Abstract
Background: Elite athletes are frequently subjected to high-intensity training regimens, which can result in cumulative physical stress, overtraining, and potential health risks. Monitoring autonomic responses to such load is essential for optimizing performance and preventing maladaptation. Objective: The present study aimed to assess changes in autonomic regulation immediately and two hours after training in athletes, using an integrated framework (combining time- and frequency-domain HRV indices with nonlinear and recurrence quantification analysis). It was investigated how repeated assessments over a 4-month period can reveal cumulative effects and identify athletes at risk. Special attention was paid to identifying signs of excessive fatigue, autonomic imbalance, and cardiovascular stress. Methods: Holter ECGs of 12 athletes (mean age 21 ± 2.22 years; males, athletes participating in competitions) over a 4-month period were recorded before, immediately after, and two hours after high-intensity training, with HRV calculated from 5-min segments. Metrics included HRV and recurrent quantitative analysis. Statistical comparisons were made between the pre-, post-, and recovery phases to quantify autonomic changes (repeated-measures ANOVA for comparisons across the three states, paired t-tests for direct two-state contrasts, post hoc analyses with Holm–Bonferroni corrections, and effect size estimates η2). Results: Immediately after training, significant decreases in SDNN (↓ 35%), RMSSD (↓ 40%), and pNN50 (↓ 55%), accompanied by increases in LF/HF (↑ 32%), were observed. DFA α1 and Recurrence Rate increased, indicating reduced complexity and more structured patterns of RR intervals. After two hours of recovery, partial normalization was observed; however, RMSSD (−18% vs. baseline) and HF (−21% vs. baseline) remained suppressed, suggesting incomplete recovery of parasympathetic activity. Indications of overtraining and cardiac risk were found in three athletes. Conclusion: High-intensity training in elite athletes induces pronounced acute autonomic changes and incomplete short-term recovery, potentially increasing fatigue and cardiovascular workload. Longitudinal repeated testing highlights differences between well-adapted, fatigued, and at-risk athletes. These findings highlight the need for individualized recovery strategies and ongoing monitoring to optimize adaptation and minimize the risk of overtraining and health complications.
1. Introduction
Heart rate variability (HRV) refers to the dynamic fluctuations in the time intervals between consecutive heartbeats [1,2], reflecting the organism’s ability to adapt to internal and external stressors. Higher HRV values are generally associated with greater physiological resilience and endurance [3]. Short, standardized resting measurements (five-minute recordings) using ECG devices are widely employed as reliable indicators for assessing the body’s responses to physical exertion [4,5].
Heart rate regulation is mediated by the autonomic nervous system (ANS) [6], where the sympathetic branch increases heart rate (HR), while the parasympathetic branch exerts the opposite effect. This interplay results in characteristic psychophysiological changes in HRV [3]. Monitoring HRV provides valuable information on the balance between the two branches of the ANS [7,8].
Previous research has demonstrated that HRV is sensitive to multiple physiological and environmental influences. Dietary factors such as omega-3 fatty acids, polyphenols, and overall nutritional status can modulate HRV both acutely and more chronically [9,10,11]. HRV is also a validated marker of stress and training status [12]. Furthermore, both ambient temperature [13,14] and body temperature can alter HRV indices [15,16].
During intense physical exercise, the activity of the sympathetic branch predominates, and it has been proven that HRV reflects adaptations to intense physical activity and training load in athletes [17]. The nervous system’s goal is to cope with the stress induced by exercise, which triggers a mechanism to reduce parasympathetic activity, leading to a decrease in many HRV indices, which defines HRV as a measure for optimizing individualized training [18].
Monitoring HRV over time provides insights into autonomic balance and recovery—particularly important in athletes, where training-induced fatigue and adaptation are critical for managing the training process. For example, endurance athletes typically exhibit faster parasympathetic tone recovery, reflecting better adaptation and fitness level [19]. In athletes, sport-specific changes in ANS regulation can lead to significant HRV variations [3,20], allowing precise management of training-induced fatigue and fine-tuning of exercise intensity. Bellenger et al., 2021 reports that following functional overreaching, post-exercise RMSSD may increase, potentially reflecting compensatory recovery or even persistent fatigue [21]. Such observations facilitate the implementation of strategies for individualized training plans [22,23,24], competition planning, optimization of training/rest schedules, and overall enhancement of athletic performance.
Previous studies have shown that HRV decreases during exercise in proportion to workload intensity, with moderate levels leading to a stable reduction and higher intensities causing minimal additional changes. This can complicate the interpretation of HRV metrics under typical training conditions [25,26,27]. Nonlinear indices, such as DFAα1 (~0.75), have been proposed to guide exercise intensity regulation [28,29]. Post-exercise HRV recovery generally occurs within one to two hours; however, higher intensity or workload can prolong HRV suppression, even up to 24 h [30,31,32]. Post-exercise metrics, such as RMSSD, have been associated with improvements in anaerobic endurance [33], while nocturnal HRV monitoring correlates with post-exercise autonomic depression [34].
Tracking individual responses to training loads enables more precise management of the training process and performance prediction, with some evidence suggesting that higher training volumes, rather than intensity, may yield superior adaptations [35]. Although HRV research has primarily focused on endurance sports, there is growing evidence of its applicability in resistance and functional training, where it can support load optimization and recovery [36]. For instance, Sánchez et al. (2024) examined psychological adaptation, training effects, individual differences, recovery, and sleep quality in basketball athletes using HRV [37]. The authors concluded that HRV represents a reliable tool for tailoring training programs to enhance athletes’ performance and development.
According to a study on HRV in athletes [38], athletes exhibit lower resting heart rates and higher HRV, indicative of enhanced autonomic regulation. The study highlights critical methodological factors that influence measurements, including body position, time of day, recording duration, baseline heart rate, sex, and age of the subjects. The authors also examine the effects of training intensity, volume, modality, and lifestyle on HRV. Turcu et al. (2023) discussed the use of HRV during or post exercise, as well as its potential for predicting physiological status [39].
During the competitive season, high-intensity training cycles occur that can induce acute autonomic disturbance and, when repeated, may lead to cumulative fatigue, overtraining, or potential cardiovascular risk. While the acute autonomic response to single training sessions has been described, less is known about how these responses evolve when athletes are repeatedly exposed to prolonged high-intensity load. Heart rate variability (HRV) analysis, including nonlinear approaches such as detrended fluctuation analysis (DFA) and recurrence quantification analysis (RQA), provides sensitive markers of both acute perturbations and adaptive versus maladaptive trends.
HRV is widely used to monitor the autonomic response to exercise, but most available data are from cross-sectional designs or short protocols (pre → post within a single session), often limited to linear measures. Nonlinear and recurrent metrics that capture changes in the structural complexity of the rhythm are less frequently applied in long-term, field-based observations of athletes. At the same time, the early recovery window after exercise (up to 1–2 h) and its incomplete normalization remain poorly characterized on a longitudinal basis. To address these shortcomings, we conducted a 4-month observation with multiple measurements before, immediately after, and 2 h after exercise in elite athletes. We combined time- and frequency-based HRV indices with fractal (DFA α1, Hurst, SampEn) and RQA metrics (REC, DET, LAM, TT), which allowed for the differentiation of adaptive versus maladaptive recovery patterns. We show that despite partial normalization by 2 h, sensitive nonlinear and recurrent measures remain aberrant in fatigued athletes (Group 2), and a small subgroup (Group 3) exhibits atypical and potentially risky profiles. Thus, we propose an integrated longitudinal framework for monitoring autonomic regulation and operational decision-making for exercise and recovery.
Based on these considerations, our study was guided by the following research question: What are the acute and short-term recovery dynamics of autonomic regulation in elite athletes undergoing prolonged high-intensity training, and how can nonlinear HRV metrics differentiate between adaptive, maladaptive, and potentially pathological responses over repeated sessions?
We hypothesized that while acute autonomic perturbations immediately after training would be present in all athletes, longitudinal monitoring across a four-month period would reveal group-specific differences: well-adapted athletes would restore HRV complexity within two hours, fatigued athletes would show cumulative suppression of nonlinear indices, and a small subgroup would present deviant patterns suggestive of cardiovascular risk.
Therefore, the purpose of the present study was to investigate changes in autonomic regulation before, immediately after, and two hours after training sessions, and to assess how repeated measurements over a 4-month period can differentiate between well-adapted, fatigued, and at-risk athletes. In addition, the study explored whether unexpected patterns could emerge, potentially revealing clinical relevance beyond training adaptation.
