Abstract
Background: Bariatric surgery is a well-established intervention for severe obesity, resulting in substantial weight loss and cardiometabolic benefits. However, the physiological mechanisms driving changes in functional capacity postoperatively remain incompletely characterized. Methods: Fourteen patients scheduled for bariatric surgery underwent serial assessments preoperatively and at 3 and 6 months postoperatively. Evaluations included body composition analysis, cardiopulmonary exercise testing (CPET), and non-invasive measurement of cardiac output (CO) and arteriovenous oxygen difference (AVDiff) using the inert gas rebreathing (IGR) method. Patients were stratified into four hemodynamic response profiles (Q1–Q4) based on directional changes in CO and AVDiff. Repeated measures changes were analyzed using Friedman and Wilcoxon signed-rank tests; correlations were assessed using Spearman’s rank method. Results: Following surgery, patients exhibited significant reductions in BMI, body fat percentage, and CO at both rest and peak exercise (all p < 0.05). VO2 peak was preserved or modestly improved. Notably, the majority of patients exhibited a response pattern of reduced CO accompanied by increased AVDiff (Q1), both at rest and during exertion. At three months, VO2 rest correlated positively with both CO rest and AVDiff rest, while VO2 peak correlated with AVDiff peak. At six months, VO2 rest and VO2 peak both correlated positively with CO peak. Conclusions: Aerobic performance following bariatric surgery is not solely determined by peak oxygen uptake but also depends on the interaction between cardiac output and oxygen extraction, as reflected by AVDiff. These findings align with the Fick principle (VO2 = CO × AVDiff) and emphasize the importance of comprehensive physiological profiling—beyond VO2 peak alone—in understanding adaptation after bariatric surgery.
1. Introduction
Obesity is a growing global epidemic and a leading cause of preventable mortality and morbidity. According to the World Health Organization, over 650 million adults worldwide are obese, with prevalence continuing to rise in both developed and developing countries. In Poland, national data from 2019 to 2020 indicate that approximately 23% of adults and 11% of children are obese, highlighting the substantial public health burden posed by this condition [1].
Beyond its metabolic consequences, obesity exerts profound effects on both the respiratory and cardiovascular systems. Respiratory complications are largely driven by the mechanical impact of adipose tissue on the chest wall and diaphragm, resulting in reduced lung compliance, decreased functional residual capacity, and increased work in breathing. These alterations contribute to increased prevalence of obstructive sleep apnea, obesity hypoventilation syndrome, and pulmonary hypertension [2].
From a cardiovascular perspective, obesity leads to an increase in blood volume and metabolic demand, necessitating an elevated cardiac output (CO). This is typically achieved through increases in stroke volume and heart rate, often resulting in left ventricular hypertrophy and diastolic dysfunction. Right ventricular function may also be compromised due to elevated pulmonary pressures and systemic volume overload, particularly in cases of obesity-related high-output heart failure [3,4].
Cardiopulmonary exercise testing (CPET) provides an integrative assessment of cardiovascular, pulmonary, and metabolic responses to physical stress, and its interpretation in individuals with obesity requires careful physiological context. Obesity is associated with a distinct pattern of exercise limitation characterized by reduced peak oxygen uptake (VO2 peak) when expressed relative to body mass, despite normal or even elevated absolute values in liters per minute [5]. Additional CPET features frequently reported in this population include an earlier onset of the anaerobic threshold, higher ventilatory equivalents of carbon dioxide (VE/VCO2 slope), and a lower oxygen pulse, all of which indicate suboptimal oxygen transport and utilization efficiency during exertion [5,6,7,8].
Bariatric surgery is the most effective long-term treatment for morbid obesity [8,9,10]. In addition to inducing substantial and sustained weight loss, it leads to resolution or improvement of numerous obesity-related comorbidities, including hypertension, type 2 diabetes mellitus, and sleep apnea. Postoperative studies demonstrate improvements in cardiac function, reduction in systemic vascular resistance, and normalization of blood pressure [11,12,13,14,15,16,17]. Simultaneously, CPET findings often show increased VO2 peak, improved ventilatory efficiency, and enhanced exercise tolerance [8,18,19,20].
While the general cardiovascular benefits of bariatric surgery are well established, the physiological mechanisms underlying these improvements—particularly during physical exertion—remain incompletely understood. Specifically, few studies have examined postoperative cardiovascular changes through the conceptual framework of the Fick principle:
(VO2 = CO × [CaO2 − CvO2]),
A cornerstone of cardiovascular physiology [21,22].
In clinical and physiological practice, this equation provides a framework to determine whether improved aerobic capacity arises primarily from central adaptations (i.e., increased cardiac output) or from peripheral mechanisms (i.e., enhanced tissue oxygen extraction). Although numerous studies have described changes in VO2 peak or CO after bariatric surgery [13,18,19,20], very few have integrated CPET with non-invasive cardiac output measurement, and virtually none have explicitly examined the arteriovenous oxygen difference (AVDiff) in this context. Even when non-invasive hemodynamic monitoring has been applied, such as in the assessment of blood pressure and cardiac output changes after gastric bypass [17], AVDiff was not considered. To our knowledge, this is among the first studies to apply inert gas rebreathing during CPET in bariatric patients, thereby enabling a direct estimation of both cardiac output and AVDiff. By combining these measurements within the framework of the Fick principle, we aim to provide a novel, integrative perspective on postoperative cardiopulmonary adaptation, addressing a previously underexplored mechanism of physiological change following surgical weight loss.
