A Review on SPECT Myocardial Perfusion Imaging Attenuation Correction Using Deep Learning
Abstract
1. Introduction
Research Questions
- Which DL architectures and algorithms have demonstrated the highest performance in AC?
- To what extent do studies integrate non-image data (e.g., patient demographics, clinical parameters) into DL models, and how does this inclusion influence model accuracy and generalizability?
- What quantitative metrics are employed to assess model performance and error?
- Do researchers utilize established MPI quantification metrics, such as Summed Stress Score (SSS), Summed Difference Score (SDS), Summed Rest Score (SRS), or Total Perfusion Deficit (TPD) to evaluate the quality of DL-generated AC images?
- What are the typical sizes and compositions of training and validation datasets reported in the literature?
- How frequently do studies employ independent external populations for additional testing?
2. Background
2.1. Single Photon Emission Computed Tomography
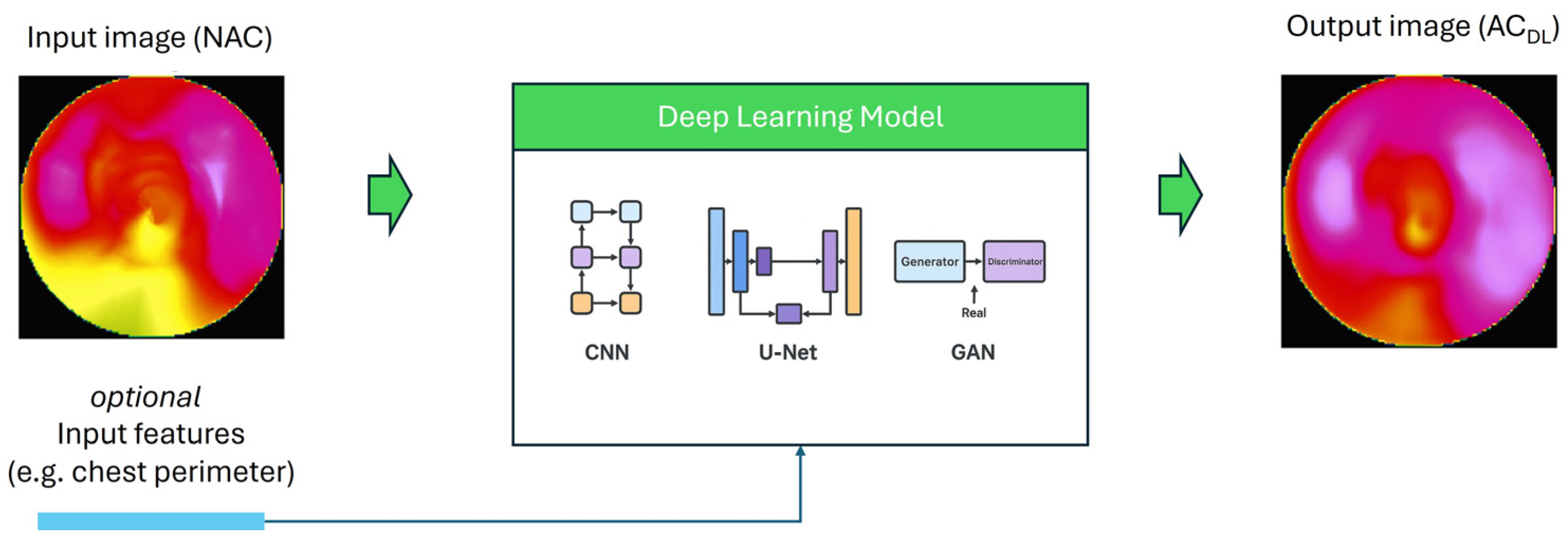
2.2. Deep Learning for Attenuation Correction
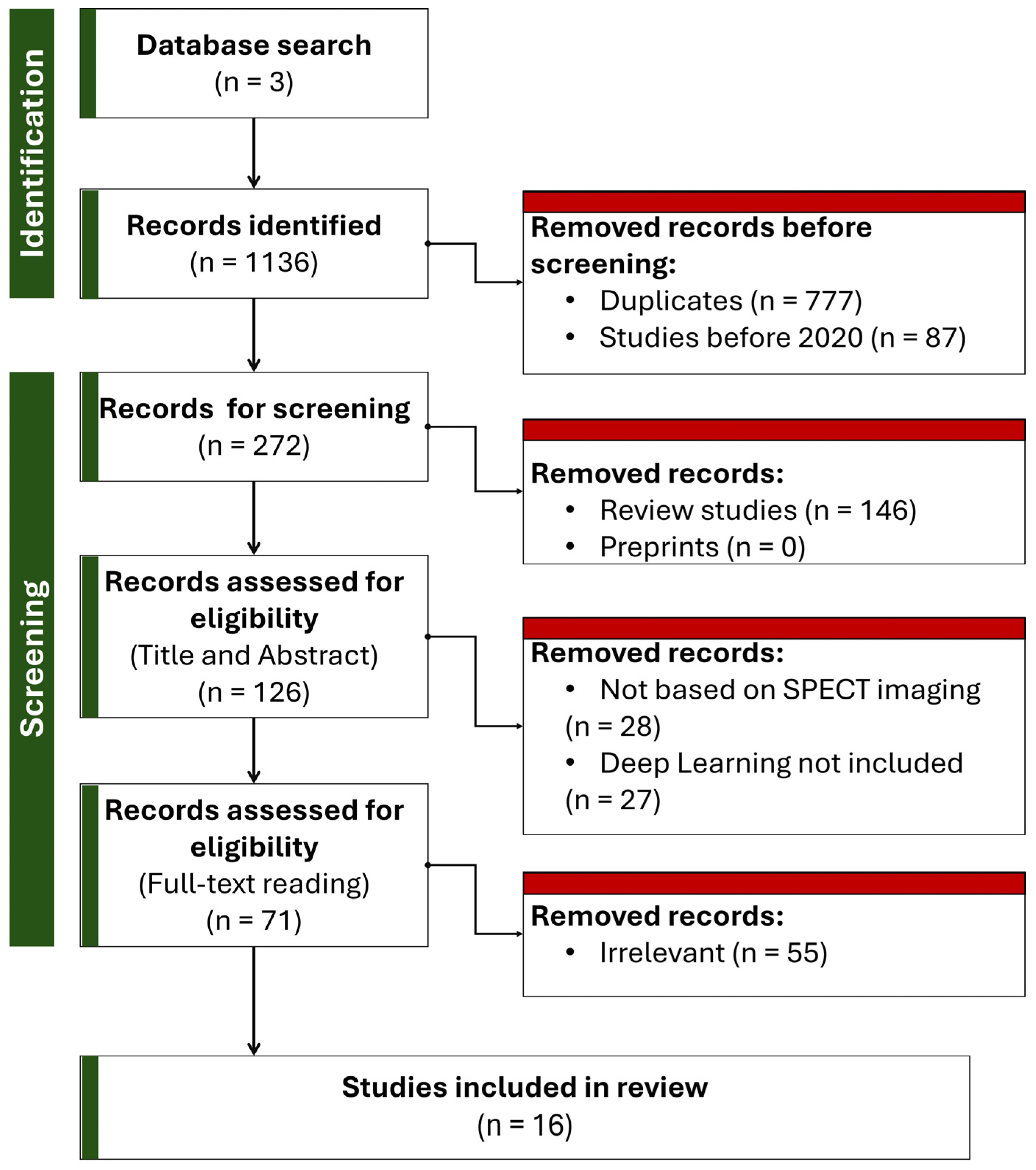
3. Methodology of Literature Review
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
- The primary objective of the study was AC in SPECT MPI.
