Synergistic Effects of Fly Ash and Oyster Shell Powder in Ternary Low-Carbon Cementitious Materials: Macro–Micro Experimental Studies and Life Cycle Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mixture Preparation and Mix Ratio Design
2.3. Test Method
2.3.1. Mechanical Properties
2.3.2. UPV
2.3.3. Surface Resistivity
2.3.4. Heat of Hydration
2.3.5. Microscopic Analysis
2.3.6. Life Cycle Assessment
3. Experimental Results
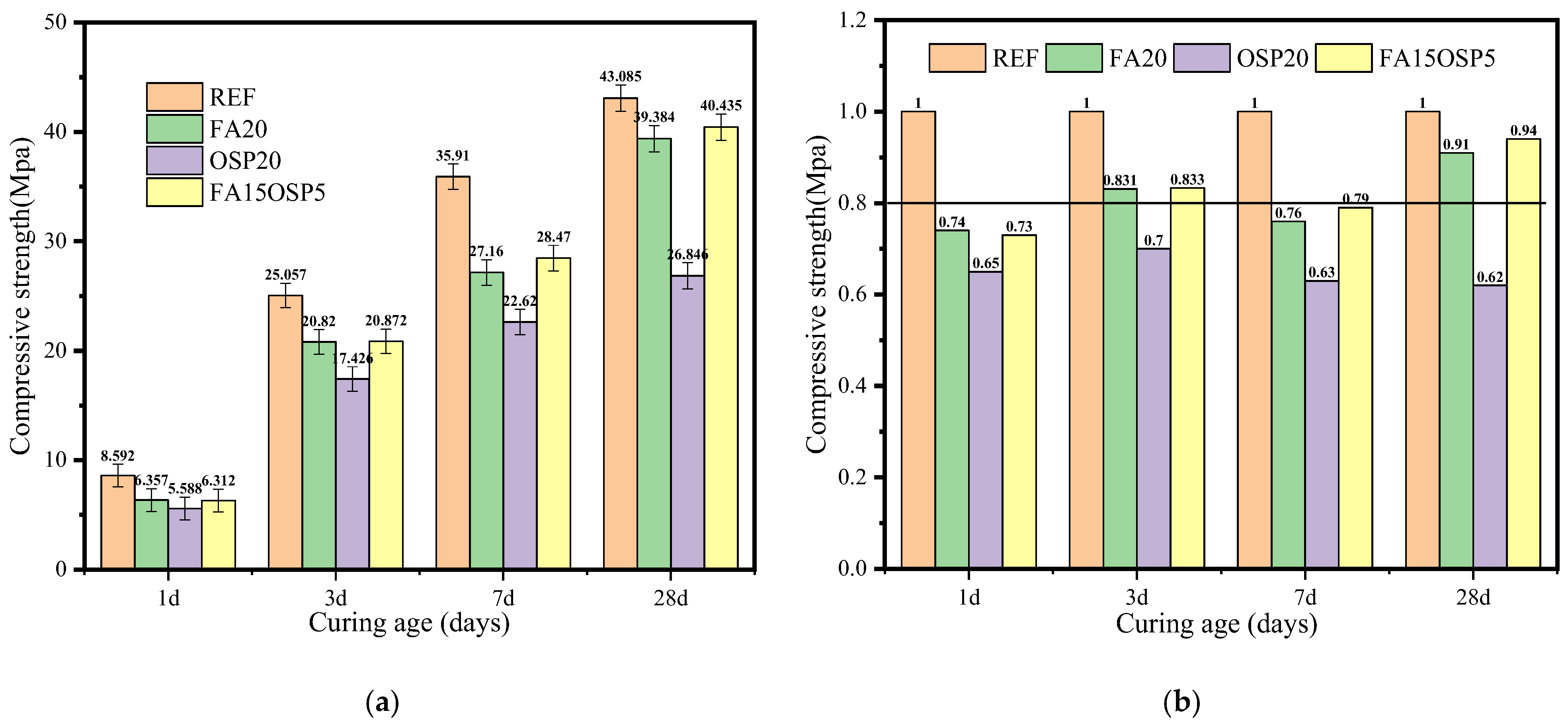
3.1. Compressive Strength
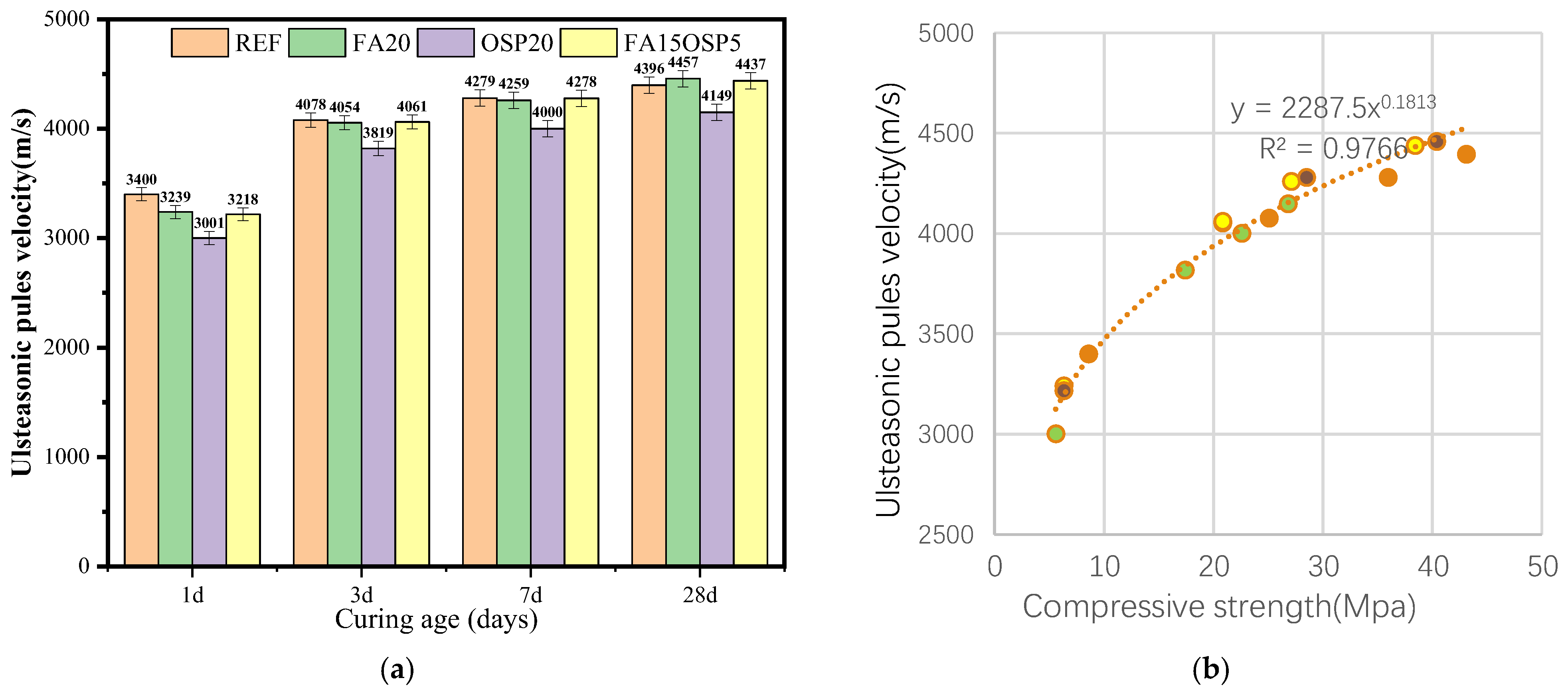
3.2. Ultrasonic Pulse Velocity
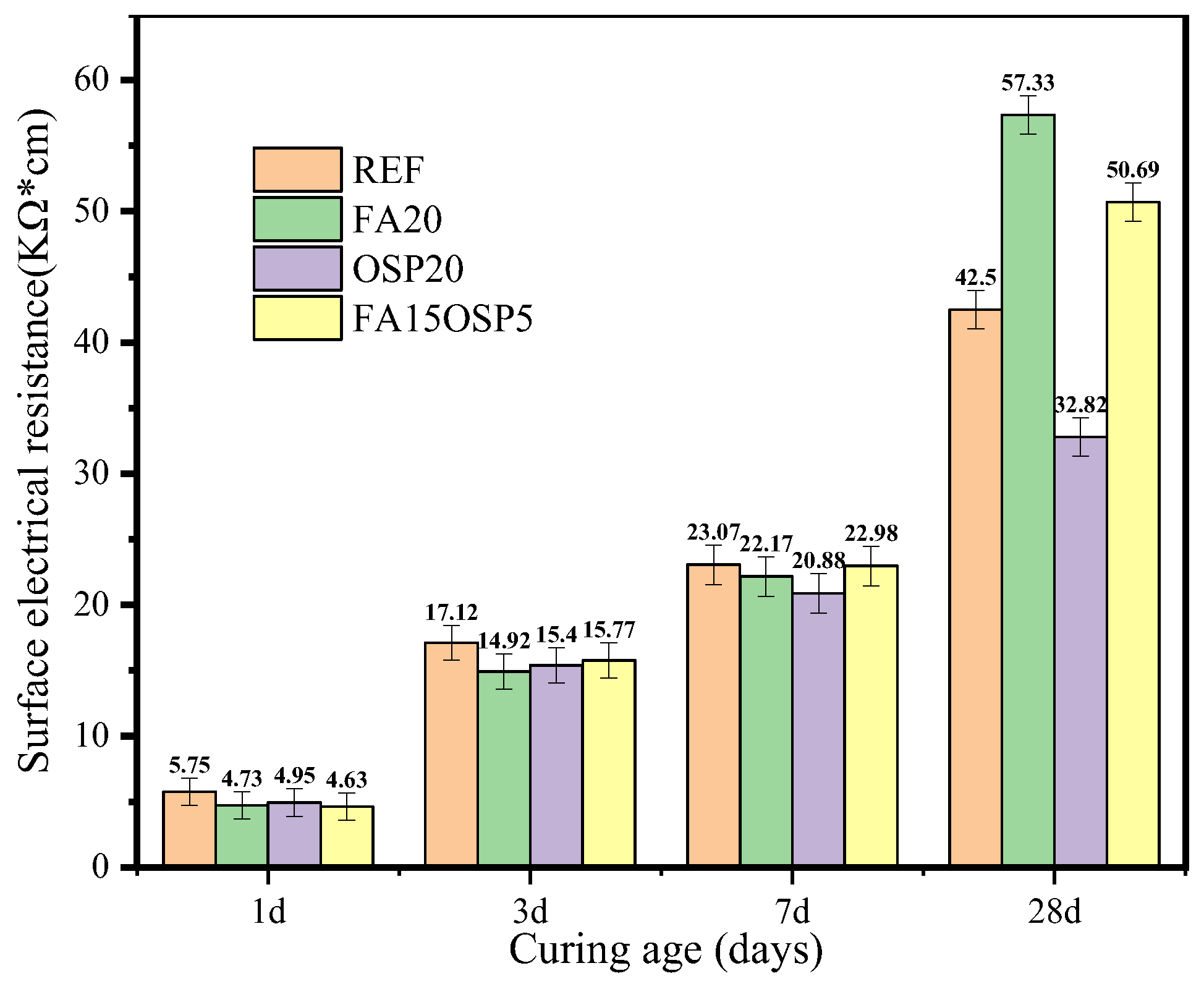
3.3. Surface Electrical Resistivity
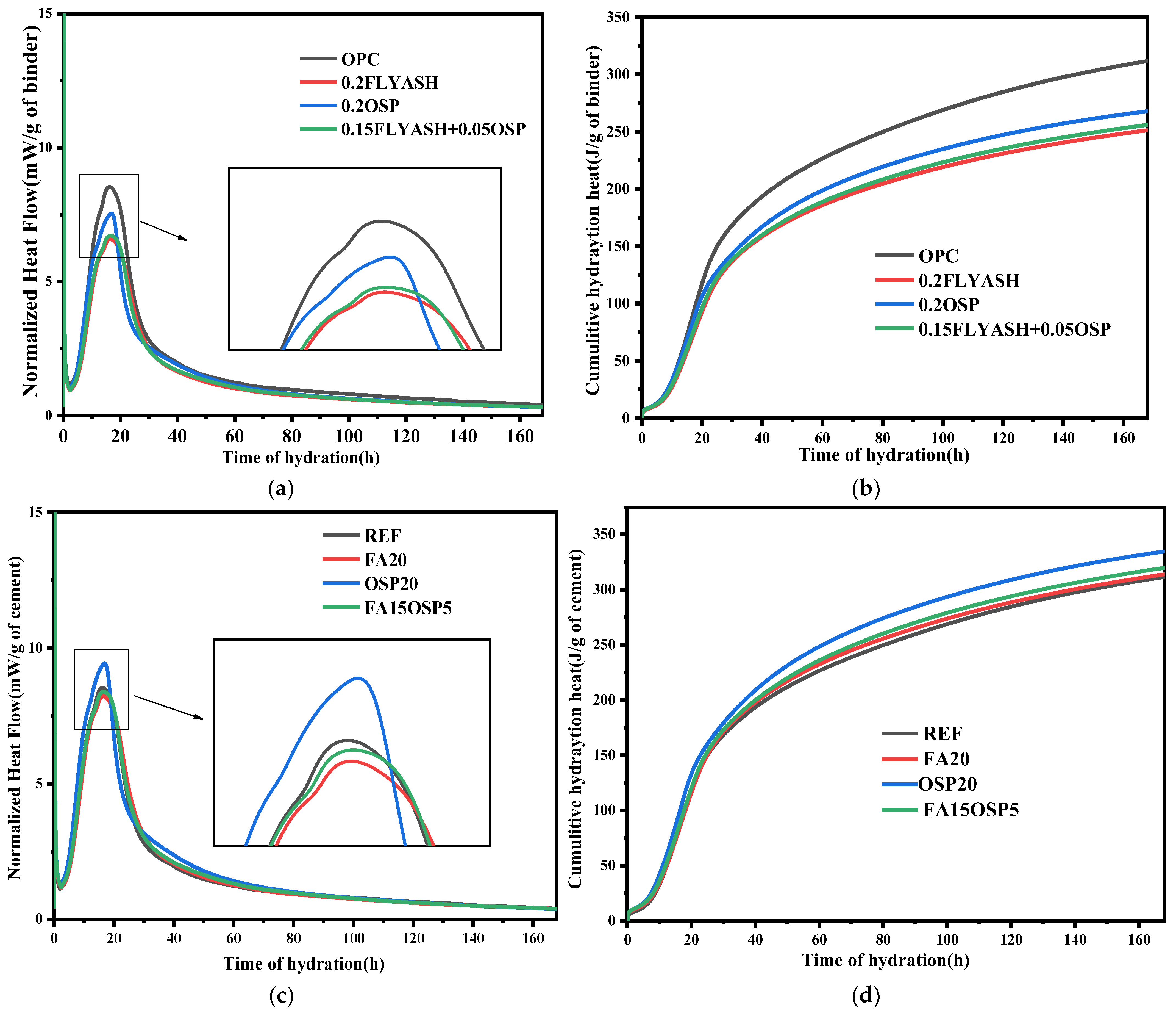
3.4. Heat of Hydration
