Abstract
This study examined engine combustion characteristics of the plastic diesel produced through pyrolysis of waste plastics as an eco-friendly source of engine fuel. We extensively measured and compared the key fuel properties based on various diesel fuel standards. Distillation and hydrotreatment processes were used to improve the quality of the pyrolysis oil, resulting in distilled plastic diesel (DPD) and hydrotreated plastic diesel (HPD). DPD and HPD were blended at 10:90 and 20:80 (vol%) ratios with commercial diesel, resulting in fuel blends termed as DPD10, DPD20, HPD10, and HPD20, respectively, to analyse their engine combustion characteristics. A full-scale 4-cylinder, 4-stroke diesel engine was used in this study. There are virtually no studies available in the literature where engine combustion characteristics have been tested with both distilled and hydrotreated plastic pyrolytic oil. This study comprehensively investigated the combustion behaviours of all four fuel blends under full-load conditions and at an engine operating speed of 1500 rpm, except engine exhaust gas temperature which was measured at varying engine speeds from 1200 rpm to 2400 rpm at an interval of 300 rpm. The study found notable differences in engine combustion characteristics between the commercial diesel and plastic diesel blends under identical operating conditions. The HPD blends had higher exhaust gas temperatures (EGTs) than the DPD blends, particularly at lower blend ratios, whereas the DPD10 and HPD10 blends had higher peak cylinder pressures than DPD20 and HPD20. The HPD10 blend exhibited the highest heat release rate (HRR) of 120.41 J/°CA. The engine combustion characteristics using a full-scale engine with distilled and hydrotreated plastic diesel and their comparison are not fully studied in the literature yet.
1. Introduction
Converting waste into energy is an appealing technology in today’s landscape, given the scarcity and elevated costs of fossil fuels, along with the mounting challenges linked to effective waste management. Plastics are lightweight, versatile, cost effective, and have a long-lasting nature; due to those advantages their use in today’s world has expanded enormously [1]. Plastic waste poses a significant contemporary concern because of its slow degradability and escalating demand for plastic products owing to their diverse user-friendly attributes. Unfortunately, plastic waste recycling remains constrained because of the substantial operational expenses and investment required in this domain [2]. Between 1950 and 2015, only 9% of waste plastics were recycled, whereas approximately 12% were incinerated. Alarmingly, a staggering 80% of waste plastics ended up in landfills during this period [3]. Waste plastics pose severe environmental hazards owing to their enduring lifespans, which persist in the environment for thousands of years. Their harmful impact extends to underground water pollution, which significantly compromises this vital resource. Research indicates an annual leakage of approximately 10 to 20 million tonnes of waste plastics into ocean waters, causing widespread damage to ecosystems and severe water pollution [4]. The escalation in plastic production has resulted in a surge in plastic waste in municipal solid waste (MSW). Effective management of this waste has become a pressing concern, prompting numerous researchers to seek sustainable solutions. To combat environmental damage, the reduction in landfilling and incineration of waste plastics has become imperative. One promising technique involves chemical recycling, specifically pyrolysis, which converts plastic waste into fuel. This process yields a fuel product with a higher calorific value as crude oil is utilised during the production phase. Pyrolysis is a potential solution for mitigating the adverse effects of waste plastics [5,6,7].
Pyrolysis is a thermal degradation process that requires high heat and pressure in an oxygen-free environment for a brief duration. In this process, long-chain hydrocarbons present in materials, such as waste plastics, are broken down into shorter-chain hydrocarbons in the absence of oxygen [2,7]. This transformation leads to the conversion of complex compounds into simpler ones, enabling the production of valuable substances, such as liquid oil, syngas, and a negligible portion of char in the case of waste plastics [8,9,10].
Researchers have investigated the effect of adding crude plastic pyrolytic oil (PPO) and diesel blends on engine combustion characteristics. Kaimal and Vijayabalan (2015) studied diesel engines’ combustion properties using PPO and its three blends with standard diesel with blend ratios of 25%, 50%, and 75% [11]. They found higher values of peak cylinder pressure, heat release, and ignition delay for neat PPO and its blends than those found when using diesel under a 100% load condition.
Geo et al. (2018) investigated engine combustion parameters using a mixture of waste PPO and commercial diesel at a ratio of 50:50 [12]. They investigated the cylinder pressure, combustion duration, and heat release rate. They found a higher peak cylinder pressure and exhaust gas temperature for PPO and diesel blends than for neat commercial diesel because of the higher ignition delay of the blends, resulting in delayed combustion. A higher heat release rate was observed during the diffusion combustion phase.
Devaraj et al. (2015) investigated engine combustion characteristics using waste PPO and PPO blends with 5% and 10% diethyl ether (DEE) [13]. In this study, they documented the cylinder pressure, delay period, and heat release rate. Their investigations revealed that the peak cylinder pressure and heat release rate were reduced when the amount of diethyl ether was increased in blends with waste PPO. However, they did not mention changes in exhaust gas temperature by adding diethyl ether with PPO and did not compare the results with neat commercial diesel usage. Similarly, Kaimal and Vijayabalan (2016) investigated the use of 5, 10, and 15% DEE blended with distilled PPO. They investigated only the peak cylinder pressure and heat release rate and did not document other vital engine combustion characteristics [14]. They found a higher ignition delay for the blends than for diesel, and the heat release rate was lower for the blends than for diesel.
Mani et al. (2010) also used crude PPO to investigate engine performance parameters and mainly focussed on the effects of exhaust gas recirculation on engine combustion parameters [15]. However, they only considered the cylinder pressure and heat release rate, did not consider other engine combustion parameters, and did not perform any post-treatment of the crude PPO for property improvements.
Engine performance, combustion, and emission parameters were also investigated by Pradeep and Gowthaman (2022) by using pyrolytic oil extracted from waste plastics [16]. The focus of the review was various types of pyrolysis processes, and the impacts of different types of plastic and catalysts on the prolysis process.