2. Materials and Methods
2.1. Study Design and Population
This prospective observational study enrolled adult patients with severe obesity (BMI ≥ 35 kg/m2), scheduled to undergo laparoscopic sleeve gastrectomy at a tertiary bariatric center. Patients who underwent other bariatric procedures, such as Roux-en-Y gastric bypass or biliopancreatic diversion, were excluded in order to maintain a homogeneous study cohort and to minimize variability in physiological outcomes related to differences in surgical technique. Inclusion criteria included age between 18 and 65 years, eligibility for surgical treatment of obesity according to national and international guidelines, and the absence of significant cardiopulmonary, oncologic, or psychiatric contraindications. All participants provided written informed consent.
Patients underwent a structured assessment at three predefined time points:
- Two weeks prior to the surgery (before);
- Three months after the surgery (3 m);
- Six months after the surgery (6 m).
Each visit included body composition analysis using bioelectrical impedance (Tanita, Tokyo, Japan), spirometry (Pony FX, Cosmed, Rome, Italy; GLI 2012 normal values), cardiopulmonary exercise testing (CPET) on cycloergometer (Ergoselect, Ergoline GmbH, Bitz, Germany), and non-invasive cardiac output (CO) measurements using inert gas rebreathing (IGR) method (Innocor®, Odense, Denmark). Oxygen uptake (VO2), CO, and arteriovenous oxygen difference (AVDiff) were evaluated both at rest and at peak exercise. Percent changes (Δ%) from baseline were calculated and allowed to stratify the patients into four hemodynamic response groups during further analysis.
2.2. Cardiopulmonary Exercise Testing (CPET)
CPET was conducted using the Innocor® system (ver. 8.10), under continuous supervision by trained personnel. The ergometer protocol was individualized based on the Wasserman prediction equation, accounting for age, sex, weight, and baseline fitness level [22]. The protocol included a 2 min unloaded pedaling phase followed by a ramp increase in workload, designed to reach symptom-limited maximal exertion within 8–12 min. Respiratory gas exchange parameters (VO2, VCO2, VE), ventilatory equivalents (etCO2, etO2), respiratory exchange ratio (RER), and heart rate were recorded breath-by-breath. Peak VO2 was determined as the average value over the final 30 s of exercise, with a respiratory exchange ratio (RER) ≥ 1.1 considered a criterion of maximal effort.
2.3. Inert Gas Rebreathing (IGR)
Non-invasive measurement of cardiac output and pulmonary blood flow was performed using the inert gas rebreathing (IGR) technique. The methodology is based on the differential absorption of an insoluble marker gas (sulfur hexafluoride, SF6) and a soluble gas (nitrous oxide, N2O) during a closed-circuit rebreathing maneuver [23].
Each session began with calibration of the gas analyzer and flow sensors using reference gas mixtures and a 3 L calibration syringe, according to manufacturer specifications.
Participants were instructed to breathe through a mouthpiece attached to a two-way valve with a bacterial filter and a nose clip. At the end of a normal expiration, the investigator manually initiated a rebreathing maneuver from a pre-inflated 3 L bag containing a mixture of 0.1% SF6 and 0.5% N2O in oxygen-enriched air. The subject rebreathed from the bag for 10–15 s at tidal volumes, typically completing 3–5 breaths, while the system continuously recorded gas concentrations using photoacoustic spectroscopy. Pulmonary blood flow (PBF) was calculated from the washout rate of N2O, as this gas is absorbed by the pulmonary capillary blood in proportion to perfusion. Cardiac output (CO) was derived from PBF under the assumption of complete pulmonary gas exchange, defined as oxygen saturation (SpO2) of at least 98%. When oxygen saturation was below this threshold, a correction for estimated pulmonary shunt flow was applied. Lung volume was determined simultaneously from the dilution of SF6 [23].
Measurements were conducted twice per visit: once in a sitting rest state, and once during peak exercise, immediately before termination of the CPET. The timing of the exercise-phase IGR was optimized to reflect the maximum physiological effort: the subject was instructed to signal the moment they were nearing exhaustion, and rebreathing was triggered within that final exertional phase. Importantly, a minimum interval of three minutes was maintained between resting and peak measurements to allow for complete washout of marker gases, in accordance with manufacturer recommendations [23].
2.4. Ethical Issues
The study protocol was approved by the Local Bioethical Committee of medical University of Bialystok (No. APK.002.262.2022) and complied with the Declaration of Helsinki.
