1. Introduction
Chronic diseases in pediatric populations pose significant challenges to modern healthcare systems, especially when the conditions are rare, complex, and require continuous monitoring. Managing these conditions not only involves timely clinical interventions but also demands active participation from families, multidisciplinary care teams, and community-based resources. In low- and middle-income countries, including Algeria, the situation is further complicated by limited access to specialized care, infrastructure gaps, and logistical barriers.
One such condition is Idiopathic Nephrotic Syndrome (INS), a rare kidney disorder that affects children and often requires long-term treatment and close monitoring. The disease is characterized by unpredictable relapses and remissions, which necessitate frequent adjustments to medication and lifestyle [
1]. Despite its impact, managing INS at home remains a daunting task for many families, particularly during public health emergencies such as the COVID-19 pandemic, which introduced unprecedented challenges to healthcare continuity [
2].
Our collaborative research with pediatric nephrologists at El-Mansoura Pediatrics Hospital in Algeria revealed several recurring obstacles in the day-to-day care of INS patients:
Limited access to regular follow-ups due to geographic or financial constraints.
A lack of standardized tools for home-based monitoring and reporting of symptoms.
Fragmented communication between caregivers and healthcare providers.
Delays in adjusting treatment plans due to insufficient real-time clinical data.
Inconsistent care personalization due to diverse patient responses to therapy [
3].
These challenges are further magnified during pandemic scenarios, where hospital visits become risky or impossible. This underlines the critical need for a digital, scalable, and intelligent system that ensures care continuity while supporting clinical decision-making remotely.
To respond to this need, we developed NS-Assist, an assistance system for pediatric decision-making designed to improve remote monitoring, enable real-time data exchange, and guide caregivers through a rule-based expert system. Built with the Internet of Medical Things (IoMT) framework and AI components, NS-Assist acts as a smart intermediary between home and hospital, supporting timely and informed clinical decisions even during crises.
This paper presents the design, development, and evaluation of NS-Assist, with a specific focus on the following:
Bridging the gap between hospital-based care and home care through a scalable telemonitoring platform.
Incorporating domain knowledge into a rule-based expert system to provide real-time, context-aware clinical suggestions.
Demonstrating the system’s robustness and usability in real-world pediatric healthcare settings in Algeria.
The rest of this paper is structured as follows:
Section 2 reviews existing technological approaches to pediatric telemonitoring and decision support.
Section 3 presents the main challenges related to idiopathic nephrotic syndrome that motivate this research.
Section 4 describes the proposed solution, NS-Assist, including its architecture and operational workflow.
Section 5 details the system implementation.
Section 6 presents the system evaluation and validation, including the field experiment and user survey results. Finally,
Section 7 concludes the paper and outlines directions for future research.
2. Related Work
Recent advances in pediatric nephrology have increasingly leveraged IoT, artificial intelligence (AI), and mobile health technologies to address the clinical and logistical challenges associated with managing chronic kidney disease (CKD) and idiopathic nephrotic syndrome (INS). This section organizes and critically reviews the existing literature across four key areas: (1) remote monitoring systems that support continuous health tracking through IoT-enabled devices and mobile applications; (2) telemedicine solutions developed to maintain care continuity during crises such as the COVID-19 pandemic; (3) predictive models that use machine learning to forecast treatment outcomes and guide clinical decisions; and (4) AI applications specifically focused on pediatric INS, aiming to enhance early detection, risk stratification, and individualized care.
Table 1 presents a comparative overview of these contributions and outlines the specific limitations that NS-Assist seeks to overcome through its integrated, pediatric-focused design.
2.1. Remote Monitoring Systems
IoT-based systems for CKD have demonstrated high predictive accuracy. Similarly, Maniam et al. [
4] developed a low-cost fuzzy logic–IoT system for CKD monitoring, attaining 99% accuracy in detecting patient status. However, both approaches target adult CKD populations and lack pediatric-specific monitoring protocols.
In the pediatric context, Wang et al. [
5] developed UrApp, a mobile application for managing childhood nephrotic syndrome. The app facilitates urine test analysis and provides educational resources, achieving a 97% agreement with standard urinalysis. While effective, UrApp lacks integration with clinical workflows and real-time alert capabilities. These systems emphasize data collection but fall short in enabling continuous, coordinated care.
2.2. Telemedicine Solutions for Pandemic Resilience
The COVID-19 pandemic underscored the need for resilient healthcare systems. Soler et al. [
6] analyzed disruptions in Spanish nephrology units, noting reduced transplant activity and increased reliance on telephone consultations. While they identified systemic gaps, their work did not propose actionable digital solutions.
Watanabe et al. [
7] observed delayed diagnoses, elevated LDL levels, and increased steroid resistance in post-pandemic pediatric INS cases. Conversely, Veltkamp et al. [
8] reported a stable INS incidence but noted reductions during school closures, suggesting links between pandemic measures and disease trends. However, current telemedicine platforms do not incorporate adaptive triage, automated alerts, or interdisciplinary coordination.
Recent research has begun addressing these gaps through more intelligent digital healthcare architectures. Liu and Wang [
9] reviewed IoT-integrated platforms for chronic disease monitoring, emphasizing personalized and real-time care via mobile sensing and ML. Mbanugo [
10] introduced AI-enhanced telemedicine models with predictive analytics, chatbots, and virtual consultations to improve access and continuity. Badidi [
11] highlighted the role of Edge AI and federated learning for low-latency, privacy-preserving diagnostics—essential in health crises.
Additionally, Rehan [
12] explored cloud-based healthcare platforms powered by AI-driven predictive analytics. His findings demonstrate how such platforms can improve early detection and risk stratification for chronic diseases, enabling proactive, scalable interventions. Collectively, these studies signify a shift toward intelligent, distributed telehealth ecosystems with enhanced pandemic resilience.
2.3. Predictive Models for Treatment Assitance
Machine learning models are increasingly used to predict treatment responses. Ye et al. [
13] applied a novel variable selection framework to predict steroid resistance, achieving 94% validation accuracy. Similarly, Zaorska et al. [
14] used neural networks for the same purpose, with 92% accuracy. Although useful for early identification of complex cases, these models often function as black boxes, limiting their interpretability in clinical settings.
Several studies focused on Tacrolimus optimization. Huang et al. [
15] integrated pharmacokinetic modeling with ML to predict drug clearance, while Mo et al. [
16,
17] used logistic regression and random forests to identify efficacy-related factors, achieving AUCs up to 80.7%. Mo et al. [
18] predicted Tacrolimus-induced nephrotoxicity using XGBoost, with 77.3% accuracy. Despite promising outcomes, these models address isolated clinical tasks and are not embedded in comprehensive decision-making frameworks.
Other works include Improta et al. [
19], who proposed a fuzzy logic-based CDSS for renal function evaluation in transplant patients, and Al-Mashhadi et al. [
20], who designed a fuzzy system for general kidney diagnosis. While valuable, these tools neither target pediatric nephrotic syndrome specifically nor integrate interdisciplinary care pathways.
2.4. Artificial Intelligence for Idiopathic Nephrotic Syndrome (INS)
Recent advances in artificial intelligence (AI) have significantly influenced pediatric nephrology by improving disease prediction, risk assessment, and clinical decision-making. In idiopathic nephrotic syndrome (INS), where early detection of treatment resistance and complications is essential, AI-based methods have shown promising applications.
Ye et al. [
13] developed machine learning models to predict steroid-resistant nephrotic syndrome (SRNS) in children, using demographic and laboratory parameters as predictive features. Their work demonstrated that gradient boosting and support vector machine classifiers can accurately identify SRNS cases before treatment initiation, allowing clinicians to individualize therapy and avoid ineffective corticosteroid exposure. This study highlights how predictive analytics can enhance early risk stratification and treatment optimization in pediatric nephrology.
Yang et al. [
21] recently introduced a machine learning–based predictive model for acute kidney injury (AKI) in hospitalized children with INS. The model, trained and externally validated on multi-center datasets, achieved strong performance in identifying high-risk patients and guiding preventive interventions. Such work illustrates the growing role of data-driven systems in continuous monitoring and early complication detection.
