Mapping 18F-FDG Positron Emission Tomography Uptake in the Aortic Wall and Thrombus: Validation and Reproducibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Image Acquisition
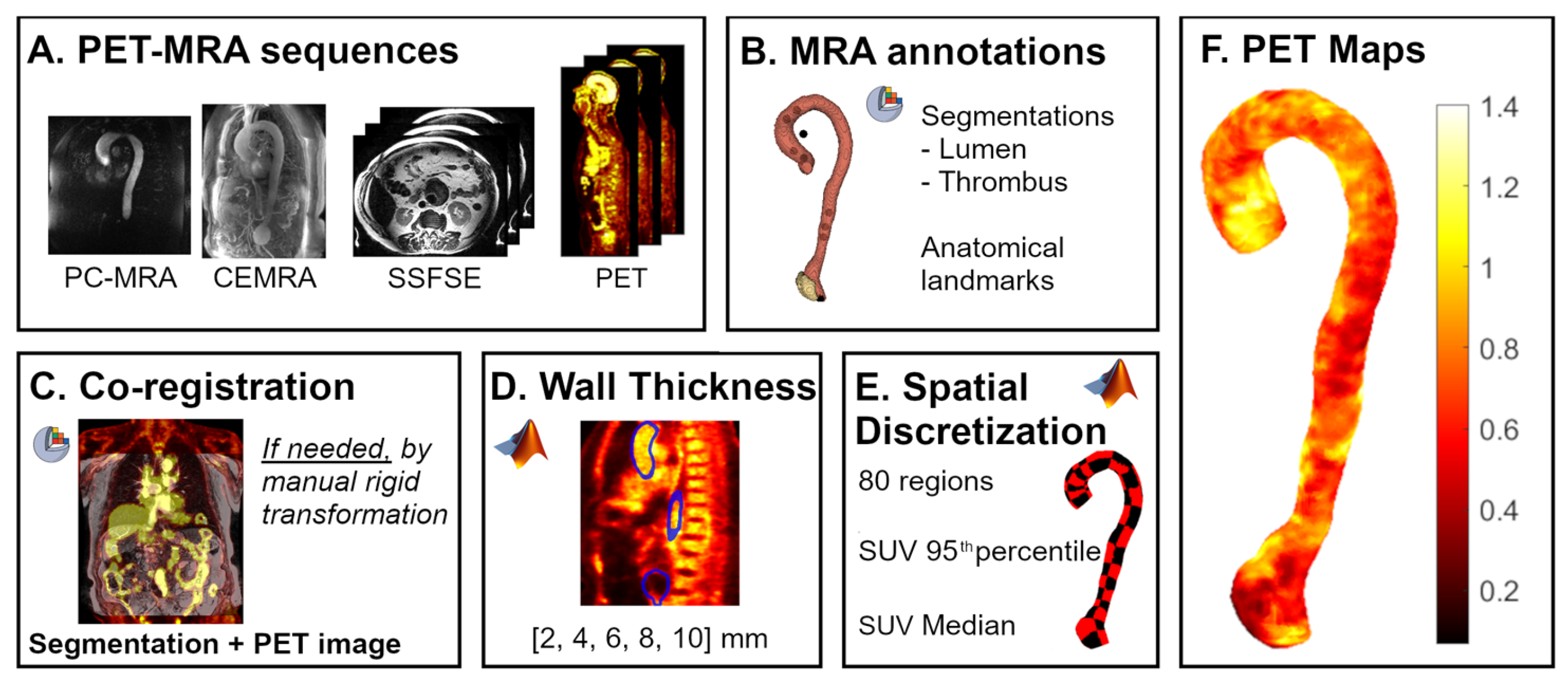
2.3. Image Analysis
2.4. Validation
2.5. Statistical Analysis
3. Results
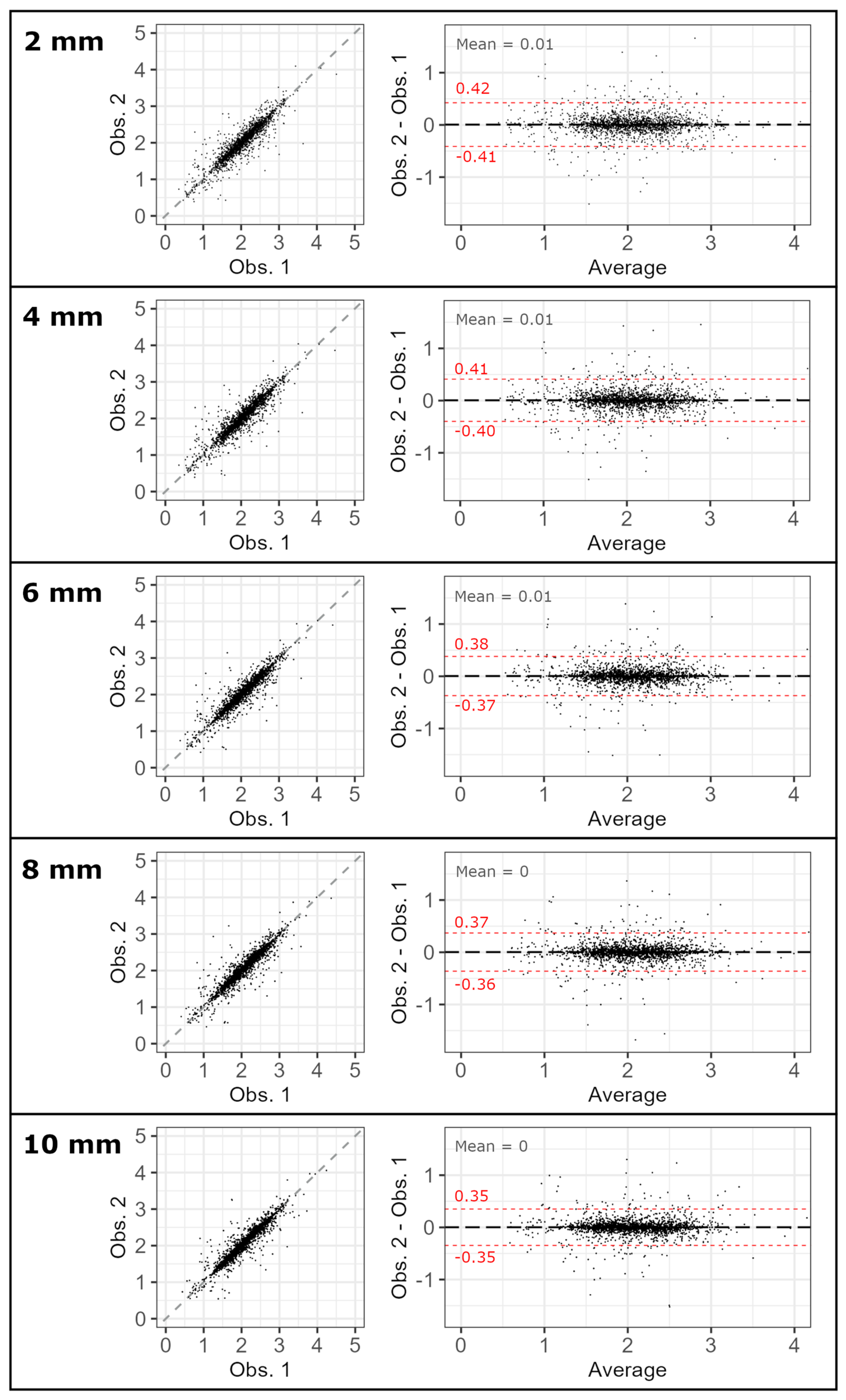
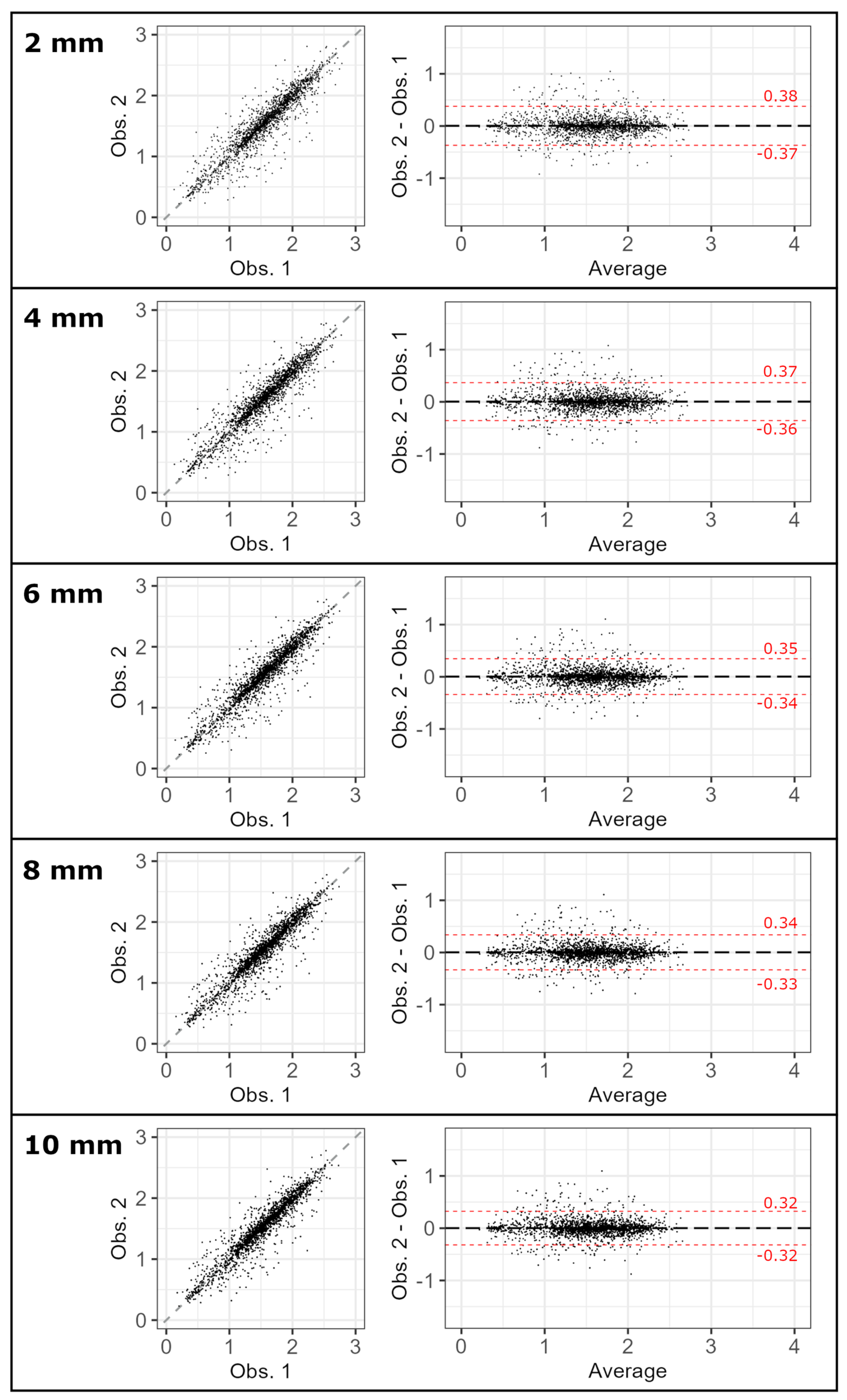
3.1. Inter-Observer Reproducibility
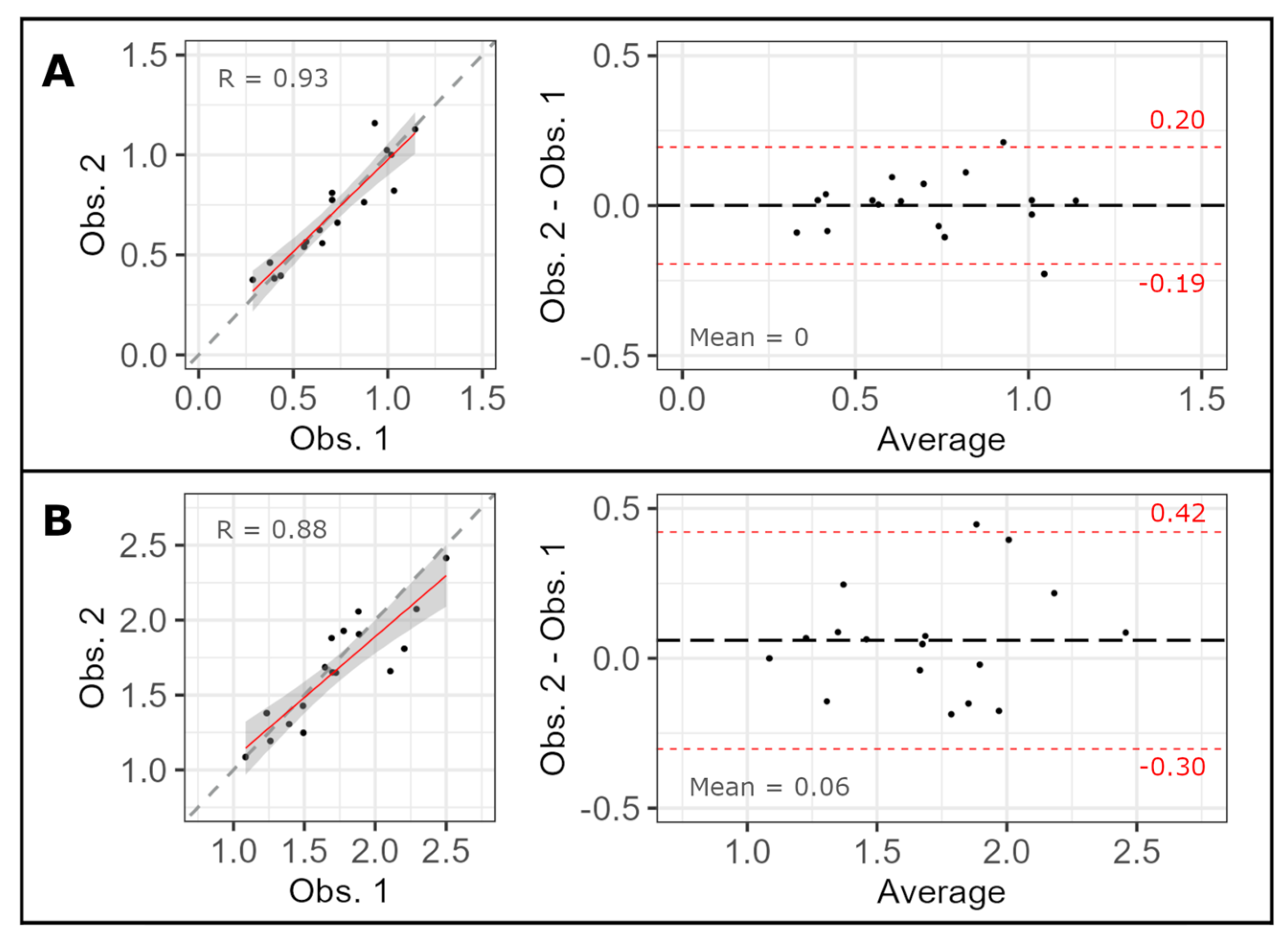
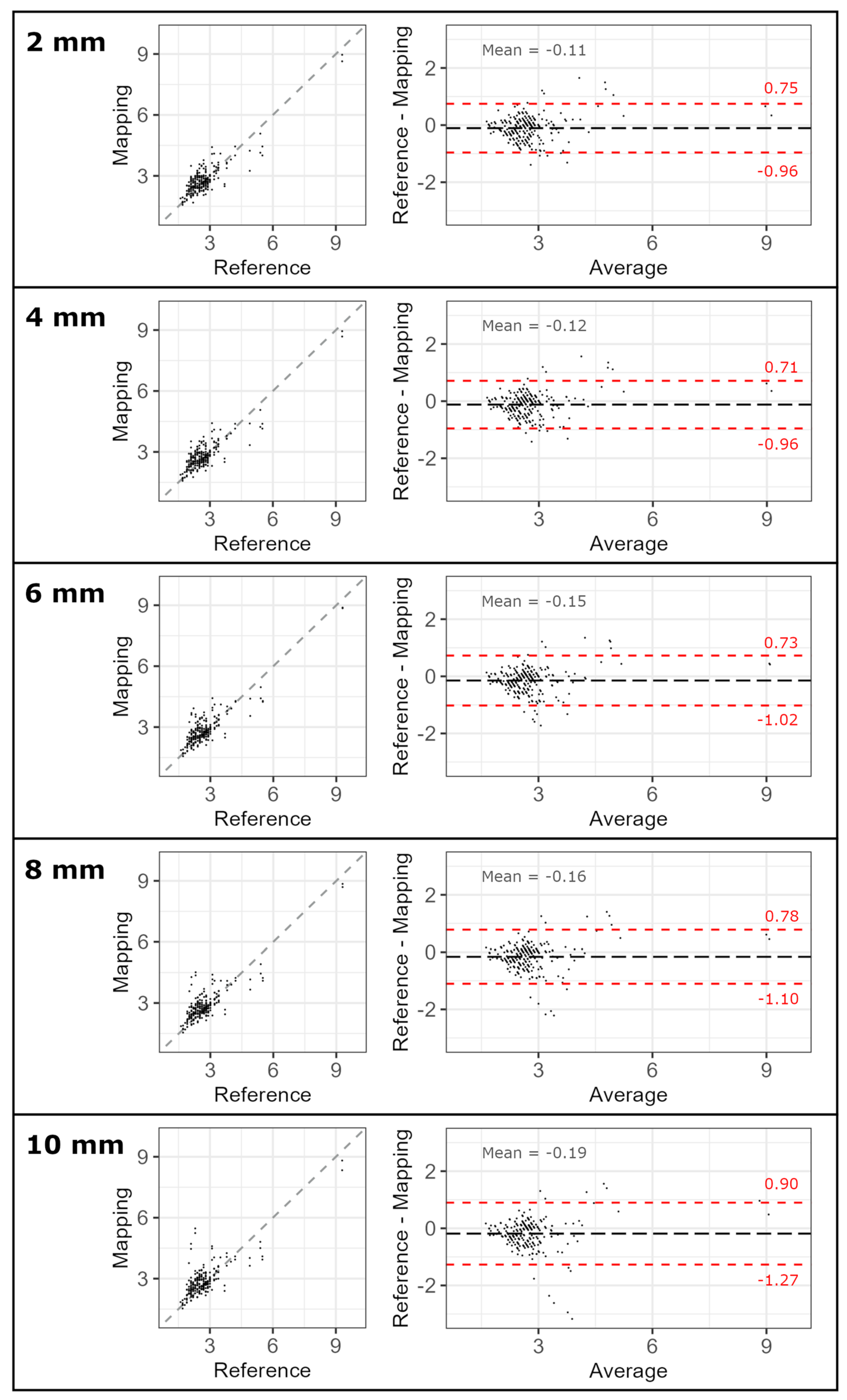
3.2. Validation
4. Discussion
5. Limitations and Future Research Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18F-FDG | 18F-fluorodeoxyglucose |
| CEMRA | Contrast-enhanced magnetic resonance angiography |
| DSC | Dice score coefficient |
| HD | Hausdorff distance |
| ICC | Intra-class correlation coefficient |
| IQR | Interquartile range |
| MR | Magnetic resonance |
| PC-MRA | Phase-contrast-enhanced magnetic resonance angiography |
| PET | Positron emission tomography |
| SSFSE | Single-shot fast-spin echo |
| SUV | Standard uptake value |
References
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Perez, Z.G.; Zafar, M.A.; Velasco, J.J.; Sonsino, A.; Ellauzi, H.; John, C.; Kalyanasundaram, A.; Ziganshin, B.A.; Elefteriades, J.A. Aortic Size at the Time of Type A and Type B Dissections. Ann. Thorac. Surg. 2023, 116, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Benedetto, S.; Sultan, D.; Wahidi, R.; Hamdi, M.; Zaghloul, M.S.; Hafezi, S.; Arif, B.; McDonald, L.K.; Harrison, K.; Thies, D.; et al. Pilot first-in-human CCR2 PET/CT to detect abdominal aortic aneurysm wall instability. Theranostics 2025, 15, 5518–5528. [Google Scholar] [CrossRef] [PubMed]