2.5. Statistical Analysis
All statistical analyses were conducted using Python (v3.11, Python Software Foundation) and R (v4.3.3, R Foundation for Statistical Computing). Data preprocessing and descriptive analyses were performed in Python with the pandas (v2.2.2) and numpy (v1.26.4) libraries, while inferential statistics employed scipy (v1.13.0). Continuous variables are reported as medians with interquartile ranges (IQR) and 95% confidence intervals (CI), reflecting the small sample size and the non-normal distribution of several variables. Distributional assumptions were assessed using the Shapiro–Wilk test. Within-subject comparisons across the three time points (baseline, 3 months, and 6 months) were evaluated using the Wilcoxon signed-rank test for paired data (baseline–3 m, 3–6 m, baseline–6 m). Results are reported as p-values, with statistical significance set at p < 0.05. No corrections for multiple comparisons were applied due to the exploratory nature of the study. To capture interindividual variability in postoperative adaptation, patients were stratified into hemodynamic response profiles (Q1–Q4) based on the direction of change in cardiac output (ΔCO) and arteriovenous oxygen difference (ΔAVDiff), separately for rest and peak exercise conditions at 3 and 6 months. Group distributions were analyzed using Fisher’s exact test. To explore associations between study variables, Spearman’s rank correlation coefficients (ρ) were calculated pairwise between parameters at 3 and 6 months. Correlation results are reported in matrix format, with significant findings (p < 0.05) marked with an asterisk. No imputation of missing data was performed.
In this study, generative artificial intelligence (GenAI) was used exclusively for the creation of illustrative graphics included in the article (chat GPT ver. 4.5).
3. Results
A total of 24 patients with severe obesity were enrolled in the study. All subjects underwent laparoscopic sleeve gastrectomy. Out of this group, 14 patients finished all planned visits and were included in the final analysis. The remaining 10 patients were classified as dropouts. The reasons for dropout included lack of willingness to continue participation in follow-up assessments, despite no medical contraindications, as well as difficulties with attending scheduled visits. No withdrawals were related to perioperative complications or adverse clinical events. The baseline characteristics of the study cohort are presented in Table 1. The analysis compared participants who completed the study protocol (n = 14) with those who withdrew prior to completion (n = 10). Statistical testing revealed no significant differences between the two groups for any measured variable (all p > 0.05).

Table 1.
Characteristics of the study cohort.
Descriptive Characteristics of the Study Population
At baseline, participants presented with severe obesity, reflected by a median body weight of 144.0 kg, BMI of 47.75 kg/m2, and fat free mass of 79.4 kg. Following bariatric surgery, substantial reductions in anthropometric indices were observed. At three months, median body weight decreased to 121,95 kg, BMI to 38.95 kg/m2, and fat free mass increased to 83.55 kg. At six months, weight further decreased to 112.3 kg, BMI to 35.52 kg/m2, and fat free mass to 77.75 kg. Body composition analysis revealed a progressive decline in body fat, both in percentage (from 51.2% at baseline to 34.9% at six months) and in absolute kilograms (from 69.7 kg to 36.5 kg). Conversely, muscle mass percentage increased from 46.4% at baseline to 61.4% at six months. Functional indices expressed relative to body weight showed parallel changes. VO2 peak per kilogram decreased from a median of 16 mL/min/kg at baseline to 10.15 mL/min/kg at three months, with partial recovery to 14.3 mL/min/kg at six months. VO2 at rest per kilogram remained stable across visits, with values of 1.8, 2.2, and 2.1 mL/min/kg, respectively. The full descriptive statistics for each parameter across the three time points are presented in Table 2.

Table 2.
Full descriptive analysis.
Analysis with the Wilcoxon signed-rank test confirmed significant reductions in body weight and BMI at both three and six months after surgery compared to baseline, with additional decreases observed between the third and sixth month (p < 0.05). Body fat percentage and absolute fat mass declined significantly across all time points (p < 0.05). Muscle mass percentage increased consistently during follow-up (p < 0.05), while absolute muscle mass rose at three months but decreased again by month six, resulting in a lower median compared to baseline (p < 0.05). Fat-free mass also increased significantly at three months (p = 0.017) and subsequently decreased at six months (p = 0.007), reflecting a similar biphasic trend. Regarding cardiopulmonary parameters, resting oxygen uptake (L/min) did not change significantly across visits (all p > 0.5). Peak oxygen uptake (L/min) was lower at both three and six months compared with baseline (p < 0.05), without further change between three and six months (p = 0.16). When normalized to body weight, VO2 rest expressed in mL/min/kg remained stable (all p > 0.05), whereas VO2 peak (mL/min/kg) decreased significantly after surgery (p < 0.05), with no difference between the two postoperative time points. Resting cardiac output decreased significantly at three and six months compared with baseline (p < 0.05), while peak cardiac output declined in all pairwise comparisons (p < 0.05). The arteriovenous oxygen difference at rest increased significantly after surgery (p < 0.05), whereas at peak exercise it remained unchanged compared with baseline (p = 0.43 and p = 0.58) but differed significantly between the third and sixth month (p < 0.05). Exact p-values are provided in Table 3, with statistically significant comparisons marked with an asterisk.

Table 3.
Pairwise comparisons of physiological and hemodynamic parameters across time points using Wilcoxon signed-rank test.
To assess interindividual variability in cardiovascular adaptation following bariatric surgery, patients were stratified into four hemodynamic response profiles based on the direction of change in cardiac output (ΔCO) and arteriovenous oxygen difference (ΔAvDiff) named groups Q1 to Q4, respectively. The classification was performed at 3 and 6 months postoperatively using the following criteria:
- Group Q1: increase in AVDiff, decrease in CO (↑AVDiff, ↓CO);
- Group Q2: increase in both AVDiff and CO (↑AVDiff, ↑CO);
- Group Q3: decrease in AVDiff, increase in CO (↓AVDiff, ↑CO);
- Group Q4: decrease in both AVDiff and CO (↓AVDiff, ↓CO).
