1. Introduction
Muscle strength, broadly defined as the capacity of the neuromuscular system to generate tension against external resistance, is a fundamental determinant of both athletic performance and musculoskeletal rehabilitation outcomes [
1,
2]. Accurate quantification of strength is essential for characterizing functional status, optimizing individualized training programs, preventing injuries, and monitoring adaptations during therapeutic interventions [
1,
2]. Consequently, valid, reliable, and feasible assessment methods are a priority in both sports’ science and clinical contexts.
Several approaches exist to evaluate muscular strength, each with specific advantages and limitations. Isokinetic dynamometry has traditionally been considered the gold standard due to its ability to assess concentric, eccentric, and isometric muscle actions with high validity [
3,
4,
5]. However, its widespread use is limited by high cost, large size, and the need for specialized operators, which restricts accessibility in field or clinical settings. Even so, isokinetic devices are not immune to measurement errors, highlighting the need for alternative tools that provide accurate and reproducible assessments of muscle strength [
3].
Portable dynamometry has emerged over the past decade as a practical approach for measuring isometric strength. These devices are easy to operate, safe during maximal voluntary contractions, and capable of delivering reproducible data in both laboratory and field conditions [
6,
7,
8,
9]. Most portable dynamometers rely on load cells, electrochemical sensors that convert applied force into electrical signals, enabling precise quantification of muscular strength [
10,
11,
12]. Their low cost, portability, and adaptability have led to increased use in sports performance monitoring, rehabilitation programs, and educational environments.
Recent advances allow load-cell systems to capture real-time force–time curves and compute multiple neuromuscular parameters, including Peak Force and maximal RFD [
10,
11,
12]. These indices provide complementary insights into explosive force production, motor unit recruitment, and neuro-muscular stability, which are relevant in both athletic and rehabilitative contexts.
However, the reproducibility of force-derived indices varies across devices and conditions, influenced by sensor design, signal acquisition, filtering, and processing algorithms [
12,
13,
14]. Therefore, the reliability of any load-cell system cannot be assumed, underscoring the need for systematic validation studies tailored to the intended application.
Currently, there is a gap in validating low-cost, accessible dynamometers capable of accurately measuring both maximal isometric strength and indices of explosive neuromuscular performance. Addressing this gap is critical for expanding strength assessment to real-world contexts without compromising measurement quality.
Accordingly, this pilot study evaluated the reliability and repeatability of the G-Force load cell system during standardized isometric hip abduction and adduction in young, physically active Chilean adults. By assessing both Peak Force and dynamic force–time variables, this study aims to establish the device’s robustness while identifying potential limitations in interpreting explosive force indices in applied and research settings.
2. Materials and Methods
2.1. Design
This observational, descriptive, cross-sectional study was conducted in 2025 at the Athan Boxing Club in Concepción, Chile. The study design and reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [
15]. The protocol was reviewed and approved by the Ethics Committee of the Central University of Chile (Protocol Code: 89/2025; Approval Date: 23 September 2025). Data collection was performed on 24 and 25 September 2025, with a 24 h interval between the test and retest sessions, following previous recommendations for reliability and validity assessments using isometric dynamometers [
16]. All participants provided written informed consent prior to enrollment, in accordance with the principles of the Declaration of Helsinki [
17].
2.2. Context
The capacity to generate rapid force is a critical component of both athletic performance and muscular rehabilitation, commonly assessed through the RFD. However, precise quantification of muscle strength parameters often requires expensive or technically demanding equipment, limiting accessibility for coaches, athletes, and practitioners in clinical or educational contexts [
18]. In this regard, the present pilot study aims to validate the G-Force system, a low-cost load cell specifically designed to measure force during hip abduction and adduction movements.
2.3. Participants
A total of 24 physically active adults (19 men and 5 women) aged 18–44 years voluntarily participated. Recruitment was performed via non-probabilistic convenience sampling through social media announcements and direct invitation. All participants signed informed consent prior to data collection. In the adduction test, one participant did not complete the second session, resulting in a final sample of 23 for that condition.
Eligibility Criteria
Inclusion criteria:
Physically active adults aged 18–44 years, able to safely perform maximal voluntary contractions.
Abstention from moderate or vigorous physical activity within 48 h prior to testing, to avoid acute exercise interference.
Capacity to understand and sign the informed consent form.