- Dux-Santoy, L.; Rodríguez-Palomares, J.F.; Teixidó-Turà, G.; Garrido-Oliver, J.; Carrasco-Poves, A.; Morales-Galán, A.; Ruiz-Muñoz, A.; Casas, G.; Valente, F.; Galian-Gay, L.; et al. Three-dimensional aortic geometry mapping via registration of non-gated contrast-enhanced or gated and respiratory-navigated MR angiographies. J. Cardiovasc. Magn. Reson. 2024, 27, 100992. [Google Scholar] [CrossRef] [PubMed]
- Dux-Santoy, L.; Rodríguez-Palomares, J.F.; Teixidó-Turà, G.; Ruiz-Muñoz, A.; Casas, G.; Valente, F.; Servato, M.L.; Galian-Gay, L.; Gutiérrez, L.; González-Alujas, T.; et al. Registration-based semi-automatic assessment of aortic diameter growth rate from contrast-enhanced computed tomography outperforms manual quantification. Eur. Radiol. 2022, 32, 1997–2009. [Google Scholar] [CrossRef]
- Singh Dhesi, S.; Adusumilli, P.; Ravikumar, N.; Waduud, M.A.; Frood, R.; Frangi, A.F.; McDermott, G.; Rudd, J.H.F.; Huang, Y.; Boyle, J.R.; et al. Development and External Validation of [18F] FDG PET-CT-Derived Radiomic Models for Prediction of Abdominal Aortic Aneurysm Growth Rate. Algorithms 2025, 18, 86. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, E334–E482. [Google Scholar] [CrossRef]