3.5. XRD
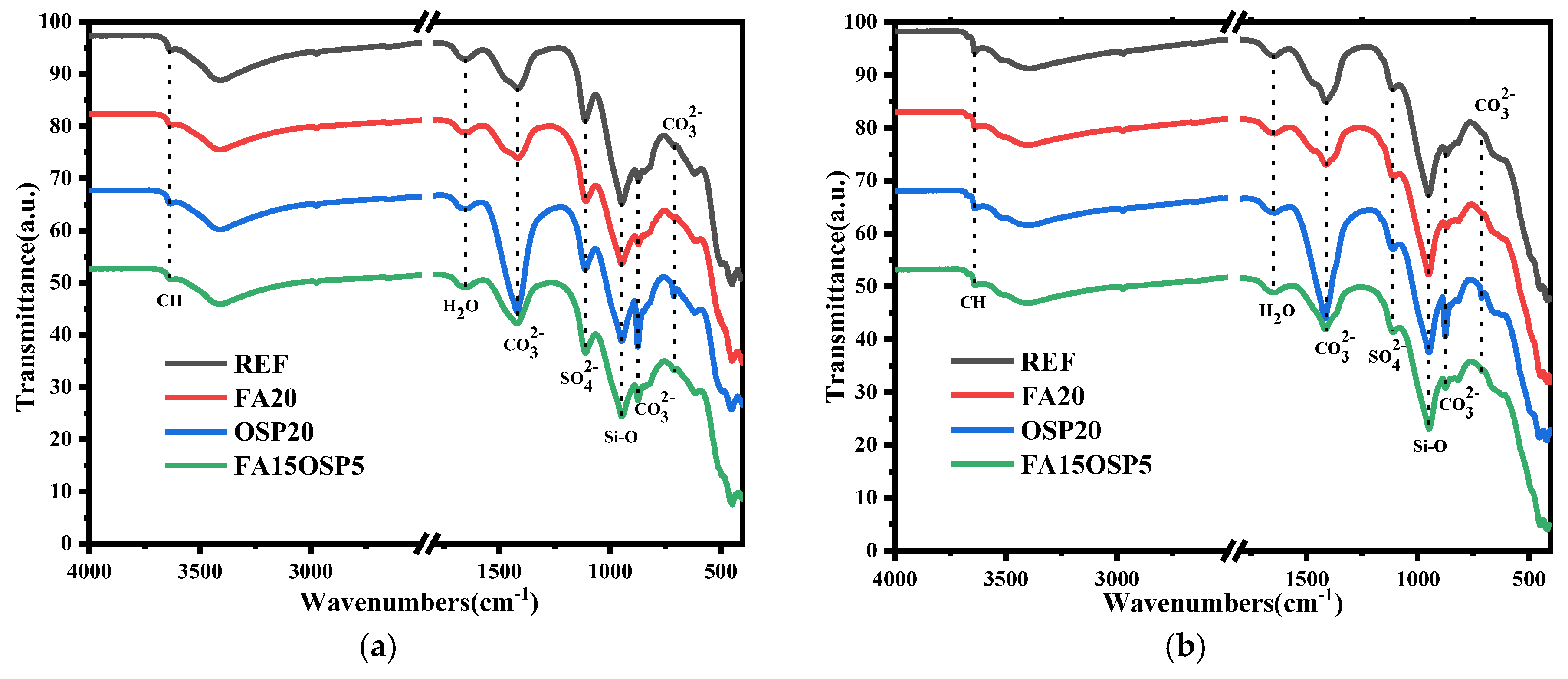
3.6. FT-IR
3.7. TGA/DTG
3.8. Life Cycle Assessment
3.8.1. Raw Materials
3.8.2. Transportation Stage of Raw Materials
3.8.3. Mixing Process of Mortar
3.8.4. Impact of Unit Compressive Strength on the Environment
3.8.5. Results of the Sustainability Analysis
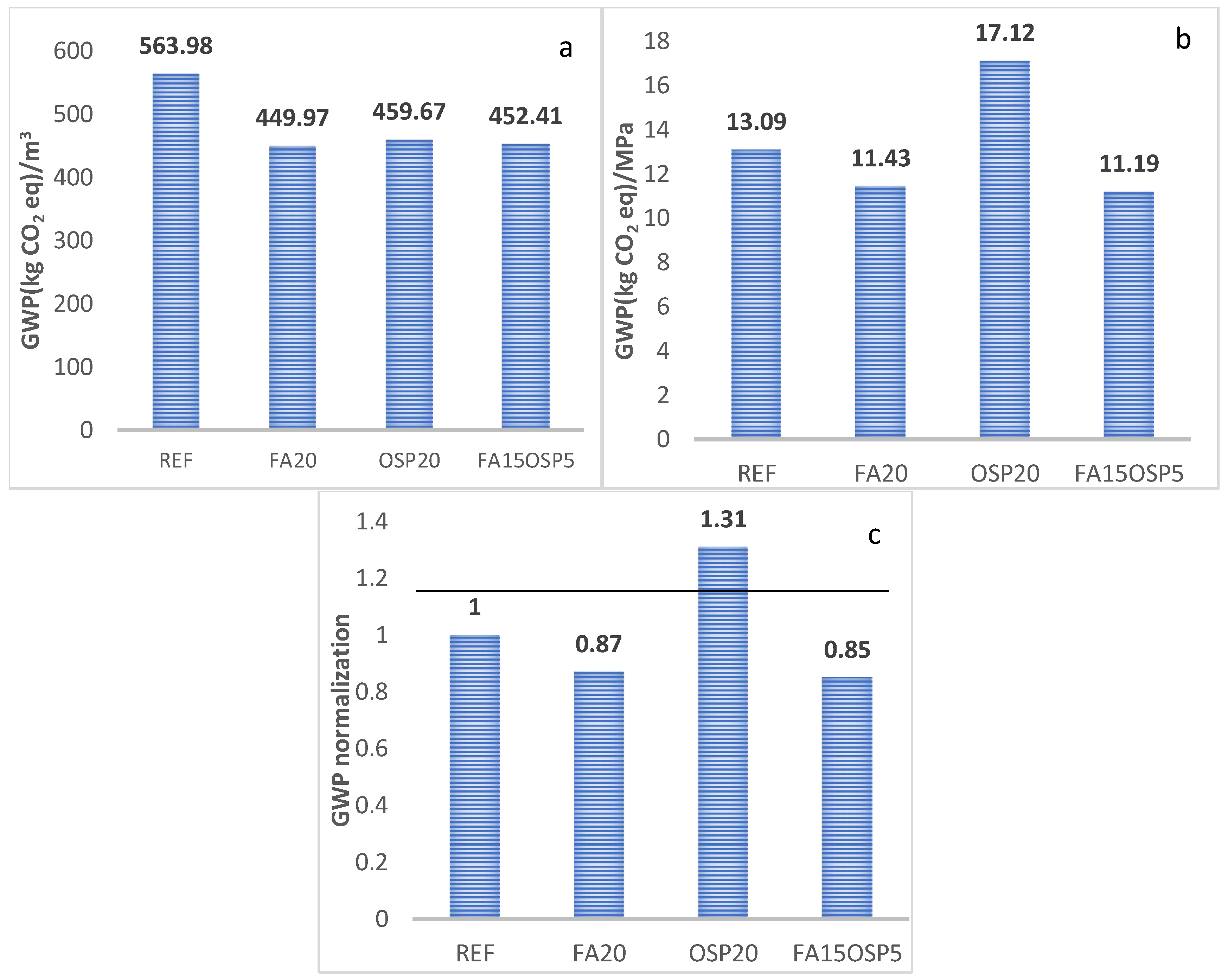
- GWP
- 2.