Exclusion criteria:
Musculoskeletal disorders or chronic non-communicable diseases (e.g., hypertension, diabetes, cardiovascular/metabolic conditions) contraindicate maximal exertion.
Use of medications influencing neuromuscular or cardiovascular function.
Inability to correctly perform the required hip abduction/adduction movements.
2.4. Load Cell System
The device employed was the G-Force dynamometry system, integrating an S-type strain gauge load cell (model YZC-516C, Guangzhou, China) manufactured from alloy steel. This sensor has a nominal capacity of 0.5 t and a rated output of 2.0 ± 0.05 mV/V. Metrological characteristics include a combined error ≤ 0.03% RO, creep ≤ 0.02% RO at 30 min, input resistance 365 ± 5 Ω, output resistance 350 ± 3 Ω, and insulation resistance ≥ 5000 MΩ at 50 V. The compensated temperature range is −10 to +40 °C, with a safe overload tolerance of 120% RO and an ultimate overload of 150% RO. Although the manufacturer provides technical specifications and an accuracy class (C2), no validation studies or publicly available calibration certificates were found for the YZC-516C model.
For this reason, the load cell was calibrated following the basic protocol described in the Chronojump Boscosystem [
19]. The calibration consisted of establishing a linear relationship between the sensor’s electrical signal and a known reference load. Specifically, a certified 5 kg (≈49.05 N) mass was used, suspended by a hanging system to simulate tensile conditions comparable to those used in hip abduction and adduction tests. This procedure allowed for verification of basic linearity within the limited force range used in the present study. Subsequently, a horizontal tare (zeroing) was performed with the sensor in a stable position to cancel the weight of the device itself and any attached accessories, in accordance with the manufacturer’s recommendations. However, no more exhaustive validation procedures were performed, such as evaluation of hysteresis, full-range linearity, or sensitivity, which is acknowledged as a limitation of this study.
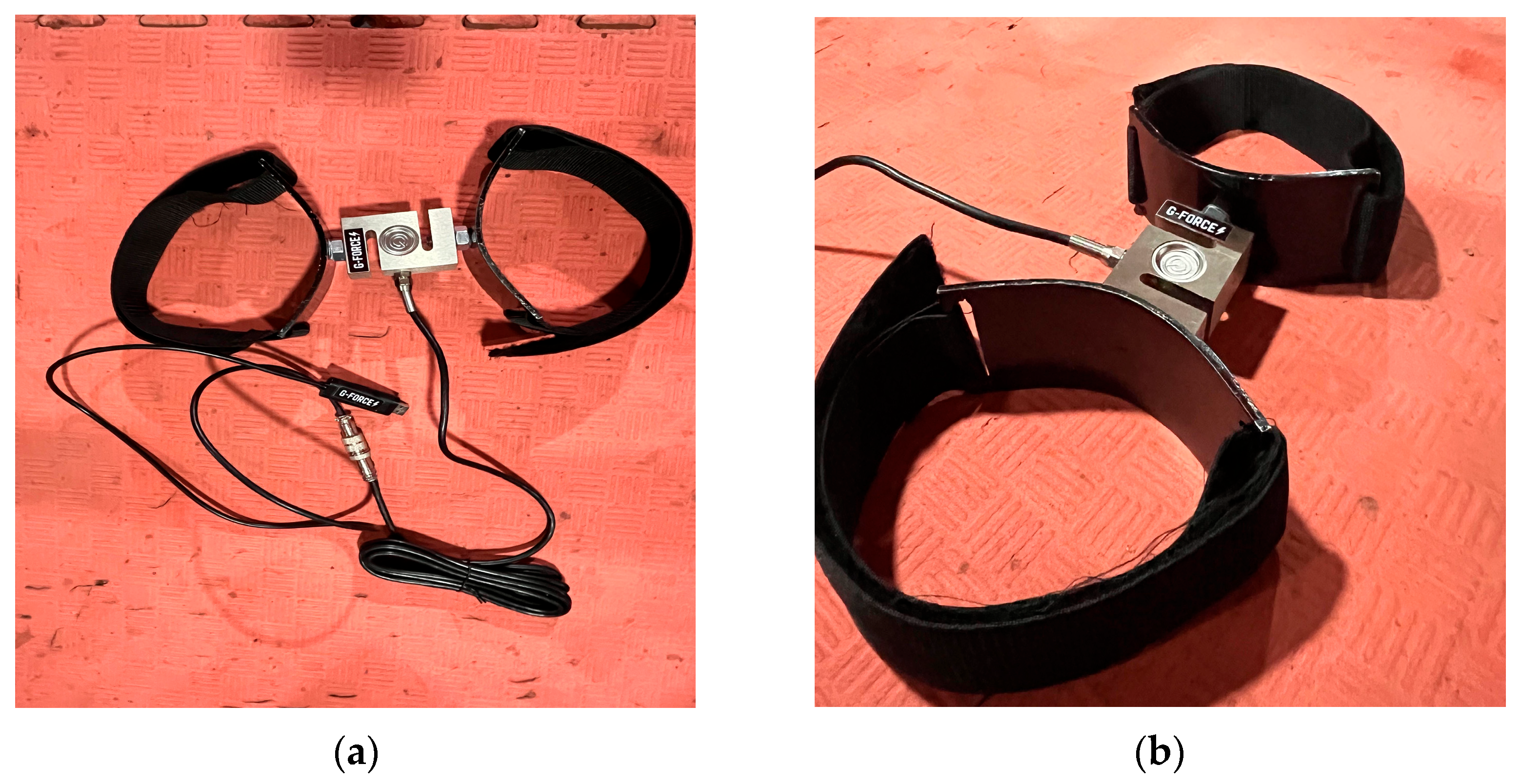