- Evangelista, A.; Galian-Gay, L.; Guala, A.; Teixido-Tura, G.; Calvo-Iglesias, F.; Sevilla, T.; Bermejo, J.; Méndez, I.; Sánchez, V.; Carmona, J.M.R.; et al. Atorvastatin Effect on Aortic Dilatation and Valvular Calcification Progression in Bicuspid Aortic Valve (BICATOR): A Randomized Clinical Trial. Circulation 2024, 149, 1938–1948. [Google Scholar] [CrossRef]
- Bruls, S.; Musumeci, L.; Courtois, A.; Hustinx, R.; Sakalihasan, S.; Namur, G.; Defraigne, J.-O.; Sakalihasan, N. Can Biomarkers and PET Imaging Predict Abdominal Aortic Aneurysm Growth Rate? J. Clin. Med. 2024, 13, 2448. [Google Scholar] [CrossRef]
- Nakahara, T.; Miyazawa, R.; Iwabuchi, Y.; Tonda, K.; Narula, N.; Strauss, H.W.; Narula, J.; Jinzaki, M. Aortic Uptake of 18 F-NaF and 18 F-FDG and Calcification Predict the Development of Abdominal Aortic Aneurysms and Is Attenuated by Drug Therapy. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1975–1985. [Google Scholar] [CrossRef]
- Robson, P.M.; Dey, D.; Newby, D.E.; Berman, D.; Li, D.; Fayad, Z.A.; Dweck, M.R. MR/PET Imaging of the Cardiovascular System. JACC Cardiovasc. Imaging 2017, 10, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Gandhi, R.; Shawer, H.; Tsoumpas, C.; Bailey, M.A. Imaging Biological Pathways in Abdominal Aortic Aneurysms Using Positron Emission Tomography. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; Sánchez-González, J.; Macías, A.; Pérez-Asenjo, B.; Zamudio, D.; Alonso-Farto, J.C.; España, S.; et al. Vascular Inflammation in Subclinical Atherosclerosis Detected by Hybrid PET/MRI. J. Am. Coll. Cardiol. 2019, 73, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef]
