, 1261. [Google Scholar] [CrossRef]
- Sun, R.; Fu, M.; Ma, L.; Zhou, Y.; Li, Q. Iron reduction in composting environment synergized with quinone redox cycling drives humification and free radical production from humic substances. Bioresour. Technol. 2023, 384, 129341. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a Soil Amendment to Remediate Heavy Metal-Contaminated Agricultural Soil: Mechanisms, Efficacy, Problems, and Strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Cui, D.; Tan, W.; Yue, D.; Yu, H.; Dang, Q.; Xi, B. Reduction capacity of humic acid and its association with the evolution of redox structures during composting. Waste Manag. 2022, 153, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gai, S.; Cheng, K.; Yang, F. Roles of humic substances redox activity on environmental remediation. J. Hazard. Mater. 2022, 435, 129070. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, X.; Wu, J.; Chen, K. Effect of compost amendment and bioaugmentation on PAH degradation and microbial community shifting in petroleum-contaminated soil. Chemosphere 2020, 256, 126998. [Google Scholar] [CrossRef]
- Wasilkowski, D.; Nowak, A.; Michalska, J.; Mrozik, A. Ecological restoration of heavy metal-contaminated soil using Na-bentonite and green compost coupled with the cultivation of the grass Festuca arundinacea. Ecol. Eng. 2019, 138, 420–433. [Google Scholar] [CrossRef]
- Nie, X.; Huang, X.; Li, M.; Lu, Z.; Ling, X. Advances in soil amendments for remediation of heavy metal-contaminated soils: Mechanisms, impact, and future prospects. Toxics 2024, 12, 872. [Google Scholar] [CrossRef]
- Klik, B.; Holatko, J.; Jaskulska, I.; Gusiatin, M.Z.; Hammerschmiedt, T.; Brtnicky, M.; Liniauskienė, E.; Baltazar, T.; Jaskulski, D.; Kintl, A.; et al. Bentonite as a functional material enhancing phytostabilization of post-industrial contaminated soils with heavy metals. Materials 2022, 15, 8331. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, A.; Li, T.; Wu, X.; Liu, Y.; Naidu, R. Immobilization of Cd and Pb in a contaminated acidic soil amended with hydroxyapatite, bentonite, and biochar. J. Soils Sediments 2021, 21, 2262–2272. [Google Scholar] [CrossRef]
- Eleraky, M.I.; Razek, T.M.A.; Hasani, I.W.; Fahim, Y.A. Adsorptive removal of lead, copper, and nickel using natural and activated Egyptian calcium bentonite clay. Sci. Rep. 2025, 15, 13050. [Google Scholar] [CrossRef]
- Schifano, V.; MacLeod, C.; Hadlow, N.; Dudeney, R. Evaluation of quicklime mixing for the remediation of petroleum contaminated soils. J. Hazard. Mater. 2007, 141, 395–409. [Google Scholar] [CrossRef]
- Lim, J.E.; Ahmad, M.; Usman, A.R.A.; Lee, S.S.; Jeon, W.-T.; Oh, S.-E.; Yang, J.W.; Ok, Y.S. Effects of natural and calcined poultry waste on Cd, Pb and As mobility in contaminated soil. Environ. Earth Sci. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, H.; Yan, H.; Li, Y.; Diao, Z.; Zhang, E.; Li, C.; Cao, Y. Study on the treatment of nickel-contaminated soil using calcium oxide. Water Air Soil Pollut. 2020, 231, 188. [Google Scholar] [CrossRef]
- Lahori, A.H.; Zhang, Z.; Guo, Z.; Mahar, A.; Li, R.; Awasthi, M.K.; Sial, T.A.; Kumbhar, F.; Wang, P.; Shen, F.; et al. Potential use of lime combined with additives on (im)mobilization and phytoavailability of heavy metals from Pb/Zn smelter contaminated soils. Ecotoxicol. Environ. Saf. 2017, 145, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, Y.; Liang, P.; Song, Q.; Zhang, H.; Wu, J.; Wu, W.; Liu, X.; Dong, C. Alkaline amendments improve the health of soils degraded by metal contamination and acidification: Crop performance and soil bacterial community responses. Chemosphere 2020, 257, 127309. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Ziółkowska, A. Effect of compost, bentonite and calcium oxide on content of some macroelements in plants from soil contaminated by petrol and diesel oil. J. Elem. 2009, 14, 405–418. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid. Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Liu, X.; Hashmi, M.Z.; Xu, J.; Brookes, P.C. Effects of inorganic and organic amendments on the uptake of lead and trace elements by Brassica chinensis grown in an acidic red soil. Chemosphere 2015, 119, 177–183. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil. Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Cappuyns, V.; Alian, V.; Vassilieva, E.; Swennen, R. pH Dependent leaching behavior of Zn, Cd, Pb, Cu and as from mining wastes and slags: Kinetics and mineralogical control. Waste Biomass Valor. 2014, 5, 355–368. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The potential for restoring the activity of oxidoreductases and hydrolases in soil contaminated with petroleum products using perlite and dolomite. Appl. Sci. 2024, 14, 3591. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Xin, Y.; Huang, T.; Liu, J. Petroleum pollution affects soil chemistry and reshapes the diversity and networks of microbial communities. Ecotoxicol. Environ. Saf. 2022, 246, 114129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).