The system was mounted on an adjustable fixation support with Velcro straps, ensuring stable and reproducible positioning and alignment of the sensitive axis with the applied force. This setup minimizes mechanical artifacts and enhances usability in both laboratory and field settings. Importantly, the G-Force system represents a low-cost and portable alternative to traditional isokinetic dynamometers, making it suitable for sports performance monitoring, rehabilitation, and applied research contexts (
Figure 1).
2.4.1. Technical Functioning: Analog–Digital Conversion and Data Processing
Data acquisition was performed via USB–serial communication using the Modbus RTU protocol (38,400 bps, 8N1) [
20], with calibration conducted using certified weights following the Chronojump basic protocol. The system included tare (zero or known load) and polarity inversion functionality, with direct export to CSV. A graphical user interface developed in Tkinter [
21] allowed for real-time visualization of force–time curves and automatic computation of neuromuscular metrics.
The S-type load cell operates in a Wheatstone bridge configuration, with relative resistance changes expressed as ΔR/R = GF·ε and output voltage Vout = (ΔR/4R)·Vexc ≈ 2 mV/V. The differential signal was amplified using a low-noise instrumentation amplifier and converted to digital format via a 24-bit nominal ADC (~21 effective bits). The system exhibited an input noise < 1.5 µV RMS, nonlinearity < 0.05% FS, and an internal analog anti-aliasing filter with a cutoff frequency ≈ 10 Hz. The load cell was excited with 5 V DC ± 0.1 V.
For digital signal conditioning, the force signals were processed in Python (SciPy 1.12) using a fourth-order zero-phase Butterworth low-pass filter (cutoff frequency = 15 Hz, filtfilt method). Optional smoothing filters—including Savitzky–Golay (window = 7 samples, polynomial order = 2) and moving average (window = 5 samples)—were applied to reduce high-frequency noise without altering contraction dynamics, optimizing the estimation of Peak Force, rate of force development (RFD), and impulse. For RFD analysis, the force–time series could be interpolated to a uniform 1000 Hz grid. The calibration factor was calculated as K = (Mref × g)/Nraw_ref, with g = 9.91 m/s2.