- The methodology involved the use of DL.
- The article was peer-reviewed and published in the English language.
- The publication date was after 2020.
- The publication was a review article, editorial, commentary, or a conference abstract without an accompanying full-text manuscript.
- The study was a duplicate of another included work.
3.3. Paper Selection
4. Review Findings
5. Comparative Review
5.1. Which DL Architecture and Algorithm Has Demonstrated the Highest Performance in AC?
5.2. To What Extent Do Studies Integrate Non-Image Data (e.g., Patient Demographics, Clinical Parameters) into DL Models, and How Does This Inclusion Influence Model Accuracy and Generalizability?
5.3. What Quantitative Metrics Are Employed to Assess Model Performance and Error?
5.4. Do Researchers Utilize Established MPI Quantification Metrics, Such as Summed Stress Score (SSS), Summed Difference Score (SDS), Summed Rest Score (SRS), or Total Perfusion Deficit (TPD) to Evaluate the Quality of DL-Generated AC Images?
5.5. What Are the Typical Sizes and Compositions of Training and Validation Datasets Reported in the Literature?
5.6. How Frequently Do Studies Employ Independent External Populations for Additional Testing?
| No. | First Author | Year | Ref. No. | Study Design | Camera | Tracer | Data Size | DL Method |
|---|---|---|---|---|---|---|---|---|
| 1 | Shi | 2024 | [66] | Single-center | Discovery NM/CT 670 (GE Healthcare, Milwaukee, WI, USA) | Tl-201 | 985 studies | U-Net |
| 2 | Hagio | 2022 | [60] | Single-center | Siemens Symbia T16 (Siemens Healthineers, Malvern, PA, USA) | 99mTc-Sestamibi | 11,532 studies | U-Net |
| 3 | Canalejo | 2023 | [61] | Single-center | Millenium Hawkeye VG SPECT/CT system (GE Healthcare, Milwaukee, WI, USA) | 99mTc-sestamibi | studies | U-Net |
| 4 | Yang | 2021 | [69] | Single-center | Discovery NM/CT 570c scanner (GE Healthcare, Milwaukee, WI, USA) | 99mTc-tetrofosmin | 100 studies | CNN |
| 5 | Huxohl | 2022 | [62] | Single-center | Symbia Intevo (Siemens Healthineers, Hoffman Estates, USA) | N/R | 150 studies | GAN, U-Net |
| 6 | Shanbhag | 2023 | [64] | Multi-center | Discovery 570c or Discovery 530c scanner (GE Healthcare, Milwaukee, WI, USA) | 99mTc-sestamibi or 99mTc-tetrofosmin | 5490 studies | GAN, U-Net |
| 7 | Shi | 2020 | [65] | Single-center | NM/CT 850 (GE Healthcare, Milwaukee, WI, USA) | 99mTc-tetrofosmin | 65 studies | GAN, U-Net |
| 8 | Yang | 2025 | [67] | Single-center | NM/CT 670 (GE Healthcare, Milwaukee, WI, USA) | 99mTc-sestamibi | 202 studies | U-Net |
| 9 | Torkaman | 2021 | [70] | Single-center | Discovery NM 570c (GE Healthcare, Milwaukee, WI, USA) | 99mTc-tetrofosmin | 100 studies | GAN, U-Net |
| 10 | Hagio | 2023 | [72] | Multi-center | Multiple | 99mTc-sestamibi or 99mTc-tetrofosmin | 722 studies | CNN |
| 11 | Chen | 2022 | [68] | Single-center | Dedicated SPECT: Alcyone Discovery NM/CT 570c (GE Healthcare, Milwaukee, WI, USA) General SPECT: NM/CT 850c | 99mTc-tetrofosmin | Dedicated SPECT: 270 studies General SPECT: 400 studies | U-Net, CNN |
| 12 | Torkaman | 2022 | [71] | Single-center | Discovery NM/CT 570c (GE Healthcare, Milwaukee, WI, USA) | 99mTc-tetrofosmin | 100 studies | U-Net, GAN |
| 13 | Mostafapour | 2022 | [63] | Single-center | Discovery NM/CT 670 (GE Healthcare, Milwaukee, WI, USA) | 99mTc-sestamibi | 99 studies | U-Net, CNN |
| 14 | Chen | 2024 | [73] | Single-center | Philips BrightView (Philips, Amsterdam, The Netherlands) | 99mTc-sestamibi | 1517 studies | U-Net |
| 15 | Chen | 2022 | [74] | Single-center | Discovery NM/CT 570c (GE Healthcare, Milwaukee, WI, USA) | 99mTc-tetrofosmin | 172 studies | CNN |
| 16 | Ochoa-Figueroa | 2024 | [75] | Single-center | D-SPECT (Spectrum Dynamics, Caesarea, Israel) | N/R | 300 studies | U-Net |
| Study | DL Method | Data Split | Metrics | Use of Patient-Specific Features | Has Visual Assessment | Uses Quantitative Analysis (Clinical Metrics) | Uses External Dataset |
|---|---|---|---|---|---|---|---|
| [66] | U-Net | 80%:20% | MAE: 0.003 SSIM: 0.99 | ✗ | ✓ | ✓ | ✗ |
| [60] | U-Net | 60%:20%:20% | R2: 0.85 | ✗ | ✓ | ✓ | ✗ |
| [61] | U-Net | 320:66 | MSSIM: 0.97 ± 0.001 NMAE: 3.08 ± 1.26 (%) | ✗ | ✓ | ✓ | ✗ |
| [69] | CNN | 10-fold CV | R2: 0.91 Segmental error: 10% | ✗ | ✓ | ✗ | ✗ |
| [62] | GAN, U-Net | 70%:15%:15% | NMAE: 0.020 ± 0.007 | ✗ | ✓ | ✗ | ✗ |
| [64] | GAN, U-Net | 4886 train (one site)–604 test (other sites) | Median Absolute Error in TPD: 1.2 | ✗ | ✓ | ✓ | ✓ |
| [65] | GAN, U-Net | 40:25 | NMAE: 3.60% ± 0.85% | ✗ | ✓ | ✗ | ✗ |
| [67] | U-Net | 5-fold CV on 167 studies—25 test | 5-fold CV: MSE: 16.94 ± 2.03 × 10−6 SSIM: 0.9955 PSNR: 43.73 ± 0.50 Test set: MSE: 11.98 × 10−6 SSIM: 0.9976 PSNR: 45.54 | ✗ | ✓ | ✓ | ✗ |
| [70] | GAN, U-Net | 5-fold CV | NRMSE: 0.1410 ± 0.0768 PSNR: 36.3823 ± 3.7424 SSIM: 0.9949 ± 0.0043 | ✗ | ✓ | ✗ | ✗ |