Nevertheless, post-treatment processes for property improvement of crude PPO was not discussed in that study. Moreover, they only considered peak cylinder pressure and heat release rate and did not consider other vital engine combustion parameters. Sushma (2018) investigated engine combustion, performance, and emission behaviour of PPO and diesel blends and PPO and oxygenated fuel blends through an Exhaust Gas Recirculation (EGR) system [17]. They found that the operating conditions (such as temperature, catalysts used, etc.) of pyrolysis affects the properties of PPO and thus the engine performance, emissions, and combustion characteristics. The detailed properties of pyrolytic oil and results compared to commercial diesel were not reported in that paper. Some researchers have investigated the effects of catalytic pyrolysis on the pyrolytic products yield; for example, metal carbonate (CoCO3) catalyst was used with dried waste high-density polyethylene plastic with different weight percentages. They found that the catalytic pyrolysis process can reduce temperature range of pyrolysis compared to pyrolyzing without catalyst [18]. Another research article utilised mineral clay (CAT 1) catalyst with mixed waste plastic (PP, LDPE, PET, PS, and HDPE) and conducted catalytic pyrolysis to find out the effects on the pyrolytic process. Highest 62.5 wt% yield was measured by thermal pyrolysis at 500 °C with the possibility of increasing it to 74.8 wt% during catalytic pyrolysis while keeping the temperature same [19]. Aguado et al. (2009) have found that catalytic pyrolysis can convert 40% of plastic, whereas less than 30% was converted without a catalyst [20]. However, in this study thermal pyrolysis was conducted.
Waste PPO shares properties with diesel, yet it tends to exhibit higher viscosity. This elevated viscosity can impact the atomisation and vapour formation of the fuel, thereby potentially leading to a notable increase in engine emissions [21]. Moreover, crude PPO (CPPO) has some disadvantages, such as a low flash point (<20 °C), calorific value (44.15 MJ/kg), and cetane index (32), along with a higher percentage of water (0.125 wt%) and sulphur contents (5.12 mg/kg), and does not follow the standard values for diesel fuel in most cases, which prevents the use of crude PPO as a substitute fuel in automobile engines [6,22]. From the authors’ comprehensive literature review, it became evident that there is a scarcity of research papers focusing on the measurement and analysis of engine combustion characteristics concerning post-treated PPO. Additionally, the review highlighted that among the researchers who have explored the potential of PPO and diesel blends in diesel engines, most have reported inferior engine combustion and performance characteristics compared with the use of neat CD.
This study introduces several unique elements compared with the existing literature. The research used a 20 L pilot-scale pyrolysis reactor, which is a departure from the usual smaller lab-scale setups. This study revealed the fuel properties and engine combustion characteristics by using plastic-derived diesel fuel and proved that if diesel engines can run with plastic-derived diesel blended with commercial diesel, then it will certainly reduce the pressure on commercial diesel. Moreover, upon receiving convincing results from this study, it will provide a sustainable solution to the increasing waste plastic generation problem, as they can be converted to PPO through pyrolysis. This study covers crude PPO production, distillation, hydrotreatment technologies, fuel characterisation, blend preparation, and a detailed analysis of engine combustion parameters, including exhaust gas temperature, cylinder pressure, and heat release rate.
2. Materials and Methods
2.1. Crude PPO Production
Mixed waste plastic (MWP) was used as the feedstock for the pyrolysis process and was sourced from a local municipal dumpsite, comprising a 1:1:1 blend of high-density polyethylene (HDPE), polypropylene (PP), and polystyrene (PS), as shown in Figure 1.

Figure 1.
Shredded waste plastic samples: (a) HDPE, (b) PP, and (c) PS.
Waste plastics contain unwanted particles, for example, dirt, food waste, soil, etc., with them, and few researchers conducted pre-treatment before the pyrolysis process. Moreover, separation of different plastic materials is vital because they are made of different resin compounds for difference in transparency and colour [23]. In this research manual sorting of unwanted wastes have been conducted. No significant pre-treatment was performed other than cleaning to remove dirt and dust and air drying. Pre-treatments of feedstock, for example, cleaning, shredding and air drying, were conducted by Olalo (2021) [7]. A photograph of the actual pilot-scale pyrolysis reactor with a 20 L capacity, which was used in the experiments in this study, is shown in Figure 2a. The schematic diagram of the process is shown in Figure 2b.
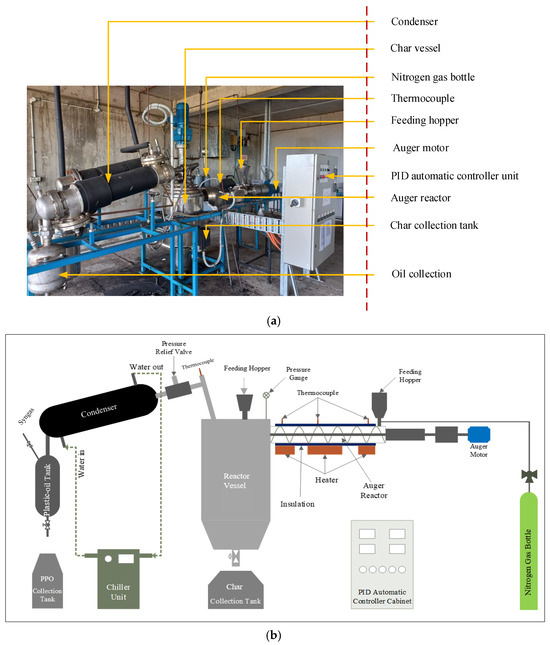
Figure 2.
(a) Waste plastic pyrolysis plant. (b) Process schematic diagram.
The stainless steel container is the main section of the pyroliser, which is called the reactor chamber. The pyroliser also consists of a condenser, oil collector, PID controller unit, condenser unit, stirrer motor, thermocouples, and char collector. Shredded MWP feedstock samples were added inside the reactor vessel using the feeding hopper, which is an opening located on top of the reactor vessel. Pyrolysis was performed at 540 °C in a completely oxygen-free environment after purging the system with nitrogen and performing leak testing. An average pyrolysis experiment takes approximately 6 h or less until no more CPPO is stored after the condenser. The vapour produced during pyrolysis in the reactor chamber made its way through the condenser units. Then, the condensable vapours were condensed as pyrolysis liquid oil, termed CPPO, and collected from the bottom of the oil collector unit. For the pyrolysis experiments, a stirrer speed of 5 rpm and 6 °C/min heating rate were used. After completing the pyrolysis experiments, the reactor was allowed to cool, and the char was stored directly by opening the underside of the reactor vessel.