- AD
- 3.
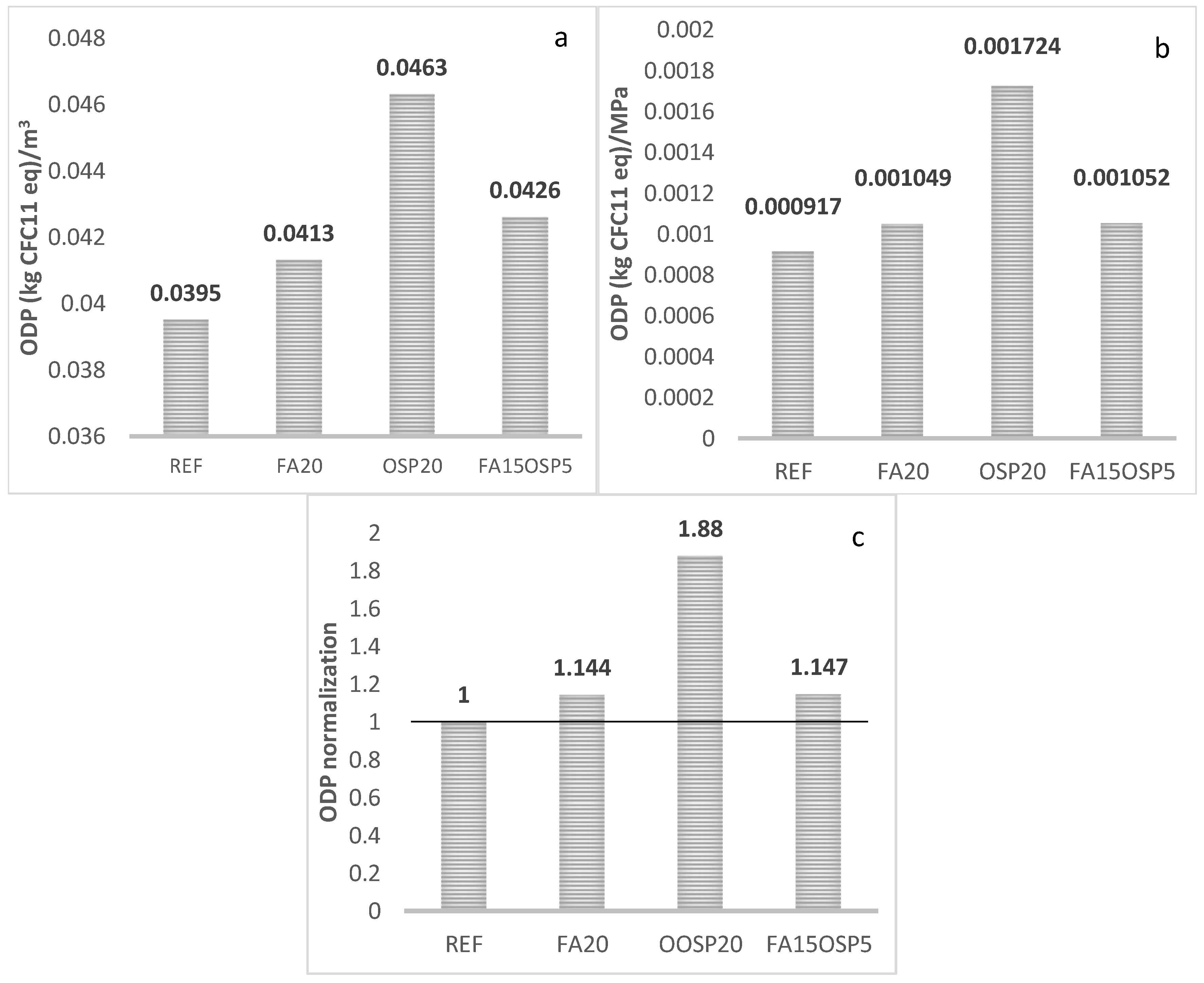
- ODP
- 4.
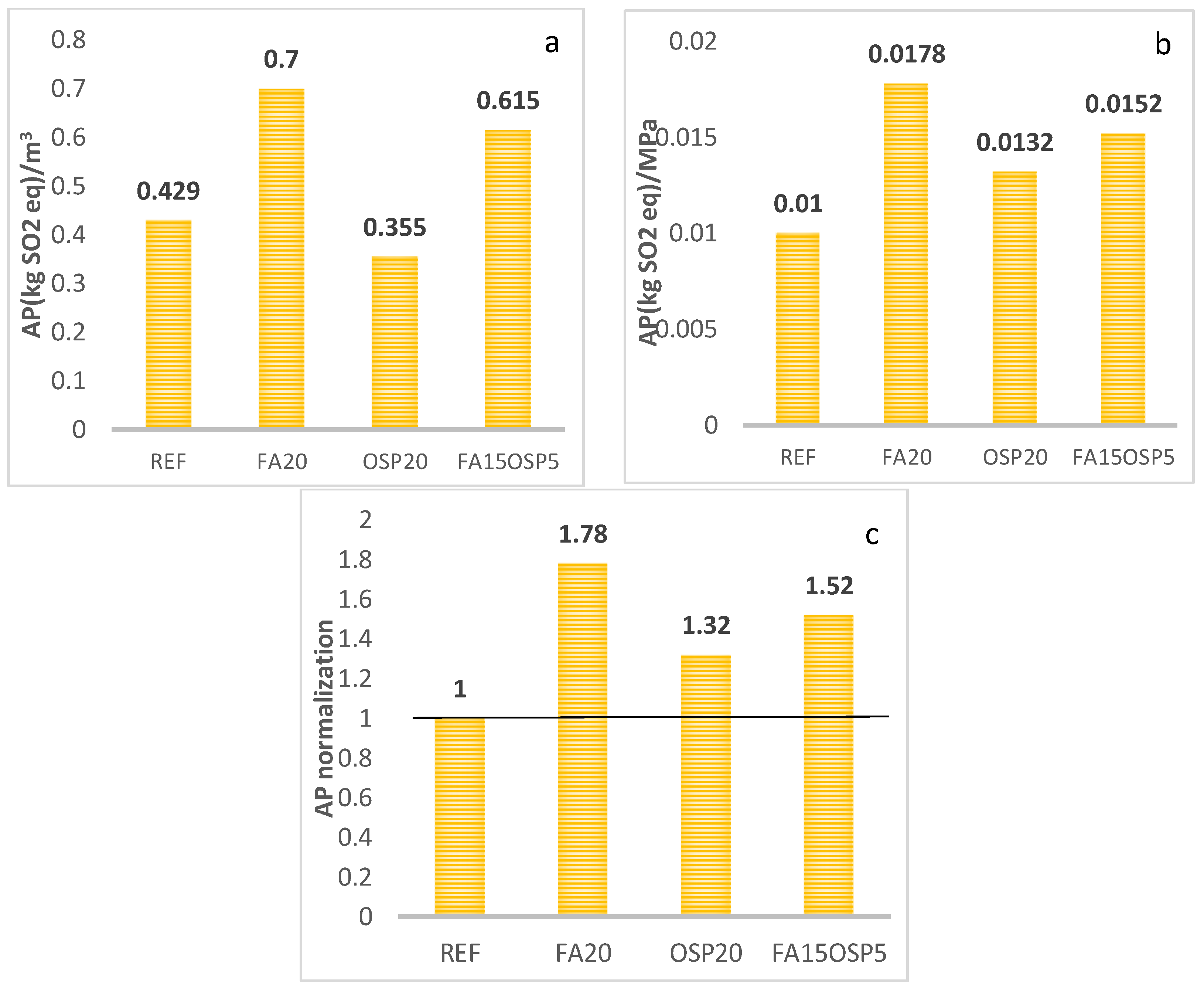
- AP
- 5.