2. Materials and Methods
2.1. Participants and Protocol
2.1.1. Participants
Twelve male elite track and field athletes participated in this study. ECG Holter monitoring was performed five days per week, before and after training sessions, over a 4-month period. The specific disciplines of the studied athletes are 200 m sprint, 400 m and long jump; they are from the same sports club (Veliko Tarnovo, Bulgaria) and perform the same training program, which is a decision of the sports club management. The research was conducted in one of the five athletics clubs in the city of Veliko Tarnovo. The observation period is from 1 March 2025 to 30 June 2025. The athletes were aged between 19 and 26 years (mean ± SD: 21 ± 2.22 years), with an average height of 1.94 ± 0.28 m. Recordings were obtained immediately before training, immediately after training, and two hours post-training.
Inclusion criteria were age over 18 years, normal health status, and absence of cardiovascular diseases. Participants were instructed to avoid alcohol, caffeine, and smoking for at least 8 h prior to training. All athletes received detailed information regarding the study protocol and provided written informed consent. Participation was voluntary and did not confer any benefits. All athletes were elite competitors participating in national-level competitions.
Exclusion criteria: athletes who did not attend training regularly and for this reason, all records were not made for them during the 4-month study period (9 athletes from the initial 21 athletes who started the study).
Initially, 21 athletes were included in the study (out of a total of 32 in the research team, who were informed about the objectives of the study and the conditions for its conduct, 6 did not consent due to restrictions on non-consumption of coffee and cigarettes, and 5 are under 18 years of age and do not meet the inclusion criteria); 9 athletes from the initial 21 athletes did not attend training regularly and, accordingly, regular records were not made for them, which is why we excluded them from the study.
2.1.2. Procedure
The HRV recordings were carried out during regular training sessions under usual training conditions, in order to capture realistic physiological responses to high-intensity exercise. Prior to each measurement, athletes rested in a seated position for at least 10 min to stabilize cardiovascular parameters. They were instructed to avoid caffeine, alcohol, and strenuous exercise for 12 h before testing. Breathing was spontaneous and not externally paced, reflecting natural conditions during training. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of the Institute of Robotics at Bulgarian Academy of Sciences (protocol code 6 and date: 2 November 2024). Written informed consent was obtained from all participants.
A 3-channel Holter ECG system with five electrodes was used for data acquisition. Each ECG recording lasted 10 min and utilized the standard Lead II configuration. Electrode placement was as follows: RA (right clavicle), LA (left clavicle), RL (right lower thorax, ground), LL (left lower thorax), and C (precordial position V5).
All ECG Holter devices were applied by the same previously trained research associate (trained by a cardiologist and a sports medicine physician). The recordings were monitored on site by the research team, who ensured compliance with the protocol. Data preprocessing and HRV analysis were performed independently by two researchers with experience in HRV analysis using Kubios software (HRV Scientific 4.1.0) and Python scripts created by the authors. The independent results obtained from the two analyses were compared and it was found that the values obtained were consistent to the second decimal place. All athletes participating in the study performed the same training in terms of duration, sequence of exercises, and intensity. All measurements were performed at the same time of day (10 min before the start of the training session, which started at 4:00 p.m. and 10 min after the end of the training session, which usually ended at 6:00 p.m.). Before each Holter recording, the athletes were asked to rest in a sitting position for 10 min. All participants were young elite athletes of similar age and training status, ensuring a homogeneous sample. Participants were also instructed to refrain from caffeine and alcohol consumption on the day of testing. This minimized confounding factors, and this standardization ensured comparable baseline conditions for all participants by stabilizing heart rate and autonomic tone and reducing variability related to postural changes or residual physical activity. We can assume that there was no influence of circadian effects, since all participating athletes performed their training at the same time of day each time. The same researcher applied the electrodes to the Holter device and two independent analysts processed all files to ensure methodological consistency.
The athletes’ training session consisted of a full cycle included the following components: 5 min of warm-up, 20 min of preparatory running over 400 m, 15 min of tempo running until reaching 160 bpm, and 40 min of athletics-specific exercises using equipment.
2.2. Methods
The Holter system allowed continuous ECG recording, from which RR intervals were directly extracted. These time series were analyzed to assess HRV using custom Python scripts, with standard analysis performed on 5-min segments from the Holter recordings [40,41]. To ensure validity, the outputs were cross-checked against Kubios HRV Scientific (v4.1.0, Kubios Oy, Kuopio, Finland), with identical results obtained for all standard parameters. This verification supports the reproducibility and methodological reliability of the custom analysis procedures.
Segment length and selection.
Each condition (pre-, post-, and 2-h recovery) was recorded for ~10 min in seated rest. HRV indices were computed from a 5-min segment taken from minute 3 to minute 8 of each recording. This choice was made to bypass initial stabilization and potential start-up noise, minimize movement or electrode-settling artifacts at the beginning, and capture a steady-state breathing pattern and autonomic tone. When a recording was slightly shorter than 10 min, the 5-min segment was centered on the middle of the trace. As a robustness test, we repeated the analyses on adjacent 5-min windows (minutes 2–7 and 4–9), which produced virtually identical effect sizes and did not alter statistical inferences.
The following methods were applied [42]:
Time- and Frequency-Domain Methods:
- Mean RR (ms): Represents the average duration between two consecutive R-peaks in the ECG (RR intervals). A reduction in Mean RR (increased heart rate) after training indicates sympathetic activation and physiological stress. Prolonged decreases during recovery may reflect incomplete recovery or overtraining.
- SDNN (ms): Standard deviation of all NN intervals, representing overall HRV and reflecting both sympathetic and parasympathetic contributions. A sharp post-training decrease may indicate increased load, while slow recovery suggests stress and limited autonomic reserve.
- RMSSD (ms): Root mean square of successive differences between NN intervals. A key marker of parasympathetic (vagal) activity. Reduced RMSSD after exercise indicates suppression of parasympathetic activity [43], commonly observed under acute stress.
- High-Frequency (HF) Power: Reflects parasympathetic tone, whereas Low-Frequency (LF) power is considered a marker of sympathetic activity [44,45].
- LF/HF Ratio: Ratio of LF to HF components, used as an index of sympathovagal balance. An increased LF/HF post-exercise indicates sympathetic dominance; persistently high values suggest stress and insufficient recovery.
Nonlinear Methods:
- Detrended Fluctuation Analysis (DFA α1, α2) [46]: DFA was applied to RR interval time series to quantify long-term correlations and assess heart rate dynamics. The scaling exponent α was calculated for short-term (α1) and long-term (α2) intervals to evaluate autonomic regulation. DFA provides insights into the fractal properties of HRV, complementing traditional time- and frequency-domain metrics. Short-term (α1) and long-term (α2) scaling exponents were calculated by plotting the log-log relationship between root-mean-square fluctuation F(n) and window size n, with α1 derived from small windows and α2 from larger windows.
- DFA α1: Measures the short-term fractal structure of heart rate. It is sensitive to changes in autonomic regulation during exercise. Elevated post-exercise values indicate loss of “physiological complexity” and increased sympathetic control.
- DFA α2: Reflects the long-term fractal structure of HRV. Reduced values are associated with accumulated fatigue, overtraining, and increased stress, while excessively high values may indicate decreased adaptability of autonomic regulation.
Fractal Methods:
- Sample Entropy (SampEn): Provides an estimate of the complexity and unpredictability of the heart rate time series [47]. A decrease in SampEn after exercise indicates a more predictable and monotonous rhythm, reflecting physiological stress. Persistently low values during recovery may indicate high levels of fatigue.
- Hurst Exponent (H): Measures long-term correlations in the RR interval time series, allowing assessment of persistent trends and overall physiological adaptation. A shift of the Hurst exponent toward or below 0.5 indicates a more chaotic rhythm, associated with fatigue and reduced physiological adaptability.
For nonlinear HRV indicators (such as DFA α1, Hurst exponent, SampEn) there are no established and generally accepted reference values in clinical or sports practice. There are individual publications in the literature with indicative data in healthy people or athletes, but there is a lack of consensus and standardization. Therefore, our study makes its contribution in this direction. The literature review shows that DFA α1 values close to 1.0 are regarded as physiologically normal, whereas values <0.75 indicate reduced complexity and sympathetic dominance [29,30]. The Hurst exponent is typically between 0.6 and 0.9 in healthy individuals, with lower values suggesting anti-correlated, less adaptive dynamics [48,49]. Sample entropy is very rarely studied and there is almost no specific data [50].
Recurrence Quantification Analysis [51,52,53,54]:
Recurrence Plot Analysis
Recurrence plots (RPs) are a nonlinear data analysis method used to visualize and quantify the recurrence of states within a dynamical system. In this study, RPs were applied to the time series of RR intervals to detect changes in cardiac dynamics induced by physical exercise.
An RP is a two-dimensional representation in which both axes correspond to time indices i and j of the same signal. Each point (i,j) in the plot is marked when the distance between two state vectors and is less than a predefined threshold ε:
where
Θ(·) is the Heaviside step function,
∥·∥ is the Euclidean norm, and
ε is chosen relative to the standard deviation of the time series. The embedding dimension m and time delay τ were selected based on the false nearest neighbors and mutual information methods, respectively.