For the derived crude PPO from the above thermal pyrolysis process using MWP, two post-treatment methods were employed: distillation and hydrotreatment, which are briefly outlined in Section 2.2 and Section 2.3.
2.2. Distillation of Crude PPO
A vacuum distillation unit (iFischer AutoDest 800/860 AC) was used for conducting the distillation of crude PPO. An automated sample collection system was integrated with the condenser of the distillation column, and all operational parameters were precisely controlled through a computerised system. The CPPO samples derived from the MWP pyrolysis process were transferred into a 10 L stainless steel flask, which was subsequently positioned on a heating mantle. The outlet of the flask was securely connected to the distillation column to maintain a closed and leak-free configuration. Distillation temperature ranges were established as follows: below 170 °C for the gasoline fraction, between 170 °C and 380 °C for the diesel fraction, and above 380 °C for the lube oil and residue fractions. Upon completion of the distillation operation, the diesel cut was collected and analysed in detail. A photograph of the vacuum distillation apparatus employed in this investigation is presented in Figure 3.
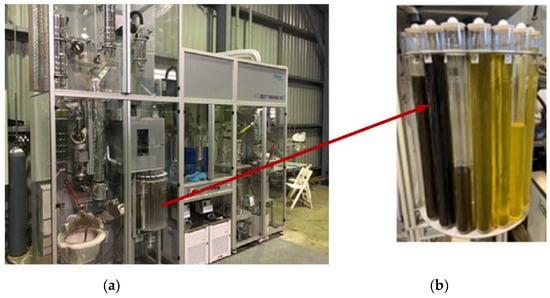
Figure 3.
(a) Vacuum distillation unit and (b) distillation column.
Following the distillation process, only the diesel fraction condensed within the temperature range of 170–380 °C was collected. This specific fraction, termed Distilled Plastic Diesel (DPD), was collected, analysed, and subsequently used for the hydrotreatment process. The hydrotreatment involved catalytic hydrodesulphurization (HDS), nitrogen removal (HDN), and hydrodeoxygenation (HDO) to further enhance the quality of the oil.
2.3. Hydrotreatment of the DPD
Hydrotreatment is a pivotal refining process to enhance the quality of pyrolytic oil by eliminating heteroatoms and saturating alkenes [24,25]. The hydrotreatment of the distilled diesel fraction (DPD) in this study was carried out in an industrial facility located in Gladstone, Australia. This process plays a crucial role in refining pyrolytic oil by reducing acidity [26] and curtailing coke production at elevated temperatures. Furthermore, hydrotreatment significantly reduces various properties of PPO, such as viscosity, density, power point, fire point, flash point, and cetane index [25].
For the hydrotreatment experiments, the temperature and pressure were set to 400 °C and 60 bar, respectively, in the presence of hydrogen gas and the NiMo/Al2O3 catalyst. The DPD oil sample exhibited clear post hydrotreatment, termed hydrotreated plastic diesel (HPD), whereas it typically appeared brown before undergoing the catalytic hydrotreatment. Figure 4 shows the physical appearance of crude PPO, DPD, and HPD.

Figure 4.
Specimens of (a) crude PPO, (b) distilled, and (c) hydrotreated plastic diesel.
When DPD is treated with hydrogen it breaks down the larger, more complex hydrocarbons into smaller and more saturated hydrocarbons. The process also removes impurities such as sulphur, nitrogen, and oxygen compounds and results in a cleaner, less viscous product. This broken down and clean oil (after removing impurities) has lower molecular weight and can flow easily and thus less viscous than both crude PPO and diesel. Moreover, the amount of paraffinic and naphthenic hydrocarbons is higher in hydrotreated PPO, which has lower viscosity than aromatic hydrocarbons. The actual photograph of the hydrotreatment processing plant is given in Figure 5 below.

Figure 5.
Hydrotreatment processing plant.
Table 1 describes the specific operating conditions used for hydrotreating DPD from waste plastics.

Table 1.
Operating conditions of the DPD hydrotreatment.
The physical and chemical characteristics of Crude PPO, DPD, and HPD were evaluated in accordance with the Australian, ASTM, and EN Quality Standard specifications for diesel fuels. Experimental fuel formulations were crafted by blending oil samples of DPD (derived after distilling crude PPO) and HPD (resulting from hydrotreating DPD) with commercial diesel (CD). The methodology of the present study that has been followed for conducting engine combustion analysis is depicted in terms of a flowchart in Figure 6.
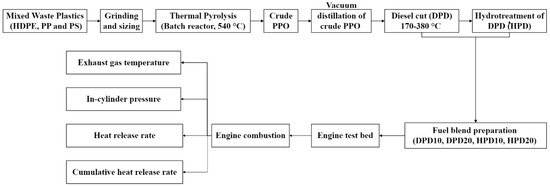
Figure 6.
Flow diagram of the methodology of the study.
3. Characterisation of CPPO, DPD, and HPD
The analysis of the standard properties of the Crude PPO, DPD, and HPD samples was accomplished in terms of flash point, density, calorific value, cetane index (CI), kinematic viscosity (KV), lubricity, sulphur content, and water content, as shown in Table 2.

Table 2.
Comparative analysis of the fuel properties of Crude PPO, DPD, and HPD in line with ASTM, EN, and Australian standard diesel.
It can be noticed that the kinematic viscosity of the oils after hydrotreatment is much lower than PPO and diesel. The reason could be the fact that when crude PPO is treated with hydrogen it breaks down the larger, more complex hydrocarbons into smaller and more saturated hydrocarbons. The process also removes impurities such as sulphur, nitrogen, and oxygen compounds and results in a cleaner, less viscous product. This broken down and clean oil (after removing impurities) has lower molecular weight and can flow easily and thus less viscous than both crude PPO and diesel. Moreover, the amount of paraffinic and naphthenic hydrocarbons is higher in hydrotreated PPO, which has lower viscosity than aromatic hydrocarbons. For a further detailed and elaborate description of the production of PPO, distillation, and hydrotreatment processes, and the characterisation of the corresponding oils, please refer to another journal article recently published by Faisal et al. [22].