- Lee, H.; Paeng, J.C.; Kim, K.H.; Cheon, G.J.; Lee, D.S.; Chung, J.-K.; Kang, K.W. Correlation of FDG PET/CT Findings with Long-Term Growth and Clinical Course of Abdominal Aortic Aneurysm. Nucl. Med. Mol. Imaging 2018, 52, 46–52. [Google Scholar] [CrossRef]
- Tegler, G.; Ericson, K.; Sörensen, J.; Björck, M.; Wanhainen, A. Inflammation in the walls of asymptomatic abdominal aortic aneurysms is not associated with increased metabolic activity detectable by 18-fluorodeoxglucose positron-emission tomography. J. Vasc. Surg. 2012, 56, 802–807. [Google Scholar] [CrossRef]
- Courtois, A.; Nusgens, B.V.; Hustinx, R.; Namur, G.; Gomez, P.; Somja, J.; Defraigne, J.-O.; Delvenne, P.; Michel, J.-B.; Colige, A.C.; et al. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J. Nucl. Med. 2013, 54, 1740–1747. [Google Scholar] [CrossRef]
- Yoder, K.K. Basic PET Data Analysis Techniques. In Positron Emission Tomography—Recent Developments in Instrumentation, Research and Clinical Oncological Practice; Misciagna, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Dashora, H.R.; Rosenblum, J.S.; Quinn, K.A.; Alessi, H.; Novakovich, E.; Saboury, B.; Ahlman, M.A.; Grayson, P.C. Comparing Semiquantitative and Qualitative Methods of Vascular 18F-FDG PET Activity Measurement in Large-Vessel Vasculitis. J. Nucl. Med. 2022, 63, 280–286. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Chareonthaitawee, P.; Treglia, G.