From the RPs, Recurrence Quantification Analysis (RQA) metrics were calculated, including:
- REC (%)—recurrence rate, proportion of recurrent points;
- DET (%)—determinism, proportion of recurrent points forming diagonal lines;
- LAM (%)—laminarity, proportion of recurrent points forming vertical lines;
- Lmax—length of the longest diagonal line (excluding the main diagonal);
- ENTR—Shannon entropy of the diagonal line length distribution.
To avoid bias related to over-reliance on single indices, we analyzed HRV across complementary domains: time-domain (SDNN, RMSSD), frequency-domain (LF, HF, LF/HF), nonlinear dynamics (DFA α1, SampEn, Hurst), and recurrence-based measures (REC, DET, LAM, TT, entropy). This multidimensional approach is recommended because each domain captures different but physiologically relevant aspects of autonomic regulation.
2.3. Statistical Analysis
All numerical and graphical results were obtained using custom Python scripts developed by the authors (Python version 3.9.13). Statistical analyses were performed using IBM SPSS Statistics (version 29.0.2.0). Data normality was assessed with the Shapiro–Wilk test. Continuous variables are presented as mean ± standard deviation (SD) and 95% confidence interval (CI). To assess the effects of training and recovery on HRV parameters, a repeated-measures ANOVA was applied. If significant effects were found, post hoc comparisons were performed using paired-samples t-tests between conditions, and a Holm–Bonferroni correction was used to control for multiple comparisons. The strength of the effect was estimated using η2.
All HRV and RQA parameters were analyzed separately using repeated-measures ANOVA with Condition (pre-training, post-training, 2-h recovery) as the within-subject factor. Since the design included only one repeated factor, no interaction terms were applicable. Given that many HRV indices are mathematically interrelated, the risk of inflated type I error was addressed by recalculating adjusted p-values using the Holm–Bonferroni step-down procedure. This approach allowed us to verify the robustness of the results across multiple comparisons. Data are presented as mean ± SD, and effect sizes (η2) are reported for significant outcomes.
Statistical significance was set at p < 0.05 after correction.
3. Results
A total of 960 records (80 days × 12 athletes) were analyzed for each condition: pre-training, post-training, and 2-h recovery. HRV analysis of all recordings revealed three distinct groups based on numerical results and graphical representations:
- Group 1: Records with slightly deviating values from normal ranges, indicating mild to moderate fatigue (695 records, 72.4%). This is the largest group, representing athletes in very good physical condition.
- Group 2: Records with moderately deviating values from normal ranges, showing strong fatigue or overreaching (211 records, 22%). This smaller group includes athletes experiencing a high level of post-training fatigue.
- Group 3: Records with markedly deviating values from normal ranges, suggesting a potential risk of cardiac issues (54 records, 5.6%). This group represents exceptions requiring close attention. These 54 recordings originated from three athletes, with atypical changes observed only in part of their sessions rather than consistently across all of their records.
Figure 1 illustrates the dynamics of RR intervals for a single athlete in the three states: pre-training Figure 1A, immediately post-training Figure 1B, and 2 h into recovery Figure 1C. In the pre-training period (A), RR intervals are stable with moderate variations, reflecting normal autonomic balance. Immediately after training Figure 1B, a pronounced fluctuation is observed initially, followed by stabilization at low values and renewed fluctuation, indicating increased sympathetic activity and suppressed parasympathetic regulation. Two hours into recovery Figure 1C, partial restoration toward baseline values and variability is seen, suggesting activation of recovery processes, although complete normalization of autonomic tone has not yet been achieved.
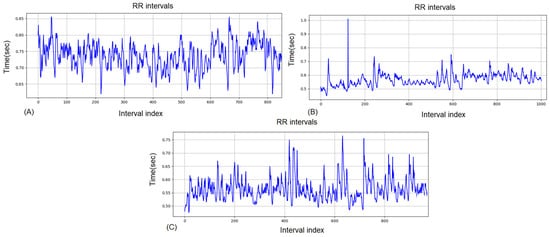
Figure 1.
RR intervals for the same competitor for Group 1: (A) pre-training, (B) post-training (C) 2-h recovery.
Results for Group 1
Table 1 presents the quantitative changes in HRV parameters across the three physiological states. The mean RR interval decreased significantly after training (p < 0.001), corresponding to a sharp increase in heart rate. Time-domain parameters, such as SDNN and RMSSD, also showed significant reductions immediately after exercise, reflecting decreased parasympathetic activity. During the 2-h recovery phase, these parameters partially returned toward baseline values (Spectral indices indicated an increase in nLF after exercise, accompanied by a decrease in nHF, demonstrating predominant sympathetic dominance. Nonlinear parameters (SD1, SD2, SD2/SD1, Hurst, DFA α1, DFA α2, SampEn) also exhibited significant changes indicating reduced complexity and adaptability of heart rate dynamics immediately after training, with partial recovery by the second hour. All parameters were statistically significant (p < 0.05).

Table 1.
HRV parameters of the 3 physiological states for Group 1 (adaptive athletes).
The ANOVA results showed that all HRV indices differed significantly across the three physiological states (p < 0.05), but the effect sizes varied. Metrics such as Mean RR, HR, SDNN, RMSSD, SD1, DFA α1, and SampEn exhibited very large effect sizes (η2 > 0.50), indicating that training and recovery had a strong influence on these parameters. Moderate to large effects were observed for SD2, SD2/SD1, Hurst, and DFA α2 (η2 ≈ 0.20–0.40), reflecting sensitivity to transitions in autonomic regulation. In contrast, frequency-domain measures (nLF, nHF, LF/HF) showed small to moderate effects (η2 < 0.10), suggesting that spectral changes were less pronounced compared to time-domain and nonlinear indices. Effect size (η2) quantifies the proportion of total variance in a parameter explained by group differences, thereby providing insight into the strength and practical significance of the observed effects beyond statistical p-values.
The graph in Figure 2 illustrates the DFA before training, immediately after, and 2 h later, clearly showing changes in the α1 and α2 scaling exponents. Before training, α1 = 0.79 and α2 = 0.06, indicating a balanced short-term correlation structure and low long-term dependency. Immediately after training, α1 dropped to 0.06, while α2 increased to 0.19, reflecting severely disrupted short-term autocorrelation and partial activation of long-term trends associated with acute exercise-induced stress. Two hours into recovery, α1 rose to 0.35 and α2 to 0.26, indicating partial restoration of short-term dynamics while structural changes in long-term cardiac regulation persist.
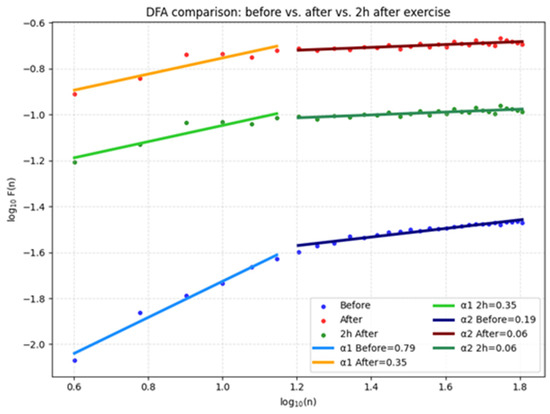
Figure 2.
DFA for the three studied conditions.
The recurrence plot analysis (Figure 3) clearly shows structural changes in heart rate dynamics across the three states. Before training Figure 3A, the structure is uniform with scattered recurrent points, reflecting stable and complex dynamics. Immediately after training Figure 3B, concentrated and more organized recurrent regions appear, indicating more deterministic and less variable behavior. After 2 h of recovery Figure 3C, the pattern approaches the pre-training state, though with slightly reduced heterogeneity still evident.
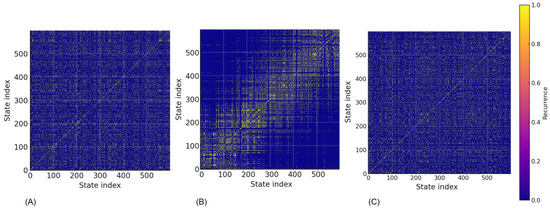
Figure 3.
Recurrence Plot for the same competitor: (A) pre-training, (B) post-trainingn (C) 2-h recovery (adaptive athletes).
The results (Table 2) show that after training, the indices of determinism (DET), laminarity (LAM) and mean retention time (TT) significantly increase, which indicates a more structured and predictable dynamics of physiological signals. The decrease in entropy reflects reduced complexity and lower variability, probably due to increased sympathetic control and fatigue. For athletes, this means that after intense exercise there is a temporary decrease in adaptability, which requires adequate recovery before the next effort. In Group 1, large effect sizes for DET, LAM, and Entropy indicate pronounced post-training increases in signal regularity with reduced complexity, partially normalizing after 2 h, while REC showed no meaningful change.