It can be noticed that the crude PPO showed a flash point of <20 °C, which is significantly lower than that of commercial diesel. This hazardous property is attributed to the presence of highly volatile light hydrocarbon fractions formed during pyrolysis. These compounds evaporate easily in ambient conditions and render the fuel extremely flammable. Hence, crude PPO requires upgrading (e.g., distillation or hydrotreating) to remove light volatiles and improve its flash point before safe handling and engine utilisation.
It is to be noted that sulphur content in the DPD sample is 87.68 mg/kg. This observation can be attributed to the higher concentration of sulphur in heavier hydrocarbon molecules, resulting in its accumulation predominantly within the diesel fraction, with smaller quantities remaining in the residue. Please note, the boiling point temperatures for the diesel cut and gasoline cut were set around 170–380 °C and below 170 °C, respectively. Residue got separated during the distillation process as well.
The WSD for HPD is higher. Higher WSD and poor lubricity in HPD is due to the hydrotreating process which saturates double bonds and removes heteroatoms (O, N, S). While this improves stability and reduces contaminants, it also removes polar compounds that naturally provide lubricity. As a result, hydrotreated fuel becomes “too clean” and lacks lubricating molecules and thus has a higher WSD.
4. FTIR Analysis of PPO
The FTIR analysis identifies the distinct functional groups of pyrolytic oil. The spectrum obtained through FTIR analysis of PPO is shown in Figure 7. The FTIR technique uses infrared light to stress chemical bonds and infrared radiation to contract chemical bonds at a specific wavelength [27].
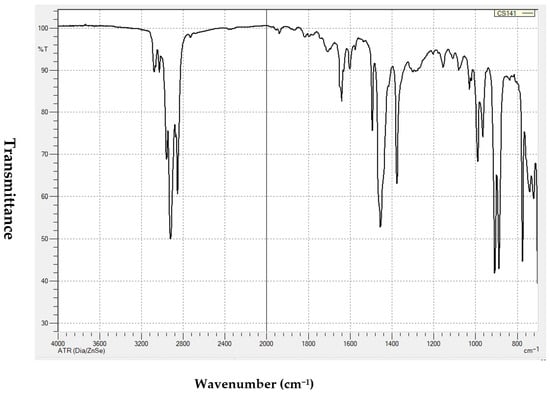
Figure 7.
FTIR spectra of PPO under optimum conditions.
It can be seen from Figure 7 that the presence of alkane at peak wavenumber of approximately 2924.65 and 2856.35 cm−1 could be due to C–H stretching. Selvaganapathy et al. (2020) reported that wavenumbers of 2919.22 cm−1 and 2854.01 cm−1 represent alkanes for PPO with C–H stretching with a strong appearance [28]. It is believed that the smaller peaks at 3082.93 cm−1 represent the functional group alkenes due to =C–H stretching, while the wavenumber 1642.26 cm−1 could represent alkene functional groups due to C=C stretching [27]. A strong appearance of a peak at 1454.31 cm−1 could represent alkane functional group due to the C–H bending and scissoring of alkane. Furthermore, the alkane functional group wavenumber of 1376.86 cm−1, could be due to CH3C–H bending. The functional group alkane was also detected at the peak of wavenumber 965.27 cm−1 could be due to the vibration of C–H bending. On the other hand, the presence of an alkene functional group can be seen at 888.66 cm−1 with =C–H bending at a strong appearance. C–H bending vibrations could also be the reason for presence of an alkene functional group at wavenumber of approximately 775.23 cm−1. It can concluded that PPO can be used as liquid hydrocarbon because it consists of alkanes, alkenes, aromatics, etc. [29,30]. Similar results were also found by Kumar and Singh [27] and Selvaganapathy et al. [28] for PPO produced from HDPE and PS, respectively.
To conduct tests for engine combustion analysis, fuel blends were created by blending the DPD and HPD fuel samples with CD at 10% and 20% volume fractions. The fuel samples were measured and mixed with CD using a magnetic stirrer (IKA C-MAG HS7) operating at 2000 rpm for 30 min, as shown in Figure 8. Two DPD and two HPD post-treated PPO diesel blends were prepared, denoted as DPD10, DPD20, HPD10, and HPD20, representing 10% DPD and 90% CD, 20% DPD and 80% CD, 10% HPD and 90% CD, and 20% HPD and 80% CD (all in volume percentages), respectively. The combustion data generated using these four experimental fuel blends were analysed and compared with the values obtained when the engine was operated with a neat CD.

Figure 8.
Blending of HPD with CD using a magnetic stirrer.
5. Test Engine and Facilities
In this study, a full-scale 4-cylinder, 4-stroke Kubota V3300 diesel engine was used to experimentally observe the engine combustion characteristics of post-treated PPO blends mixed with neat CD, namely, DPD10, DPD20, HPD10, and HPD20. The experiments were conducted at the thermodynamic laboratory of Central Queensland University, Australia. Figure 9a illustrates the schematic layout, and Figure 9b shows an actual photograph of the engine test bed.
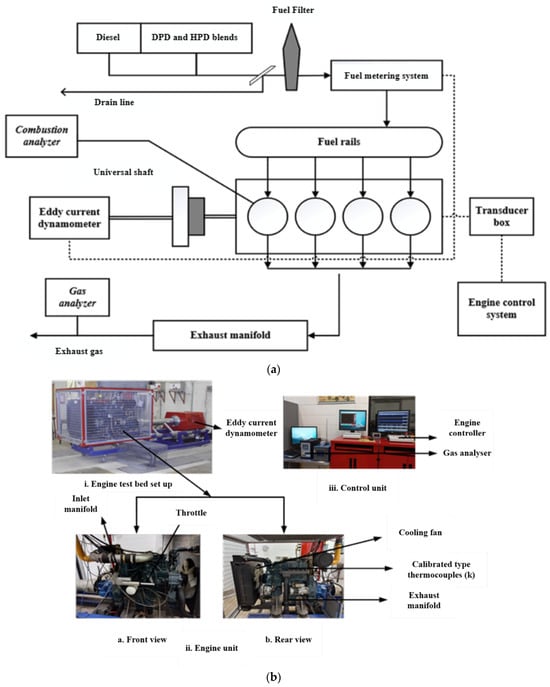
Figure 9.