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Boellaard, R.; Bucerius, J.; Carril, J.M.; et al. FDG-PET/CT (A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 47, 1250–1269. [Google Scholar] [CrossRef]
- McBride, O.M.B.; Joshi, N.; Robson, J.; MacGillivray, T.; Gray, C.; Fletcher, A.; Dweck, M.; van Beek, E.; Rudd, J.; Newby, D.; et al. Positron Emission Tomography and Magnetic Resonance Imaging of Cellular Inflammation in Patients with Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 518–526. [Google Scholar] [CrossRef]
- Minderhoud, S.C.S.; Fletcher, A.J.; MacNaught, G.; Cadet, S.; Korteland, S.-A.; Kardys, I.; Rizopoulos, D.; Slomka, P.; Newby, D.E.; Roos-Hesselink, J.W.; et al. Vascular biomechanics and molecular disease activity in the thoracic aorta: A novel imaging method. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Kadian-Dodov, D.; Seo, P.; Robson, P.M.; Fayad, Z.A.; Olin, J.W. Inflammatory Diseases of the Aorta: JACC Focus Seminar, Part 2. J. Am. Coll. Cardiol. 2022, 80, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Loecher, M.; Schrauben, E.; Johnson, K.M.; Wieben, O. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J. Magn. Reson. Imaging 2016, 43, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.B.; Sheen, H.; Lee, H.Y.; Kang, J.; Yoon, D.K.; Suh, T.S. Digital Imaging and Communications in Medicine (DICOM) information conversion procedure for SUV calculation of PET scanners with different DICOM header information. Phys. Medica 2017, 44, 243–248. [Google Scholar] [CrossRef][Green Version]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Robson, P.M.; Kaufman, A.; Pruzan, A.; Dweck, M.R.; Trivieri, M.