Filler et al. [
22] provided a comprehensive review of artificial intelligence applications in pediatric nephrology, emphasizing the need for explainable and ethically aligned models that integrate with clinical workflows. They underline that transparent, clinician-guided AI tools are crucial for real-world adoption, particularly in sensitive pediatric contexts.
2.5. Gaps Addressed by NS-Assist
Table 1 summarizes the limitations identified across the reviewed studies. NS-Assist is designed to address these gaps through a unified, pediatric-centered digital health framework:
Fragmentation: Existing tools function in silos and are not integrated into unified care workflows.
Pediatric Limitations: Most CKD tools are adult-focused and do not support pediatric-specific management (e.g., corticosteroid tapering).
Lack of Explainability: ML models often lack interpretability, reducing trust in clinical use.
Limited Integration Between Predictive Models and Clinical Decision Support: Most AI approaches focus on risk prediction (e.g., steroid resistance, AKI) but remain disconnected from operational decision-making and telemonitoring systems.
Pandemic Inadaptability: While many telemedicine studies identify needs, few propose actionable technical frameworks.
NS-Assist fills these gaps through the following:
An integrated IoMT and rule-based Decision Support System (DSS): Real-time monitoring via IoMT is coupled with interpretable, guideline-driven decision logic (e.g., proteinuria thresholds).
Pandemic-resilient features: Virtual triage and automated alerts reduce unnecessary hospital visits and prioritize urgent cases.
Interdisciplinary care support: Dedicated interfaces promote collaboration among pediatricians, nurses, and families to ensure adherence to treatment protocols.
Compared with existing pediatric decision support tools like UrApp [
5] and generic IoT-based CKD monitors, NS-Assist distinguishes itself with its rule-based engine tailored to INS-specific protocols, real-time data integration, and pandemic-ready triage. Unlike black-box ML models [
13,
14], NS-Assist provides transparent recommendations aligned with clinical guidelines. Its ability to link physiological data with actionable alerts bridges the gap between isolated monitoring tools and coordinated medical decision-making platforms.
3. Identified Problems in Idiopathic Nephrotic Syndrome Management
This paper presents collaborative research on managing idiopathic nephrotic syndrome (INS), a chronic condition that affects children and often recurs over time. These recurrences impact emotional well-being, academic engagement, and long-term care, underscoring the need for personalized therapeutic strategies. Effective management depends on close collaboration between the child, family, and an interdisciplinary healthcare team to adapt both lifestyle and treatment plans.
A key aspect of this approach is addressing the syndrome’s distinct clinical stages: the initial episode, where corticosteroid therapy and early monitoring are introduced; early relapse, which demands timely treatment adjustments and follow-up; and post-treatment monitoring, focused on tapering medications, detecting remission, and preventing future relapses. These stages represent critical decision points that structure therapeutic strategies, ensuring interventions remain both personalized and clinically consistent.
Interviews with pediatric practitioners highlighted the main challenges of managing INS, particularly the importance of early relapse detection, parental involvement, and consistent long-term monitoring. Frequent medical visits and hospitalizations disrupt children’s routines and place a heavy burden on families, especially those living far from specialized healthcare facilities. Tailoring treatment approaches to individual needs is therefore essential, and ongoing research continues to seek improved outcomes for complex cases.
Overall, this study underscores the necessity of adaptive healthcare strategies and resilient pediatric management models to better support children with INS and their families in dynamic care environments.
3.1. Current Treatment Process
The treatment protocol for Idiopathic Nephrotic Syndrome (INS) typically begins with a fifteen-day hospitalization for confirmed cases, involving clinical and biological diagnosis, symptomatic management (e.g., edema control, dietary adjustments), and daily monitoring by pediatricians and nurses. After discharge, caregivers transition to home-based care, with parents performing daily physiological measurements and regular proteinuria tests. Follow-up consultations are scheduled every two weeks initially, then extended to monthly and eventually quarterly checkups over a two-year period. Relapses require re-hospitalization and therapeutic adjustments (e.g., corticosteroid dosing). The process emphasizes interdisciplinary collaboration among pediatricians, nurses, and nutritionists, alongside active parental involvement. However, challenges remain in managing frequent hospital visits and complications, particularly for families living in remote areas or during health crises such as pandemics.
3.2. Current Process Challenges
The management of pediatric idiopathic nephrotic syndrome (INS) faces multifaceted challenges, particularly in daily home monitoring for families distant from hospitals, where delayed parental reporting of physiological and biological data (often weekly or biweekly) risks complications and treatment delays. Key issues include managing acute complications (e.g., infections, thromboses) during treatment, addressing pre-existing comorbidities (e.g., hypertension), and handling frequent relapses requiring specialized care for corticosteroid-dependent/resistant cases. These challenges were compounded by pandemic-related difficulties, including limited healthcare access, delayed follow-ups, and heightened viral exposure risks during hospital visits. To mitigate these barriers, adaptive strategies such as integrating telemedicine for remote monitoring, virtual consultations, and dynamic treatment adjustments were prioritized to ensure continuity of care and reduce systemic vulnerabilities during health crises.
4. The Proposed Solution: NS-Assist
The proposed NS-Assist system presents a robust solution for improving the management of idiopathic nephrotic syndrome (INS). Developed in collaboration with pediatricians from the Pediatrics Department at El-Mansoura Hospital, NS-Assist is a computerized decision support system designed to transform disease management through continuous patient monitoring and timely clinical intervention. The following section outlines its core functionalities and operational flow.
During pandemics, NS-Assist emphasizes virtual triage to minimize hospital visits while ensuring continuity of care. Remote alerts identify high-risk situations (e.g., infections, elevated creatinine levels), allowing clinicians to prioritize in-person consultations effectively. By integrating IoMT-enabled data collection with a transparent decision support system, NS-Assist enhances clinical accuracy and reinforces healthcare system resilience under crisis conditions.