Engine test bed setup: (a) schematic and (b) photographic view.
To study the combustion features of the engine, the initial phase involved running the engine for 20 min with CD fuel until a stable state was achieved. The engine was then transitioned to DPD and HPD fuel blends for the primary experiments, each of which was tested for 30 min per load condition. To conclude the study, the engine was once again operated with a CD to purge the fuel flow lines for another 15 min before being shut down. Detailed engine specifications can be found in Table 3.

Table 3.
Operating conditions of the Kubota V3300 diesel engine.
During the combustion tests, the engine speed was systematically varied from 1200 to 2400 rpm at intervals of 300 rpm. No modifications or adjustments were made to the engine during experimental procedures. All the data presented in this study were obtained under a specific set of conditions: 1500 rpm speed and full (100%) load.
The Dyno Dynamics engine dynamometer, a device that applies a load to the engine and measures the power generated against that load, was employed for testing. Controlled by a computer and integrated with an engine test bed, this dynamometer facilitates the experimentation process. A flow meter (Coriolis-type, model CMF025M319NQEIEZZZ) was used for measuring flow rate of the fuel supplied from the main fuel supply line through nozzle systems. Two volumetric flowmeters positioned in the supply and return lines were employed to calculate fuel consumption. In addition, parameters such as the peak pressure inside the cylinder, the HRR, the ignition delay, and the CHRR were measured in this study.
Uncertainty in a measurement refers to the degree of doubt regarding the accuracy of the results obtained from a given instrument. Precision of the instrument is the most important parameter to achieve accurate measurements. Measurement accuracy can be determined with the help of uncertainty analysis of the instruments used. Uncertainty analysis of some of the instruments used in this study has been conducted at 95% confidence level using standard deviation at a p value of 0.05 [31]. Experiments were repeated at least three times, and the mean value was taken into consideration, and the values are presented in Table 4.

Table 4.
Uncertainties of instruments used for the present study.
6. Results and Discussion
Four vital engine combustion parameters, namely exhaust gas temperature, peak cylinder pressure, heat release rate, and cumulative heat release rate, are described in the following sections.
6.1. Exhaust Gas Temperature (EGT)
The performance of engine combustion could be measured using important parameters, of which the EGT is fundamental. The low exhaust temperature indicates efficient burning of fuel and better combustion. On the other hand, a high exhaust temperature or high heat loss convert less heat energy to work [32]. A literature review showed a higher EGT for PPO than for diesel. A higher heat release rate, increased ignition delay resulting in better diffusion combustion, and higher oxygen content in the fuel may be the reasons for the higher EGT for PPO compared with diesel [21]. Moreover, a higher air–fuel ratio can result in a higher EGT [33].
Figure 10 illustrates the variation in the engine exhaust gas temperature at different engine operating speeds. The speed varied between 1200 and 2400 rpm under full-load conditions with a gap of 300 rpm. Commercial diesel fuel has a higher EGT than all four of the test fuel blends at most operating speeds, except for the HPD10 and HPD20 blends at 2400 rpm. The reason for this behaviour could be the higher heat release rate of the fuel blends than that of neat commercial diesel. The reason for the discrepancy with the literature review, where higher EGT has been reported for PPO than diesel, could be the fact that most of them used crude PPO without any fuel property improvement. Distillation and hydrotreatment were performed for improving fuel properties of PPO in this research. Significant improvements in fuel properties were achieved, resulting in a lower EGT for the test fuel blends than for commercial diesel.
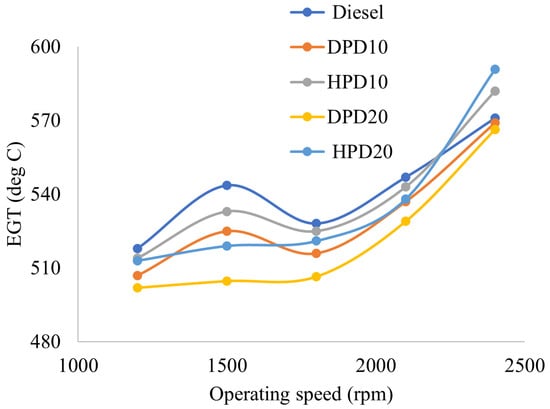
Figure 10.
Variation in exhaust gas temperature with engine operating speed.
For the DPD10 blend, the EGT was lower than diesel by an average of 1.99% throughout the operating speed range. A similar decreasing trend was observed for the DPD20 blend, where EGT was lower than diesel by an average of 3.68% over the operating speed range. An average reduction in EGT by 2.07% and 3.87% was also observed using HPD10 and HPD20 blends, respectively, compared to commercial diesel fuel. Analysis of the experimental data also revealed that EGT was higher for DPD10 than for DPD20 at all operating speeds. A similar behaviour was observed between the HPD10 and HPD20 blends, where a decrease in EGT was observed at operating speeds of 1200, 1500, and 2100 rpm for HPD20 compared with HPD10. However, the opposite scenario was observed at speeds of 1800 and 2400 rpm, where EGT was found to be higher for HPD20 than for HPD10. Another observation that was found from the results analysis was that EGT was higher in the hydrotreated fuel blends HPD10 and HPD20 than in the DPD10 and DPD20 blends, respectively. The reason for this behaviour can be the fact that the combustion temperature was higher when using HPD fuel blends as more complete combustion occurred; hence, the EGT was also higher.
6.2. In-Cylinder Pressure
The in-cylinder pressure is highly dependent on the ignition delay and on the formation of the air–fuel mixture in the ignition delay period. If ignition delay is greater, the uncontrolled combustion can occur; thus, the in-cylinder pressure also increases.