| [72] | CNN | 722 test (the model was trained with 11,532 studies of [60]) | No reported comparison metrics. AUC was 0.752 for identification of obstructive stenosis using the model’s AC images | ✗ | ✓ | ✓ | ✓ |
| [68] | U-Net, CNN | Dedicated SPECT: 100:20:150 General SPECT: 240:60:100 | Dedicated SPECT: NMSE: 1.20 ± 0.72% APE: 3.24 ± 2.79% R2 = 0.9499 General SPECT: NMSE: 2.57 ± 1.06% | ✓ | ✓ | ✗ | ✗ |
| [71] | U-Net, GAN | leave-one-subject-out cross-validation | NRMSE: 0.135 ± 0.064 PSNR: 36.615 ± 3.45 SSIM: 0.995 ± 0.004 | ✗ | ✓ | ✗ | ✗ |
| [63] | U-Net, CNN | 99:19 | ME: −4.41 ± 11.8 SSIM: 0.98 ± 0.05 | ✗ | ✓ | ✓ | ✗ |
| [73] | U-Net | 1131:386 | NMSE: 0.5% | ✗ | ✓ | ✗ | ✗ |
| [74] | CNN | 100:30:42 | NMSE: 2.01 ± 1.01% | ✓ | ✓ | ✗ | ✗ |
| [75] | U-Net | 300 studies (the model is pretrained by the vendor) | No reported comparison metrics. | ✗ | ✓ | ✗ | ✗ |
6. Clinical Applicability and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full form |
| AC | Attenuation Correction |
| ACM | Attenuation Correction Map |
| APE | Absolute Percentage Error |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CI | Confidence Interval |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| CTAC | CT-based Attenuation Correction |
| CTA | Computed Tomography Angiography |
| CZT | Cadmium–Zinc–Telluride |
| cGAN | Conditional Generative Adversarial Network |
| CycleGAN | Cycle-Consistent Generative Adversarial Network |
| DeepAC | Deep Learning-based Attenuation Correction |
| DL | Deep Learning |
| DLAC | Deep Learning Attenuation Correction |
| DLACS | Deep Learning Attenuation Correction Software |
| DuRDN | Dual Squeeze-and-Excitation Residual Dense Network |
| FA-ACNet | Feature-Aligned Attenuation Correction Network |
| FDA | Food and Drug Administration |
| FOV | Field of View |
| GAN | Generative Adversarial Network |
| GENAC | Generated Attenuation-Corrected |
| ICA | Invasive Coronary Angiography |
| ICC | Intraclass Correlation Coefficient |
| IQ·SPECT | Siemens IQ·SPECT Collimator System |
| LEHR | Low-Energy High-Resolution |
| LoA | Limits of Agreement |
| MAE | Mean Absolute Error |
| ME | Mean Error |
| MPI | Myocardial Perfusion Imaging |
| MSSIM | Mean Structural Similarity Index |
| MSE | Mean Square Error |
| NMAE | Normalized Mean Absolute Error |
| NMSE | Normalized Mean Square Error |
| NAC | Non-Attenuation Corrected |
| NRMSE | Normalized Root Mean Square Error |
| PET | Positron Emission Tomography |
| PSNR | Peak Signal-to-Noise Ratio |
| PRAC | Post-Reconstruction Attenuation Correction |
| ResNet | Residual Neural Network |
| ResUNet | Residual U-Net |
| ROC | Receiver Operating Characteristic |
| R2 | Coefficient of Determination |
| SDS | Summed Difference Score |
| SRS | Summed Rest Score |
| SPECT | Single-Photon Emission Computed Tomography |
| SSS | Summed Stress Score |
| SSIM | Structural Similarity Index |
| TPD | Total Perfusion Deficit |
References
- Huck, D.M.; Weber, B.; Schreiber, B.; Pandav, J.; Parks, S.; Hainer, J.; Brown, J.M.; Divakaran, S.; Blankstein, R.; Dorbala, S.; et al. Comparative Effectiveness of PET and SPECT MPI for Predicting Cardiovascular Events After Kidney Transplant. Circ. Cardiovasc. Imaging 2024, 17, e015858. [Google Scholar] [CrossRef] [PubMed]
- Brindis, R.G.; Douglas, P.S.; Hendel, R.C.; Peterson, E.D.; Wolk, M.J.; Allen, J.M.; Patel, M.R.; Raskin, I.E.; Hendel, R.C.; Bateman, T.M.; et al. ACCF/ASNC Appropriateness Criteria for Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging (SPECT MPI). J. Am. Coll. Cardiol. 2005, 46, 1587–1605. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bateman, T.M.; Case, J.A.; Heller, G. Attenuation Artifact, Attenuation Correction, and the Future of Myocardial Perfusion SPECT. J. Nucl. Cardiol. 2007, 14, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Pazhenkottil, A.P.; Ghadri, J.-R.; Nkoulou, R.N.; Wolfrum, M.; Buechel, R.R.; Küest, S.M.; Husmann, L.; Herzog, B.A.; Gaemperli, O.; Kaufmann, P.A. Improved Outcome Prediction by SPECT Myocardial Perfusion Imaging After CT Attenuation Correction. J. Nucl. Med. 2011, 52, 196–200. [Google Scholar] [CrossRef]
- Sharma, P.; Patel, C.D.; Karunanithi, S.; Maharjan, S.; Malhotra, A. Comparative Accuracy of CT Attenuation-Corrected and Non–Attenuation-Corrected SPECT Myocardial Perfusion Imaging. Clin. Nucl. Med. 2012, 37, 332–338. [Google Scholar] [CrossRef]
- Hsieh, J.; Flohr, T. Computed Tomography Recent History and Future Perspectives. J. Med. Imaging 2021, 8, 052109. [Google Scholar] [CrossRef]
- Shao, W.; Rowe, S.P.; Du, Y. Artificial Intelligence in Single Photon Emission Computed Tomography (SPECT) Imaging: A Narrative Review. Ann. Transl. Med. 2021, 9, 820. [Google Scholar] [CrossRef]