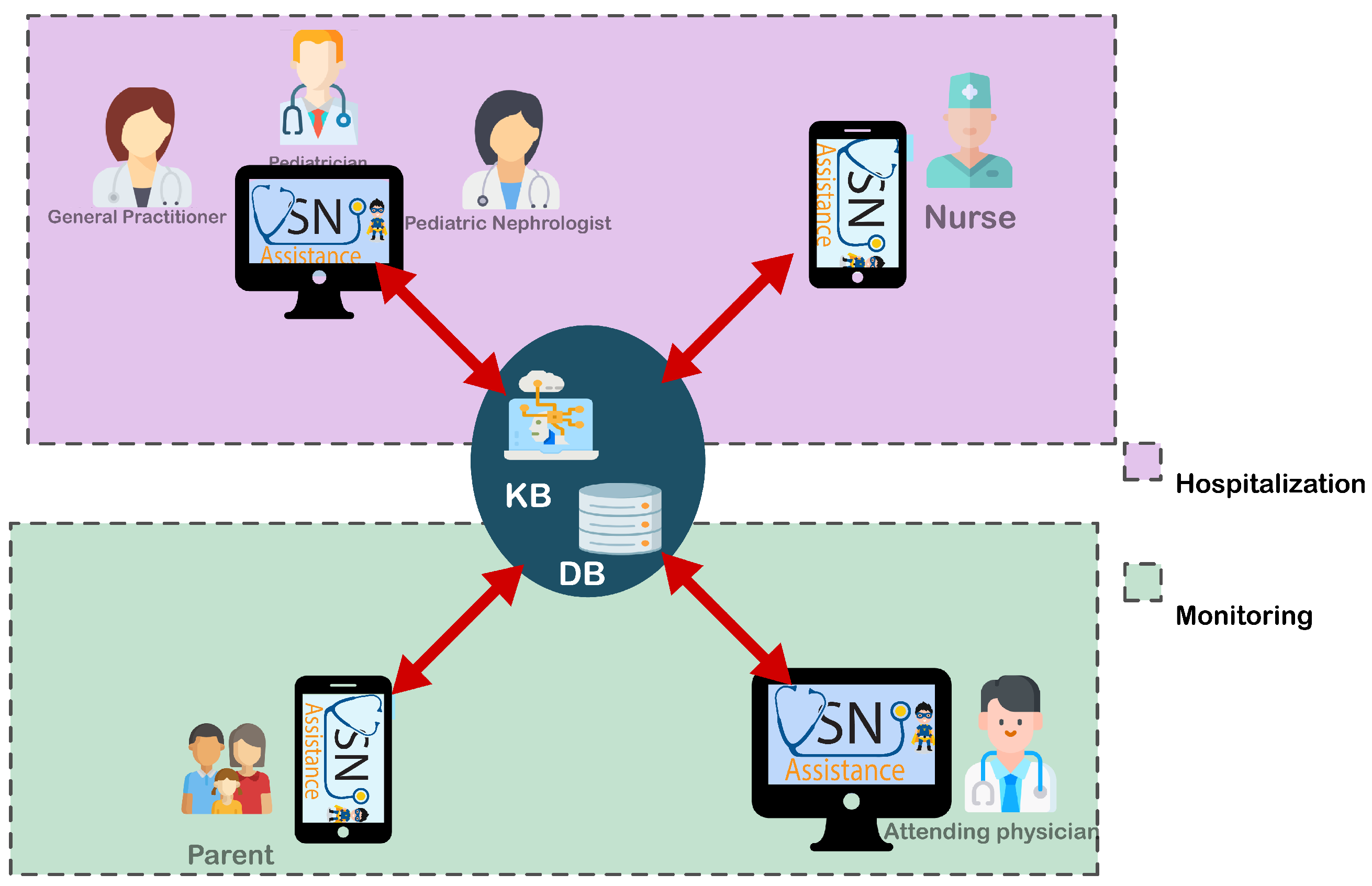
4.1. General Architecture of NS-Assist
NS-Assist’s architecture (
Figure 1) is structured to optimize the management of pediatric idiopathic nephrotic syndrome (INS) through a decision support system (DSS) that combines intelligent clinical reasoning with remote monitoring capabilities. The system enables continuous interaction between healthcare professionals and patient families, supporting diagnosis, treatment adaptation, and long-term follow-up through an integrated digital platform. From a deployment perspective, NS-Assist adopts a hybrid client–server architecture in which the decision support engine—comprising the rule-based and fuzzy inference modules—is deployed on a secure web server using a Spring Boot backend connected to a MySQL database. Physicians access the system through a ReactJS-based web interface, while parents and caregivers interact with a Flutter (Dart) mobile application that communicates with the same server via RESTful APIs. This configuration ensures centralized reasoning, synchronized data management, and seamless accessibility across desktop and mobile devices. To further clarify the software structure of the developed applications,
Figure 2 illustrates the architecture of the NS-Assist web and mobile applications. The backend, implemented in Spring Boot, hosts the business logic, authentication, and the DSS modules responsible for data acquisition, knowledge management, and inference. It exposes RESTful APIs that allow secure communication with both user interfaces. The web application (ReactJS) provides physicians with tools to manage patient files, visualize alerts, and adjust treatment plans, whereas the mobile application (Flutter) enables caregivers to record daily measurements, receive recommendations, and follow up on treatment progress. Both clients communicate with the backend through HTTPS and JSON messages, ensuring data confidentiality and integrity, while the MySQL database maintains centralized and synchronized medical records. This modular software architecture supports scalability, maintainability, and easy integration of future AI components. The overall system is organized into two interconnected layers, described below, which collectively support clinical decision-making and continuous patient monitoring.
In the hospitalization layer, patient files are created and continuously updated with clinical information, treatment history, and decision support outputs such as relapse protocols and corticosteroid adjustments. Nurses collect real-time physiological data, including blood pressure and urine chemistry, using IoMT devices. Any detected anomalies automatically trigger alerts, enabling rapid clinician intervention and ensuring timely adjustments in patient management.
In the monitoring layer, post-discharge care is supported through remote tracking of the patient’s health status. Parents record daily physiological measurements such as proteinuria and edema, which are transmitted to a central database for continuous oversight. Physicians then review this information to monitor progress, detect early signs of relapse, and make evidence-based adjustments to treatment plans during follow-up consultations.
Knowledge Base: the core of the DSS, hosts rules and protocols (e.g., 60 mg/m
2/day corticosteroid dosing for relapses) to guide diagnoses, treatment adjustments, and complication management. This integration of real-time data and rules-based guidance ensures consistent, proactive care across hospitalization and home monitoring phases. This knowledge base will be detailed in
Section 4.3.
4.2. NS-Assist Process
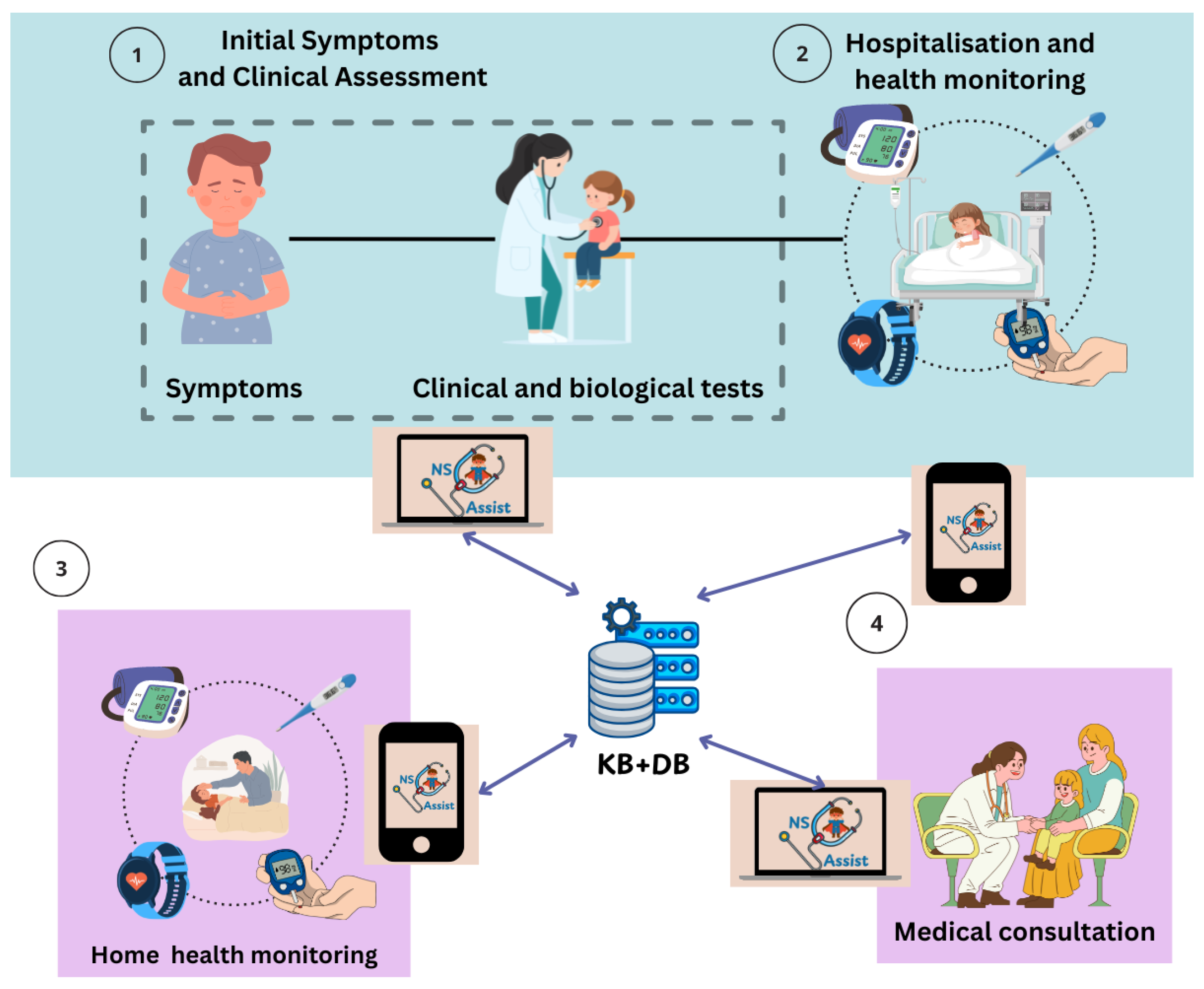
NS-Assist is a knowledge-driven decision support system (DSS) specifically designed to assist both healthcare professionals and patient families in the management of idiopathic nephrotic syndrome (INS) during hospitalization and home monitoring phases. The system serves two primary user groups: (1) physicians and pediatric nephrologists, who use the web application to manage patient records, receive rule-based and fuzzy-assisted decision support, and monitor treatment progress; and (2) patients’ parents or caregivers, who use the mobile application to record daily clinical parameters, such as proteinuria, weight, and edema, and to communicate with the medical team. By integrating Internet of Medical Things (IoMT) sensors for real-time data collection, NS-Assist enables continuous collaboration between doctors and families, supporting early relapse detection, timely intervention, and improved continuity of pediatric care through the following operational phases (
Figure 3):
Clinical Assessment and Initial Symptoms
The process begins with the onset of symptoms, where NS-Assist structures clinical and biological evaluations to assess the patient’s condition. By organizing this information, the system guides physicians in making accurate initial treatment decisions (
Figure 3➀).