Table 2.
RQA Metrics of the 3 physiological states for Group 1.
The post hoc analysis using Tukey HSD showed that for the REC (%) parameter, there were no significant differences between the three states (p > 0.6). For DET (%), significant differences were observed between Post and Pre (decrease, p < 0.01) and between Pre and Recovery (increase, p < 0.01), while Post vs. Recovery was not significant (p = 0.0685). For LAM (%), all pairwise comparisons showed significant differences (p < 0.0001). TT also demonstrated significant changes between Post and Pre (increase) and between Pre and Recovery (increase), whereas Post vs. Recovery was not significant (p = 0.985). For Entropy, all comparisons between states were significant (p < 0.001).
Results for Group 2
Figure 4 presents the RR interval dynamics for a fatigued athlete in the three conditions: pre-training (A), immediately post-training (B), and after 2 h of recovery (C).
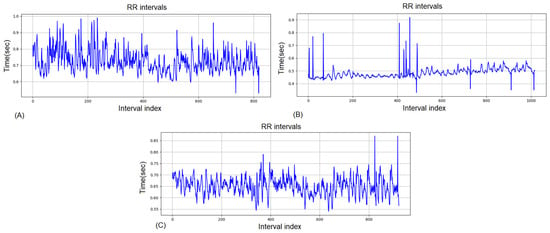
Figure 4.
RR intervals for the same competitor for Group 2: (A) pre-training, (B) post-training, (C) 2-h recovery.
In the pre-training state Figure 4A, RR intervals show larger fluctuations and reduced stability compared to Group 1, reflecting signs of accumulated fatigue. Immediately post-training Figure 4B, there is a sharp reduction in RR interval length and a pattern of irregular variability, consistent with strong sympathetic dominance and suppressed parasympathetic tone. Even after 2 h of recovery Figure 4C, variability remains attenuated and the intervals are less stable than in Group 1, indicating incomplete autonomic restoration and a delayed recovery process.
In athletes with moderate fatigue (Table 3), Post-training measurements showed further suppression of vagal tone and pronounced sympathetic dominance. After 2-h recovery, HRV parameters indicated partial but incomplete return to baseline values, suggesting a prolonged autonomic imbalance. In Group 2, large effect sizes for RR, HR, RMSSD, DFA α1, and SampEn (η2 = 0.5–0.6) indicate pronounced autonomic perturbations and a marked loss of HRV complexity after training. Moderate effects in SDNN, SD1, and SD2 further support persistent fatigue, while small effects in LF/HF suggest that nonlinear metrics are more sensitive markers of maladaptation.

Table 3.
HRV parameters of the 3 physiological states for Group 2 (fatigued athletes).
DFA analysis of fatigued athletes (Group 2) highlights a significantly different recovery profile compared to Group 1 (Figure 5). Before training, α1 values were already reduced, suggesting pre-existing signs of autonomic overload, α2 was low, reflecting weak long-term correlation. Immediately after training, α1 decreased further, indicating a loss of short-term fractal correlation, and α2 showed only a slight increase, insufficient to stabilize long-term dynamics. Two hours after the start of recovery, α1 partially recovered but remained below normal resting levels, while α2 increased moderately, suggesting incomplete normalization. This pattern demonstrates slow recovery and persistent maladaptation of short-term autonomic regulation, consistent with cumulative fatigue and overload processes.
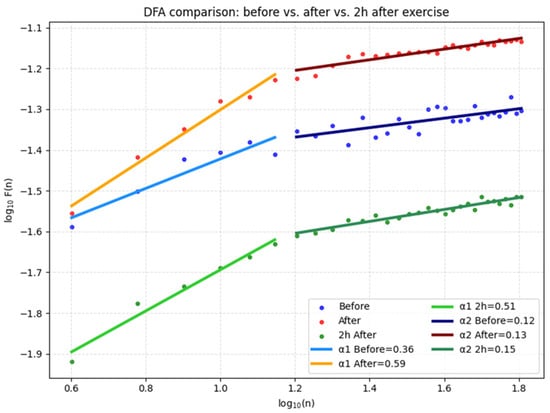
Figure 5.
DFA for the three conditions studied.
The recurrence plots for Group 2 (Figure 6) reveal marked differences compared to Group 1. Before training Figure 6A, the plots already show denser and more clustered recurrence regions, suggesting reduced complexity and a tendency toward more rigid dynamics. Immediately after training Figure 6B, the recurrence structures become strongly organized with long diagonal and vertical lines, indicative of highly deterministic and laminar patterns consistent with strong sympathetic dominance and suppressed variability. Even after 2 h of recovery Figure 6C, the plots fail to return to the pre-training state; recurrent structures remain overly regular, reflecting incomplete parasympathetic reintegration and persistent autonomic imbalance. These findings confirm that fatigued athletes display slower and less efficient recovery, with recurrence analysis capturing maladaptive patterns that are not fully visible in conventional HRV indices.
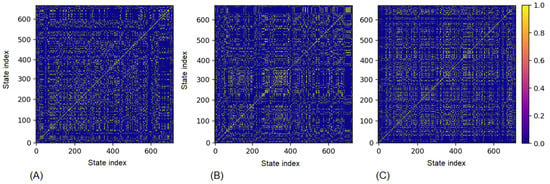
Figure 6.
Recurrence Plot for the same competitor: (A) pre-training, (B) post-training (C) 2-h recovery (fatigued athletes).
In lightly fatigued athletes, baseline RQA parameters Pre-training (Table 4) already showed signs of autonomic imbalance compared to “fresh” athletes—higher DET and LAM, slightly increased TT, and slightly lower Entropy, reflecting reduced heart rate complexity and more structured recurring patterns. Post-training dynamics were similar to those in fresh athletes, with a marked increase in determinism (DET) and laminarity (LAM), prolonged trapping times (TT), and decreased Entropy, but absolute values remained less favorable in the lightly fatigued group. During the 2-h recovery phase, parameters improved toward better values but remained distant from the baseline of fresh athletes, indicating incomplete recovery and persistent structural rhythm patterns. In Group 2, the large effects for DET and LAM (η2 ≈ 0.46–0.50) indicate more structured and predictable heart rate dynamics after training, accompanied by a decrease in entropy (η2 = 0.31), reflecting persistent fatigue and reduced autonomic complexity.

Table 4.
RQA Metrics of the 3 physiological states for Group 2.
Results for Group 3 (group of exceptions)
The RR interval series shown in Figure 7A is a typical example of low HRV. The variability of the second graph (B) is also low. After training, the RR intervals of the graph are very compact around 0.5–0.6 s with rare irregular deviations, which means that the differences between successive beats are minimal. This is a sign of decreased HRV, i.e., dominance of the sympathetic nervous system and suppression of parasympathetic activity. In the context of sports and physiology, this is usually associated with:
- Acute stress after exercise;
- Temporary inability to recover quickly;
- Potential risk of too frequent or intense training without sufficient rest.
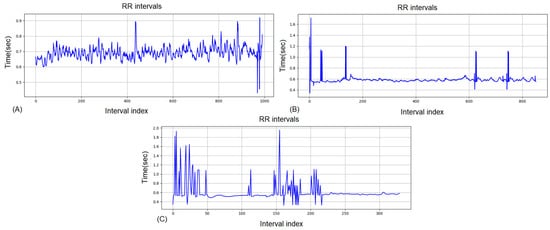
Figure 7.
RR intervals for the same athlete for Group 3: (A) pre-training, (B) post-training (C) 2-h recovery.
The RR interval graph in Figure 7C shows several important signs of fatigue and potential risks: Large fluctuations at the beginning and around the middle—sharp peaks and dips (from ~0.4 s to ~2 s) suggest an unstable rhythm, which may be the result of high stress on the cardiovascular system or rhythm disturbances (extrasystoles, missed beats). Subsequent strongly narrowed variability (~0.5–0.6 s)—the second half of the recording is with low HRV, which indicates sympathetic dominance and insufficient recovery. Possible risks—in an athlete this condition may indicate overload or fatigue, and in predisposed people—the potential for arrhythmias and reduced adaptive reserve.
In athletes with a high degree of fatigue (Table 5) Pre-training values are characterized by lower RMSSD, SDNN and nHF, as well as higher LF/HF compared to “fresh” athletes, which is an indicator of incomplete recovery of parasympathetic activity even after a 24-h rest. At a higher degree of fatigue and after training, HR remains elevated, and variability (SDNN, RMSSD) partially recovers, without reaching baseline levels. The LF/HF ratio and reduced DFA α1 indicate continued dominance of sympathetic activity and incomplete recovery of autonomic balance. This combination of indicators indicates accumulated training stress and a risk of deterioration of functional status with continued load without sufficient rest. The strongest effect sizes in Group 3 were observed for nonlinear indices (DFA α1, SampEn, SD2/SD1), indicating marked alterations in HRV complexity that differentiate these athletes and may reflect potential cardiovascular risk.