- Berker, Y.; Li, Y. Attenuation Correction in Emission Tomography Using the Emission Data—A Review. Med. Phys. 2016, 43, 807–832. [Google Scholar] [CrossRef]
- Hutton, B.F. The Origins of SPECT and SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 3–16. [Google Scholar] [CrossRef]
- Patton, J.A.; Turkington, T.G. SPECT/CT Physical Principles and Attenuation Correction. J. Nucl. Med. Technol. 2008, 36, 1–10. [Google Scholar] [CrossRef]
- Welch, A.; Clack, R.; Natterer, F.; Gullberg, G.T. Toward Accurate Attenuation Correction in SPECT without Transmission Measurements. IEEE Trans. Med. Imaging 1997, 16, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, G.F.; Geramifar, P.; Tegaw, E.M.; Ay, M.R. Techniques for Generating Attenuation Map Using Cardiac SPECT Emission Data Only: A Systematic Review. Ann. Nucl. Med. 2019, 33, 1–13. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hajianfar, G.; Gharibi, O.; Sabouri, M.; Mohebi, M.; Amini, M.; Yasemi, M.J.; Chehreghani, M.; Maghsudi, M.; Mansouri, Z.; Edalat-Javid, M.; et al. Artificial Intelligence-Powered Coronary Artery Disease Diagnosis from SPECT Myocardial Perfusion Imaging: A Comprehensive Deep Learning Study. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 3019–3035. [Google Scholar] [CrossRef] [PubMed]
- Balaji, V.; Song, T.-A.; Malekzadeh, M.; Heidari, P.; Dutta, J. Artificial Intelligence for PET and SPECT Image Enhancement. J. Nucl. Med. 2024, 65, 4–12. [Google Scholar] [CrossRef]
- Kawakubo, M.; Nagao, M.; Kaimoto, Y.; Nakao, R.; Yamamoto, A.; Kawasaki, H.; Iwaguchi, T.; Matsuo, Y.; Kaneko, K.; Sakai, A.; et al. Deep Learning Approach Using SPECT-to-PET Translation for Attenuation Correction in CT-Less Myocardial Perfusion SPECT Imaging. Ann. Nucl. Med. 2024, 38, 199–209. [Google Scholar] [CrossRef]
- Kwon, K.; Oh, D.; Kim, J.H.; Yoo, J.; Lee, W.W. Deep-Learning-Based Attenuation Map Generation in Kidney Single Photon Emission Computed Tomography. EJNMMI Phys. 2024, 11, 84. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Akhavanallaf, A.; Fessler, J.A.; Dewaraja, Y.K. 90Y SPECT Scatter Estimation and Voxel Dosimetry in Radioembolization Using a Unified Deep Learning Framework. EJNMMI Phys. 2023, 10, 82. [Google Scholar] [CrossRef]
- Le, T.D.; Shitiri, N.C.; Jung, S.-H.; Kwon, S.-Y.; Lee, C. Image Synthesis in Nuclear Medicine Imaging with Deep Learning: A Review. Sensors 2024, 24, 8068. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Tokodi, M. Automated Interpretation of Myocardial Perfusion Images. JACC Cardiovasc. Imaging 2022, 15, 1103–1106. [Google Scholar] [CrossRef]
- Saboury, B.; Bradshaw, T.; Boellaard, R.; Buvat, I.; Dutta, J.; Hatt, M.; Jha, A.K.; Li, Q.; Liu, C.; McMeekin, H.; et al. Artificial Intelligence in Nuclear Medicine: Opportunities, Challenges, and Responsibilities Toward a Trustworthy Ecosystem. J. Nucl. Med. 2023, 64, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, D.; Akiyama, T.; Hashimoto, M.; Iwabuchi, Y.; Katsuki, T.; Kimura, M.; Akiba, Y.; Sawada, H.; Inohara, T.; Yuasa, S.; et al. A Deep Learning-Based Automated Diagnosis System for SPECT Myocardial Perfusion Imaging. Sci. Rep. 2024, 14, 13583. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, G.F.; Geramifar, P.; Abbasi, M.; Tsegaw, E.M.; Amin, M.; Salimi, A.; Mohammadi, M.; Teimourianfard, B.; Ay, M.R. Attenuation Correction for Dedicated Cardiac SPECT Imaging Without Using Transmission Data. Mol. Imaging Radionucl. Ther. 2023, 32, 42–53. [Google Scholar] [CrossRef]
- LeCun, Y.; Kavukcuoglu, K.; Farabet, C. Convolutional networks and applications in vision. In Proceedings of the 2010 IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 253–256. [Google Scholar]
- Dong, S.; Wang, P.; Abbas, K. A Survey on Deep Learning and Its Applications. Comput. Sci. Rev. 2021, 40, 100379. [Google Scholar] [CrossRef]
- Elasri, M.; Elharrouss, O.; Al-Maadeed, S.; Tairi, H. Image Generation: A Review. Neural Process. Lett. 2022, 54, 4609–4646. [Google Scholar] [CrossRef]
- Baraheem, S.S.; Le, T.-N.; Nguyen, T.V. Image Synthesis: A Review of Methods, Datasets, Evaluation Metrics, and Future Outlook. Artif. Intell. Rev. 2023, 56, 10813–10865. [Google Scholar] [CrossRef]
- Apostolopoulos, D.J.; Savvopoulos, C. What Is the Benefit of CT-Based Attenuation Correction in Myocardial Perfusion SPET? Hell. J. Nucl. Med. 2016, 19, 89–92. [Google Scholar]
- Verberne, H.J.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bengel, F.; De Bondt, P.; Buechel, R.R.; Cuocolo, A.; Van Eck-Smit, B.L.F.; Flotats, A.; et al. EANM Procedural Guidelines for Radionuclide Myocardial Perfusion Imaging with SPECT and SPECT/CT: 2015 Revision. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1929–1940. [Google Scholar] [CrossRef]