Hospitalization and Clinical Monitoring
During hospitalization, NS-Assist centralizes and continuously updates patient records. It tracks key clinical parameters and generates timely alerts in case of critical changes, supporting physicians in providing appropriate interventions (
Figure 3➁).
Home Health Monitoring
In the home phase, NS-Assist ensures continuity of care by managing daily health data and maintaining structured follow-up. The system highlights early warning signs of relapse or complications, enabling physicians to intervene promptly when necessary (
Figure 3➂).
Medical Consultation and Decision Support
In the final phase, NS-Assist leverages its knowledge base (Table 3) to provide evidence-based decision support. It assists physicians in diagnosis and therapeutic planning, including the adjustment of treatment strategies during relapse episodes (
Figure 3➃).
In addition, NS-Assist automatically raises alarms when critical conditions are predicted. The system evaluates real-time physiological data against predefined clinical rules and triggers visual and push notifications to both physicians and caregivers, ensuring rapid response to potential relapses or complications.
4.3. Knowledge Base
Machine learning has shown remarkable progress in healthcare, offering valuable insights and predictive capabilities. However, in our work we adopt a knowledge-based system, as it provides specific advantages that align with the critical requirements of clinical practice. Knowledge-based systems are built on explicit, clinically validated rules (e.g., “IF symptom X AND biomarker Y, THEN diagnosis Z”), which makes their reasoning process transparent, traceable, and easy to communicate. This clarity is especially important in healthcare, where decisions must be justified to patients, regulatory authorities, and medical teams.
Another key strength is flexibility: the knowledge base can be continuously updated, with rules added, refined, or removed as medical expertise and treatment guidelines evolve. This flexibility ensures that the system remains clinically relevant and adaptable to new evidence.
Our collaboration with pediatric nephrologists to design a knowledge base for INS therapeutic management highlights these strengths. Critically, INS treatment follows a well-defined clinical schema—a stepwise protocol for diagnosis, relapse management, and dose adjustments—that maps directly to rule-based logic. The following are examples:
Diagnostic criteria (e.g., proteinuria thresholds, hypoalbuminemia) translate into clear “IF-THEN” rules for hospitalization or renal biopsy.
Treatment escalation steps (e.g., corticosteroid resistance → immunosuppressive therapy) become actionable decision pathways.
This schema-to-rules alignment ensures the system mirrors real-world clinical workflows, minimizing adoption barriers and maximizing physician trust.
4.3.1. Knowledge Base Key Attributes
The proposed NS-Assist knowledge base evaluates three critical biomarkers to guide clinical decisions for pediatric INS [
23]:
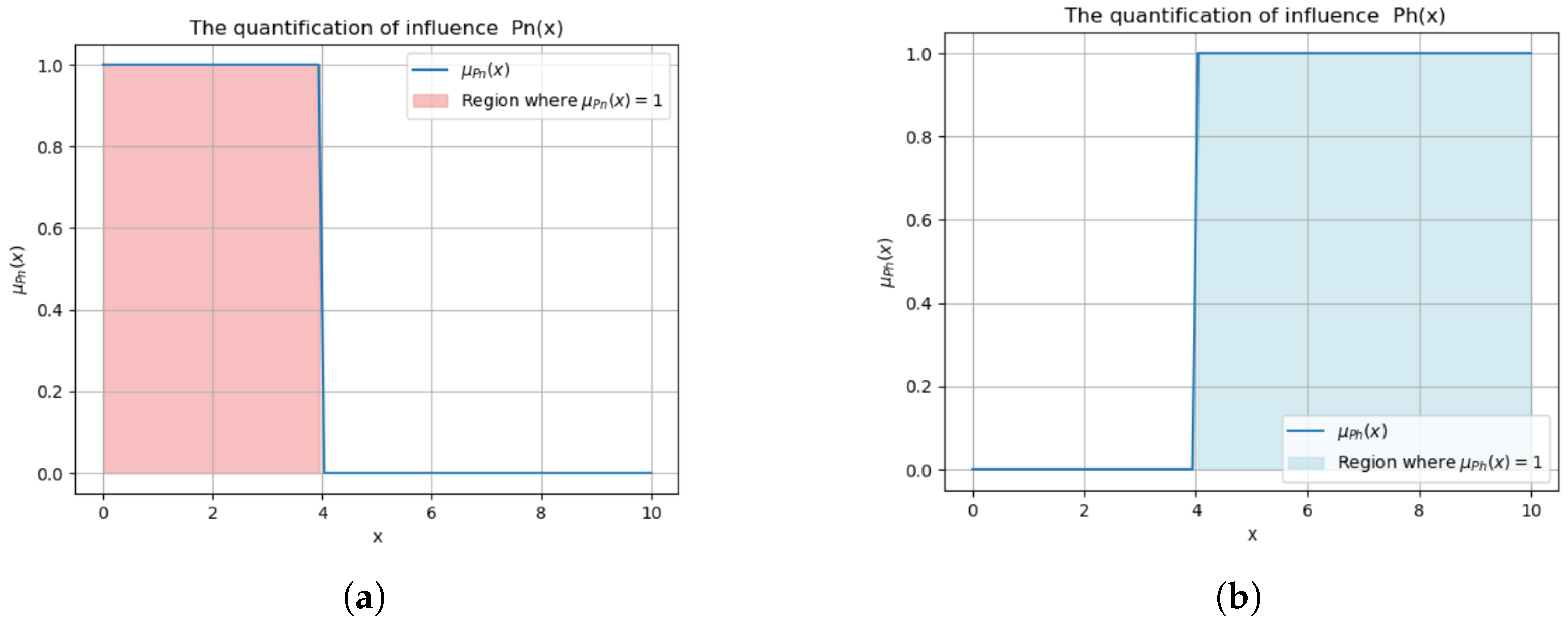
Proteinuria:
Normal threshold:
. Exceeding this indicates relapse risk [
24].
This binary classification (
Figure 4) triggers alerts for abnormal levels, prompting interventions like corticosteroid adjustments (e.g.,
).
Urine Dipstick:
Negative (−) results are considered normal; positive results (+, , etc.) indicate proteinuria.
In the knowledge base, positive readings are coded as 1 (abnormal) and negative readings as 0 (normal), supporting relapse detection.
Creatinine:
These proposed rules are embedded within the DSS logic and operate in conjunction with data streams from IoMT devices. As the system continuously receives patient data (e.g., blood pressure, proteinuria levels), it applies rule-based thresholds and decision logic to detect anomalies. When deviations are identified, such as a sudden increase in creatinine, the corresponding rule is triggered, leading to automated alerts and context-specific recommendations for clinicians.