The resulting distributions are summarized in Table 4.

Table 4.
Hemodynamic Response Groups (Q1–Q4) with Fisher’s Exact Test.
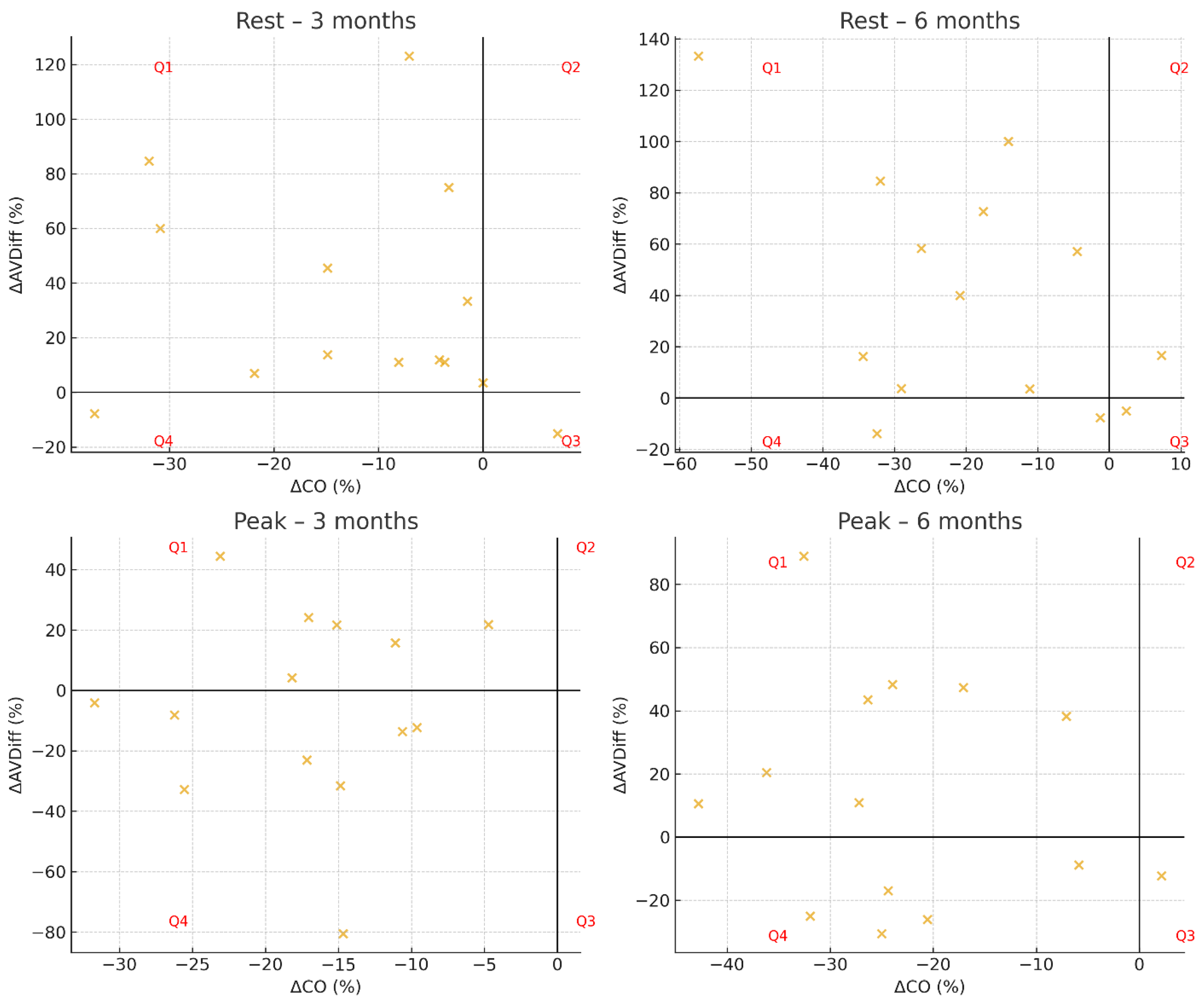
At three months after surgery, the distribution of patients across hemodynamic response groups differed significantly from uniformity at rest (p = 0.048), whereas no significant difference was observed at peak exercise (p = 0.075). At six months, the distribution did not differ significantly from uniformity, either at rest (p = 0.097) or at peak exercise (p = 0.130). Individual patient trajectories are presented in Figure 1, which displays scatterplots of ΔCO versus ΔAVDiff for each time point and condition.
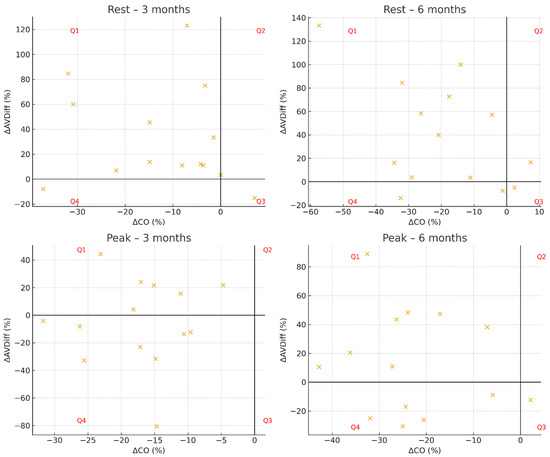
Figure 1.