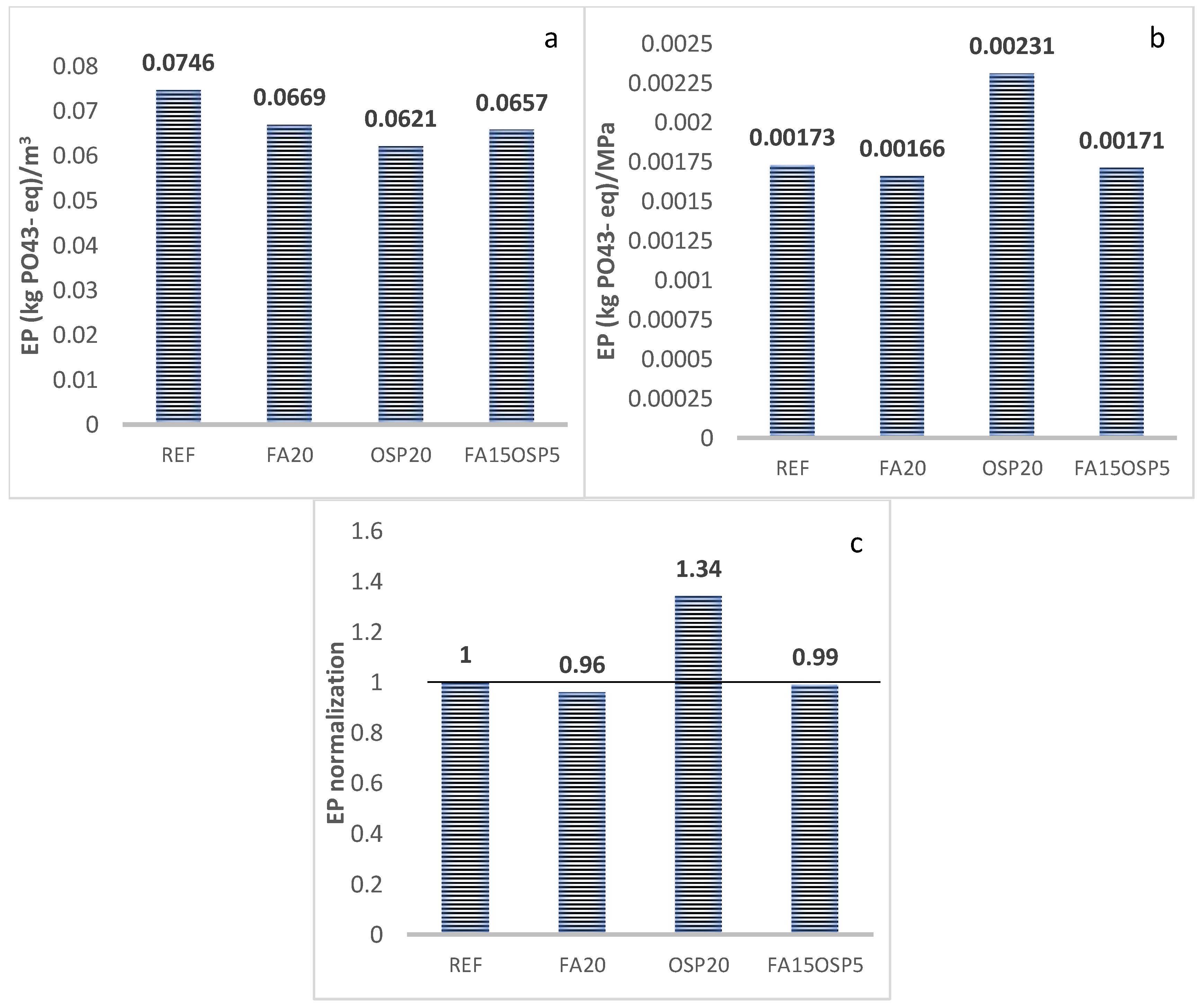
- EP
- 6.
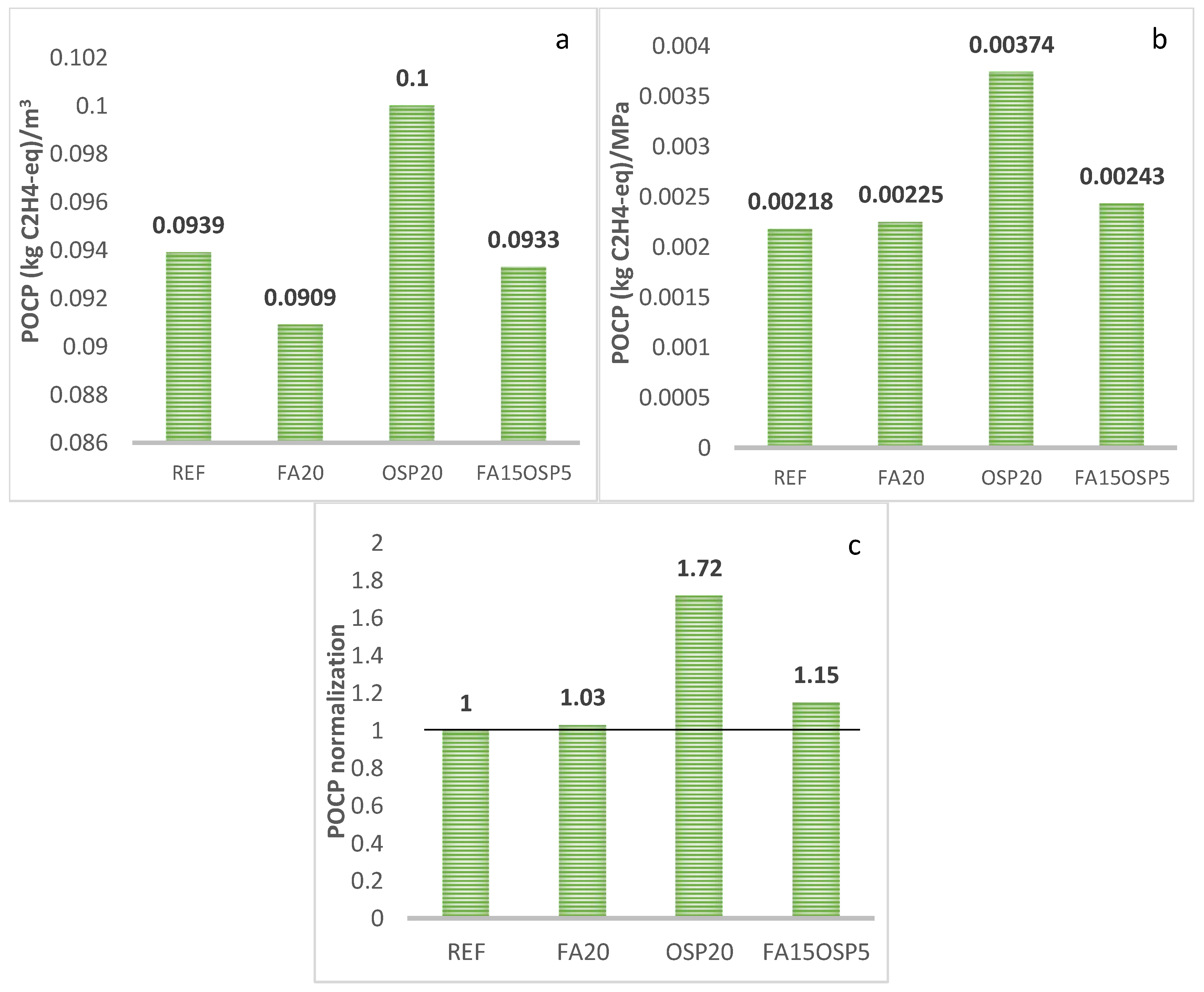
- POCP
- 7.