4.3.2. Example of Knowledge Base Rules
To operationalize the therapeutic schema for idiopathic nephrotic syndrome (INS), we developed a structured knowledge base by translating clinical protocols into IF–THEN rules. These rules, derived from established treatment guidelines [
23,
26], encode the key decision points across the three main clinical stages: the initial episode, early relapse, and post-treatment monitoring.
Table 2 defines the main variables used in the knowledge base, representing measurable indicators (e.g., proteinuria levels), clinical milestones (e.g., day of treatment), and specific actions (e.g., initiating corticosteroid therapy or referring to a nephrologist).
For illustration,
Table 3 presents an example of the rule set for the post-treatment relapse stage. These rules guide corticosteroid tapering schedules, nephrology referrals, and remission criteria based on elapsed time and biomarker trends. These rules guide corticosteroid tapering schedules, nephrology referrals, and remission criteria based on time elapsed and biomarker trends. This rule-based encoding ensures that therapeutic interventions remain consistent with clinical best practices and are automatically triggered by incoming patient data.
4.3.3. Fuzzy Inference Extension
In addition to the crisp rule base presented above, NS-Assist integrates a fuzzy inference component to handle uncertainty and gradual clinical variations that are not captured by binary thresholds. This module focuses on biomarkers such as proteinuria, edema, and creatinine, which are represented as linguistic variables with terms like Low, Moderate, and High. Triangular and trapezoidal membership functions were defined according to pediatric nephrology reference ranges to model these fuzzy sets.
During the fuzzification stage, continuous input values (e.g., proteinuria level or edema degree) are converted into fuzzy memberships for the respective terms. A Mamdani-type inference process then applies fuzzy rules such as: (1) IF proteinuria is High AND edema is Severe THEN relapse risk is Critical; (2) IF proteinuria is Moderate AND edema is Mild THEN relapse risk is Warning; (3) IF proteinuria is Low AND edema is None THEN relapse risk is Normal.
These fuzzy rules are aggregated to form a combined fuzzy output that represents the relapse-risk level. Defuzzification is carried out using the centroid (center-of-gravity) method to yield a crisp risk index in the range [0, 1]. This value is then mapped to the discrete alert categories Normal, Warning, and Critical, used by NS-Assist to notify clinicians and caregivers. Diagnostic simulations performed on representative patient patterns confirmed strong consistency between fuzzy outputs and physician assessments, supporting the reliability and interpretability of the decision-support process.
5. Implementation
This section presents the implementation of the NS-Assist system, designed to improve the management of pediatric Idiopathic Nephrotic Syndrome (INS) through complementary web and mobile applications. The web application integrates a rule-based clinical decision support system (DSS) that provides evidence-driven diagnostic and therapeutic recommendations. The mobile application enables real-time monitoring of patient data, supporting the early detection of anomalies. Together, these components strengthen clinical decision-making, reduce diagnostic delays, and promote treatment adherence, particularly in resource-limited or crisis environments.
5.1. NS-Assist Process Through the Web Application
The web application plays a central role in the management of idiopathic nephrotic syndrome, supporting clinicians across several stages of care. In the first stage (Clinical Assessment and Initial Symptoms), it is used to register clinical symptoms and laboratory test results, ensuring structured and accessible patient records. In the second stage (Hospitalization and Clinical Monitoring), it centralizes medical information, providing physicians with a comprehensive view of patient history, relapse episodes, and previous treatments to support informed clinical decisions. Finally, in the fourth stage (Medical Consultation and Decision Support), the web application interacts with the system’s knowledge base to generate evidence-based recommendations, offering diagnostic suggestions, treatment plans, and appropriate medical actions.
Figure 5,
Figure 6 and
Figure 7 illustrate the role of the web application during the
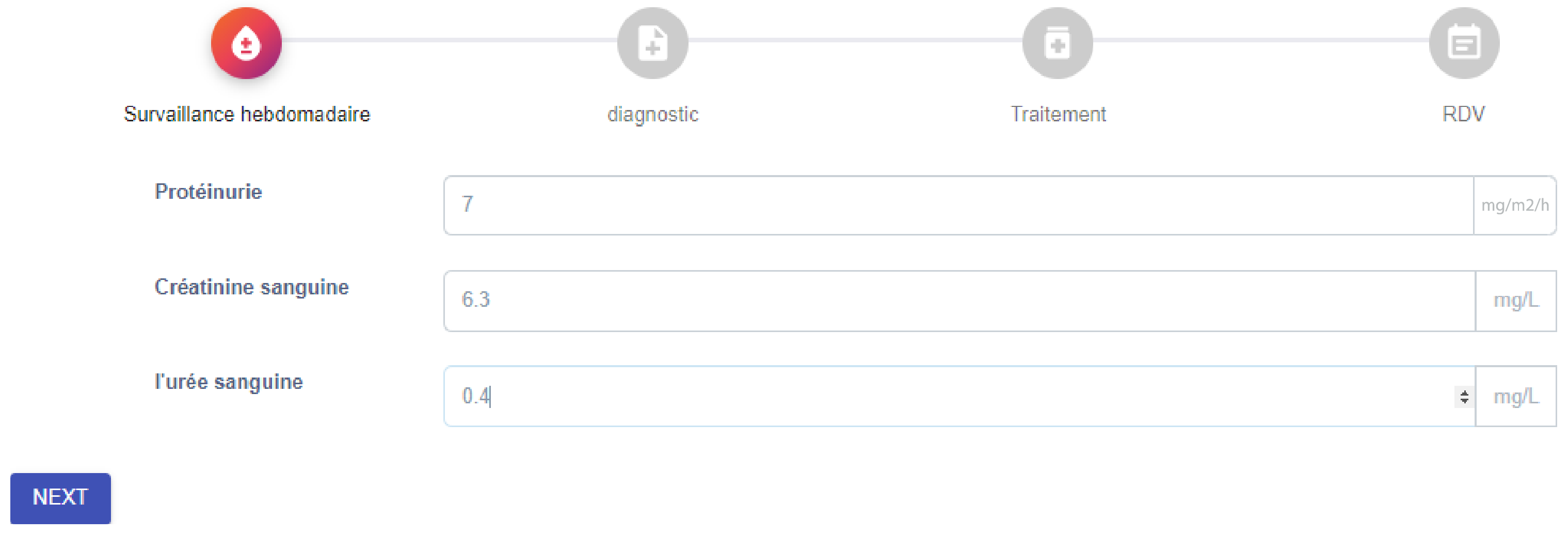
Medical Consultation and Decision Support.