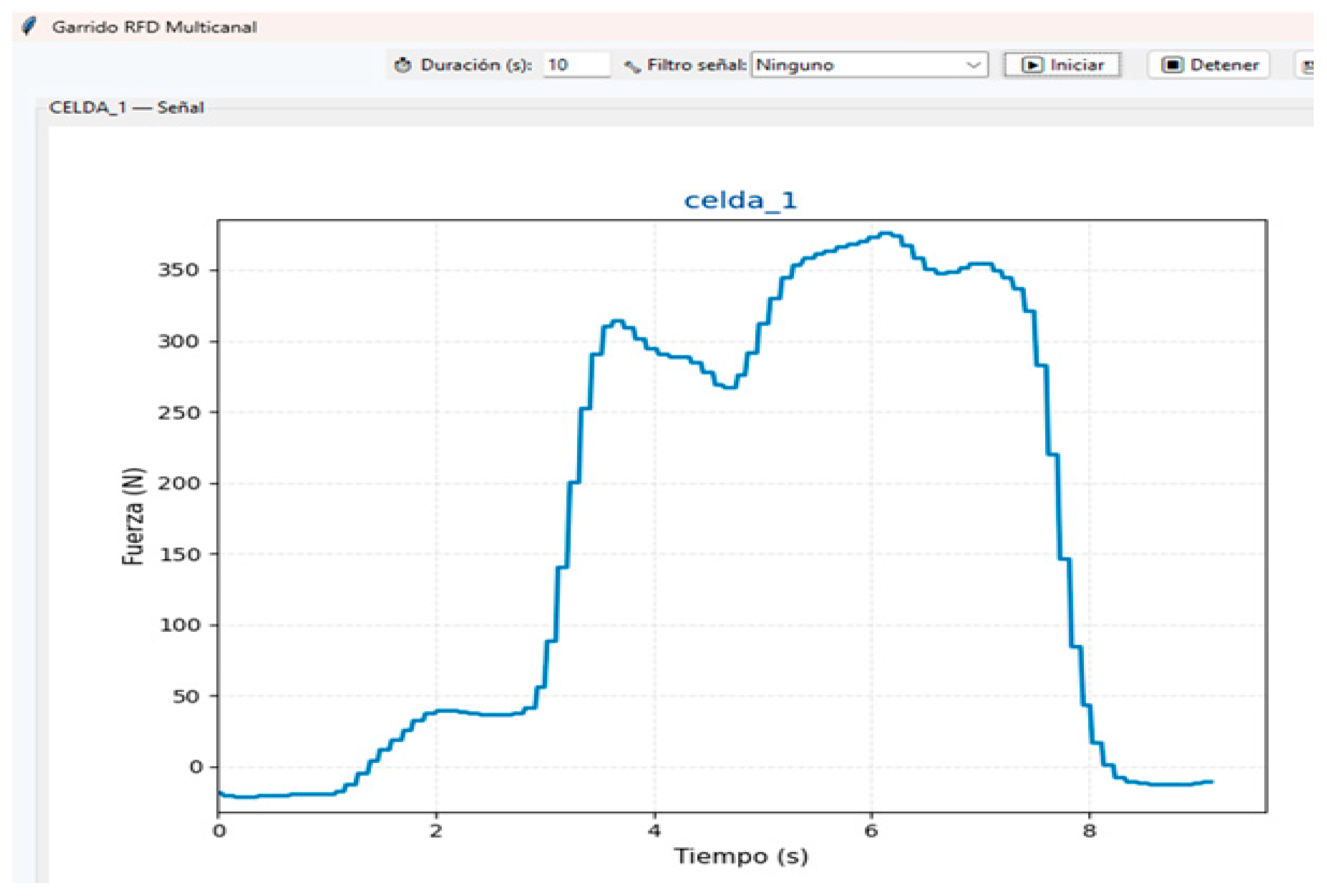
Signal processing and metric computation (Peak Force, instantaneous and windowed RFD, and maximum Jerk) were performed as described in
Supplement S1, which provides the full code and stepwise methodology for reproducibility, including filtering, interpolation, contraction onset detection, and export to CSV (
Figure 2)
2.4.2. Neuromuscular Metrics Computation
Peak RFD reflects the neuromuscular system’s capacity to generate explosive force within very short time windows (<50 ms) and is particularly sensitive to signal stability [
22]. In this study, RFD at predefined time intervals was derived from the force–time curve following contraction onset (50, 100 and 200 ms) [
23]. Maximum jerk refers to the rate of rapid changes in the slope of the force–time curve, capturing the stability and quality of neuromuscular activation [
24]. Peak Force is defined as the highest force achieved during the contraction, considering directionality (negative for adduction, positive for abduction). The time to reach peak force was also recorded. These measures provide insights into movement symmetry, directional consistency, and measurement reproducibility [
25]. The computational details of neuromuscular metrics are provided
Supplement S1.
2.5. Sociodemographic and Anthropometric Variables
Age, stature, and body weight were measured using standardized procedures. Height was assessed with a portable stadiometer (Cescorf, São Paulo, Brazil; max 200 cm), and body weight with a digital scale (SECA model 803). Both instruments comply with the International Society for the Advancement of Kinanthropometry (ISAK) recommendations [
26]. Body mass index (BMI) was calculated using the following standard equation [
27]:
2.6. Warm-Up Protocol
Participants completed a standardized 5 min warm-up consisting of dynamic stretching (flexion, extension, abduction, adduction of major joints) and mobility drills, followed by three submaximal isometric hip contractions at ~50–70% of estimated maximal effort. This procedure ensured neuromuscular preparation and familiarization with the device [
28,
29].