- Summary of the LCA
4. Discussion
4.1. Mechanism of Action
4.1.1. OSP Mechanism
4.1.2. FA Mechanism
4.1.3. Synergistic Effects of FA and OSP
4.2. Significance of FA and OSP to Sustainable Development
5. Conclusions
- When OSP replaced 20% of OPC, the relative compressive strength (RVS) from 1 to 28 days decreased significantly (from 0.65 to 0.62), primarily due to impurities in OSP negatively impacting the hydration process and structural density. When FA replaced 20% of OPC, the strength decreased from 1 to 7 days (0.74–0.76), but recovered to 0.94 at 28 days due to the pozzolanic reaction. For the FA + OSP ternary system, the relative strength ranged from 0.73 to 0.89 at various ages, slightly higher than the FA-only system at early ages and slightly lower at 28 days. This suggests that the nucleation and filling effects of OSP partially offset the dilution effect of FA at early ages, but may slightly inhibit the FA reaction in terms of long-term strength.
- The UPV test results are consistent with the compressive strength trend. With increasing FA and OSP dosages, the UPV decreased slightly, reflecting a decrease in hydration products. A good power function relationship (R2 = 0.9766) was observed between strength and UPV, validating the effectiveness of UPV as a nondestructive testing indicator.
- Due to its pozzolanic reaction, FA significantly increased the resistivity of concrete in the late hydration stage. However, in the early hydration stage, reducing the OPC dosage resulted in a slight decrease in surface resistivity. From 1 to 7 days, compressive strength and resistivity showed a strong exponential correlation (R2 = 0.9298). By 28 days, although resistivity increased significantly, the correlation with strength weakened (R2 = 0.8486), indicating that the predictive relationship weakened over time. This is because the FA reaction had a greater impact on surface resistivity than on compressive strength.
- Hydration heat results showed that the addition of FA and OSP resulted in a dilution effect, reducing the hydration rate. OSP promoted early hydration to a certain extent, while FA had a certain inhibitory effect in the early stages. The order of C3S heat flux peak intensity was REF > OSP20 > FA15OSP5 > FA20.
- The order of CH thermal decomposition peaks is REF > OSP20 > FA20 > FA15OSP5, consistent with the calculated CH content. The volcanic ash reaction of FA significantly consumes CH, while the effect of OSP is relatively small. The chemically bound water content in the mixed system decreases and exhibits a good power function relationship with the compressive strength (R2 = 0.891).
- The synergistic application of FA and OSP reduces the life cycle greenhouse gas emissions (GWP) and abiotic resource depletion (AD) of the ternary system to 0.85 and 0.96, respectively, compared to the control group. However, the acidification potential (AP), ozone depletion potential (ODP), and photochemical ozone creation potential (POCP) increase to 1.52, 1.14, and 1.15, respectively, compared to the control, while the eutrophication potential (EP) remains essentially unchanged (0.99).
- The LCA parameters used in this study are based on local Korean geographical conditions and raw material processing. Factors such as transportation distance and energy structure may differ significantly in other regions, affecting the applicability of the LCA results. It is recommended that other researchers adjust this method by combining it with local databases (such as KLCID or Ecoinvent) to improve the versatility and scientificity of the model.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Impact Factor | OPC | FA | OSP | Sand | Water |
|---|---|---|---|---|---|
| GWP (kg eq/kg) | 9.3 × 10−1 | 1.98 × 10−3 | 1.96 × 10−2 | 2.6 × 10−3 | 1.68 × 10−4 |
| EP (kg eq/kg) | 1.146 × 10−4 | 5.53 × 10−5 | 4.58 × 10−7 | 1.097 × 10−6 | 7.89 × 10−8 |
| ODP (kg eq/kg) | 1.189 × 10−8 | 5.59 × 10−9 | 1.11 × 10−9 | 1.257 × 10−10 | 1.92 × 10−12 |
| AP (kg eq/kg) | 7.238 × 10−4 | 3.2 × 10−3 | 1.03 × 10−4 | 6.286 × 10−6 | 4.1 × 10−7 |
| AD (kg eq/kg) | 2.4 × 10−3 | 3.37 × 10−4 | 3.3 × 10-3 | 4.983 × 10−6 | 1.39 × 10−6 |
| POCP (kg eq/kg) | 7.210 × 10−5 | 3.22 × 10−5 | 5.55 × 10−5 | 1.171 × 10−6 | 1.8 × 10−8 |
| Raw Materials | OPC | FA | OSP | Sand | Water |
|---|---|---|---|---|---|
| Transport distance | 10 | 80 | 200 | 80 | 0 |
| GWP ( eq/kg *km) | AD ( eq/kg* km) | ODP ( eq/kg* km) | AP ( eq/kg* km) | EP ( eq/kg* km) | POCP ( eq/kg* km) | |
|---|---|---|---|---|---|---|
| Impact Factor |
| Impact Factor | GWP (kg eq/kg*kWh) | AD ( eq/kg*kWh) | ODP ( eq/kg*kWh) | AP ( eq/kg*kWh) | EP ( eq/kg*kWh) | POCP ( eq/kg*kWh) |
|---|---|---|---|---|---|---|
| Electricity | 4.951 × 10−1 | 3.13 × 10−3 | 1.368 × 10−11 | 8.327 × 10−4 | 1.558 × 10−4 | 3.526 × 10−6 |
References
- Zhao, Y.; Wang, T.; Yi, W. Emergy-accounting-based comparison of carbon emissions of solid waste recycled concrete. Constr. Build. Mater. 2023, 387, 131674. [Google Scholar] [CrossRef]
- Korczak, K.; Kochański, M.; Skoczkowski, T. Mitigation options for decarbonization of the non-metallic minerals industry and their impacts on costs, energy consumption and GHG emissions in the EU—Systematic literature review. J. Clean. Prod. 2022, 358, 132006. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Mamatha, B.S.; Sujatha, D.; Uday, D.N.; Kiran, M.C. Properties of flyash based wood geopolymer composite. Low-Carbon Mater. Green Constr. 2023, 1, 29. [Google Scholar] [CrossRef]
- Şahmaran, M.; Li, V.C. Durability properties of micro-cracked ECC containing high volumes fly ash. Cem. Concr. Res. 2009, 39, 1033–1043. [Google Scholar] [CrossRef]
- Thwe, K.S.; Ayawanna, J.; Mase, L.Z.; Chaiyaput, S. Utilization of ladle furnace slag and fly ash as partially replacement of cement. Clean. Eng. Technol. 2025, 25, 100910. [Google Scholar] [CrossRef]
- Yin, S.; Yan, Z.; Chen, X.; Wang, L. Effect of fly-ash as fine aggregate on the workability and mechanical properties of cemented paste backfill. Case Stud. Constr. Mater. 2022, 16, e01039. [Google Scholar] [CrossRef]
- Bhat, R.; Han, T.; Sant, G.; Neithalath, N.; Kumar, A. A comprehensive analysis of hydration kinetics and compressive strength development of fly ash-Portland cement binders. J. Build. Eng. 2024, 88, 109191. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Maghfouri, M.; Alimohammadi, V.; Asadi, I.; Roychand, R. The acid and chloride permeability resistance of masonry cement plaster mortar incorporating high-volume fly ash content. J. Build. Eng. 2024, 86, 108783. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; Schutter, G.D.; Yu, Z. Chemical sulfate attack performance of partially exposed cement and cement + fly ash paste. Constr. Build. Mater. 2012, 28, 230–237. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, P.; Pan, T.; Liao, Y.; Zhao, H. Evaluation of the eco-friendly crushed waste oyster shell mortars containing supplementary cementitious materials. J. Clean. Prod. 2019, 237, 117811. [Google Scholar] [CrossRef]
- ASTM F1736-21e1; Standard Guide for Irradiation of Shellfish for Pathogen and Spoilage Control. ASTM International: West Conshohocken, PA, USA, 2021.