Table 5.
HRV parameters of the 3 physiological states for Group 3.
The graphs in Figure 8 show that after exercise (red and orange lines) DFA α1 values decrease compared to before exercise (blue line), which is a sign of increased sympathetic control and stress on the autonomic nervous system. Even 2 h after exercise (green line) α1 and α2 remain lower than baseline, indicating incomplete recovery and sustained cardiovascular stress. Such a pattern is associated with higher risk in sensitive or vulnerable individuals, especially those with a predisposition to cardiac problems.
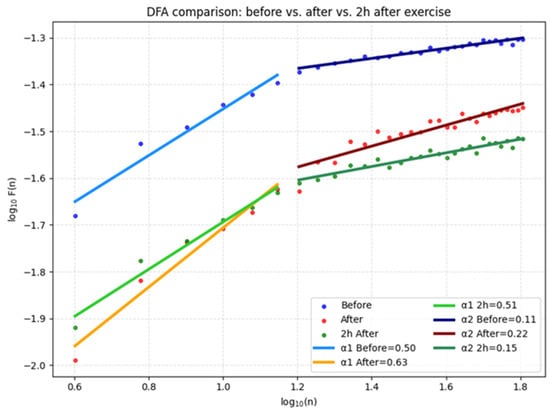
Figure 8.
DFA for fatigue and stress.
The recurrence plots for Group 3 (Figure 9) highlight atypical heart rate dynamics. Before training Figure 9A, the structure already deviates from the typical heterogeneous patterns, with irregular clusters indicating unstable baseline regulation. Immediately post-training Figure 9B, the recurrence map displays highly fragmented and block-like structures, reflecting abnormal determinism and potential pathological rigidity in cardiac dynamics. Unlike in Groups 1 and 2, the 2-h recovery Figure 9C does not restore the variability; instead, recurrent regions remain irregular and poorly organized, suggesting persistent autonomic dysregulation. These atypical recurrence patterns may serve as early warning signs of maladaptation or even increased cardiovascular risk, requiring individual monitoring and clinical attention.
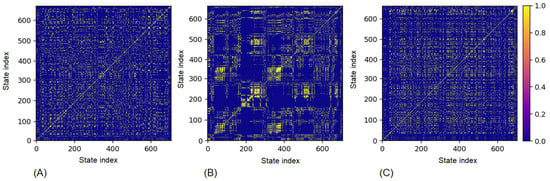
Figure 9.
Recurrence Plot for fatigue and stress: (A) pre-training, (B) post-training (C) 2-h recovery.
In the athletes from Group 3 (Table 6), even before the load, signs of incomplete recovery were observed—high values of DET and LAM and reduced entropy, which indicate reduced variability and increased structuring of the heart rate. After training, these indicators worsened further, indicating a strong stress on the autonomic nervous system and predominant sympathetic activity. Two hours later, the lack of significant improvement in the metrics confirms a state of overfatigue and a high risk of delayed recovery. All p value (Post/2-h) values are >0.05, which means that there is no statistically significant difference between the “Post-Training” and “2-h recovery” states for any of the studied indicators. Large effect sizes for DET, LAM, TT, and Entropy (η2 = 0.31–0.48) indicate substantial post-training increases in signal regularity and reduced complexity, suggesting atypical autonomic rigidity in Group 3 athletes.

Table 6.
RQA Metrics Before and After Training for competitors Group 3.
Under significant physical or psycho-emotional stress, the sympathetic nervous system is activated, leading to increased heart rate, shortened refractory period, and heightened myocardial electrical excitability. These changes can trigger premature ventricular contractions (PVCs), even in healthy athletes. The presence of PVCs during or immediately after exercise is an important indicator of cardiac adaptation and a potential marker of increased risk for arrhythmias, particularly in cases of incomplete recovery or overtraining. Therefore, a PVC analysis was performed on these recordings as part of the assessment of cardiac risk. The analysis was conducted on ECG recordings.
Detection of PVCs in the ECG (Figure 10) was achieved through a combined analysis of temporal intervals and QRS complex morphology (including QRS width assessment). PVCs are characterized by a preceding shortened RR interval followed by a compensatory pause, as well as an elongated QRS complex and morphological differences compared to the normal rhythm. The mathematical definition of a PVC is given by the condition:
where pi is the correlation coefficient between the current QRS and the pattern of a normal complex. This approach provides reliable discrimination of PVCs from normal heartbeats while maintaining the clinical validity of the analysis.
PVC ⟺ (QRSwidth > 0.12 s)∧(RRi−1 < 0.8⋅RRmean)∧(pi < 0.9)
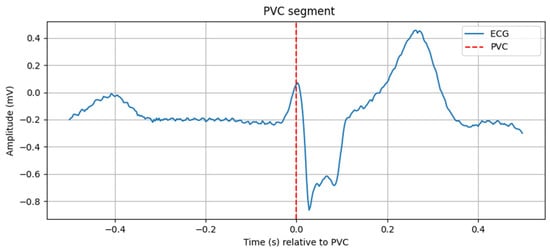
Figure 10.
PVC segment.
The graph in Figure 10 shows a one-second segment of the ECG recording, centered around the first detected PVC. The horizontal axis represents time in seconds relative to the PVC event (t = 0 s), while the vertical axis represents the ECG signal amplitude in millivolts. The red dashed vertical line marks the exact moment of the extrasystole. The QRS complex at this point is widened and morphologically different from normal complexes, which is characteristic of a PVC. The segment clearly illustrates the shortened preceding RR interval and the compensatory pause following the event, thereby visually confirming the automatic detection of the arrhythmia.
As a result of the analysis, PVCs were detected in 73% of the recordings, indicating significant fatigue/stress that may negatively impact cardiac function. Therefore, athletes with this profile should be referred to a cardiologist for consultation, prolonged medical monitoring, and treatment if necessary.
In the remaining 27% of athletes, signs of physiological fatigue were observed following intensive exercise, without immediate indications of pathological cardiac regulation. The observed incomplete recovery of parasympathetic activity and the persistently elevated LF/HF ratio within two hours post-exercise highlight the need for an extended recovery period (at least 24–48 h) before the next high-intensity training session, along with the use of active regeneration techniques (light aerobic activity, breathing exercises, adequate hydration) to prevent the accumulation of fatigue and reduce the risk of overtraining.
Based on the conducted studies, the following conclusions were drawn:
Group 1: Athletes in this group are in the best physical condition and have the highest likelihood of excellent competition performance.
Group 2: Athletes in this group are at risk of developing fatigue and overtraining.
Group 3: Athletes in this group should be referred to a cardiologist for evaluation or further monitoring of their health status.
4. Discussion
This study revealed significant changes in HRV parameters following intensive sports training, indicating an acute autonomic imbalance and a shift toward sympathetic dominance immediately post-exercise. The marked reduction in SDNN, RMSSD, and pNN50, alongside an increase in recurrence frequency, reflects diminished parasympathetic modulation and reduced overall heart rate variability. These findings align with previous studies showing that intense exercise causes immediate suppression of vagal activity and enhanced sympathetic output, which can persist for several hours post-exercise [55,56,57]. These changes are observed in athletes from both Groups 1 and 2.
The partial recovery observed within two hours—evident from parameters such as SDNN and recurrence frequency approaching baseline, while RMSSD and pNN50 remain suppressed—suggests that full autonomic recovery may require a longer period, as reported in other studies on endurance and sprint athletes (e.g., [16,58,59]). Our data indicate that although some markers normalize relatively quickly, parameters related to short-term parasympathetic activity (RMSSD, HF power) recover more slowly, consistent with Buchheit et al. (2008), who highlighted prolonged vagal suppression after high-intensity training [34]. In well-trained and fit athletes (Group 1), partial recovery occurs relatively quickly, while a longer-lasting suppression of vagal activity is a normal adaptive response, not a sign of fatigue.
In fatigued or overreached athletes (Group 2), the recovery of HRV parameters is considerably delayed. SDNN and recurrence frequency remain suppressed for extended periods, while RMSSD, pNN50, and HF power—markers of short-term parasympathetic activity—recover much more slowly, sometimes taking hours or even days to return toward baseline. This prolonged suppression reflects sustained sympathetic dominance and delayed vagal reactivation, indicating insufficient recovery and accumulated fatigue rather than a normal adaptive response. Consequently, autonomic regulation remains impaired for longer durations, reducing cardiovascular adaptability and increasing susceptibility to additional stress or training loads. These patterns underscore the importance of monitoring HRV to detect early signs of overreaching or maladaptation in athletes exposed to high or cumulative training demands.