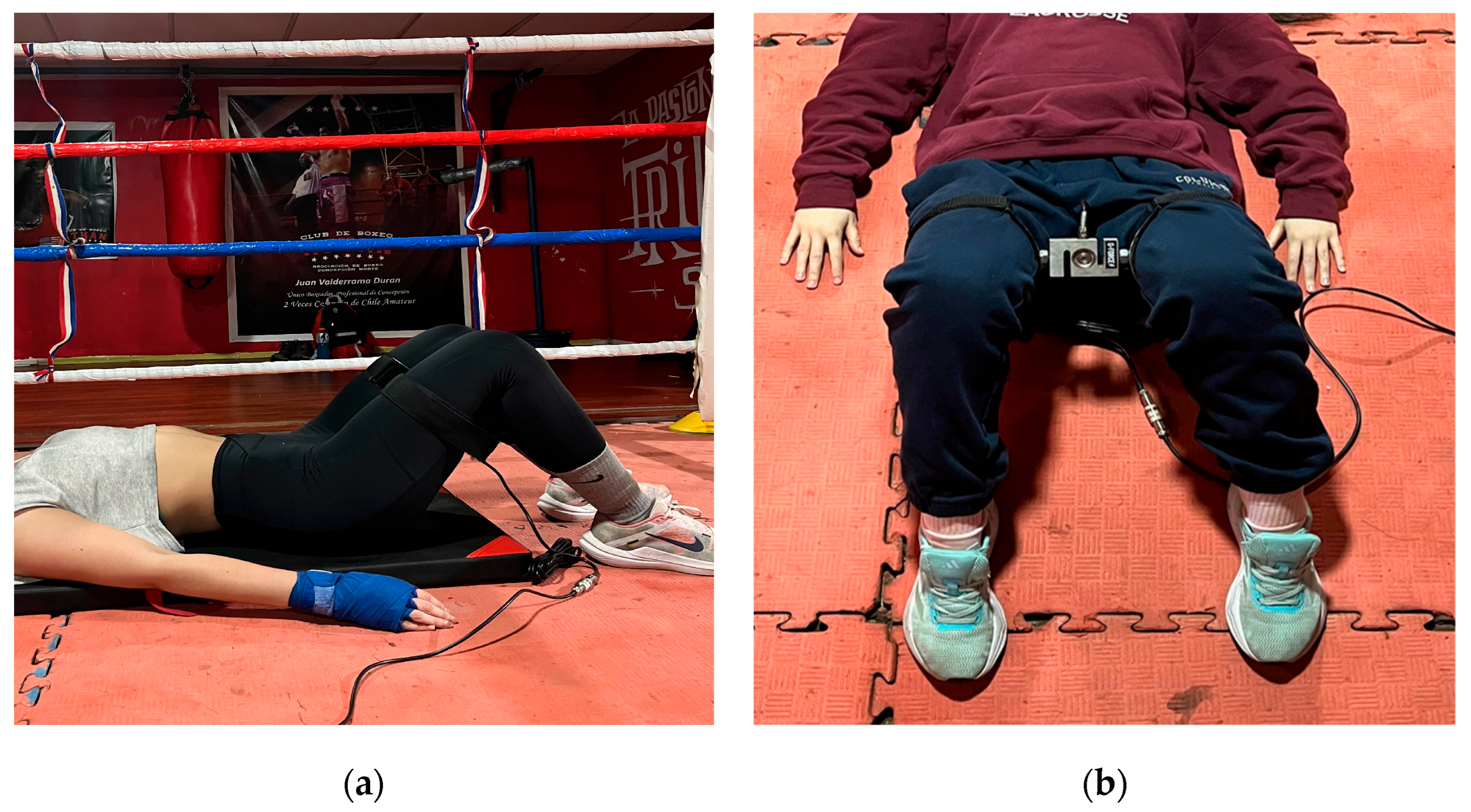
Isometric Hip Abduction and Adduction Tests
Testing was conducted with the hip and knee flexed at 90°, ensuring trunk stability and femoral alignment. Each participant first performed three familiarization contractions per movement. The order of abduction and adduction was alternated to minimize fatigue bias. Subsequently, two maximal voluntary contractions (MVCs) of each movement were executed, each sustained for 10 s with 2–3 min rest between efforts [
23,
30,
31,
32]. Reproducibility was evaluated by repeating the same protocol after 24 h under identical conditions [
16] (
Figure 3).