Hemodynamic response profiles in Groups Q1–Q4.
At both three and six months, patients were predominantly classified into Group Q1 (↑AVDiff, ↓CO), with fewer individuals assigned to Group Q4 (↓AVDiff, ↓CO). At rest, Group Q1 generally showed higher VO2 rest and CO rest compared with Group Q4, whereas fat free mass and absolute muscle mass were comparable. Muscle mass percentage was slightly higher in Q4, while body fat percentage remained lower in Q1 across follow-up visits. During peak exercise, Group Q1 demonstrated higher VO2 peak in absolute values, as well as when expressed relative to body weight (mL/min/kg), compared with Q4 at both time points. Peak cardiac output was similar between the groups, while Group Q4 tended to show a relatively higher muscle mass percentage. Detailed descriptive statistics for each parameter, including BMI, body composition, VO2 (absolute and relative), cardiac output, and arteriovenous oxygen difference, are presented in Table 5.

Table 5.
Group Q1 vs. Group Q4 Comparison (Rest and Peak).
To explore the associations between body composition and functional performance, Spearman correlation analyses were performed using a pairwise approach for each time point. For clarity, Table 6a,b present only statistically significant correlations (p < 0.05). At three months (Table 6a), VO2 rest correlated positively with both CO rest (ρ = 0.73, p = 0.011) and AVDiff rest (ρ = 0.77, p = 0.005), while VO2 peak correlated positively with AVDiff peak (ρ = 0.75, p = 0.007). At six months (Table 6b), VO2 rest correlated positively with CO peak (ρ = 0.63, p = 0.027), and VO2 peak correlated strongly with CO peak (ρ = 0.83, p < 0.05). No significant correlations were observed between fat free mass, relative oxygen uptake (mL/min/kg), or other body composition indices and cardiopulmonary parameters at either time point.

Table 6.
Correlation Analysis: Body Composition and Cardiopulmonary Parameters—(a) 3 months, (b) 6 months.
4. Discussion
The present study explored cardiopulmonary and hemodynamic adaptations after bariatric surgery using an integrative framework based on the Fick principle. Consistently with previous reports, substantial postoperative weight reduction was accompanied by a significant decline in both resting and peak cardiac output, while functional capacity, assessed by oxygen uptake, was preserved or improved. This dissociation suggests that early postoperative gains in performance are predominantly mediated by enhanced peripheral oxygen extraction rather than central circulatory output. Such findings fit within the physiological context of obesity-related circulatory overload, in which weight loss reduces metabolic demand and blood volume, thereby alleviating central load and facilitating muscular oxidative adaptations. To capture interindividual variability in adaptation, patients were stratified into hemodynamic response profiles (Q1–Q4) according to the direction of change in cardiac output and arteriovenous oxygen difference. The majority of patients were classified into Q1 (↑AVDiff, ↓CO) or Q4 (↓AVDiff, ↓CO), whereas groups characterized by an increase in cardiac output (Q2, Q3) were rarely observed. Comparative analysis between Q1 and Q4 showed that Q1 patients generally exhibited higher VO2 rest and CO rest, and tended to achieve higher VO2 peak, whereas Q4 was characterized by relatively higher muscle mass percentage despite lower oxygen uptake. These patterns indicate that although both groups shared reductions in central output, their compensatory capacity via peripheral extraction differed, underscoring heterogeneity in postoperative adaptation. In the whole cohort, VO2 rest correlated positively with both CO rest and AVDiff rest at three months, while VO2 peak correlated with AVDiff peak. At six months, VO2 rest and VO2 peak were both positively correlated with CO peak. By contrast, most associations between anthropometric parameters and hemodynamic outcomes were not statistically significant.
An apparent increase in absolute muscle mass and fat-free mass at three months, followed by a subsequent decline at six months, was observed in our cohort. This trajectory differs from most reports in the literature, where muscle mass usually decreases continuously after bariatric surgery [24,25,26]. Several factors may explain this discrepancy. First, bioimpedance analysis is highly sensitive to fluid status, and postoperative shifts in hydration—common during the early recovery period—can transiently elevate estimates of lean compartments. Second, the rapid and substantial fat loss occurring in the first months may alter the relative distribution of body compartments in prediction algorithms, yielding an apparent increase in lean mass despite the absence of true hypertrophy. Third, the relatively small sample size makes the analysis vulnerable to the influence of outliers, so that a few individuals with higher lean mass at follow-up could disproportionately affect group medians.