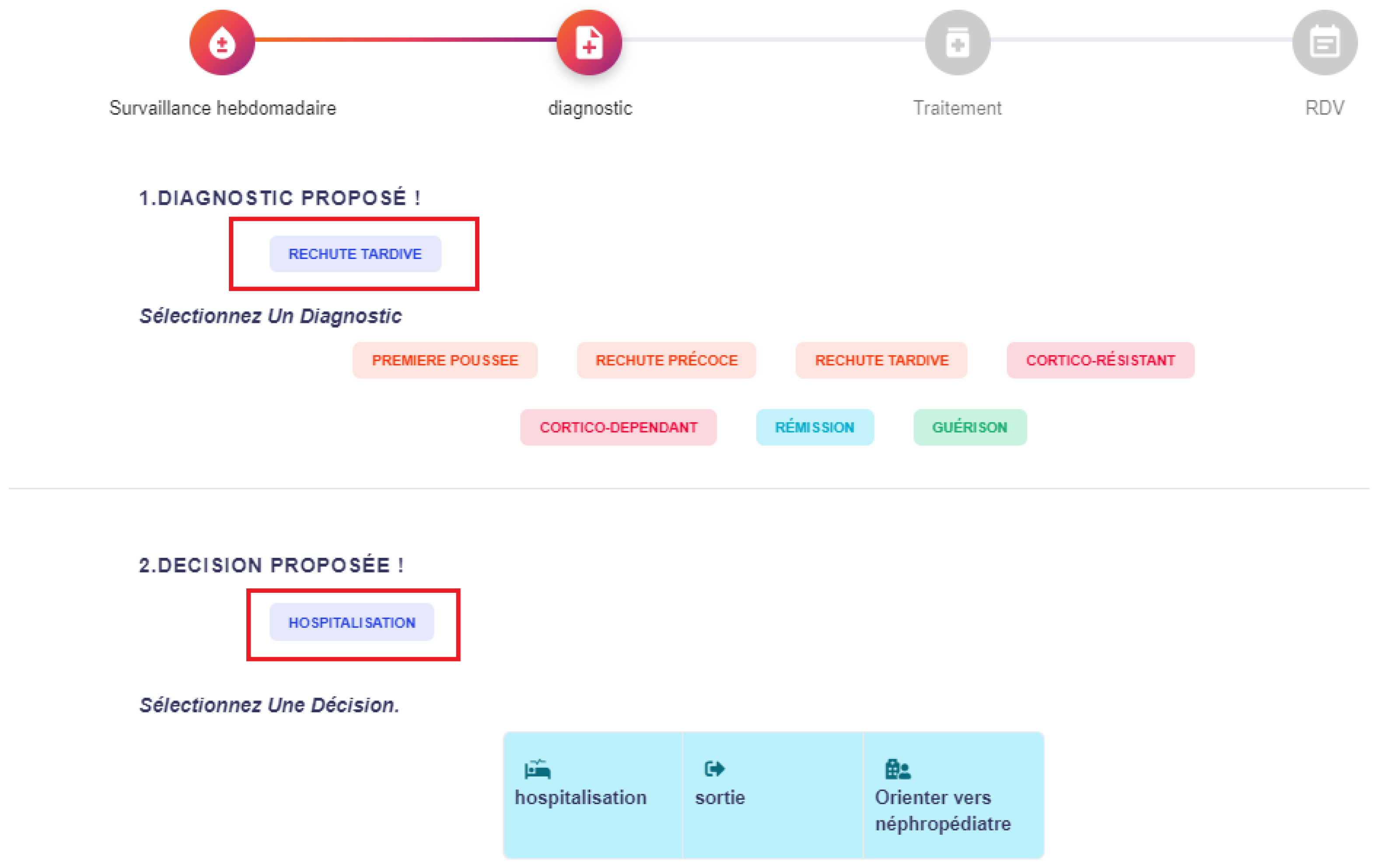
Figure 5 shows the data entry interface for weekly monitoring, where clinicians record biological indicators such as proteinuria, creatinine, and urea levels. Based on these inputs, the system provides diagnostic support as shown in
Figure 6, suggesting possible clinical outcomes (e.g., late relapse) together with corresponding actions (e.g., hospitalization). Finally,
Figure 7 demonstrates the treatment recommendation interface, where the system proposes corticosteroid dosage, frequency, and duration in line with clinical guidelines.
5.2. NS-Assist Process Through the Mobile Application
Complementing the web application, the mobile application is primarily used during the second (Hospitalization and Clinical Monitoring) and third (Home Health Monitoring) phases. During hospitalization, caregivers and nurses can input daily physiological parameters (e.g., blood pressure, weight) and biomarker values (e.g., proteinuria, creatinine) directly through IoMT-connected devices. At home, parents can perform daily follow-up of their child’s condition, with data transmitted in real time to the central database. The Knowledge Base processes these inputs by applying predefined clinical rules to identify anomalies and trigger alerts when necessary.
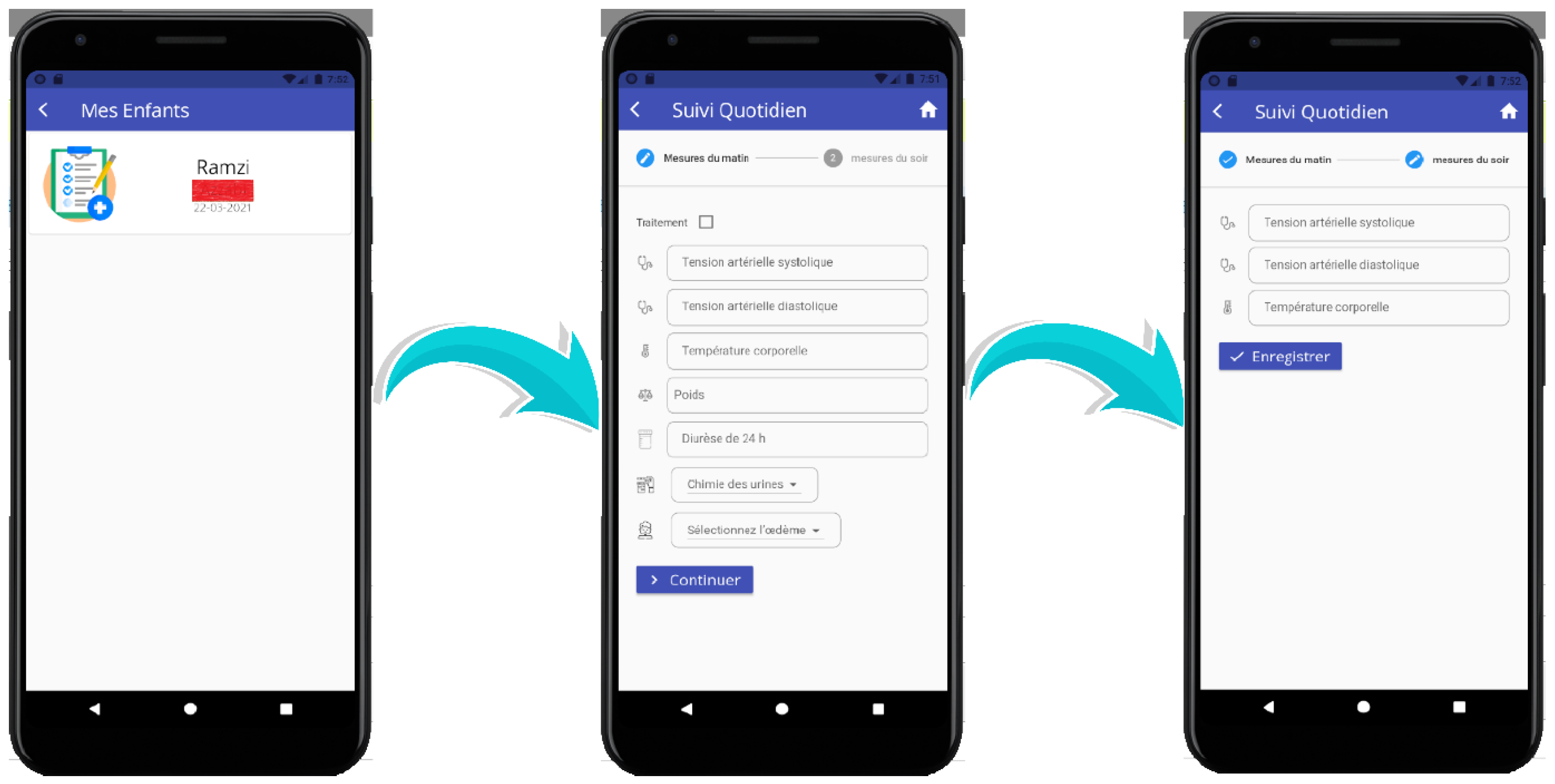
Figure 8 illustrates this functionality during the
Home Health Monitoring phase. The interface enables parents to record daily measurements such as systolic and diastolic blood pressure, body temperature, weight, urine chemistry, and the presence of edema. Once submitted, these values are analyzed automatically, and alerts are generated for pediatricians when abnormalities are detected (e.g., elevated blood pressure or appearance of edema). This integration of parent-reported data with automated monitoring ensures proactive follow-up, timely interventions, and continuous communication between families and physicians, even outside the hospital setting.
6. System Evaluation and Validation
This section presents the evaluation of the NS-Assist system through a field experiment and a complementary survey designed to assess its usability, clinical impact, and workflow efficiency.
