Impact of Metal Screw Cap Closures on Trace Element Profiles in White Wines After One Year in Bottle
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Elemental Analysis
2.3. Statistical Analysis
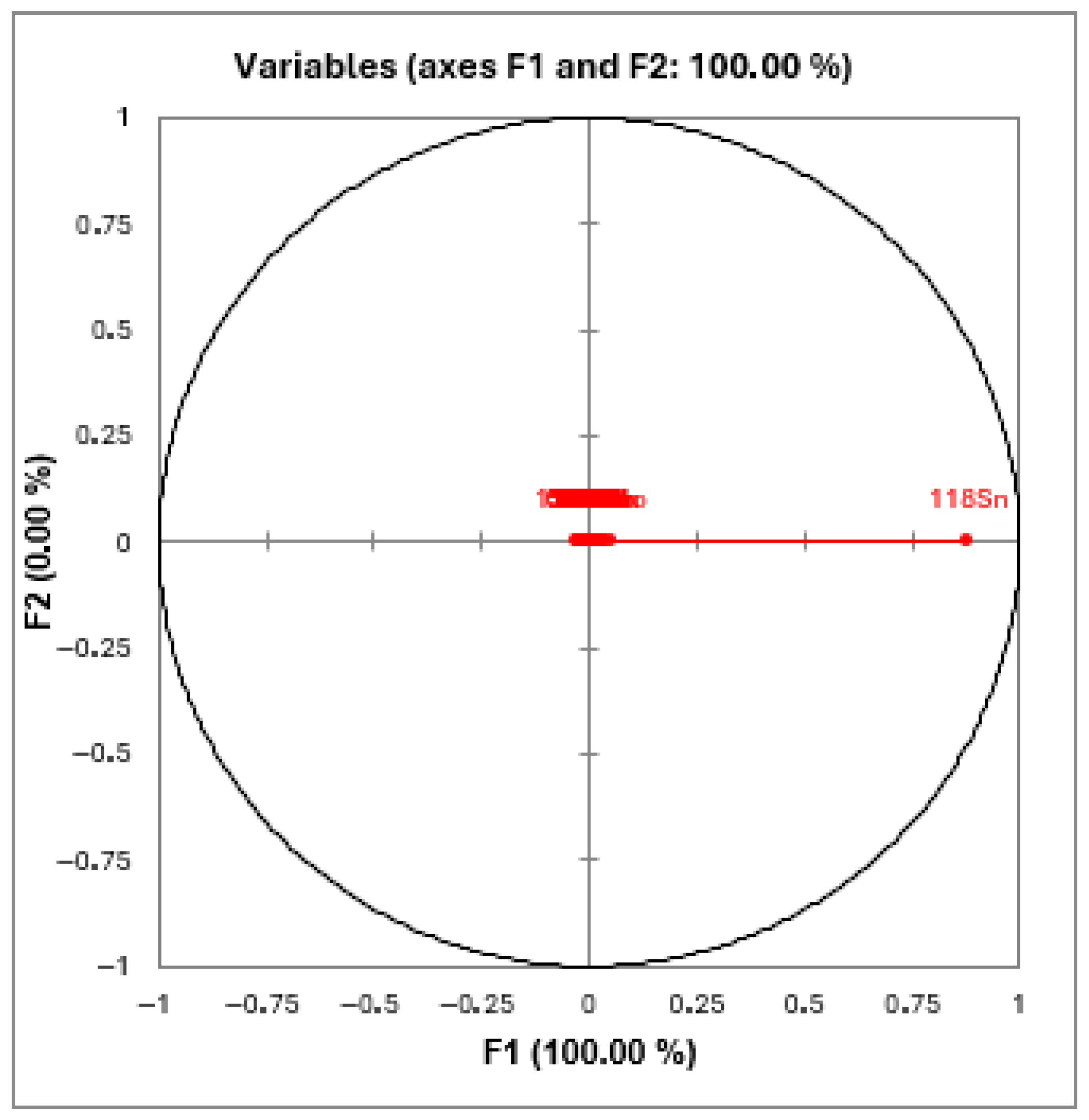
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohana-Levi, N.; Netzer, Y. Long-Term Trends of Global Wine Market. Agriculture 2023, 13, 224. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. State of the World Vine and Wine Sector in 2023. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (accessed on 24 November 2024).