Taken together, these findings suggest that early functional recovery is determined by a dynamic balance between central and peripheral components of oxygen transport, rather than by linear relationships with body composition indices. The observed postoperative trajectory is consistent with earlier work showing reductions in cardiac output after bariatric surgery, most likely related to decreased blood volume and metabolic demand. In a meta-analysis by Reddy et al. [13], weight loss interventions were shown to significantly decrease cardiac output, stroke volume, and left ventricular mass, supporting the concept that reduced circulatory load is a key mechanism of hemodynamic improvement. Sorimachi et al. [27] demonstrated long-term remodeling after bariatric surgery, with reductions in left ventricular volumes and mass accompanied by improvements in diastolic function, further highlighting the beneficial effects of sustained weight loss on cardiac structure and performance. Similarly, Alpert et al. [28] reported that weight reduction is associated with normalization of cardiac morphology and improvements in systolic and diastolic function, while Koschker et al. [29], in a randomized controlled trial, confirmed that bariatric surgery leads to significant improvements in cardio-metabolic outcomes, including reductions in cardiac output and blood pressure. Our results provide additional insight by showing that despite a consistent postoperative decline in cardiac output, functional capacity was preserved through enhanced oxygen extraction. The dissociation between reduced cardiac output and preserved or even improved VO2 peak has important clinical implications. In obese individuals, chronically elevated cardiac output reflects increased metabolic demand and expanded blood volume, contributing to high-output heart failure and adverse cardiac remodeling. The postoperative reduction in cardiac output therefore likely represents a beneficial unloading of the heart, decreasing circulatory stress without impairing functional capacity. Preservation of VO2 peak despite lower central output suggests that peripheral adaptations—improved skeletal muscle oxygen extraction and metabolic efficiency—compensate for reduced cardiac work. This mechanism resonates with the clinical context of obesity-related high-output heart failure (HOHF), a condition characterized by elevated cardiac output secondary to increased metabolic requirements and expanded plasma volume [4]. Chronic volume overload in HOHF can drive ventricular remodeling, diastolic dysfunction, and eventually systolic impairment. The marked reduction in cardiac output observed in our cohort, together with preserved or improved functional capacity, may represent a partial reversal of this maladaptive hemodynamic state. Profiling into Q1–Q4 subgroups further emphasizes that such adaptations are not uniform and may be clinically relevant for individualized prognosis and management. From a clinical standpoint, these results underline the importance of evaluating not only peak oxygen uptake but also its physiological determinants-cardiac output and arteriovenous oxygen difference-when monitoring postoperative recovery. The integration of CPET with non-invasive hemodynamic monitoring provides a multidimensional view of adaptation that may inform perioperative risk stratification and tailored rehabilitation. For instance, patients with insufficient peripheral extraction may benefit from targeted exercise programs aimed at enhancing muscle oxidative capacity.
Several limitations should be acknowledged. First, the relatively small sample size limits the generalizability of the findings and reduces the statistical power, particularly for subgroup analyses. Moreover, attrition was considerable, with nearly half of the initially enrolled participants not completing the study. Although baseline comparisons suggested no major differences between completers and dropouts, the risk of attrition bias cannot be excluded. Another limitation is that no correction for multiple comparisons or post hoc testing was performed. While this approach increases the risk of type I error, it was considered acceptable in the context of an exploratory pilot study with limited sample size, and the findings should therefore be interpreted with caution. The short-term follow-up captures only the early phase of adaptation, and longer-term trajectories remain to be defined. Finally, while inert gas rebreathing offers a non-invasive method for estimating cardiac output, accuracy may be influenced by technical and physiological factors, particularly at peak exercise. Future studies should therefore include larger, more diverse cohorts, apply more rigorous statistical frameworks, and extend follow-up to better delineate temporal patterns of cardiopulmonary adaptation.
5. Conclusions
Bariatric surgery in patients with obesity resulted in early and significant improvements in body composition, accompanied by a marked reduction in cardiac output at both rest and peak exercise. Despite these hemodynamic changes, aerobic performance—as measured by VO2 peak—was largely preserved or modestly improved. Most patients exhibited a favorable hemodynamic adaptation pattern, characterized by reduced cardiac output and increased arteriovenous oxygen difference (AVDiff), indicative of enhanced peripheral oxygen extraction. These findings emphasize the dominant role of peripheral mechanisms in early postoperative functional adaptation and support the application of comprehensive cardiopulmonary exercise testing (CPET) with non-invasive hemodynamic profiling. Importantly, they advocate for a multidimensional physiological evaluation—beyond VO2 peak alone—to more accurately capture meaningful adaptations following bariatric surgery. Future research should confirm these observations in larger cohorts with longer follow-up and explore whether such physiological profiling can improve clinical outcomes and guide personalized rehabilitation after bariatric surgery.