Figure 11 reveals the comparison of in-cylinder pressure among four of the test fuel blends, namely DPD10, DPD20, HPD10, and HPD20, with commercial diesel. All data were calculated at 1500 rpm and full (100%)-load conditions. The highest increase in pressure inside the cylinder was low for commercial diesel and was only 65.67 bar at 380 °CA. It was also noticeable from the data analysis that the highest amount of pressure rise inside the cylinder was in the range of 378–386 °CA, whereas the highest pressure generated varied in magnitude. The experimental data analysis revealed that the DPD blends (both 10 and 20 vol%) had an in-cylinder pressure profile like neat commercial diesel. The peak cylinder pressures generated by DPD10 and DPD20 were observed to be 66.11 bar and 65.68 bar, respectively, at 381 °CA. In Figure 9, the pressure graphs for DPD10 and DPD20 almost overlapped. It can be said that the highest value of the generated in-cylinder pressure was slightly higher for DPD10 than for DPD20. Similar results were documented by Geo et al. (2018), who used a 50:50 blend of distilled PPO and diesel and obtained a peak cylinder pressure of 69 bar, whereas it was 67 bar when using neat commercial diesel under full load conditions [12]. Another study reported a higher peak cylinder pressure for neat PPO and its 25%, 50%, and 75% blends with diesel [11]. They found the peak cylinder pressure for neat PPO to be 71 bar, whereas for the 25%, 50%, and 75% blends, it was 67, 68, and 69 bar, respectively, which were higher than those of neat diesel (67 bar) at the 100% load condition.
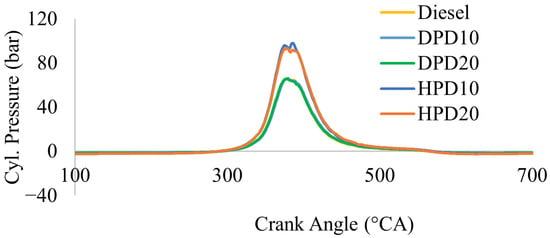
Figure 11.
Peak cylinder pressure versus crank angle.
For HPD10 and HPD20, the pressures developed inside the cylinder were also similar. It is visible from Figure 10 that ignition starts around the crank angle position of 290 °CA, and the highest in-cylinder pressure reached was 97.88 bar at 386 °CA for HPD10 and 93.27 bar at 378 °CA for HPD20 blend, and in the graph they almost coincided. The higher values of peak cylinder pressure for the HPD fuel blends were probably due to the higher caloric value, higher cetane index, and higher viscosity of the HPD blends than those of DPD and neat commercial diesel. However, experimental data have provided evidence that as the amount of HPD in the blend with commercial diesel increases, the in-cylinder pressure decreases.
Higher peak cylinder pressure for crude PPO than for commercial diesel was found in the literature, and the reasons were documented as a higher delay period, air–fuel mixture, higher viscosity, and higher calorific value of PPO [11,33,34]. Moreover, increased peak cylinder pressure was also observed for PPO and diesel blends by other researchers as a result of higher ignition delay, resulting in a higher heat release rate, viscosity, calorific value, and cetane number of crude PPO [6,12,21,35]. The higher oxygen content in PPO contributes to better combustion and higher peak cylinder pressure [11,21].
6.3. Heat Release Rate (HRR)
The heat release rate is a vital engine combustion parameter for determining the combustion start and end times and the ignition delay. It is a measurement of the rate at which heat is added to the combustion chamber due to the burning of fuel. The heat release rate for different crank angles was determined using Equation (1),
where heat release rate = (J/°CA), cylinder pressure = P (Pa), specific heat ratio for air = = Cp/Cv = 1.35, and instantaneous volume = V (m3).
Figure 12 describes the differences in HRR with varying crank angles. The experimental data implies that the HRR for diesel is comparable to that of DPD and diesel blends with both 10 and 20 vol percentages. Investigational results have shown a higher HRR for diesel (73.93 J/°CA) than for DPD10 (68.87 J/°CA) and DPD20 (69.67 J/°CA). However, the HRR of the hydrotreated PPO blends was higher than those of the diesel and distilled PPO blends. HRR of HPD10 was higher than HPD20 and was found to be 120.41 J/°CA and 106.00 J/°CA for HPD10 and HPD20, respectively.
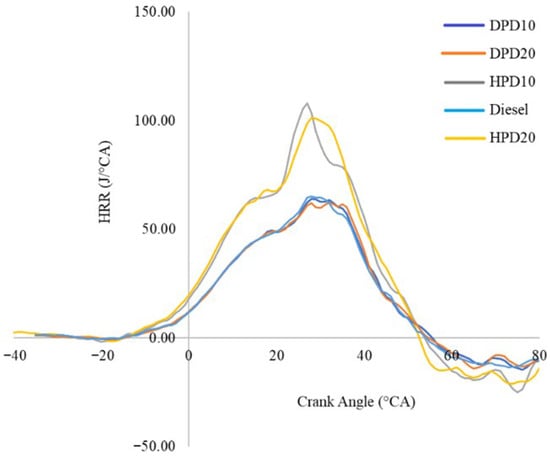
Figure 12.
Variation in HRR with crank angle.
A literature review also provided evidence of a lower heat release rate for neat commercial diesel than for blends of PPO and diesel [11,12,35]. The reason for this phenomenon was documented as the higher ignition delay, increased calorific value, and higher oxygen content of the blends compared to diesel. Kumar and Sankaranarayanan (2016) documented the maximum HRR for diesel as 71.56 J/°CA, which is lower than the highest HRR of plastic oil and found as 91.93 J/°CA because of the longer ignition delay of PPO than commercial diesel [36].
Negative values of the apparent HRR were observed during the compression and expansion strokes. This occurs because the HRR calculation incorporates both heat release and heat transfer to the cylinder walls. When the latter exceeds the chemical energy release, the net heat release becomes negative. Such behaviour is consistent with previous diesel combustion studies and reflects wall heat transfer rather than reverse combustion.
6.4. Cumulative Heat Release Rate (CHRR)
The CHRR is another vital parameter that can determine the combustion characteristics. CHRR can be determined by Equation (2) mentioned below [37]:
here, is the cumulative heat release rate, P = cylinder pressure (Pa), = specific heat ratio for air = Cp/Cv = 1.35, and V = instantaneous volume (m3).