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Han, Y.; Lin, R.; Wang, X.Y. Sustainable mixtures using waste oyster shell powder and slag instead of cement: Performance and multi-objective optimization design. Constr. Build. Mater. 2022, 348, 128642. [Google Scholar] [CrossRef]
- Yang, B.; Han, Y.; Kong, Z.; Wang, X.Y. Effect of belite-rich cement on the micro/macro properties and sustainability of slag–oyster powder–cement-based ternary materials. Constr. Build. Mater. 2025, 468, 140460. [Google Scholar] [CrossRef]
- Her, S.; Im, S.; Liu, J.; Suh, H.; Kim, G.; Sim, S.; Wi, K.; Park, D.; Bae, S. Exploring the potential of pulverized oyster shell as a limestone substitute in limestone calcined clay cement (LC3) and its implications for performance. Constr. Build. Mater. 2024, 425, 135918. [Google Scholar] [CrossRef]
- Han, Y.; Lin, R.S.; Wang, X.Y. Compressive Strength Estimation and CO2 Reduction Design of Fly Ash Composite Concrete. Buildings 2022, 12, 139. [Google Scholar] [CrossRef]
- Wang, X.Y. Evaluation of the hydration heat and strength progress of cement–fly ash binary composite. J. Ceram. Process. Res. 2020, 21, 622–631. [Google Scholar] [CrossRef]
- Yang, B.; Han, Y.; Kong, Z.; Wang, X.Y. Effect of waste oyster shell powder on the micro- and macroproperties and sustainable performance of cement-based materials. J. Build. Eng. 2024, 92, 109800. [Google Scholar] [CrossRef]
- Wang, X.Y. Optimal Design of Low-Carbon Concrete Containing Fly Ash and Limestone Powder. Ceram.—Silik. 2022, 66, 202–210. [Google Scholar] [CrossRef]
- Xuan, M.Y.; Cho, H.K.; Wang, X.Y. Performance improvement of waste oyster-shell powder–cement binary system via carbonation curing. J. Build. Eng. 2023, 70, 106336. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kuo, W.T.; Lin, C.C.; Po-Yo, C. Study of the material properties of fly ash added to oyster cement mortar. Constr. Build. Mater. 2013, 41, 532–537. [Google Scholar] [CrossRef]
- Massoumi Nejad, B.; Enferadi, S.; Andrew, R. A comprehensive analysis of process-related CO2 emissions from Iran’s cement industry. Clean. Environ. Syst. 2025, 16, 100251. [Google Scholar] [CrossRef]
- Sawa, D.; Yamashita, N.; Tanikawa, H.; Daigo, I.; Maruyama, I. CO2 uptake estimation in Japan’s cement lifecycle. J. Clean. Prod. 2025, 486, 144542. [Google Scholar] [CrossRef]
- Han, Y.; Yang, B.; Meng, L.Y.; Cho, H.K.; Lin, R.; Wang, X.Y. Optimization of the life cycle environmental impact of shell powder and slag concrete using response surface methodology. Process Saf. Environ. Prot. 2025, 194, 272–288. [Google Scholar] [CrossRef]
- ASTM C305-20; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM-C109/C109M_21; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C597-22; Standard Test Method for Ultrasonic Pulse Velocity Through Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Half-cell potential measurements—Potential mapping on reinforced concrete structures. Mater. Struct. Mater. Constr. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Lin, R.S.; Han, Y.; Wang, X.Y. Macro–meso–micro experimental studies of calcined clay limestone cement (LC3) paste subjected to elevated temperature. Cem. Concr. Compos. 2021, 116, 103871. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, X.Y. Damage evolution in limestone calcined clay cement (LC3) paste containing recycled glass powder exposed to elevated temperatures. Constr. Build. Mater. 2025, 464, 140140. [Google Scholar] [CrossRef]
- Lin, R.S.; Lee, H.S.; Han, Y.; Wang, X.Y. Experimental studies on hydration–strength–durability of limestone-cement-calcined Hwangtoh clay ternary composite. Constr. Build. Mater. 2021, 269, 121290. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Oliveira, A.P.; Vieira, J.D.; Junior, A.N.; Cascudo, O. Study of the development of hydration of ternary cement pastes using X-ray computed microtomography, XRD-Rietveld method, TG/DTG, DSC, calorimetry and FTIR techniques. J. Build. Eng. 2023, 64, 105616. [Google Scholar] [CrossRef]
- Liao, Y.; Fan, J.; Li, R.; Da, B.; Chen, D.; Zhang, Y. Influence of the usage of waste oyster shell powder on mechanical properties and durability of mortar. Adv. Powder Technol. 2022, 33, 103503. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, M.; Jiang, Q.; Wang, S.; Zhuo, X. Evaluation of using oyster shell as a complete replacement for aggregate to make eco-friendly concrete. J. Build. Eng. 2024, 84, 108587. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, C.; Wang, B.; Han, J.; Guo, L.; Tang, N. Study on the micro-rheological properties of fly ash-based cement mortar. Constr. Build. Mater. 2024, 442, 137664. [Google Scholar] [CrossRef]
- Trtnik, G.; Kavčič, F.; Turk, G. Prediction of concrete strength using ultrasonic pulse velocity and artificial neural networks. Ultrasonics 2009, 49, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sathiparan, N.; Jayasundara, W.G.B.S.; Samarakoon, K.S.D.; Banujan, B. Prediction of characteristics of cement stabilized earth blocks using non-destructive testing: Ultrasonic pulse velocity and electrical resistivity. Materialia 2023, 29, 101794. [Google Scholar] [CrossRef]
- Mendes, S.E.S.; Oliveira, R.L.N.; Cremonez, C.; Pereira, E.; Pereira, E.; Medeiros-Junior, R.A. Electrical resistivity as a durability parameter for concrete design: Experimental data versus estimation by mathematical model. Constr. Build. Mater. 2018, 192, 610–620. [Google Scholar] [CrossRef]
- Feng, G.; Wang, Z.; Qi, T.; Du, X.; Guo, J.; Wang, H.; Shi, X.; Wen, X. Effect of velocity on flow properties and electrical resistivity of cemented coal gangue-fly ash backfill (CGFB) slurry in the pipeline. Powder Technol. 2022, 396, 191–209. [Google Scholar] [CrossRef]
- Canbek, O.; Washburn, N.R.; Kurtis, K.E. Relating LC3 microstructure, surface resistivity and compressive strength development. Cem. Concr. Res. 2022, 160, 106920. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]
- Wang, Y.S.; Meng, L.Y.; Chen, L.; Wang, X.Y. An innovative strategy for CO2 conversion and utilization: Semi-wet carbonation pretreatment of wollastonite to prepare carbon-fixing products and produce LC3. Cem. Concr. Compos. 2025, 160, 106050. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Wu, J.F.; Qi, F.Y.; Zhang, J.; Chen, Z.W.; Wang, H.L.; Liu, Q.F. Modeling of effect of fly ash amount on microstructure and chloride diffusivity of blended fly ash-cement systems. Constr. Build. Mater. 2024, 443, 137711. [Google Scholar] [CrossRef]
- Yang, K.H.; Jung, Y.B.; Cho, M.S.; Tae, S.H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
- Life Cycle Assessment (LCA) Data of Korea. 2025. Available online: https://www.keiti.re.kr/site/keiti/02/10202100000002023101610.jsp (accessed on 1 September 2025).