-G.; Abgral, R.; Karakatsanis, N.A.; Brunner, P.M.; Guttman, E.; Fayad, Z.A.; et al. Scan-rescan measurement repeatability of 18F-FDG PET/MR imaging of vascular inflammation. J. Nucl. Cardiol. 2022, 29, 1660–1670. [Google Scholar] [CrossRef]
- Laurent, C.; Ricard, L.; Fain, O.; Buvat, I.; Adedjouma, A.; Soussan, M.; Mekinian, A. PET/MRI in large-vessel vasculitis: Clinical value for diagnosis and assessment of disease activity. Sci. Rep. 2019, 9, 12388. [Google Scholar] [CrossRef]
- Kuzniar, M.; Tegler, G.; Wanhainen, A.; Ahlström, H.; Mani, K.; Hansen, T. Feasibility of Assessing Inflammation in Asymptomatic Abdominal Aortic Aneurysms with Integrated 18F-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 464–471. [Google Scholar] [CrossRef]
- Mulorz, J.; Mazrekaj, A.; Sehl, J.; Arnautovic, A.; Garabet, W.; Krott, K.-J.; Schelzig, H.; Elvers, M.; Wagenhäuser, M.U. Relative Thrombus Burden Ratio Reveals Overproportioned Intraluminal Thrombus Growth—Potential Implications for Abdominal Aortic Aneurysm. J. Clin. Med. 2024, 13, 962. [Google Scholar] [CrossRef]
- Huang, Y.; Teng, Z.; Elkhawad, M.; Tarkin, J.M.; Joshi, N.; Boyle, J.R.; Buscombe, J.R.; Fryer, T.D.; Zhang, Y.; Park, A.Y.; et al. High Structural Stress and Presence of Intraluminal Thrombus Predict Abdominal Aortic Aneurysm 18F-FDG Uptake: Insights from Biomechanics. Circ. Cardiovasc. Imaging 2016, 9, e004656. [Google Scholar] [CrossRef]