Author Contributions
Conceptualization, A.C. and Ł.M.; methodology, Ł.M.; software, Ł.M.; validation, A.C., R.M.M.; formal analysis, H.R.H.; investigation, A.C.; resources, A.C.; data curation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, Ł.M., H.R.H. and R.M.M.; visualization, A.C.; supervision, H.R.H., R.M.M.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the subsidy from the Medical University of Białystok, Poland. Funding numbers: B.SUB.23.178, B.SUB.24.107, B.SUB.25.146.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AT | Anaerobic Threshold |
| AVDiff | Arteriovenous Oxygen Difference |
| BMI | Body Mass Index |
| BPD-DS | Biliopancreatic Diversion with Duodenal Switch |
| CaO2 | Arterial Oxygen Content |
| CO | Cardiac Output |
| CPET | Cardiopulmonary Exercise Testing |
| CvO2 | Venous Oxygen Content |
| DASI | Duke Activity Status Index |
| ESS | Epworth Sleepiness Scale |
| GLP-1 | Glucagon-Like Peptide-1 |
| HOHF | High-Output Heart Failure |
| IGR | Inert Gas Rebreathing |
| IQR | Interquartile Range |
| LVH | Left Ventricular Hypertrophy |
| MRC | Medical Research Council Dyspnoea Scale |
| N2O | Nitrous Oxide |
| NoSAS | NoSAS Score (Sleep Apnea Screening Score) |
| OWLQOL | Obesity and Weight-Loss Quality of Life Instrument |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PBF | Pulmonary Blood Flow |
| RER | Respiratory Exchange Ratio |
| RYGB | Roux-en-Y Gastric Bypass |
| SF6 | Sulphur Hexafluoride |
| SpO2 | Peripheral Oxygen Saturation |
| STOP-Bang | Snoring, Tiredness, Observed Apnea, high blood Pressure, BMI, Age, Neck circumference, Gender questionnaire |
| T2DM | Type 2 Diabetes Mellitus |
| TNF-α | Tumor Necrosis Factor Alpha |
| VE | Minute Ventilation |
| VE/VCO2 slope | Ventilatory Equivalent for Carbon Dioxide Slope |
| VO2 | Oxygen Uptake |
| VO2 peak | Peak Oxygen Uptake |
| VSAQ | Veterans Specific Activity Questionnaire |
| WHO | World Health Organization |
| WHR | Waist-to-Hip Ratio |
References
- Fijałkowska, A.; Dzielska, A.; Mazur, J.; Korzycka, M.; Breda, J.; Oblacińska, A. Childhood Obesity Surveillance Initiative (COSI) in Poland: Implementation of Two Rounds of the Study in the Context of International Methodological Assumptions. J. Mother Child 2020, 24, 2. [Google Scholar]
- Parameswaran, K.; Todd, D.C.; Soth, M. Altered respiratory physiology in obesity. Can. Respir. J. 2006, 13, 203. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Bostick, B.P. Effects of Obesity on Cardiovascular Hemodynamics, Cardiac Morphology, and Ventricular Function. Curr. Obes. Rep. 2016, 5, 424–434. Available online: https://pubmed.ncbi.nlm.nih.gov/27744513/ (accessed on 4 May 2023). [CrossRef] [PubMed]
- Shen, Q.; Hiebert, J.B.; Rahman, F.K.; Krueger, K.J.; Gupta, B.; Pierce, J.D. Understanding Obesity-Related High Output Heart Failure and Its Implications. Int. J. Heart Fail. 2021, 3, 160. [Google Scholar] [CrossRef] [PubMed]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.L.; Muls, E. Exercise capacity in lean versus obese women. Scand. J. Med. Sci. Sports 2001, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.É.; Phillips, D.B.; Brotto, A.R.; Rampuri, Z.H.; Stickland, M.K. Ventilatory efficiency in athletes, asthma and obesity. Eur. Respir. Rev. 2021, 30, 200206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schraufnagel, D.E.; Agostoni, P. Cardiopulmonary Exercise Testing. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S1–S2. Available online: https://pubmed.ncbi.nlm.nih.gov/28746821/ (accessed on 11 June 2023). [CrossRef]
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Whipp, B. (Eds.) Principles of Exercise Testing and Interpretation. Can. J. Cardiol. 2007, 23, 274. [Google Scholar]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res. 2016, 118, 1844. [Google Scholar] [CrossRef]
- Dadan, J.; Iwacewicz, P.; Hady, H.R. New approaches in bariatric surgery. Wideochirurgia Inne Tech. Maloinwazyjne 2008, 3, 66–70. [Google Scholar]
- Wojciak, P.A.; Pawłuszewicz, P.; Diemieszczyk, I.; Komorowska-Wojtunik, E.; Czerniawski, M.; Krętowski, A.; Błachnio-Zabielska, A.; Dadan, J.; Ładny, J.R.; Hady, H.R. Laparoscopic sleeve gastrectomy: A study of efficiency in treatment of metabolic syndrome components, comorbidities and influence on certain biochemical markers. Videosurgery Other Miniinvasive Tech. 2019, 15, 136–147. [Google Scholar] [CrossRef]
- Schauer, P.; Mingrone, G.; Ikramuddin, S.; Wolfe, B. Clinical Outcomes of Metabolic Surgery: Efficacy of Glycemic Control, Weight Loss, and Remission of Diabetes. Diabetes Care 2016, 39, 902–911. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=ovftr&NEWS=N&AN=00003458-201606000-00009 (accessed on 8 December 2022). [CrossRef] [PubMed]