The field evaluation was conducted in collaboration with the Pediatrics Department of El-Mansoura Hospital (Algeria). Fifteen pediatric patients with idiopathic nephrotic syndrome (INS) in stable, non-relapse condition and their families participated after providing informed consent. Patients were selected from ongoing follow-up cases managed at the hospital. The study was observational and focused on assessing system usability and workflow efficiency; therefore, no randomization or control group was applied. All patient data were anonymized in accordance with ethical standards.
The experiment compared the temporal distribution of healthcare processes in two settings: traditional methods and system-assisted consultations. Figures were developed based on feedback from medical professionals and parents of children diagnosed with INS.
We begin with a typical case involving a child without complications (
Figure 9a). Here, the system reduces the time needed for health parameter measurements (2–5 min) and discussions with parents or children (5–10 min), while communication with other physicians and decision-making remain unchanged.
The second case compares a routine consultation for a child with unrelated health concerns (
Figure 9b). In this scenario, the system reduces time across all processes: parameter measurements (5–10 min), parent or child discussions (10–15 min), communication with other physicians (5–10 min), and decision-making (1–10 min).
Figure 9c illustrates the relapse case, where significant time savings are observed in measurements (5–10 min), discussions (10–20 min), inter-physician communication (5–30 min), and decision-making (1–10 min).
Overall,
Figure 9 consolidates these findings, emphasizing the system’s ability to streamline clinical workflows and support more personalized care. Making time allocations visible across scenarios also helps physicians plan consultations more effectively.
To further validate the system among users, a survey was conducted involving 25 healthcare professionals (physicians and medical students) and 15 parents. The 13-item questionnaire evaluated four dimensions: (1) usability of the clinician and parent interfaces, (2) clinical utility in improving care quality through real-time monitoring, (3) workflow efficiency and coordination, and (4) potential limitations such as cost or reduced personal interaction. The survey aimed to assess how NS-Assist enhances decision-making, streamlines workflows, and supports patient-centered care.
6.1. Survey Results
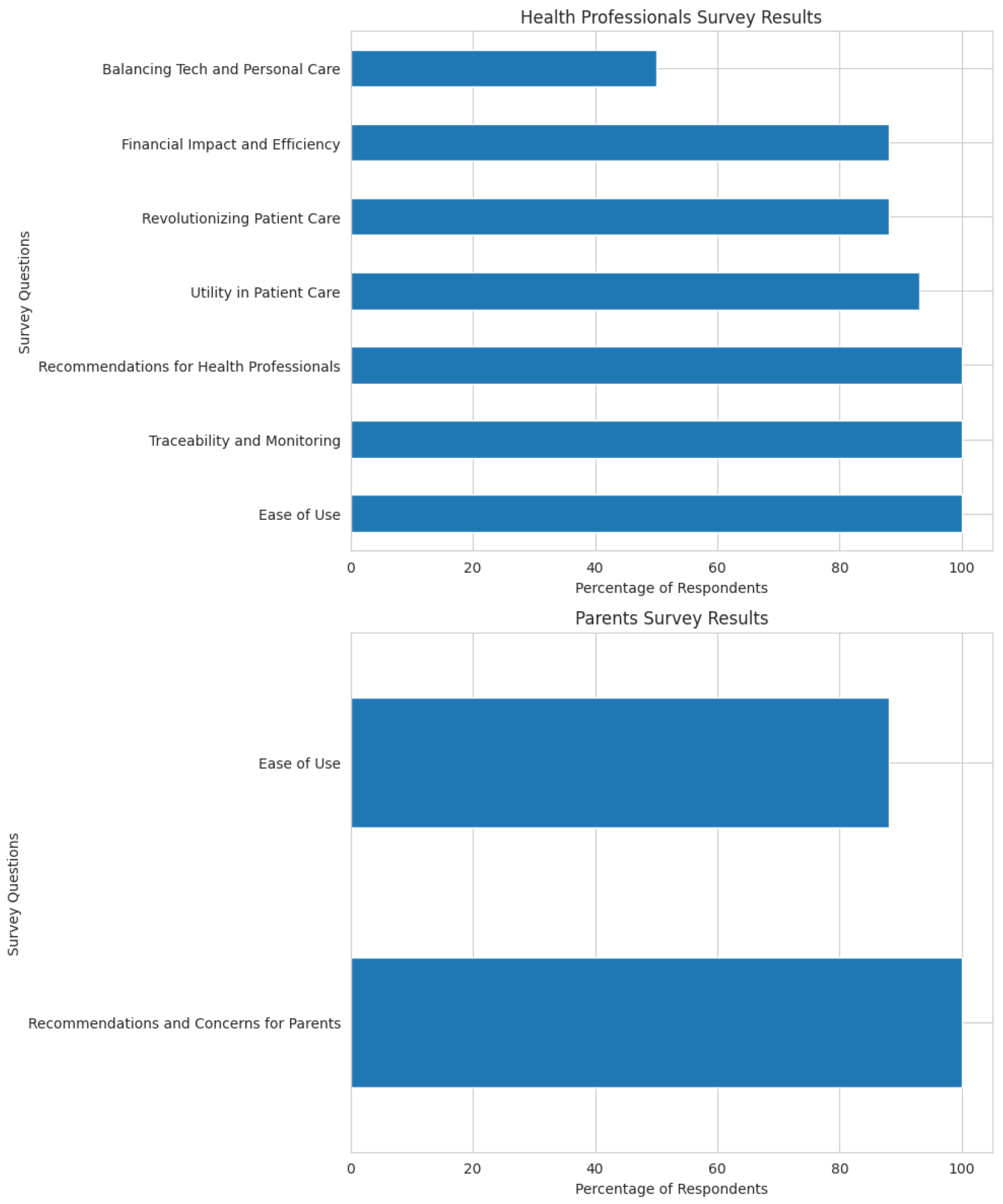
The results show strong endorsement of the NS-Assist system by both healthcare professionals and parents, while also offering valuable feedback for improvement (
Figure 10).
6.1.1. For Health Professionals
Ease of Use: 100% of professionals found the web interface easy to use.
Clinical Utility: All respondents agreed that the system improves care for INS patients; 93% noted better coordination among providers.
Innovative Impact: 88% believe the system can revolutionize therapeutic care; 97% valued real-time monitoring.
Traceability and Monitoring: All respondents confirmed complete traceability of patient measures.
Financial Efficiency: 88% indicated no additional costs; 90% highlighted time savings in daily tasks.
Recommendations: 100% would recommend NS-Assist to colleagues.
Personal Interaction: 50% expressed concern that digital tools might reduce clinician–patient contact.
6.1.2. For Parents
Overall, NS-Assist demonstrates strong potential to enhance INS management through improved usability, efficiency, and real-time insights. The positive reception from healthcare professionals and parents reinforces its value as a reliable, patient-centered telemonitoring and decision-support solution.
7. Conclusions
In this paper, we introduced NS-Assist, a smart pediatric monitoring and decision-support system specifically designed to manage Idiopathic Nephrotic Syndrome (INS) in children. Developed and tested in collaboration with pediatric teams in Algeria, the system integrates a rule-based expert engine with Internet of Medical Things (IoMT) technologies to provide real-time monitoring and medical decision support in both normal and crisis conditions.
NS-Assist demonstrates strong potential to assist healthcare professionals and parents through intelligent alerts, streamlined workflows, and resilient remote supervision. Its structured knowledge base and context-aware architecture make it well-suited for pediatric care, particularly in resource-constrained environments.
Although initially implemented in Algeria, the system’s modular and flexible design allows adaptation to other healthcare contexts and pediatric diseases worldwide. Future directions include incorporating machine learning for enhanced diagnostic accuracy and expanding its use to additional chronic pediatric conditions. NS-Assist thus offers a scalable model for improving care continuity and resilience across different regions, especially during global health emergencies.
Furthermore, NS-Assist emphasizes explainability through its hybrid reasoning approach, whereby each recommendation can be traced to explicit clinical rules or fuzzy inference logic. This transparency strengthens physician trust and facilitates clinical validation. Nevertheless, the current validation scope remains limited, highlighting the need for broader, multi-center evaluations. Future work will also focus on integrating NS-Assist with electronic health record systems and other medical assistance platforms to ensure seamless data exchange, adaptive knowledge updates, and full interoperability within hospital infrastructures.