2.7. Bias
This study has several limitations and potential sources of bias that should be considered when interpreting the findings [
33,
34].
▪ Population bias: This study was conducted exclusively in young, physically active adults, which limits the generalizability of the results to other age groups, fitness levels, or individuals with musculoskeletal or neuromuscular disorders.
▪ Learning or fatigue bias: Although familiarization trials and rest periods were included, performance during MVCs may still have been affected by learning effects or residual fatigue between trials, particularly in the initial and final attempts of each session.
▪ Measurement bias: Although calibrated load cells were used, variability in RFD parameters may partly reflect the intrinsic sensitivity of the device and the signal processing algorithms, rather than true physiological differences.
2.8. Sample Size
The sample size was primarily determined considering the exploratory nature of this pilot study and the recommendations for reliability studies [
35,
36,
37]. Bonett’s formula was applied to estimate the intraclass correlation coefficient (ICC) with a desired precision 95% confidence intervals (95% CI), width = 0.1 and assuming a true ICC of 0.9 with two repeated measurements per participant. To ensure an 80% probability that the CI would fall within the desired range, the minimum required sample size was 24 participants, a figure that aligns with the recommendations of Koo et al. [
36] and Bonett [
37], who suggest samples of 20 to 30 individuals as appropriate sizes for pilot reproducibility studies.
2.9. Statistical Analysis
Data analysis was performed using SPSS v27.0 (IBM Corp., Armonk, NY, USA). Normality was assessed with the Shapiro–Wilk test, and homogeneity of variances was evaluated using Levene’s test. Parametric variables (e.g., height, peak force) were analyzed using Student’s t-tests, while non-parametric variables (e.g., age, weight, BMI, RFDs and maximum jerk) were analyzed using Wilcoxon tests. Descriptive statistics included mean (X), standard deviation (SD), and 95% CI.
Repeatability (intra-session) was assessed by comparing MVC1 and MVC2 within the same session (Day 1), reflecting measurement consistency under identical conditions. Reliability (inter-session) was evaluated by comparing measurements between different days (Day 1 vs. Day 2), reflecting stability over time. The ICC (two-way mixed-effects, absolute agreement, single measures) with 95% CI was calculated and interpreted as poor (<0.50), moderate (0.50–0.75), good (0.75–0.90), or excellent (>0.90) [
35,
36,
37,
38].
The coefficient of variation (CV%) while a CV < 15% was used as a criterion for minimum acceptable reliability in MCVs protocols [