The post-exercise increase in recurrence frequency and DFA α1 indicates reduced complexity and more regular RR interval patterns, reflecting decreased cardiovascular adaptability. Similar changes have been reported in fractal and recurrence analyses of HRV in both athletes and clinical populations [29,30]. Such alterations may serve as early indicators of insufficient recovery if they persist over extended periods. A Gronwald et al. even suggest using DFA α1 diagnostically as a marker of physiological demand and as a “global” biomarker for determining training zones and load [30]. Such changes (increase in recurrence frequency and DFA α1, reduced complexity of RR intervals) apply to athletes after acute training, regardless of whether they are fit or fatigued and are an acute effect of the intense load. If these changes are recovered quickly, the athlete is fit and adapted (Group 1). Interpreting the significance of these changes depends on the dynamics of recovery. If these changes persist for a long time, this may be a signal of fatigue or insufficient recovery. Thus, in group 2 (fatigued or overtrained athletes) these changes in recurrence frequency and DFA α1 remain altered for a longer period, reflecting prolonged autonomic dysregulation. This leads to lower cardiovascular adaptability and an increased risk of fatigue accumulation in subsequent training. The persistence of these indicators can be used as an early indicator of insufficient recovery or the initial phase of overtraining.
RQA parameters further support these findings: decreased determinism indicates reduced predictability of heart rhythm, while increased laminarity points to longer episodes of quasi-stable, less adaptive RR interval patterns. These changes are characteristic of transient destabilization of autonomic regulation under high training loads and represent a normal acute response in well-adapted athletes from Group 1, rather than a sign of fatigue.
In fatigued or overreached athletes from Group 2, RQA parameters show prolonged alterations: decreased determinism reflects continued reduced predictability of heart rhythm, while increased laminarity indicates extended episodes of quasi-stable, less adaptive RR interval patterns. These sustained changes are indicative of prolonged autonomic dysregulation under high or cumulative training loads, contrasting with the transient destabilization observed in well-recovered athletes.
In our study, heart rate (HR) increased significantly after exercise, from 82.87 ± 12.64 bpm at rest to 115.20 ± 14.52 bpm post-exercise (Δ = +32.33 bpm, +39%). Even after 2 h of recovery, HR remained above baseline (100.50 ± 21.86 bpm, Δ = +17.63 bpm, +21.3%), reflecting slower recovery and greater cardiovascular strain. In comparison, in Buchheit et al. (2008) in the HIT group of adolescents showed stable HR—%HRmean practically did not change (77.6 ± 1.9 → 77.5 ± 1.9), and %HRpeak even decreased by about 5% (88.7 ± 2.7 → 83.8 ± 1.6) [31], reflecting faster autonomic readaptation and better cardiovascular fitness. This emphasizes that the participants in our study are less adapted to the load compared to trained adolescents in [34].
Notably, HRV analysis of one athlete revealed pronounced changes between the two post-training states. For accuracy, it should be noted that pre-training HRV parameters were already outside normal ranges, suggesting incomplete recovery from previous sessions and the potential for cumulative fatigue. Compared to other athletes, the magnitude of changes observed in this athlete—particularly the large drop in RMSSD and pNN50—suggests excessive training load relative to the athlete’s recovery capacity. Although not necessarily pathological, persistently reduced vagal markers are associated with overtraining and increased risk of injury [4].
Therefore, individualized monitoring and adjustment of this athlete’s training program are recommended, including recovery strategies such as extended rest intervals, to optimize performance and reduce the risk of overtraining or cardiac complications. Cardiological consultation is strongly advised, with further follow-up if necessary.
These results can be viewed in the context of Yu et al. (2021), who showed that delayed recovery of HR after exercise in healthy subjects was associated with a higher risk of developing metabolic syndrome (HRR3 ≤ 45 bpm; HR = 1.304, 95% CI 1.061–1.602, p = 0.001) [60]. Although the time points of measurement differed (1–3 min in [60] vs. 10 min in our study), the slower recovery observed in our participants supports the concept that slower cardiovascular readaptation after exercise may reflect greater stress on the cardiovascular system and potentially greater health risk.
Novelty and Longitudinal Insights
The acute increase in HR and suppression of vagally mediated HRV parameters immediately after high-intensity exercise has been documented in the literature, but in addition, the present study conducted a longitudinal study and applied nonlinear methods for HRV analysis. We followed athletes in different training states for 4 months, which allowed us to distinguish acute autonomic responses from cumulative effects associated with prolonged exercise.
The results showed clear differences between the three groups:
Group 1 (well-adapted athletes): demonstrated recovery of HRV complexity (DFA α1, SampEn, RQA parameters) within 2 h after exercise, which is a sign of effective autonomic adaptation. Furthermore, comparison of baseline HRV parameters on the day before the first training session (observed with Holter) with those on the day before the last training session showed a modest improvement in resting HRV in well-adapted athletes (e.g., higher RMSSD, higher parasympathetic activity).
Group 2 (fatigued athletes): showed persistent suppression of nonlinear indices within the studied training sessions. This is an indicator of accumulated fatigue and potential risk of maladaptive responses and overtraining. Also, after a 4-month period, a trend of a slight decrease in nonlinear indices was observed in the comparative analysis.
Group 3 (athletes with exceptions): represents a small subgroup with deviating values, including the presence of PVCs, suggesting a possible cardiac risk. This group does not follow typical recovery patterns and emphasizes the practical importance of HRV monitoring to identify athletes in whom additional medical monitoring is necessary.
During the 4-month training period, we observed a persistent attenuation of vagal markers in the fatigued subgroup (Group 2), while well-adapted athletes (Group 1) demonstrated a faster recovery of HRV complexity indices (DFA α1, SampEn, RQA indicators). This suggests that HRV dynamics over multiple sessions can distinguish adaptive from maladaptive training responses.
These findings highlight the dual contribution of the study: on the one hand, they confirm acute autonomic changes after high-intensity exercise; on the other hand, they provide new longitudinal evidence that HRV dynamics can be used to distinguish adaptive from maladaptive responses, as well as to detect potential health risks. Thus, nonlinear HRV measures emerge as particularly sensitive markers for both monitoring the training process and for early diagnosis of conditions requiring medical intervention.
Limitations
This study has several limitations that should be acknowledged. First, the sample size was relatively small, which reduces the generalizability of the findings and limits the statistical power to detect subtle effects. Second, the study did not include a control group, making it difficult to distinguish training-induced changes from normal physiological fluctuations over time. Third, only male athletes were recruited, which prevents conclusions about potential sex-related differences in autonomic responses and recovery dynamics. Finally, although standardized procedures were applied before each measurement, potential confounding variables such as sleep quality, nutritional status, and psychological stress were not fully controlled. These external factors may have influenced HRV and RQA outcomes, and should be addressed in future studies to strengthen the robustness of the results.
An important methodological limitation concerns the interpretation of frequency-domain indices obtained from short-term HRV recordings. While high-frequency power (HF, 0.15–0.40 Hz) is considered a reliable marker of vagal activity even in 5-min segments, low-frequency power (LF, 0.04–0.15 Hz) and the LF/HF ratio require cautious interpretation. Their physiological significance remains debated, as LF oscillations reflect slower modulations related to the baroreflex, which are more robustly captured in long-term (24-h) recordings. Therefore, although LF and LF/HF values are reported in this study for completeness, they should not be overly interpreted as accurate indicators of sympathetic-parasympathetic balance in the context of short-term measurements.
A limitation of the present study is the relatively small number of participants (n = 12). However, the longitudinal repeated-measures design provided a large number of recordings per athlete, which increased the effective power to detect within-subject changes. Still, the limited cohort size restricts the generalizability of our findings, and future studies on larger athlete populations are warranted.
Practical Implications
The present findings carry important implications for individualized recovery management in elite athletes. The observation that well-adapted athletes (Group 1) restored HRV complexity within two hours after training suggests that standard recovery protocols may be sufficient for this subgroup. In contrast, fatigued athletes (Group 2) exhibited persistent suppression of nonlinear HRV indices across repeated sessions, indicating that additional recovery time, reduced training load, or active recovery interventions may be necessary to prevent maladaptation and overtraining. Importantly, the identification of athletes with abnormal HRV dynamics and frequent PVCs (Group 3) emphasizes the role of HRV monitoring as an early warning tool for cardiovascular risk, highlighting the need for medical evaluation rather than purely training-related adjustments.
From a practical standpoint, these findings suggest that incorporating nonlinear HRV metrics such as DFA α1, SampEn, and RQA into regular athlete monitoring could provide coaches and sports physicians with sensitive markers for tailoring training prescriptions. By differentiating between adaptive, maladaptive, and potentially pathological responses, HRV monitoring can contribute to safer and more effective performance optimization strategies.
Future Research Directions
The authors plan to validate and implement a photoplethysmography (PPG)-based device [49,50,61,62] for monitoring the physiological status of the body, which is expected to be particularly useful for athletes. In recent years, PPG sensors have significantly improved, becoming more accurate, compact, affordable, and accessible for everyday use, thanks to advances in biotechnology [51,52,53,63,64,65].