Figure 13 describes the CHRR at different crank angles and conducted at an engine operating speed of 1500 rpm and 100% load. Experimental results have provided evidence that the highest CHRR is comparable for the diesel and distilled plastic diesel blends, DPD10 and DPD20. The highest CHRR values for diesel, DPD10, and DPD20 were very close. However, a higher CHRR was observed for the hydrotreated plastic diesel blends HPD10 and HPD20. CHRR at the early stages of combustion was slightly higher for HPD10 than for HPD20; however, as the combustion progressed, CHHR for HPD20 exceeded that for HPD10. Another observation from the experimental results was that as the amount of plastic diesel increased in the blend with commercial diesel, the CHRR increased (for both DPD and HPD blends). Thus, the highest CHRR for DPD10 is higher than that for DPD20 and is found to be 431.77 J and 427.56 J, respectively. The CHRR for diesel was found to be 452.83 J. Similarly, the highest value of CHRR for HPD20 was lower than that of HPD10 and was observed to be 649.91 J and 799.72 J, respectively. The lower CHRR for diesel and DPD blends can be attributed to improper air–fuel mixing and a lower cetane index than that of HPD blends. One study found higher CHRR for crude PPO than for neat diesel, PPO, and diesel blends [11].
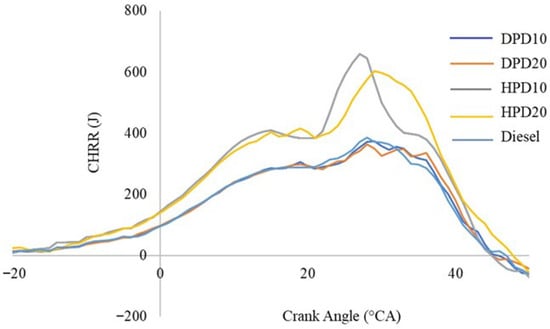
Figure 13.
Cumulative heat release rate with varying crank angles.
The higher CHRR for HPD blends compared with diesel could be because of the higher heating value of the hydrotreated plastic diesel blends than for neat diesel [37] and the availability of a more combustible air–fuel mixture during the pre-mixed combustion phase [11].
7. Conclusions and Recommendations
This study investigated the combustion characteristics of distilled and hydrotreated diesels in blends with commercial diesel, and the results were compared with only commercial diesel. The exhaust gas temperature (EGT) for the fuel blends of DPD10, DPD20, HPD10, and HPD20 were mostly lower than those of commercial diesel, with average decreases of 1.99%, 3.68%, 2.07%, and 3.87%, respectively. The results indicated lower heat loss and better combustion performance for the experimental fuel blends compared with neat commercial diesel. Comparable cylinder pressures were found for diesel, DPD10, and DPD20. However, higher in-cylinder pressure was found for HPD10 and HPD20 compared to neat commercial diesel and DPD blends owing to their higher calorific value and higher viscosity, with the highest value obtained for HPD10 being 978.76 bar, which provides evidence of more complete combustion.
The HRR for commercial diesel was observed to be 73.93 J/°CA, which was higher than both DPD10 and DPD20 but lower than those of HPD10 and HPD20. The CHRR for diesel and DPD blends with both 10 and 20 vol percentages were similar. However, HPD blends possess higher CHRR values than neat commercial diesel, DPD10, and DPD20.
Thus, the analysis revealed that HPD blends performed better than DPD blends and, in some cases, diesel blends in terms of achieving better combustion characteristics when used in diesel engines. Thus, it is evident that refining pyrolysis oil, especially with the hydrotreatment process, improves the quality of pyrolysis oil and improves combustion performance.
In this research, quite a few topics were recognised for further investigation. Each recommendation is a significant piece of work as outlined below.
- In this study thermal pyrolysis of waste plastics has been conducted. Future research could be to investigate the oil yield from the process if catalytic pyrolysis is considered. Then, it would be thought provoking to investigate the differences in liquid oil yield between thermal and catalytic pyrolysis processes.
- The highest temperature that was chosen in this study for conducting thermal pyrolysis was 540 °C because of the limitation of our pyrolysis instrument. It would be worth considering higher temperatures and seeing how it affects the pyrolysis process.
- In this study a mixture of HDPE, PP, and PS plastic types have been chosen for the pyrolysis process. However, it would be interesting to see how the pyrolytic oil yield is impacted if other plastic type combinations were used, for example, low density polyethylene (LDPE), polyethylene terephthalate (PET), etc. The impact of the mixture of PP and PET on oil yield from catalytic pyrolysis was investigated by Sembiring et al. (2018) at 400 °C [38]. They found that when the amount of PET plastic increases in the blend, production of wax increases significantly; however, liquid oil reduces in amount. Thus, the authors believe using other types of plastic mixtures would both reduce and deteriorate the quality of the produced fuel.
- The pyrolysis reactor vessels were heated by using an internal electrical heating mechanism, which is essential for breaking down waste plastics. However, these heating methods come with drawbacks, namely they are expensive and add complexity to the reactor system. Integrating a solar heating system into the pyrolysis setup can present a solution to mitigate these heating challenges. By doing so, the reliance on costly and environmentally harmful heating methods can be reduced.
- Distilled and hydrotreated plastic diesels were blended with commercial diesel of up to 20 volume percentages in our study to observe the combustion characteristics. It would be beneficial to investigate how these parameters vary while using higher blend ratios (greater than 20 vol%). It would be interesting to see the effects of adding oxygenated compounds with distilled PPO, for example, diethyl ether (DEE), algae biodiesel, and emulsions, and investigating how it influences engine combustion characteristics.