39]. The standard error of measurement (SEM) and the minimum detectable change (MDC) were also calculated according to a previous recommendation for functional testing in athletes [
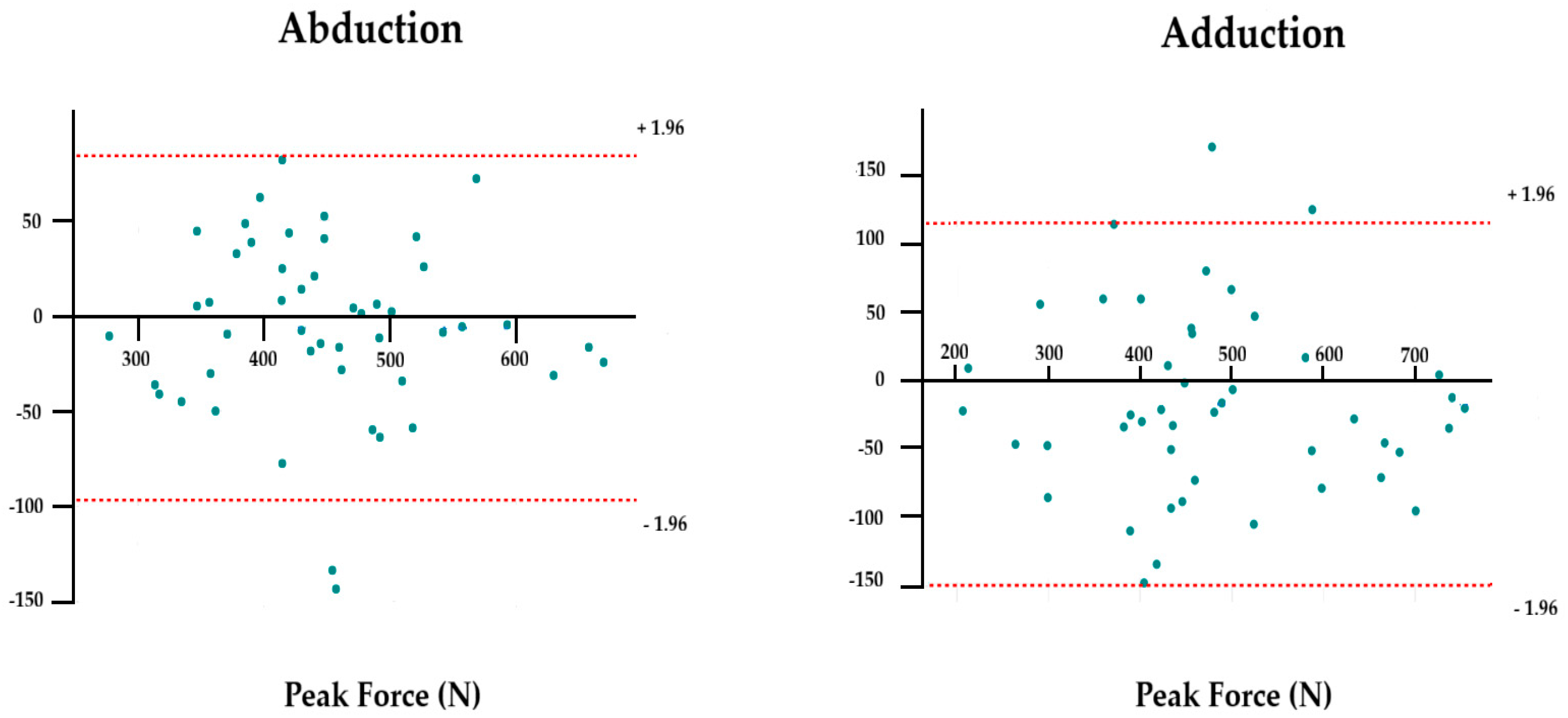
40]. Based on these metrics, a Bland–Altman plot was generated for peak force, which was considered an uncensored variable due to its high levels of absolute and relative reliability obtained in our study [
41]. Statistical significance for all analyses was set at an alpha level of <0.05.
4. Discussion
The present study assessed the reliability and repeatability of the G-Force load cell system during isometric hip abduction and adduction tests in young, physically active adults in Chile, a context where access to commercial, low-cost, and calibrated dynamometers is very limited. According to standard reproducibility classifications [
35,
36,
37,
38,
39], Peak Force demonstrated excellent intra- and inter-session reliability (ICC = 0.87–0.96), although the coefficients of variation were relatively high (20.2–30.5%), which exceeds the generally accepted thresholds (<10–15%) for strength testing. Courel-Ibáñez et al. (2024) [
43] suggest that such elevated variability may result from protocols with a limited MVCs per participant, which reduce movement familiarization and increase interaction effects.
Bland–Altman analysis revealed negligible bias values for peak force in both abduction (−6.54 N) and adduction (−17.57 N), confirming the absence of systematic error and supporting the stability of this parameter [
41]. However, adduction exhibited greater bias and wider dispersion (SD = 68.1 N) compared with abduction (SD = 46 N), likely due to higher biomechanical complexity and the recruitment of deeper stabilizing muscles [
44,
45,
46,
47].
In contrast, abduction tends to be more stable and less prone to compensatory strategies, resulting in more consistent measurements. These results suggest that abduction measurements may be more reproducible, while adduction requires stricter standardization of body positioning to reduce variability. From a clinical perspective, this also underscores the relevance of adductor testing in sports such as soccer and hockey, where strength deficits are strongly associated with groin pain and increased injury risk [
44,
45,
46,
47].
In contrast, the curve-derived force–time metrics (peak RFD, RFD50, RFD100, RFD200) and maximum Jerk demonstrated only moderate reproducibility within sessions (ICC = 0.55–0.67) and low-to-moderate reproducibility between sessions (ICC = 0.39–0.70). These metrics were associated with higher variability than Peak Force, reflecting their greater sensitivity to signal processing, contraction onset detection, and computational algorithms [
10,
11,
22,
37,
47,
48,
49,
50]. Consequently, RFD and Jerk should be considered exploratory outcomes rather than primary endpoints in small cross-sectional studies.