- Bouchareb, Y.; AlSaadi, A.; Zabah, J.; Jain, A.; Al-Jabri, A.; Phiri, P.; Shi, J.Q.; Delanerolle, G.; Sirasanagandla, S.R. Technological Advances in SPECT and SPECT/CT Imaging. Diagnostics 2024, 14, 1431. [Google Scholar] [CrossRef]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100. [Google Scholar] [CrossRef]
- Abbott, B.G.; Case, J.A.; Dorbala, S.; Einstein, A.J.; Galt, J.R.; Pagnanelli, R.; Bullock-Palmer, R.P.; Soman, P.; Wells, R.G. Contemporary Cardiac SPECT Imaging—Innovations and Best Practices: An Information Statement from the American Society of Nuclear Cardiology. Circ. Cardiovasc. Imaging 2018, 11, e000020. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C. Deep-Learning-Based Methods of Attenuation Correction for SPECT and PET. J. Nucl. Cardiol. 2023, 30, 1859–1878. [Google Scholar] [CrossRef]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; La Fougère, C.; Mariani, G.; Massalha, S.; et al. Two Decades of SPECT/CT—The Coming of Age of a Technology: An Updated Review of Literature Evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef]
- Seo, Y.; Mari, C.; Hasegawa, B.H. Technological Development and Advances in Single-Photon Emission Computed Tomography/Computed Tomography. Semin. Nucl. Med. 2008, 38, 177–198. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, P.; Yan, J. A Review of State-of-the-Art Resolution Improvement Techniques in SPECT Imaging. EJNMMI Phys. 2025, 12, 9. [Google Scholar] [CrossRef]
- Van Audenhaege, K.; Van Holen, R.; Vandenberghe, S.; Vanhove, C.; Metzler, S.D.; Moore, S.C. Review of SPECT Collimator Selection, Optimization, and Fabrication for Clinical and Preclinical Imaging. Med. Phys. 2015, 42, 4796–4813. [Google Scholar] [CrossRef]
- Shiba, S.; Sagara, H. Passive Gamma Emission Tomography with Ordered Subset Expectation Maximization Method. Ann. Nucl. Energy 2021, 150, 107823. [Google Scholar] [CrossRef]
- Trevisan, A.C.; Raed, M.D.; Tumas, V.; Alexandre-Santos, L.; Pitella, F.A.; Itikawa, E.N.; Silvah, J.H.; Kato, M.; Martinez, E.Z.; Achcar, J.A.; et al. Comparison between OSEM and FBP Reconstruction Algorithms for the Qualitative and Quantitative Interpretation of Brain DAT-SPECT Using an Anthropomorphic Striatal Phantom: Implications for the Practice. Res. Biomed. Eng. 2020, 36, 77–88. [Google Scholar] [CrossRef]
- Dong, X.; Lei, Y.; Wang, T.; Higgins, K.; Liu, T.; Curran, W.J.; Mao, H.; Nye, J.A.; Yang, X. Deep learning-based attenuation correction in the absence of structural information for whole-body positron emission tomography imaging. Phys. Med. Biol. 2020, 65, 055011. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sohn, J.H.; Behr, S.C.; Gullberg, G.T.; Seo, Y. CT-Less Direct Correction of Attenuation and Scatter in the Image Space Using Deep Learning for Whole-Body FDG PET: Potential Benefits and Pitfalls. Radiol. Artif. Intell. 2021, 3, e200137. [Google Scholar] [CrossRef]
- Arabi, H.; Zaidi, H. Deep Learning-Guided Estimation of Attenuation Correction Factors from Time-of-Flight PET Emission Data. Med. Image Anal. 2020, 64, 101718. [Google Scholar] [CrossRef]
- Krokos, G.; MacKewn, J.; Dunn, J.; Marsden, P. A Review of PET Attenuation Correction Methods for PET-MR. EJNMMI Phys. 2023, 10, 52. [Google Scholar] [CrossRef]
- Jahangir, R.; Kamali-Asl, A.; Arabi, H.; Zaidi, H. Strategies for Deep Learning-based Attenuation and Scatter Correction of Brain18 F-FDG PET Images in the Image Domain. Med. Phys. 2024, 51, 870–880. [Google Scholar] [CrossRef]
- Murata, T.; Yokota, H.; Yamato, R.; Horikoshi, T.; Tsuneda, M.; Kurosawa, R.; Hashimoto, T.; Ota, J.; Sawada, K.; Iimori, T.; et al. Development of Attenuation Correction Methods Using Deep Learning in Brain-perfusion Single-photon Emission Computed Tomography. Med. Phys. 2021, 48, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Kaida, H.; Yoshida, S.; Ishii, K. Attenuation Correction Using Deep Learning for Brain Perfusion SPECT Images. Ann. Nucl. Med. 2021, 35, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- LeCun, Y.; Bengio, Y. Convolutional Networks for Images, Speech, and Time Series. In The Handbook of Brain Theory and Neural Networks; MIT Press: Cambridge, MA, USA, 1995; Volume 3361. [Google Scholar]
- Apostolopoulos, I.D.; Papandrianos, N.I.; Apostolopoulos, D.J.; Papageorgiou, E. Between Two Worlds: Investigating the Intersection of Human Expertise and Machine Learning in the Case of Coronary Artery Disease Diagnosis. Bioengineering 2024, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, D.J.; Apostolopoulos, I.D.; Papathanasiou, N.D.; Spyridonidis, T.; Panayiotakis, G.S. Explainable Artificial Intelligence Method (ParaNet+) Localises Abnormal Parathyroid Glands in Scintigraphic Scans of Patients with Primary Hyperparathyroidism. Algorithms 2023, 16, 435. [Google Scholar] [CrossRef]