- Seo, J.; Park, S. Developing an official program to calculate heavy-duty vehicles CO2 emissions in Korea. Transp. Res. Part D Transp. Environ. 2023, 120, 103774. [Google Scholar] [CrossRef]
- Shao, S.; Zhu, H.; Guo, M.; Zhang, Y. Application of waste oyster shells in construction: Overview, constitutive modeling, and life cycle assessment. J. Build. Eng. 2024, 87, 108965. [Google Scholar] [CrossRef]
- Peceño, B.; Alonso-Fariñas, B.; Vilches, L.F.; Leiva, C. Study of seashell waste recycling in fireproofing material: Technical, environmental, and economic assessment. Sci. Total Environ. 2021, 790, 148102. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A.; Ventura, A. LCA allocation procedure used as an incitative method for waste recycling: An application to mineral additions in concrete. Resour. Conserv. Recycl. 2010, 54, 1231–1240. [Google Scholar] [CrossRef]
- Lee, M.; Tsai, W.S.; Chen, S.T. Reusing shell waste as a soil conditioner alternative? A comparative study of eggshell and oyster shell using a life cycle assessment approach. J. Clean. Prod. 2020, 265, 121845. [Google Scholar] [CrossRef]
- Han, Y.; Lin, R.; Lim, J.; Wang, X.Y. Enhancing the properties of alkali-activated slag through dry ice addition: Investigation of hydration products and performance characteristics. J. Mater. Res. Technol. 2023, 26, 8983–8996. [Google Scholar] [CrossRef]







| Chemical Composition | OPC (wt%) | OSP (wt%) | FA (wt%) |
|---|---|---|---|
| Al2O3 | 4.64 | 0.190 | 22.10 |
| SiO2 | 18.80 | 0.577 | 49.50 |
| Fe2O3 | 2.73 | 0.652 | 6.78 |
| CaO | 62.50 | 55.10 | 5.77 |
| MgO | 2.24 | 0.535 | 0.866 |
| TiO2 | 0.237 | 0 | 1.28 |
| Na2O | 0.145 | 0.747 | 0.621 |
| K2O | 0.908 | 0.312 | 1.23 |
| SO3 | 3.61 | 0.577 | 0.641 |
| P2O5 | 0.177 | 0.210 | 0.431 |
| SrO | 0.082 | 0.167 | 0.122 |
| Other | 3.45 | 0.594 | 10.31 |
| LOI. | 0.482 | 40.90 | 0.329 |
| Sample Name | OPC (%) | FA (%) | OSP (%) | Sand (%) | Water (%) | w/b | Sand/Binder |
|---|---|---|---|---|---|---|---|
| REF | 100 | 0 | 0 | 250 | 50 | 0.5 | 2.5 |
| FA20 | 80 | 20 | 0 | 250 | 50 | 0.5 | 2.5 |
| OSP20 | 80 | 0 | 20 | 250 | 50 | 0.5 | 2.5 |
| FA15OSP5 | 80 | 15 | 5 | 250 | 50 | 0.5 | 2.5 |
| OSP | OPC | FA | Sand | Water | |
|---|---|---|---|---|---|
| (kg/m3) | 2670 | 3150 | 2350 | 2600 | 1000 |
| Mass (kg/m3) | |||||
|---|---|---|---|---|---|
| Sample Name | OPC | FA | OSP | Sand | Water |
| REF | 575.2 | 0 | 0 | 1438.1 | 287.6 |
| FA20 | 451.3 | 112.8 | 0 | 1410.4 | 282.1 |
| OSP20 | 456.4 | 0 | 114.1 | 1426.2 | 285.2 |
| FA15OSP5 | 452.6 | 84.9 | 28.3 | 1414.3 | 282.9 |
| Sample Name | OPC (%) | FA (%) | OSP (%) | Water (%) |
|---|---|---|---|---|
| REF | 100 | 0 | 0 | 50 |
| FA20 | 80 | 20 | 0 | 50 |
| OSP20 | 80 | 0 | 20 | 50 |
| FA15OSP5 | 80 | 15 | 5 | 50 |
| Mass (kg/m3) | ||||
|---|---|---|---|---|
| Sample Name | OPC | FA | OSP | Water |
| REF | 1230.8 | 0 | 0 | 615.4 |
| FA20 | 944.9 | 236.2 | 0 | 590.6 |
| OSP20 | 967.4 | 0 | 241.9 | 604.7 |
| FA15OSP5 | 950.5 | 178.2 | 59.4 | 594.1 |
| Number | Test Method | Test Age (Days) | Standard Sizes/Types | Purpose | |||
|---|---|---|---|---|---|---|---|
| 1 | Compressive strength | 1 | 3 | 7 | 28 | Mortar (50 mm × 50 mm × 50 mm) | Mechanical properties |
| 2 | UPV | √ | √ | √ | √ | Mortar (40 mm × 40 mm × 160 mm) | Mechanical properties |
| 3 | Surface resistivity | √ | √ | √ | √ | Mortar (40 mm × 40 mm × 160 mm) | Durability |
| 4 | Heat of hydration | √ | √ | √ | - | Pure paste (powder) | Hydration kinetics |
| 5 | XRD | √ | - | - | √ | Pure paste (powder) | Phase identification |
| 6 | TGA | √ | - | - | √ | Pure paste (powder) | Phase identification |
| 7 | FT-IR | √ | - | - | √ | Pure paste (powder) | Phase identification |
| 8 | LCA | - | - | - | √ | - | Sustainability |
| Name/Day | 1 | 28 |
|---|---|---|
| REF | 11.14% | 23.63% |
| FA20 | 8.90% | 19.31% |
| OSP20 | 9.94% | 19.90% |
| FA15OSP5 | 9.12% | 19.21% |
| Name/Day | 1 | 28 |
|---|---|---|
| REF | 10.86% | 20.06% |
| FA20 | 8.24% | 16.76% |
| OSP20 | 8.97% | 16.87% |
| FA15OSP5 | 8.03% | 16.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-J.; Park, K.-B.; Wang, X.-Y. Synergistic Effects of Fly Ash and Oyster Shell Powder in Ternary Low-Carbon Cementitious Materials: Macro–Micro Experimental Studies and Life Cycle Evaluation. Appl. Sci. 2025, 15, 11319. https://doi.org/10.3390/app152111319
Wang K-J, Park K-B, Wang X-Y. Synergistic Effects of Fly Ash and Oyster Shell Powder in Ternary Low-Carbon Cementitious Materials: Macro–Micro Experimental Studies and Life Cycle Evaluation. Applied Sciences. 2025; 15(21):11319. https://doi.org/10.3390/app152111319
Chicago/Turabian StyleWang, Kang-Jia, Ki-Bong Park, and Xiao-Yong Wang. 2025. "Synergistic Effects of Fly Ash and Oyster Shell Powder in Ternary Low-Carbon Cementitious Materials: Macro–Micro Experimental Studies and Life Cycle Evaluation" Applied Sciences 15, no. 21: 11319. https://doi.org/10.3390/app152111319
APA StyleWang, K.-J., Park, K.-B., & Wang, X.-Y. (2025). Synergistic Effects of Fly Ash and Oyster Shell Powder in Ternary Low-Carbon Cementitious Materials: Macro–Micro Experimental Studies and Life Cycle Evaluation. Applied Sciences, 15(21), 11319. https://doi.org/10.3390/app152111319