| Characteristic | n = 82 |
|---|---|
| Age (years) | 66 [53–73] |
| Male sex (n (%)) | 67 (81.7%) |
| Body surface area (m2) | 1.9 [1.8–2.1] |
| Aortic valve (n (%)) | |
| Bicuspid aortic valve | 39 (47.6%) |
| Tricuspid aortic valve | 43 (52.4%) |
| Aneurysm location (n (%)) | |
| Aortic root | 1 (1.2%) |
| Thoracic ascending | 43 (52.5%) |
| Thoracic descending | 2 (2.4%) |
| Abdominal | 36 (43.9%) |
| Thrombus (n (%)) | 29 (35.4%) |
| Thickness | 2 [mm] | 4 [mm] | 6 [mm] | 8 [mm] | 10 [mm] | |
|---|---|---|---|---|---|---|
| Interobserver reproducibility | ||||||
| ICC | Median | 0.959 [0.955, 0.962] | 0.960 [0.958, 0.963] | 0.964 [0.962, 0.965] | 0.964 [0.963, 0.966] | 0.966 [0.965, 0.968] |
| 95th percentile | 0.950 [0.946, 0.955] | 0.953 [0.949, 0.956] | 0.958 [0.956, 0.960] | 0.959 [0.957, 0.961] | 0.962 [0.960, 0.963] | |
| Validation with manual assessment | ||||||
| ICC | Maximum | 0.848 [0.803, 0.882] | 0.852 [0.803, 0.888] | 0.836 [0.774, 0.879] | 0.810 [0.739, 0.860] | 0.755 [0.669, 0.817] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragulat-Arévalo, M.; Ferrer-Cornet, M.; Dux-Santoy, L.; Oliveró-Soldevila, R.; Garcia-Reyes, M.; Teixidó-Turà, G.; Garrido-Oliver, J.; Galian-Gay, L.; Lopez-Gutierrez, P.; Catalá-Santarrufina, A.; et al. Mapping 18F-FDG Positron Emission Tomography Uptake in the Aortic Wall and Thrombus: Validation and Reproducibility. Appl. Sci. 2025, 15, 10685. https://doi.org/10.3390/app151910685