- Feleki, A.; Apostolopoulos, I.D.; Moustakidis, S.; Papageorgiou, E.I.; Papathanasiou, N.; Apostolopoulos, D.; Papandrianos, N. Explainable Deep Fuzzy Cognitive Map Diagnosis of Coronary Artery Disease: Integrating Myocardial Perfusion Imaging, Clinical Data, and Natural Language Insights. Appl. Sci. 2023, 13, 11953. [Google Scholar] [CrossRef]
- Feleki, A.; Apostolopoulos, I.D.; Papageorgiou, K.; Papageorgiou, E.I.; Apostolopoulos, D.J.; Papandrianos, N.I. A Fuzzy Cognitive Map Learning Approach for Coronary Artery Disease Diagnosis in Nuclear Medicine. In Fuzzy Logic and Technology, and Aggregation Operators; Massanet, S., Montes, S., Ruiz-Aguilera, D., González-Hidalgo, M., Eds.; Lecture Notes in Computer Science; Springer Nature: Cham, Switzerland, 2023; Volume 14069, pp. 14–25. ISBN 978-3-031-39964-0. [Google Scholar]
- Papandrianos, N.I.; Apostolopoulos, I.D.; Feleki, A.; Apostolopoulos, D.J.; Papageorgiou, E.I. Deep Learning Exploration for SPECT MPI Polar Map Images Classification in Coronary Artery Disease. Ann. Nucl. Med. 2022, 36, 823–833. [Google Scholar] [CrossRef]
- Samaras, A.-D.; Moustakidis, S.; Apostolopoulos, I.D.; Papageorgiou, E.; Papandrianos, N. Uncovering the Black Box of Coronary Artery Disease Diagnosis: The Significance of Explainability in Predictive Models. Appl. Sci. 2023, 13, 8120. [Google Scholar] [CrossRef]
- Domingues, I.; Pereira, G.; Martins, P.; Duarte, H.; Santos, J.; Abreu, P.H. Using Deep Learning Techniques in Medical Imaging: A Systematic Review of Applications on CT and PET. Artif. Intell. Rev. 2020, 53, 4093–4160. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 9351, pp. 234–241. ISBN 978-3-319-24573-7. [Google Scholar]
- Ali, S.; Abuhmed, T.; El-Sappagh, S.; Muhammad, K.; Alonso-Moral, J.M.; Confalonieri, R.; Guidotti, R.; Del Ser, J.; Díaz-Rodríguez, N.; Herrera, F. Explainable Artificial Intelligence (XAI): What We Know and What Is Left to Attain Trustworthy Artificial Intelligence. Inf. Fusion 2023, 99, 101805. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.-C. Visual interpretability for deep learning: A survey. Front. Inf. Technol. Electron. Eng. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Hagio, T.; Poitrasson-Rivière, A.; Moody, J.B.; Renaud, J.M.; Arida-Moody, L.; Shah, R.V.; Ficaro, E.P.; Murthy, V.L. “Virtual” Attenuation Correction: Improving Stress Myocardial Perfusion SPECT Imaging Using Deep Learning. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Prieto Canalejo, M.; Palau San Pedro, A.; Geronazzo, R.; Minsky, D.; Juárez-Orozco, L.; Namías, M. Synthetic Attenuation Correction Maps for SPECT Imaging Using Deep Learning: A Study on Myocardial Perfusion Imaging. Diagnostics 2023, 13, 2214. [Google Scholar] [CrossRef]
- Huxohl, T.; Patel, G.; Zabel, R.; Burchert, W. Deep Learning Approximation of Attenuation Maps for Myocardial Perfusion SPECT with an IQ SPECT Collimator. EJNMMI Phys. 2023, 10, 49. [Google Scholar] [CrossRef]
- Mostafapour, S.; Gholamiankhah, F.; Maroufpour, S.; Momennezhad, M.; Asadinezhad, M.; Zakavi, S.R.; Arabi, H.; Zaidi, H. Deep Learning-Guided Attenuation Correction in the Image Domain for Myocardial Perfusion SPECT Imaging. J. Comput. Des. Eng. 2022, 9, 434–447. [Google Scholar] [CrossRef]
- Shanbhag, A.D.; Miller, R.J.H.; Pieszko, K.; Lemley, M.; Kavanagh, P.; Feher, A.; Miller, E.J.; Sinusas, A.J.; Kaufmann, P.A.; Han, D.; et al. Deep Learning–Based Attenuation Correction Improves Diagnostic Accuracy of Cardiac SPECT. J. Nucl. Med. 2023, 64, 472–478. [Google Scholar] [CrossRef]
- Shi, L.; Onofrey, J.A.; Liu, H.; Liu, Y.-H.; Liu, C. Deep Learning-Based Attenuation Map Generation for Myocardial Perfusion SPECT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2383–2395. [Google Scholar] [CrossRef]
- Lim, S.; Park, Y.-J.; Lee, S.J.; An, Y.-S.; Yoon, J.-K. Clinical Feasibility of Deep Learning–Based Attenuation Correction Models for Tl-201 Myocardial Perfusion SPECT. Clin. Nucl. Med. 2024, 49, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, Z.; Wei, J.; Jiang, L.; Yu, L.; Cai, H.; Li, L.; Guo, Q.; Zhao, Z. Deep Learning-Based CT-Free Attenuation Correction for Cardiac SPECT: A New Approach. BMC Med. Imaging 2025, 25, 38. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Xie, H.; Shi, L.; Liu, H.; Holler, W.; Lin, M.; Liu, Y.-H.; Miller, E.J.; Sinusas, A.J.; et al. Direct and Indirect Strategies of Deep-Learning-Based Attenuation Correction for General Purpose and Dedicated Cardiac SPECT. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3046–3060. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, L.; Wang, R.; Miller, E.J.; Sinusas, A.J.; Liu, C.; Gullberg, G.T.; Seo, Y. Direct Attenuation Correction Using Deep Learning for Cardiac SPECT: A Feasibility Study. J. Nucl. Med. 2021, 62, 1645–1652. [Google Scholar] [CrossRef]