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Zhai, H.-Y.; Li, S.-Y.; Zhao, X.; Lan, Y.-B.; Zhang, X.-K.; Shi, Y.; Duan, C.-Q. The compositional characteristics, influencing factors, effects on wine quality and relevant analytical methods of wine polysaccharides: A review. Food Chem. 2023, 403, 134467. [Google Scholar] [CrossRef]
- Pohl, P. What do metals tell us about wine? TrAC 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef] [PubMed]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Akamatsu, F.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Variation in the mineral composition of wine produced using different winemaking techniques. J. Biosci. Bioeng. 2020, 130, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.D.; Teodosiu, C.; Morosanu, I.; Jitar, O.; Cotea, V.V. Quantification of toxic metals during different winemaking stages. BIO Web Conf. 2019, 15, 02024. [Google Scholar] [CrossRef]
- Mladenova, E.; Bakardzhiyski, I.; Dimitrova, E. Investigation of Elemental Composition in White Wine Treated with Varying Doses of Bentonite. Beverages 2024, 10, 114. [Google Scholar] [CrossRef]
- Harding, J.; Robinson Obe, J.; Thomas, T.Q. The Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Leite, C.; Pereira, H. Cork-Containing Barks—A Review. Front. Mater. 2017, 3, 63. [Google Scholar] [CrossRef]
- Gardner, D. Innovative Packaging for the Wine Industry: A Look at Wine Closures; Virginia Tech Food Science and Technology: Blacksburg, VA, USA, 2008. [Google Scholar]
- Furtado, I.; Lopes, P.; Oliveira, A.S.; Amaro, F.; Bastos, M.L.; Cabral, M.; Guedes de Pinho, P.; Pinto, J. The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods 2021, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Hopfer, H.; Nelson, J.; Mitchell, A.E.; Heymann, H.; Ebeler, S.E. Profiling the trace metal composition of wine as a function of storage temperature and packaging type. J. Anal. At. Spectrom. 2013, 28, 1288–1291. [Google Scholar] [CrossRef]
- Schäfer, S.G.; Femfert, U. Tin—A toxic heavy metal? A review of the literature. Regul. Toxicol. Pharmacol. 1984, 4, 57–69. [Google Scholar] [CrossRef]
- Agency for Toxic Substances Disease Registry (ATSDR). Toxicological Profile for Tin and Tin Compounds; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2005.
- EFSA. Panel on Dietetic Products, Nutrition and Allergies, Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [NDA] related to the tolerable upper intake level of tin. EFSA J. 2005, 3, 26. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 13 June 2023 on maximum levels for certain contaminants in food. In Official Journal of the European Union; Publications Office of the European Union: Luxembourg, 2023; Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 28 November 2024).
- Tariba, B. Metals in wine—Impact on wine quality and health outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef] [PubMed]

| Element | LD | Element | LD | Element | LD |
|---|---|---|---|---|---|
| Li | 0.347 | Cu | 1.33 | Sn | 0.061 |
| Be | 0.007 | Zn | 0.435 | Sb | 0.051 |
| V | 0.883 | As | 0.135 | Cs | 0.021 |
| Cr | 3.04 | Se | 0.502 | Ba | 0.258 |
| Mn | 0.294 | Rb | 0.040 | Tl | 0.003 |
| Fe | 22.1 | Sr | 0.513 | Pb | 0.016 |
| Co | 0.043 | Mo | 0.020 | ||
| Ni | 0.137 | Cd | 0.018 |
| Element | Content | Element | Content | Element | Content |
|---|---|---|---|---|---|
| Li | <0.027 | Cu | 0.057 (0.031) | Sn | 0.015 (0.007) |
| Be | <0.001 | Zn | 3.54 (0.35) | Sb | <0.002 |
| V | <0.242 | As | <0.082 | Cs | <0.008 |
| Cr | <0.343 | Se | <0.088 | Ba | 0.924 (0.113) |
| Mn | <0.242 | Rb | <0.029 | Tl | <0.001 |
| Fe | <13.8 | Sr | 1.29 (0.21) | Pb | <0.058 |
| Co | <0.008 | Mo | <0.033 | ||
| Ni | <0.015 | Cd | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, R.; Almeida, A. Impact of Metal Screw Cap Closures on Trace Element Profiles in White Wines After One Year in Bottle. Appl. Sci. 2025, 15, 11486. https://doi.org/10.3390/app152111486
Azevedo R, Almeida A. Impact of Metal Screw Cap Closures on Trace Element Profiles in White Wines After One Year in Bottle. Applied Sciences. 2025; 15(21):11486. https://doi.org/10.3390/app152111486
Chicago/Turabian StyleAzevedo, Rui, and Agostinho Almeida. 2025. "Impact of Metal Screw Cap Closures on Trace Element Profiles in White Wines After One Year in Bottle" Applied Sciences 15, no. 21: 11486. https://doi.org/10.3390/app152111486
APA StyleAzevedo, R., & Almeida, A. (2025). Impact of Metal Screw Cap Closures on Trace Element Profiles in White Wines After One Year in Bottle. Applied Sciences, 15(21), 11486. https://doi.org/10.3390/app152111486