Bragulat-Arévalo M, Ferrer-Cornet M, Dux-Santoy L, Oliveró-Soldevila R, Garcia-Reyes M, Teixidó-Turà G, Garrido-Oliver J, Galian-Gay L, Lopez-Gutierrez P, Catalá-Santarrufina A, et al. Mapping 18F-FDG Positron Emission Tomography Uptake in the Aortic Wall and Thrombus: Validation and Reproducibility. Applied Sciences. 2025; 15(19):10685. https://doi.org/10.3390/app151910685
Chicago/Turabian StyleBragulat-Arévalo, Mireia, Marta Ferrer-Cornet, Lydia Dux-Santoy, Ruper Oliveró-Soldevila, Marvin Garcia-Reyes, Gisela Teixidó-Turà, Juan Garrido-Oliver, Laura Galian-Gay, Pere Lopez-Gutierrez, Alba Catalá-Santarrufina, and et al. 2025. "Mapping 18F-FDG Positron Emission Tomography Uptake in the Aortic Wall and Thrombus: Validation and Reproducibility" Applied Sciences 15, no. 19: 10685. https://doi.org/10.3390/app151910685
APA StyleBragulat-Arévalo, M., Ferrer-Cornet, M., Dux-Santoy, L., Oliveró-Soldevila, R., Garcia-Reyes, M., Teixidó-Turà, G., Garrido-Oliver, J., Galian-Gay, L., Lopez-Gutierrez, P., Catalá-Santarrufina, A., García-Garzón, J. R., Martinez-Esquerda, N., Solsona, J., Ferreira-González, I., Bellmunt-Montoya, S., Rodriguez-Palomares, J., & Guala, A. (2025). Mapping 18F-FDG Positron Emission Tomography Uptake in the Aortic Wall and Thrombus: Validation and Reproducibility. Applied Sciences, 15(19), 10685. https://doi.org/10.3390/app151910685