These results confirm previous findings showing that Peak Force is more stable than explosive force metrics. For instance, Merino-Muñoz et al. [
32] reported good to excellent reliability for hip adductor Peak Force (ICC = 0.706–0.819) and lower consistency for RFD (ICC = 0.367–0.660). Similarly, Pichardo et al. [
11] and Montoro-Bombú et al. [
12] confirmed the validity of load cell-based systems for maximal force assessment while emphasizing the limitations in explosive force measurements. Together, these findings support the use of Peak Force for both research and applied contexts while highlighting that caution is needed when interpreting RFD and Jerk.
From a practical standpoint, the findings of our study and reports in the literature support the use of load cell systems as a low-cost, portable, and reliable tool for maximal isometric hip strength assessment, particularly in Chile and similar contexts where access to commercial isokinetic equipment is restricted, suitable for sports, clinical, and rehabilitation settings where access to isokinetic dynamometry is limited [
10,
11,
12,
23,
48,
49,
50,
51]. RFD and maximum Jerk may provide complementary information on neuromuscular activation but should be interpreted cautiously and preferably in longitudinal studies where intra-individual changes can yield more meaningful trends [
23,
49,
50].
Moreover, the MDC values of 90–130 N observed for peak force represent a relevant functional threshold. In young, physically active adults, this magnitude of change corresponds to approximately 20–25% of average force values, suggesting that only substantial adaptations—such as multi-week strength training or rehabilitation programs—would surpass the measurement error and qualify as true improvements [
51]. This implies that small early gains (<100 N), expected during the first weeks of rehabilitation, may not be reliably detected, limiting sensitivity in short-term recovery assessment. Conversely, in athletic populations, a change of 100 N can translate into meaningful performance gains in acceleration, change in direction, and joint stability, reinforcing the device’s value for high-performance monitoring [
51]. Importantly, given that adductor strength deficits are strongly linked to groin pain and hip-related injuries in sports such as soccer and hockey, a measurable gain of ≥100 N could signify not only improved performance but also a clinically meaningful reduction in injury risk.
Nevertheless, the relatively high CV values observed in peak force (20–30%) indicate that measurement variability is a limitation. Although peak force was the most stable metric in this study, these values exceed the <10–15% CV thresholds often cited as benchmarks for strength testing reliability, thereby constraining its capacity to detect subtle physiological or clinical changes [
39]. This highlights the importance of multiple MVC trials and strict protocol standardization, particularly for reducing random variability for adduction testing, where the high test demands limit performance.
Another key limitation relates to external validity. Since this study involved only young, physically active adults, the results cannot be generalized to older, sedentary, or clinical populations. This lack of representativeness, combined with the limitations inherent to observational designs, must be considered when extrapolating the use of the G-Force system beyond the study group [
52,
53].
Additionally, the YZC-516C model lacks publicly available scientific certification, introducing uncertainty regarding absolute measurement accuracy. External factors such as sleep, nutrition, and fatigue were not controlled and could influence RFD and Jerk metrics, despite familiarization and rest periods [
54,
55,
56]. Finally, the cross-sectional design precludes evaluation of sensitivity to long-term training or intervention-induced changes [
57].
Future research should focus on validating the G-Force system in diverse populations, comparing outcomes with reference standards (e.g., isokinetic dynamometry), and improving signal processing for RFD and Jerk. Longitudinal studies are also needed to determine if the 90–130 N MDC threshold is consistent and whether smaller but clinically meaningful changes can be reliably detected.
5. Conclusions
The G-Force load cell system reliably measures maximal isometric hip strength assessment, particularly for Peak Force, while force–time variables (RFD and Jerk) demonstrated moderate reproducibility. These findings indicate that the G-Force system can serve as a practical, low-cost, and portable alternative for evaluating maximal strength in sports and rehabilitation contexts, especially when access to isokinetic equipment is limited.
However, due to the relatively high variability observed and the absence of external calibration, the results should be interpreted with caution, and further research is required to refine signal processing, improve measurement precision, and confirm the system’s external validity. Future longitudinal and comparative studies will help establish the broader applicability and sensitivity of this tool for detecting meaningful neuromuscular adaptations.