- Torkaman, M.; Yang, J.; Shi, L.; Wang, R.; Miller, E.J.; Sinusas, A.J.; Liu, C.; Gullberg, G.T.; Seo, Y. Direct Image-Based Attenuation Correction Using Conditional Generative Adversarial Network for SPECT Myocardial Perfusion Imaging. In Proceedings of the Medical Imaging 2021: Biomedical Applications in Molecular, Structural, and Functional Imaging, Online Only, 12–15 February 2021; Gimi, B.S., Krol, A., Eds.; SPIE: Washington, DC, USA, 2021; p. 27. [Google Scholar]
- Torkaman, M.; Yang, J.; Shi, L.; Wang, R.; Miller, E.J.; Sinusas, A.J.; Liu, C.; Gullberg, G.T.; Seo, Y. Data Management and Network Architecture Effect on Performance Variability in Direct Attenuation Correction via Deep Learning for Cardiac SPECT: A Feasibility Study. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 755–765. [Google Scholar] [CrossRef]
- Hagio, T.; Moody, J.B.; Poitrasson-Rivière, A.; Renaud, J.M.; Pierce, L.; Buckley, C.; Ficaro, E.P.; Murthy, V.L. Multi-Center, Multi-Vendor Validation of Deep Learning-Based Attenuation Correction in SPECT MPI: Data from the International Flurpiridaz-301 Trial. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1028–1033. [Google Scholar] [CrossRef]
- Chen, Y.; Pretorius, P.H.; Yang, Y.; King, M.A.; Lindsay, C. Investigation of Scatter Energy Window Width and Count Levels for Deep Learning-Based Attenuation Map Estimation in Cardiac SPECT/CT Imaging. Phys. Med. Biol. 2024, 69, 225009. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Shi, L.; Liu, H.; Pang, Y.; Wang, R.; Miller, E.J.; Sinusas, A.J.; Liu, C. CT-Free Attenuation Correction for Dedicated Cardiac SPECT Using a 3D Dual Squeeze-and-Excitation Residual Dense Network. J. Nucl. Cardiol. 2022, 29, 2235–2250. [Google Scholar] [CrossRef]
- Ochoa-Figueroa, M.; Valera-Soria, C.; Pagonis, C.; Ressner, M.; Norberg, P.; Sanchez-Rodriguez, V.; Frias-Rose, J.; Good, E.; Davidsson, A. Diagnostic Performance of a Novel Deep Learning Attenuation Correction Software for MPI Using a Cardio Dedicated CZT Camera. Experience in the Clinical Practice. Rev. Esp. Med. Nucl. Imagen Mol. Engl. Ed. 2024, 43, 23–30. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the Short-Term Survival Benefit Associated with Revascularization Compared with Medical Therapy in Patients with No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photon Emission Computed Tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Berman, D.S.; Shaw, L.J.; Kiat, H.; Cohen, I.; Cabico, J.A.; Friedman, J.; Diamond, G.A. Incremental Prognostic Value of Myocardial Perfusion Single Photon Emission Computed Tomography for the Prediction of Cardiac Death: Differential Stratification for Risk of Cardiac Death and Myocardial Infarction. Circulation 1998, 97, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lei, B.; Zhou, Z.; Song, T.-A.; Balaji, V.; Dutta, J. AI in SPECT Imaging: Opportunities and Challenges. Semin. Nucl. Med. 2025, 55, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Slomka, P.J. Artificial Intelligence in Nuclear Cardiology: An Update and Future Trends. Semin. Nucl. Med. 2024, 54, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Sciagrà, R.; Valente, S.; Dominietto, M. Artificial Intelligence in Nuclear Cardiology. J. Clin. Med. 2025, 14, 6416. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Caobelli, F.; Huang, Y.; Li, Y.; Zhang, J.; Shi, K.; Yu, F. The Role of Deep Learning in Myocardial Perfusion Imaging for Diagnosis and Prognosis: A Systematic Review. iScience 2024, 27, 111374. [Google Scholar] [CrossRef]
- Gould, K.L.; Pan, T.; Loghin, C.; Johnson, N.P.; Guha, A.; Sdringola, S. Frequent Diagnostic Errors in Cardiac PET/CT Due to Misregistration of CT Attenuation and Emission PET Images: A Definitive Analysis of Causes, Consequences, and Corrections. J. Nucl. Med. 2007, 48, 1112–1121. [Google Scholar] [CrossRef]
- Gong, H.; Tao, S.; Rajendran, K.; Zhou, W.; McCollough, C.H.; Leng, S. Deep-learning-based Direct Inversion for Material Decomposition. Med. Phys. 2020, 47, 6294–6309. [Google Scholar] [CrossRef]
- Ghosh, S.; Varshney, N.; Al Hasan, M.M.; Roy, A.; Craig, P.; Koppal, S.J.; Dalir, H.; Asadizanjani, N. Exploring Physics-Informed Machine Learning for System Matrix Formulation in x-Ray Imaging Forward Models. In Proceedings of the Developments in X-Ray Tomography XV; Müller, B., Wang, G., Eds.; SPIE: San Diego, CA, USA, 2024; p. 66. [Google Scholar]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Reinke, A.; Godau, P.; Tizabi, M.D.; Buettner, F.; Christodoulou, E.; Glocker, B.; Isensee, F.; Kleesiek, J.; Kozubek, M.; et al. Metrics Reloaded: Recommendations for Image Analysis Validation. Nat. Methods 2024, 21, 195–212. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Damen, J.A.A.; Kaul, T.; Hooft, L.; Andaur Navarro, C.; Dhiman, P.; Beam, A.L.; Van Calster, B.; Celi, L.A.; Denaxas, S.; et al. PROBAST+AI: An Updated Quality, Risk of Bias, and Applicability Assessment Tool for Prediction Models Using Regression or Artificial Intelligence Methods. BMJ 2025, 388, e082505. [Google Scholar] [CrossRef]