Author Contributions
N.Z.: Conceptualization, Methodology, Software, Writing—original draft, Writing—review and editing, Visualization, and Project administration; N.B. (Nardjes Bouchemal): Validation, Formal analysis, Writing—review and editing, and Supervision; I.B.: Methodology and Writing—original draft; N.B. (Naila Bouchemal): Validation and Formal analysis; G.I.: Methodology, Validation, and Formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union—NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BGRRP-2.013-0001-C03.
Institutional Review Board Statement
Not applicable. The study did not involve direct experimentation on human participants.
Informed Consent Statement
Patient data were anonymized prior to analysis. Informed consent was obtained from all subjects or their legal guardians involved in the study.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors gratefully acknowledge the financial support of the European Union—NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BGRRP-2.013-0001-C03. The authors would also like to thank all contributors and institutions that supported this work. Special thanks are extended to Saad Harous and Mounia Talbi for their valuable guidance and constructive feedback during the development of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bagga, A.; Mantan, M. Nephrotic syndrome in children. Indian J. Med. Res. 2005, 122, 13. [Google Scholar]
- Orphanet. Idiopathic Nephrotic Syndrome. 2023. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Expert=357502&lng=EN (accessed on 11 February 2024).
- UpToDate. Treatment of Idiopathic Nephrotic Syndrome in Children. 2023. Available online: https://www.uptodate.com/contents/treatment-of-idiopathic-nephrotic-syndrome-in-children (accessed on 11 February 2024).
- Maniam, G.; Sampe, J.; Jaafar, R.; Ibrahim, M.F. Smart monitoring system for chronic kidney disease patients based on fuzzy logic and IoT. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 324–333. [Google Scholar] [CrossRef]
- Wang, C.; Boyd, R.; Mitchell, R.; Wright, W.D.; McCracken, C.; Escoffery, C.; Patzer, R.E.; Greenbaum, L.A. Development of a novel mobile application to detect urine protein for nephrotic syndrome disease monitoring. BMC Med. Inform. Decis. Mak. 2019, 19, 105. [Google Scholar] [CrossRef]
- Soler, M.J.; Heras, M.M.; Ortiz, A.; Pino, M.D.D.P.Y.; Lazo, M.S. Impact of COVID-19 pandemic in Spanish Nephrology Services. Nefrologia (Engl. Ed.) 2020, 40, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Onuki, Y.; Watanabe, T.; Ono, T.; Oyake, C.; Ikeda, H. Pediatric idiopathic nephrotic syndrome during COVID-19 omicron variant pandemic. Pediatr. Int. 2024, 66, e15779. [Google Scholar] [CrossRef]
- Veltkamp, F.; Thenot, V.; Mussies, C.; van Lieshout, B.; Peters-Sengers, H.; Kers, J.; Khan, D.H.; Hogan, J.; Florquin, S.; Bouts, A.H.M.; et al. Incidence of idiopathic nephrotic syndrome during the COVID 19 pandemic in the Paris area (France) and in The Netherlands. Pediatr. Nephrol. 2023, 38, 3681–3692. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B. Advanced applications in chronic disease monitoring using IoT mobile sensing device data, machine learning algorithms and frame theory: A systematic review. Front. Public Health 2025, 13, 1510456. [Google Scholar] [CrossRef]
- Mbanugo, O.J. AI Enhanced Telemedicine: A Common Sense Approach to Chronic Disease Management and a Tool to Bridging the Gap in Healthcare Disparities. Int. J. Res. Publ. Rev. 2025, 6, 3223–3241. [Google Scholar] [CrossRef]
- Badidi, E. Edge AI for early detection of chronic diseases and the spread of infectious diseases: Opportunities, challenges, and future directions. Future Internet 2023, 15, 370. [Google Scholar] [CrossRef]
- Rehan, H. Enhancing early detection and management of chronic diseases with AI driven predictive analytics on healthcare cloud platforms. J. Assist. Sci. Discov. 2024, 4, 1–38. [Google Scholar]
- Ye, Q.; Li, Y.; Liu, H.; Mao, J.; Jiang, H. Machine learning models for predicting steroid resistant nephrotic syndrome. Front. Immunol. 2023, 14, 1090241. [Google Scholar] [CrossRef]
- Zaorska, K.; Zawierucha, P.; Świerczewska, M.; Ostalska-Nowicka, D.; Zachwieja, J.; Nowicki, M. Prediction of steroid resistance and steroid dependence in nephrotic syndrome children. J. Transl. Med. 2021, 19, 130. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Wang, Y.; Chen, X.; Zheng, W.; Zhong, X.; Shang, D.; Huang, M.; Gao, X.; Deng, H.; et al. Tacrolimus pharmacokinetics in pediatric nephrotic syndrome: A combination of population pharmacokinetic modelling and machine learning approaches to improve individual prediction. Front. Pharmacol. 2022, 13, 942129. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Zeng, H.; Zheng, W.; Ieong, C.; Li, H.; Huang, Q.; Xu, Z.; Yang, J.; Liang, Q.; et al. Tacrolimus in the treatment of childhood nephrotic syndrome: Machine learning detects novel biomarkers and predicts efficacy. Pharmacotherapy 2023, 43, 43–52. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Wang, X.; Zhong, X.; Liang, H.; Wei, Y.; Deng, H.; Hu, R.; Zhang, T.; Chen, Y.; et al. Prediction of tacrolimus dose/weight adjusted trough concentration in pediatric refractory nephrotic syndrome: A machine learning approach. Pharmacogenom. Pers. Med. 2022, 15, 143–155. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Ieong, C.; Gao, X.; Li, Y.; Liao, X.; Yang, H.; Li, H.; He, F.; He, Y.; et al. Early prediction of tacrolimus induced tubular toxicity in pediatric refractory nephrotic syndrome using machine learning. Front. Pharmacol. 2021, 12, 638724. [Google Scholar] [CrossRef] [PubMed]
- Improta, G.; Mazzella, V.; Vecchione, D.; Santini, S.; Triassi, M. Fuzzy logic based clinical decision support system for the evaluation of renal function in post Transplant Patients. J. Eval. Clin. Pract. 2020, 26, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Al Mashhadi, H.M.; Khudhair, A.L. Design AI platform using fuzzy logic technique to diagnose kidney diseases. Telkomnika 2023, 21, 324–332. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Li, Y.; Wang, A.; Chen, Z.; Wang, L.; Xiao, L.; Yu, S.; Chen, H.; Zhang, F.; et al. Development and external validation of a machine learning based predictive model for acute kidney injury in hospitalized children with idiopathic nephrotic syndrome. BMC Med. Inform. Decis. Mak. 2025, 25, 364. [Google Scholar]
- Filler, G.; Gipson, D.S.; Iyamuremye, D.; de Ferris, M.E.D.G. Artificial intelligence in pediatric nephrology—A call for action. Adv. Kidney Dis. Health 2023, 30, 17–24. [Google Scholar] [CrossRef]
- Gruppen, M.P.; Bouts, A.H.; der Weide, M.C.J.; Merkus, M.P.; Zurowska, A.; Maternik, M.; Massella, L.; Emma, F.; Niaudet, P.; Cornelissen, E.A.M.; et al. A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid sensitive idiopathic nephrotic syndrome. Kidney Int. 2018, 93, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Skandhan, A. Proteinuria: A Guide to Diagnosis and Assessment. Proteins 2020, 2, 10. [Google Scholar]
- University of Iowa. Creatinine. 2024. Available online: https://www.healthcare.uiowa.edu/path_handbook/handbook/test530.html (accessed on 11 February 2024).
- Boyer, O.; Baudouin, V.; Bérard, E.; Dossier, C.; Audard, V.; Guigonis, V.; Vrillon, I. Aspects cliniques du syndrome néphrotique idiopathique de l’enfant. Arch. Pédiatrie 2017, 24, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).