The device is anticipated to assess the user’s condition on a scale of normal, mild fatigue, and overfatigue, while providing alerts in the presence of potential health concerns. Upon detecting more serious indications, a review of individual training plans, extended rest periods, and, if necessary, consultation with a cardiologist would be recommended. This approach would enable earlier identification of the risk of overtraining and cardiac disturbances, while simultaneously optimizing recovery and ensuring the safety of athletes.
5. Conclusions
This study indicates that high-intensity training in athletes leads to acute alterations in HRV, with reduced parasympathetic activity and signs of sympathetic predominance immediately post-exercise. Partial recovery was seen after two hours, but several parameters remained below baseline, suggesting incomplete autonomic restoration.
These findings should be interpreted with caution, given the small sample size, the absence of a control group, and possible influence of uncontrolled external factors.
Future research with larger and more diverse cohorts, inclusion of female athletes, and control of variables such as sleep, nutrition, and stress is needed to validate these preliminary observations and to develop robust composite indices for individualized training and recovery management.
Author Contributions
Conceptualization, G.G.-T.; methodology, G.G.-T.; software G.G.-T. and Y.-A.T.; validation, G.G.-T.; formal analysis, G.G.-T.; investigation, data curation, G.G.-T.; writing—original draft preparation, G.G.-T. and Y.-A.T.; writing—review and editing, P.L.; supervision, P.L.; project administration, P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Fund of Bulgaria (scientific project “Research, mathematical analysis and assessment of the impact of stress on cardiac data”), grant number KP-06-M72/1, 5 December 2023.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Institute of Robotics at Bulgarian Academy of Sciences (protocol code 6 and date: 2 November 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The Holter data recorded before and immediately after and 2 h after can be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| HR | heart rate |
| RR | RR interval |
| SDNN | standard deviation of normal-to-normal intervals |
| RMSSD | root mean square of successive differences |
| LF | low-frequency power |
| HF | high-frequency power |
| nLF, nHF | normalized low- and high-frequency power |
| LF/HF | low-frequency to high-frequency ratio |
| SD1, SD2 | Poincaré plot indices |
| DFA α1 | short-term detrended fluctuation analysis exponent |
| DFA α2 | long-term detrended fluctuation analysis exponent |
| SampEn | sample entropy |
| RQA | recurrence quantification analysis |
| REC | recurrence rate |
| DET | determinism |
| LAM | laminarity |
| TT | trapping time |
References
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring athletic training status through autonomic heart rate regulation: A systematic review and meta-analysis. Sports Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef]
- Nakamura, F.Y.; Gantois, P.; Giannaki, C.; Vlahoyiannis, A.; Bogdanis, G.C. Heart rate variability as a means of assessing workload effects and recovery during exercise training. In Fundamentals of Recovery, Regeneration, and Adaptation to Exercise Stress: An Integrated Approach; Apostolopoulos, N.C., Bogdanis, G.C., Seagrave, L.R., Plyley, M.J., Eds.; Springer: Cham, Switzerland, 2025; pp. 1–25. [Google Scholar] [CrossRef]
- Chiang, J.K.; Lin, Y.C.; Hung, T.Y.; Kao, H.H.; Kao, Y.H. The impact on autonomic nervous system activity during and following exercise in adults: A meta-regression study and trial sequential analysis. Medicina 2024, 60, 1223. [Google Scholar] [CrossRef]
- Addleman, J.S.; Lackey, N.S.; DeBlauw, J.A.; Hajduczok, A.G. Heart rate variability applications in strength and conditioning: A narrative review. J. Funct. Morphol. Kinesiol. 2024, 9, 93. [Google Scholar] [CrossRef]
- Sundas, A.; Contreras, I.; Navarro-Otano, J.; Soler, J.; Beneyto, A.; Vehi, J. Heart rate variability over the decades: A scoping review. PeerJ 2025, 13, e19347. [Google Scholar] [CrossRef]
- Young, H.A.; Benton, D. Heart-rate variability: A biomarker to study the influence of nutrition on physiological and psychological health? Behav. Pharmacol. 2018, 29, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yoo, S.D. Nutritional Biomarkers and Heart Rate Variability in Patients with Subacute Stroke. Nutrients 2022, 14, 5320. [Google Scholar] [CrossRef]
- Dikariyanto, V.; Smith, L.; Chowienczyk, P.J.; Berry, S.E.; Hall, W.L. Snacking on Whole Almonds for Six Weeks Increases Heart Rate Variability during Mental Stress in Healthy Adults: A Randomized Controlled Trial. Nutrients 2020, 12, 1828. [Google Scholar] [CrossRef]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J., 3rd; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; O’Neill, M.S.; Park, S.K.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am. J. Epidemiol. 2011, 173, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Damoun, N.; Amekran, Y.; Taiek, N.; Hangouche, A.J.E. Heart rate variability measurement and influencing factors: Towards the standardization of methodology. Glob. Cardiol. Sci. Pract. 2024, 2024, e202435. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, J.A.; Cleveland, E.L.; Barkstrom, E.; Gonzalez, J.E.; Carter, J.R. Core body temperature changes before sleep are associated with nocturnal heart rate variability. J. Appl. Physiol 2023, 135, 136–145. [Google Scholar] [CrossRef]
- Mowery, N.T.; Morris, J.A., Jr.; Jenkins, J.M.; Ozdas, A.; Norris, P.R. Core temperature variation is associated with heart rate variability independent of cardiac index: A study of 278 trauma patients. J. Crit. Care. 2011, 26, e9–e534. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart rate variability in elite triathletes: Is variation in variability the key to effective training? A case comparison. Eur. J. Appl. Physiol. 2012, 112, 3729–3741. [Google Scholar] [CrossRef]
- Carrasco-Poyatos, M.; González-Quílez, A.; Altini, M.; Granero-Gallegos, A. Heart rate variability-guided training in professional runners: Effects on performance and vagal modulation. Physiol. Behav. 2022, 244, 113654. [Google Scholar] [CrossRef]
- Dupuy, A.; Birat, A.; Maurelli, O.; Garnier, Y.M.; Blazevich, A.J.; Rance, M.; Ratel, S. Post-exercise heart rate recovery and parasympathetic reactivation are comparable between prepubertal boys and well-trained adult male endurance athletes. Eur. J. Appl. Physiol. 2022, 122, 345–355. [Google Scholar] [CrossRef]
- DeBlauw, J.A.; Drake, N.B.; Kurtz, B.K.; Crawford, D.A.; Carper, M.J.; Wakeman, A.; Heinrich, K.M. High-intensity functional training guided by individualized heart rate variability results in similar health and fitness improvements as predetermined training with less effort. J. Funct. Morphol. Kinesiol. 2021, 6, 102. [Google Scholar] [CrossRef]
- Bellenger, C.R.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. The impact of functional overreaching on post-exercise parasympathetic reactivation in runners. Front. Physiol. 2021, 11, 614765. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Hautala, A.J.; Kinnunen, H.; Nissilä, J.; Virtanen, P.; Karjalainen, J.; Tulppo, M.P. Daily exercise prescription on the basis of HR variability among men and women. Med. Sci. Sports Exerc. 2010, 42, 1355–1363. [Google Scholar] [CrossRef]
- Vesterinen, V.; Nummela, A.; Heikura, I.; Laine, T.; Hynynen, E.; Botella, J.; Häkkinen, K. Individual endurance training prescription with heart rate variability. Med. Sci. Sports Exerc. 2016, 48, 1347–1354. [Google Scholar] [CrossRef]
- Ruiz, J.P.M.; Rubio-Arias, J.; Clemente-Suarez, V.J.; Ramos-Campo, D.J. Effectiveness of training prescription guided by heart rate variability versus predefined training for physiological and aerobic performance improvements: A systematic review and meta-analysis. Appl. Sci. 2020, 10, 8532. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Hautala, A.J.; Kinnunen, H.; Tulppo, M.P. Endurance training guided individually by daily heart rate variability measurements. Eur. J. Appl. Physiol. 2007, 101, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac Autonomic Responses during Exercise and Post-Exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, G.R.H.; Brodie, D.A. The Use of Heart Rate Variability Measures to Assess Autonomic Control during Exercise. Scand. J. Med. Sci. Sports 2006, 16, 302–313. [Google Scholar] [CrossRef] [PubMed]