- Reddy, Y.N.; Anantha-Narayanan, M.; Obokata, M.; Koepp, K.E.; Erwin, P.; Carter, R.E.; Borlaug, B.A. Hemodynamic Effects of Weight Loss in Obesity: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2019, 7, 678–687. Available online: https://pubmed.ncbi.nlm.nih.gov/31302042/ (accessed on 5 June 2023). [CrossRef]
- Sandoval, D.A.; Patti, M.E. Glucose metabolism after bariatric surgery: Implications for T2DM remission and hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. Available online: https://pubmed.ncbi.nlm.nih.gov/36289368/ (accessed on 5 June 2023). [CrossRef]
- Schlottmann, F.; Galvarini, M.M.; Dreifuss, N.H.; Laxague, F.; Buxhoeveden, R.; Gorodner, V. Metabolic Effects of Bariatric Surgery. J. Laparoendosc. Adv. Surg. Tech A 2018, 28, 944–948. Available online: https://pubmed.ncbi.nlm.nih.gov/30004821/ (accessed on 5 June 2023). [CrossRef] [PubMed]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. Available online: https://pubmed.ncbi.nlm.nih.gov/32870301/ (accessed on 7 June 2023). [CrossRef] [PubMed]
- van Brussel, P.M.; van den Bogaard, B.; de Weijer, B.A.; Truijen, J.; Krediet, C.P.; Janssen, I.M.; van de Laar, A.; Kaasjager, K.; Fliers, E.; Van Lieshout, J.J.; et al. Blood pressure reduction after gastric bypass surgery is explained by a decrease in cardiac output. J. Appl. Physiol. 2017, 122, 223–229. [Google Scholar] [CrossRef]
- Remígio, M.I.; Santa Cruz, F.; Ferraz, Á.; Remígio, M.C.; Parente, G.; Nascimento, I.; Brandão, D.; Dornelas de Andrade, A.D.F.; de Moraes Neto, F.; Campos, J. The Impact of Bariatric Surgery on Cardiopulmonary Function: Analyzing VO2 Recovery Kinetics. Obes. Surg. 2018, 28, 4039–4044. [Google Scholar] [CrossRef]
- Zavorsky, G.S.; Kim, D.J.; Christou, N.V. Compensatory exercise hyperventilation is restored in the morbidly obese after bariatric surgery. Obes. Surg. 2008, 18, 549–559. Available online: https://pubmed.ncbi.nlm.nih.gov/18360754/ (accessed on 23 May 2023). [CrossRef]
- Borasio, N.; Neunhaeuserer, D.; Gasperetti, A.; Favero, C.; Baioccato, V.; Bergamin, M.; Busetto, L.; Foletto, M.; Vettor, R.; Ermolao, A. Ventilatory Response at Rest and During Maximal Exercise Testing in Patients with Severe Obesity Before and After Sleeve Gastrectomy. Obes. Surg. 2021, 31, 694–701. [Google Scholar] [CrossRef]
- Inadomi, C.; Terao, Y.; Yamashita, K.; Fukusaki, M.; Takada, M.; Sumikawa, K. Comparison of oxygen consumption calculated by Fick’s principle (using a central venous catheter) and measured by indirect calorimetry. J. Anesth. 2008, 22, 163–166. Available online: https://pubmed.ncbi.nlm.nih.gov/18500614/ (accessed on 8 August 2025). [CrossRef] [PubMed]
- Bizouarn, P.; Soulard, D.; Blanloeil, Y.; Guillet, A.; Goarin, Y. Oxygen consumption after cardiac surgery -a comparison between calculation by Fick’s principle and measurement by indirect calorimetry. Intensive Care Med. 1992, 18, 206–209. Available online: https://pubmed.ncbi.nlm.nih.gov/1430583/ (accessed on 8 August 2025). [CrossRef]
- Chwiedź, A.; Minarowski, Ł.; Mróz, R.M.; Razak Hady, H. Non-Invasive Cardiac Output Measurement Using Inert Gas Rebreathing Method during Cardiopulmonary Exercise Testing—A Systematic Review. J. Clin. Med. 2023, 12, 7154. [Google Scholar] [CrossRef]
- Vaurs, C.; Diméglio, C.; Charras, L.; Anduze, Y.; Chalret du Rieu, M.; Ritz, P. Determinants of changes in muscle mass after bariatric surgery. Diabetes Metab. 2015, 41, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.; Meli, E.F.; Candia, F.P.; Filippi, F.; Vilallonga, R.; Cordero, E.; Hernández, I.; Eguinoa, A.Z.; Burgos, R.; Vila, A.; et al. The Impact of Bariatric Surgery on the Muscle Mass in Patients with Obesity: 2-Year Follow-up. Obes. Surg. 2022, 32, 625–633. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorimachi, H.; Obokata, M.; Omote, K.; Reddy, Y.N.; Takahashi, N.; Koepp, K.E.; Ng, A.C.; Rider, O.J.; Borlaug, B.A. Long-Term Changes in Cardiac Structure and Function Following Bariatric Surgery. J. Am. Coll. Cardiol. 2022, 80, 1501–1512. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Mehra, A.; Ardhanari, S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog. Cardiovasc. Dis. 2014, 56, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Koschker, A.C.; Warrings, B.; Morbach, C.; Seyfried, F.; Jung, P.; Dischinger, U.; Edelmann, F.; Herrmann, M.J.; Stier, C.; Frantz, S.; et al. Effect of bariatric surgery on cardio-psycho-metabolic outcomes in severe obesity: A randomized controlled trial. Metabolism 2023, 147, 155655. Available online: https://pubmed.ncbi.nlm.nih.gov/37393945/ (accessed on 14 November 2023). [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).