Author Contributions
Methodology, M.I.J.; Formal analysis, F.F.; Investigation, A.A.C.; Data curation, M.I.J.; Writing—original draft, F.F.; Writing—review & editing, M.G.R., M.I.J. and A.A.C.; Supervision, M.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Khatha, W.; Ekarong, S.; Somkiat, M.; Jiraphon, S. Fuel Properties, Performance and Emission of Alternative Fuel from Pyrolysis of Waste Plastics. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 9–11 November 2019; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Mcnutt, M. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 19, e1700782. [Google Scholar] [CrossRef]
- Gourmelon, G. Global plastic production rises, recycling lags. Vital Signs 2015, 22, 91–95. [Google Scholar]
- Kumar, R.; Mishra, M.; Singh, S.; Kumar, A. Experimental evaluation of waste plastic oil and its blends on a single cylinder diesel engine. J. Mech. Sci. Technol. 2016, 30, 4781–4789. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef]
- Olalo, J. Characterization of Pyrolytic Oil Produced from Waste Plastic in Quezon City, Philippines Using Non-catalytic Pyrolysis Method. Chem. Eng. Trans. 2021, 86, 1495–1500. [Google Scholar]
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.-C.; Karonis, D. Alternative diesel from waste plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef]
- Desai, S.B.; Galage, C. Production and analysis of pyrolysis oil from waste plastic in Kolhapur city. Int. J. Eng. Res. Gen. Sci. 2015, 3, 590–595. [Google Scholar]
- Siddiqui, M.N.; Redhwi, H.H. Pyrolysis of mixed plastics for the recovery of useful products. Fuel Process. Technol. 2009, 90, 545–552. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Convers. Manag. 2015, 105, 951–956. [Google Scholar] [CrossRef]
- Geo, V.E.; Sonthalia, A.; Aloui, F.; Josephin, J.F. Study of engine performance, emission and combustion characteristics fueled with diesel-like fuel produced from waste engine oil and waste plastics. Front. Environ. Sci. Eng. 2018, 12, 8. [Google Scholar] [CrossRef]
- Devaraj, J.; Robinson, Y.; Ganapathi, P. Experimental investigation of performance, emission and combustion characteristics of waste plastic pyrolysis oil blended with diethyl ether used as fuel for diesel engine. Energy 2015, 85, 304–309. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. An investigation on the effects of using DEE additive in a DI diesel engine fuelled with waste plastic oil. Fuel 2016, 180, 90–96. [Google Scholar] [CrossRef]
- Mani, M.; Nagarajan, G.; Sampath, S. An experimental investigation on a DI diesel engine using waste plastic oil with exhaust gas recirculation. Fuel 2010, 89, 1826–1832. [Google Scholar] [CrossRef]
- Pakiya Pradeep, A.; Gowthaman, S. Combustion and emission characteristics of diesel engine fuelled with waste plastic oil–a review. Int. J. Ambient. Energy 2022, 43, 1269–1287. [Google Scholar] [CrossRef]
- Sushma, P. Waste plastic oil as an alternative fuel for diesel engine–A Review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Telangana, India, 13–14 July 2018; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Phanisankar, B.S.S.; Vasudeva Rao, N.; Manikanta, J.E. Conversion of waste plastic to fuel products. Mater. Today Proc. 2020, 33, 5190–5195. [Google Scholar] [CrossRef]
- Ghodke, P.K. High-quality hydrocarbon fuel production from municipal mixed plastic waste using a locally available low-cost catalyst. Fuel Commun. 2021, 8, 100022. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Peral, A. Catalytic cracking of polyethylene over zeolite mordenite with enhanced textural properties. J. Anal. Appl. Pyrolysis 2009, 85, 352–358. [Google Scholar] [CrossRef]
- Mani, M.; Nagarajan, G.; Sampath, S. Characterisation and effect of using waste plastic oil and diesel fuel blends in compression ignition engine. Energy 2011, 36, 212–219. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.; Chowdhury, A.A.; Schaller, D.; Jahirul, M. Uncovering the differences: A comparison of properties of crude plastic pyrolytic oil and distilled and hydrotreated plastic diesel produced from waste and virgin plastics as automobile fuels. Fuel 2023, 350, 128743. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Sebastian, J. Pyrolysis process to produce fuel from different types of plastic–a review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 396, 012062. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A.; Dimitriadis, A. Catalyst evaluation for waste cooking oil hydroprocessing. Fuel 2012, 93, 638–641. [Google Scholar] [CrossRef]
- Belbessai, S.; Azara, A.; Abatzoglou, N. Recent Advances in the Decontamination and Upgrading of Waste Plastic Pyrolysis Products: An Overview. Processes 2022, 10, 733. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Ardiyanti, A.; Venderbosch, R.; Heeres, H. Characterization of hydrotreated fast pyrolysis liquids. Energy Fuels 2010, 24, 5264–5272. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Optimization of process parameters by response surface methodology (RSM) for catalytic pyrolysis of waste high-density polyethylene to liquid fuel. J. Environ. Chem. Eng. 2014, 2, 115–122. [Google Scholar] [CrossRef]
- Selvaganapathy, T.; Muthuvelayudham, R.; Jayakumar, M. Process parameter optimization study on thermolytic polystyrene liquid fuel using response surface methodology (RSM). Mater. Today Proc. 2020, 26, 2729–2739. [Google Scholar] [CrossRef]
- Hasan, M.; Rasul, M.; Jahirul, M.; Khan, M. Fast pyrolysis of macadamia nutshell in an auger reactor: Process optimization using response surface methodology (RSM) and oil characterization. Fuel 2023, 333, 126490. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Makarem, M.; Rahimpour, M. Light olefins/bio-gasoline production from biomass. In Bioenergy Systems for the Future; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–148. [Google Scholar]
- Imdadul, H.; Masjuki, H.; Kalam, M.; Zulkifli, N.; Alabdulkarem, A.; Rashed, M.; Teoh, Y.; How, H. Higher alcohol–biodiesel–diesel blends: An approach for improving the performance, emission, and combustion of a light-duty diesel engine. Energy Convers. Manag. 2016, 111, 174–185. [Google Scholar] [CrossRef]
- Aydin, H. Combined effects of thermal barrier coating and blending with diesel fuel on usability of vegetable oils in diesel engines. Appl. Therm. Eng. 2013, 51, 623–629. [Google Scholar] [CrossRef]
- Mani, M.; Subash, C.; Nagarajan, G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl. Therm. Eng. 2009, 29, 2738–2744. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed investigation of the combustion characteristics of a DI diesel engine fuelled with plastic oil and rice bran methyl ester. J. Energy Inst. 2017, 90, 324–330. [Google Scholar] [CrossRef]
- Singh, R.; Ruj, B.; Sadhukhan, A.; Gupta, P.; Tigga, V. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sankaranarayanan, G. Investigation on environmental factors of waste plastics into oil and its emulsion to control the emission in DI diesel engine. Ecotoxicol. Environ. Saf. 2016, 134, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.T. Combustion of biodiesel in CI engine. Int. J. Appl. Res. 2017, 3, 145–149. [Google Scholar]
- Sembiring, F.; Purnomo, C.W.; Purwono, S. Catalytic Pyrolysis of Waste Plastic Mixture. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 24–27 July 2017; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).