- Liu, X.; Rivera, S.C.; Moher, D.; Calvert, M.J.; Denniston, A.K. Reporting Guidelines for Clinical Trial Reports for Interventions Involving Artificial Intelligence: The CONSORT-AI Extension. BMJ 2020, 370, m3164. [Google Scholar] [CrossRef]
- Rivera, S.C.; Liu, X.; Chan, A.-W.; Denniston, A.K.; Calvert, M.J. Guidelines for Clinical Trial Protocols for Interventions Involving Artificial Intelligence: The SPIRIT-AI Extension. BMJ 2020, 370, m3210. [Google Scholar] [CrossRef]
- Vasey, B.; Nagendran, M.; Campbell, B.; Clifton, D.A.; Collins, G.S.; Denaxas, S.; Denniston, A.K.; Faes, L.; Geerts, B.; Ibrahim, M.; et al. Reporting Guideline for the Early-Stage Clinical Evaluation of Decision Support Systems Driven by Artificial Intelligence: DECIDE-AI. Nat. Med. 2022, 28, 924–933. [Google Scholar] [CrossRef]
- Mongan, J.; Moy, L.; Kahn, C.E. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A Guide for Authors and Reviewers. Radiol. Artif. Intell. 2020, 2, e200029. [Google Scholar] [CrossRef] [PubMed]
- Norgeot, B.; Quer, G.; Beaulieu-Jones, B.K.; Torkamani, A.; Dias, R.; Gianfrancesco, M.; Arnaout, R.; Kohane, I.S.; Saria, S.; Topol, E.; et al. Minimum Information about Clinical Artificial Intelligence Modeling: The MI-CLAIM Checklist. Nat. Med. 2020, 26, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Boussard, T.; Bozkurt, S.; Ioannidis, J.P.A.; Shah, N.H. MINIMAR (MINimum Information for Medical AI Reporting): Developing Reporting Standards for Artificial Intelligence in Health Care. J. Am. Med. Inform. Assoc. 2020, 27, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Saboury, B.; Ghesani, M. Artificial Intelligence in Nuclear Medicine: Point—More Reality Than Hype Today. Am. J. Roentgenol. 2024, 223, e2431288. [Google Scholar] [CrossRef]
- Shanbhag, A.D.; Miller, R.J.H.; Lemley, M.; Kavanagh, P.; Liang, J.X.; Marcinkiewicz, A.M.; Builoff, V.; Van Kriekinge, S.; Ruddy, T.D.; Fish, M.B.; et al. General Purpose Deep Learning Attenuation Correction Improves Diagnostic Accuracy of SPECT MPI. JACC Cardiovasc. Imaging 2025, S1936878X25003316. [Google Scholar] [CrossRef]
- Gichoya, J.W.; Banerjee, I.; Bhimireddy, A.R.; Burns, J.L.; Celi, L.A.; Chen, L.-C.; Correa, R.; Dullerud, N.; Ghassemi, M.; Huang, S.-C.; et al. AI Recognition of Patient Race in Medical Imaging: A Modelling Study. Lancet Digit. Health 2022, 4, e406–e414. [Google Scholar] [CrossRef]
| In Short | Description |
|---|---|
| External Evaluation | Most studies rely only on internal validation; very few use independent external cohorts, making generalizability uncertain. |
| Integration | No models have been prospectively integrated or tested in routine clinical workflows, so real-world usability is unproven. |
| Compatibility | Models are often trained on data from a single vendor (e.g., GE cameras), and robustness across different scanner types (Siemens, CZT, IQ·SPECT) is unknown. |
| Multi-center studies | There is a lack of large-scale, multi-institutional collaborations to test robustness across diverse patient populations and acquisition protocols. |
| CT error propagation | Since CT-based AC is used as ground truth, errors from misregistration or artifacts may be reproduced by DL models. Physics-informed methods could mitigate this but are underexplored. |
| Non-image data | Patient-specific features (e.g., BMI, gender, chest size) are rarely incorporated, despite potential benefits for contextual accuracy. |
| Standardized metrics | No consensus exists on evaluation metrics; studies use heterogeneous criteria (SSIM, PSNR, AUC, TPD, etc.), hindering comparisons and clinical adoption. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolopoulos, I.D.; Papandrianos, N.Ι.; Papageorgiou, E.I.; Apostolopoulos, D.J. A Review on SPECT Myocardial Perfusion Imaging Attenuation Correction Using Deep Learning. Appl. Sci. 2025, 15, 11287. https://doi.org/10.3390/app152011287
Apostolopoulos ID, Papandrianos NΙ, Papageorgiou EI, Apostolopoulos DJ. A Review on SPECT Myocardial Perfusion Imaging Attenuation Correction Using Deep Learning. Applied Sciences. 2025; 15(20):11287. https://doi.org/10.3390/app152011287
Chicago/Turabian StyleApostolopoulos, Ioannis D., Nikolaοs Ι. Papandrianos, Elpiniki I. Papageorgiou, and Dimitris J. Apostolopoulos. 2025. "A Review on SPECT Myocardial Perfusion Imaging Attenuation Correction Using Deep Learning" Applied Sciences 15, no. 20: 11287. https://doi.org/10.3390/app152011287
APA StyleApostolopoulos, I. D., Papandrianos, N. Ι., Papageorgiou, E. I., & Apostolopoulos, D. J. (2025). A Review on SPECT Myocardial Perfusion Imaging Attenuation Correction Using Deep Learning. Applied Sciences, 15(20), 11287. https://doi.org/10.3390/app152011287