- McNarry, M.A.; Lewis, M.J. Heart Rate Variability Reproducibility during Exercise. Physiol. Meas. 2012, 33, 1123–1133. [Google Scholar] [CrossRef]
- Rogers, B.; Giles, D.; Draper, N.; Hoos, O. A New Detection Method Defining the Aerobic Threshold for Endurance Exercise and Training Prescription Based on Fractal Correlation Properties of Heart Rate Variability. Front. Physiol. 2021, 11, 1806. [Google Scholar] [CrossRef]
- Gronwald, T.; Rogers, B.; Hoos, O. Fractal Correlation Properties of Heart Rate Variability: A New Biomarker for Intensity Distribution in Endurance Exercise and Training Prescription? Front. Physiol. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Seiler, S.; Haugen, O.; Kuffel, E. Autonomic Recovery after Exercise in Trained Athletes: Intensity and Duration Effects. Med. Sci. Sports Exerc. 2007, 39, 1366–1373. [Google Scholar] [CrossRef]
- Perkins, S.E.; Jelinek, H.F.; Al-Aubaidy, H.A.; De Jong, B.; Fell, J. Immediate and Long-Term Effects of Endurance and High-Intensity Interval Exercise on Linear and Nonlinear Heart Rate Variability. J. Sci. Med. Sport. 2017, 20, 312–316. [Google Scholar] [CrossRef]
- Nuuttila, O.-P.; Kyröläinen, H.; Häkkinen, K. Acute Physiological Responses to Four Running Sessions Performed at Different Intensity Zones. Int. J. Sports Med. 2021, 42, 513–522. [Google Scholar] [CrossRef]
- Buchheit, M.; Millet, G.P.; Parisy, A.; Pourchez, S.; Laursen, P.B.; Ahmaidi, S. Supramaximal Training and Postexercise Parasympathetic Reactivation in Adolescents. Med. Sci. Sports Exerc. 2008, 40, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, E.; Vesterinen, V.; Rusko, H.; Nummela, A. Effects of Moderate and Heavy Endurance Exercise on Nocturnal HRV. Int. J. Sports Med. 2010, 31, 428–432. [Google Scholar] [CrossRef]
- Davletyarova, K.; Vacher, P.; Nicolas, M.; Basset, F.A.; Bieuzen, F. Associations between Heart Rate Variability-Derived Indexes and Training Load. J. Strength. Cond. Res. 2022, 36, 2005–2010. [Google Scholar] [CrossRef]
- Sánchez, R.P.; Alonso-Pérez-Chao, E.; Calleja-González, J.; Jiménez Sáiz, S.L. Heart Rate Variability in Basketball: The Golden Nugget of Holistic Adaptation? Appl. Sci. 2024, 14, 10013. [Google Scholar] [CrossRef]
- Lundstrom, C.J.; Foreman, N.A.; Biltz, G. Practices and Applications of Heart Rate Variability Monitoring in Endurance Athletes. Int. J. Sports Med. 2023, 44, 9–19. [Google Scholar] [CrossRef]
- Turcu, A.M.; Ilie, A.C.; Ștefăniu, R.; Țăranu, S.M.; Sandu, I.A.; Alexa-Stratulat, T.; Pîslaru, A.I.; Alexa, I.D. The Impact of Heart Rate Variability Monitoring on Preventing Severe Cardiovascular Events. Diagnostics 2023, 13, 2382. [Google Scholar] [CrossRef] [PubMed]
- De Maria, B.; Dalla Vecchia, L.A.; Porta, A.; La Rovere, M.T. Autonomic Dysfunction and Heart Rate Variability with Holter Monitoring: A Diagnostic Look at Autonomic Regulation. Herzschrittmacherther. Elektrophysiol. 2021, 32, 315–319. [Google Scholar] [CrossRef]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Cheshmedzhiev, K. Examination of Cardiac Activity with ECG Monitoring Using Heart Rate Variability Methods. Diagnostics 2024, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Gospodinov, M.; Cheshmedzhiev, K. Cardio-Diagnostic Assisting Computer System. Diagnostics 2020, 10, 322. [Google Scholar] [CrossRef]
- Schmitt, L.; Regnard, J.; Millet, G.P. Monitoring Fatigue Status with HRV Measures in Elite Athletes: An Avenue beyond RMSSD? Front. Physiol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Li, K.; Rüdiger, H.; Ziemssen, T. Spectral Analysis of Heart Rate Variability: Time Window Matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Mateo-March, M.; Moya-Ramón, M.; Javaloyes, A.; Sánchez-Muñoz, C.; Clemente-Suárez, V.J. Validity of Detrended Fluctuation Analysis of Heart Rate Variability to Determine Intensity Thresholds in Elite Cyclists. Eur. J. Sport. Sci. 2022, 23, 580–587. [Google Scholar] [CrossRef]
- Rogers, B.; Gronwald, T. Fractal correlation properties of heart rate variability as a biomarker for intensity distribution and training prescription in endurance exercise: An update. Front. Physiol. 2022, 13, 879071. [Google Scholar] [CrossRef]
- Bakhchina, A.V.; Arutyunova, K.R.; Sozinov, A.A.; Demidovsky, A.V.; Alexandrov, Y.I. Sample entropy of the heart rate reflects properties of the system organization of behaviour. Entropy 2018, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Zimatore, G.; Serantoni, C.; Gallotta, M.C.; Meucci, M.; Mourot, L.; Ferrari, D.; Baldari, C.; De Spirito, M.; Maulucci, G.; Guidetti, L. Recurrence quantification analysis-based methodology in automatic aerobic threshold detection: Applicability and accuracy across age groups, exercise protocols and health conditions. Appl. Sci. 2024, 14, 9216. [Google Scholar] [CrossRef]
- Błażkiewicz, M.; Hadamus, A.; Borkowski, R. Recurrence quantification analysis as a form of postural control assessment: A systematic review. Appl. Sci. 2023, 13, 5587. [Google Scholar] [CrossRef]
- Gospodinova, E.; Lebamovski, P.; Georgieva-Tsaneva, G.; Negreva, M. Evaluation of the Methods for Nonlinear Analysis of Heart Rate Variability. Fractal Fract. 2023, 7, 388. [Google Scholar] [CrossRef]
- Martinis, M. Changes in the Hurst exponent of heart rate variability during physical activity. Phys. Rev. E 2004, 70, 012903. [Google Scholar] [CrossRef]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Entropy analysis of heart rate variability and its application to recognize major depressive disorder: A pilot study. Technol. Health Care 2019, 27 (Suppl. 1), 407–424. [Google Scholar] [CrossRef]
- Zimatore, G.; Falcioni, L.; Gallotta, M.C.; Bonavolontà, V.; Campanella, M.; De Spirito, M.; Maulucci, G.; Guidetti, L. Recurrence quantification analysis of heart rate variability to detect both ventilatory thresholds. PLoS ONE 2021, 16, e0249504. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, D.; Saperova, E.V.; Dimitriev, A.; Karpenko, Y. Recurrence quantification analysis of heart rate during mental arithmetic stress in young females. Front. Physiol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Kaikkonen, P.; Nummela, A.; Rusko, H. Heart rate variability dynamics during early recovery after different endurance exercises. Eur. J. Appl. Physiol. 2007, 102, 79–86. [Google Scholar] [CrossRef]
- Gronwald, T.; Hoos, O. Correlation properties of heart rate variability during endurance exercise: A systematic review. Ann. Noninvasive Electrocardiol. 2020, 25, e12697. [Google Scholar] [CrossRef]
- Abad, C.C.C.; Pereira, L.A.; Zanetti, V.; Kobal, R.; Loturco, I.; Nakamura, F.Y. Short-Term Cardiac Autonomic Recovery after a Repeated Sprint Test in Young Soccer Players. Sports 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Špenko, M.; Potočnik, I.; Edwards, I.; Potočnik, N. Training History, Cardiac Autonomic Recovery from Submaximal Exercise and Associated Performance in Recreational Runners. Int. J. Environ. Res. Public. Health. 2022, 19, 9797. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Hong, W.J.; Jin, S.M.; Hur, K.Y.; Jee, J.H.; Bae, J.C.; Kim, J.H.; Lee, M.K. Delayed heart rate recovery after exercise predicts development of metabolic syndrome: A retrospective cohort study. J. Diabetes Investig. 2022, 13, 167–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgieva-Tsaneva, G.; Cheshmedzhiev, K.; Tsanev, Y.-A.; Dechev, M.; Popovska, E. Healthcare monitoring using an Internet of Things-based cardio system. IoT 2025, 6, 10. [Google Scholar] [CrossRef]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Cheshmedzhiev, K. Cardiodiagnostics based on photoplethysmographic signals. Diagnostics 2022, 12, 412. [Google Scholar] [CrossRef]
- Alugubelli, N.; Abuissa, H.; Roka, A. Wearable devices for remote monitoring of heart rate and heart rate variability—What we know and what is coming. Sensors 2022, 22, 8903. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The accuracy of acquiring heart rate variability from portable devices: A systematic review and meta-analysis. Sports Med. 2019, 49, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; White, G.; Armstrong, N. Wearable devices suitable for monitoring twenty-four-hour heart rate variability in military populations. Sensors 2021, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).