Incorporation of Protein Alternatives in Bakery Products: Biological Value and Techno-Functional Properties
Abstract
1. Introduction
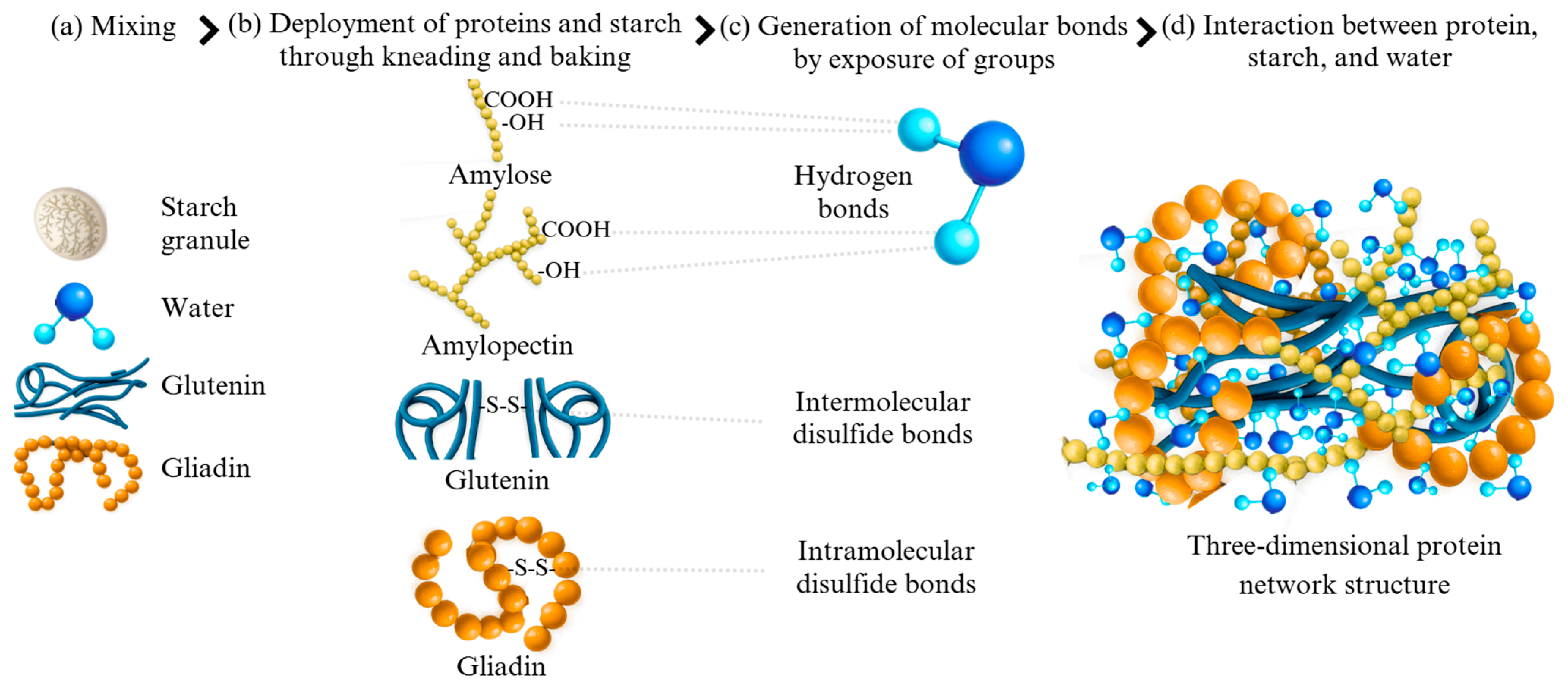
2. Role of Proteins in Bakery Products
3. Techno-Functional Properties of Wheat Proteins
4. Alternative Protein Sources in Bakery Products
4.1. Cereals
4.1.1. Oat
4.1.2. Rye
4.1.3. Rice
4.1.4. Quinoa
4.2. Legumes
4.3. Oilseeds
5. Other Protein Sources
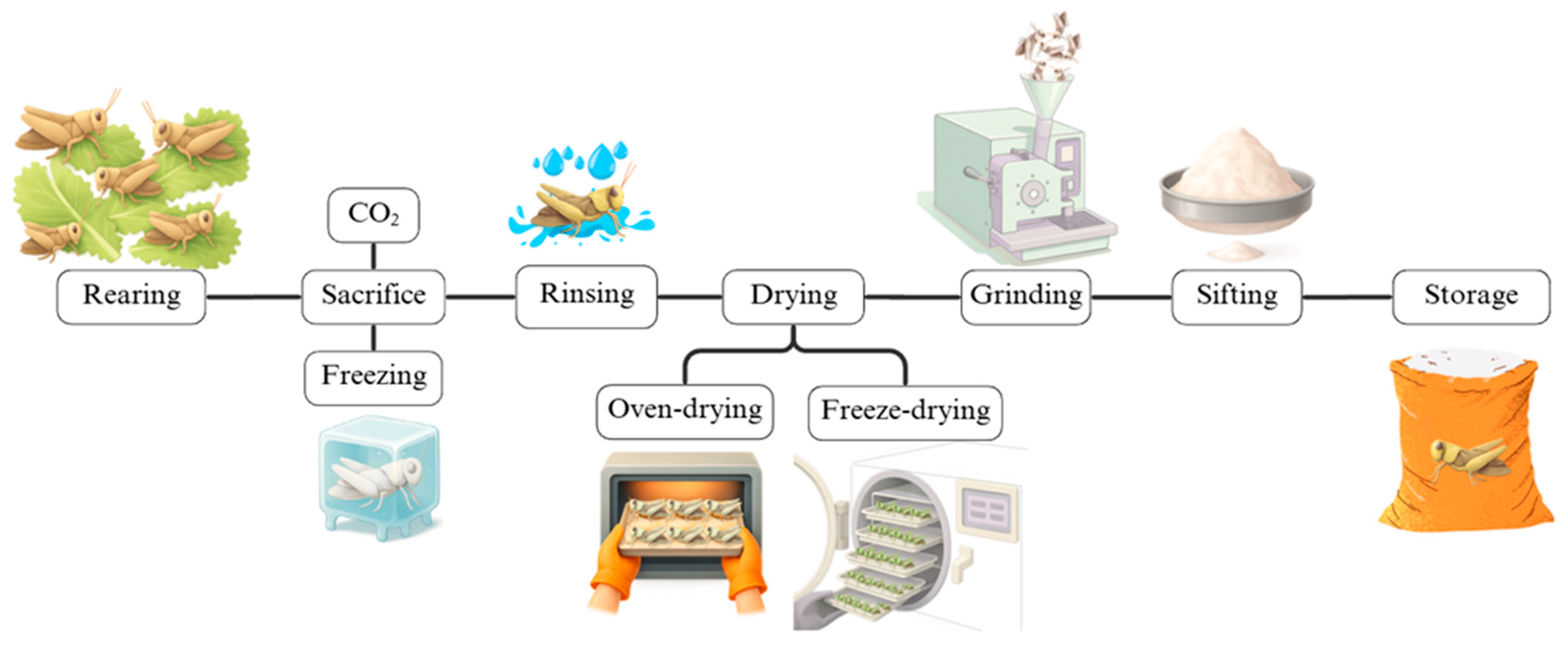
5.1. Insects
5.2. Cheese Whey
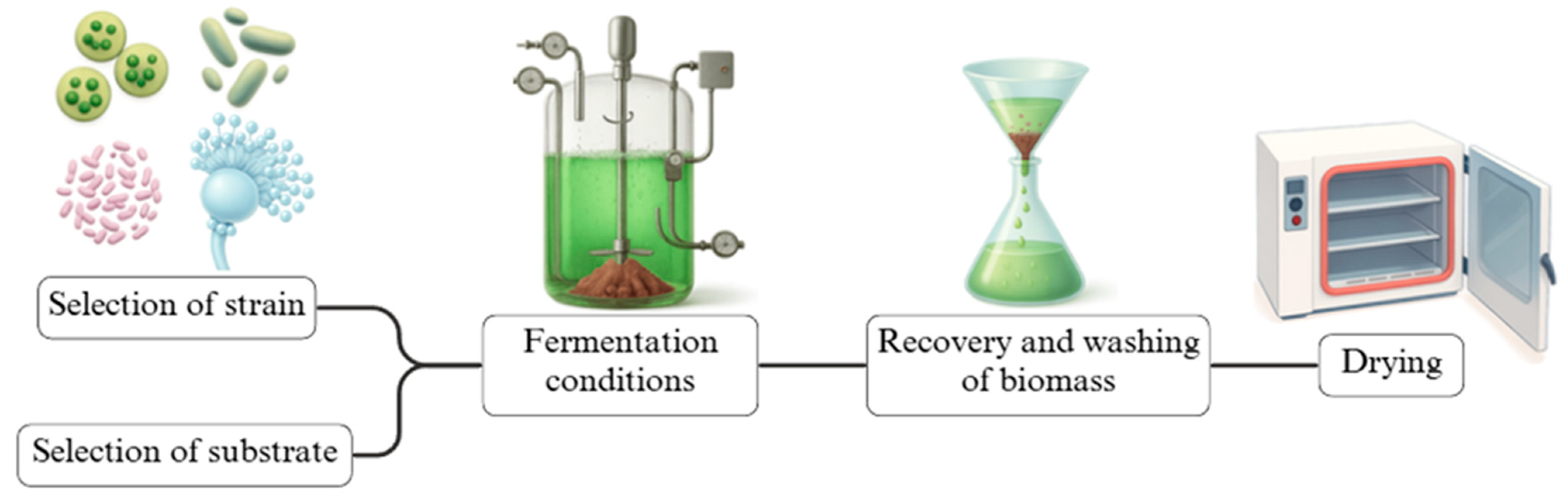
5.3. Single-Cell Protein
6. Challenges in the Use of Alternative Protein Ingredients
7. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2019, 10, 541–563. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Ribeiro, S.F.; Pereira, J.C.V.; Antunes, A.E.C.; Bezerra, R.M.N.; da Cunha, D.T. Consumer perceptions of functional foods: A scoping review focusing on non-processed foods. Food Rev. Int. 2025, 41, 1738–1756. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Varzakas, T. High protein substitutes for gluten in gluten-free bread. Foods 2021, 10, 1997. [Google Scholar] [CrossRef]
- Hajzer, Z.E.; Alibrahem, W.; Kharrat Helu, N.; Oláh, C.; Prokisch, J. Functional foods in clinical trials and future research directions. Foods 2025, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Casolani, N.; Ruggeri, M.; Spizzirri, U.G.; Aiello, F.; Chiodo, E.; Martuscelli, M.; Restuccia, D.; Mastrocola, D. Sensory evaluation and consumers’ acceptance of a low glycemic and gluten-free carob-based bakery product. Foods 2024, 13, 2815. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer acceptance toward functional foods: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Yuan, X.; Zhong, M.; Huang, X.; Hussain, Z.; Ren, M.; Xie, X. Industrial production of functional foods for human health and sustainability. Foods 2024, 13, 3546. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Physicochemical properties and consumer acceptance of bread enriched with alternative proteins. Foods 2020, 9, 933. [Google Scholar] [CrossRef]
- Maleky, F.; Ahmadi, L. Adhering to recommended dietary protein intake for optimizing human health benefits versus exceeding levels. RSC Adv. 2025, 15, 9230–9242. [Google Scholar] [CrossRef]
- Nunes, E.A.; Colenso-Semple, L.; McKellar, S.R.; Yau, T.; Ali, M.U.; Fitzpatrick-Lewis, D.; Sherifali, D.; Gaudichon, C.; Tomé, D.; Atherton, P.J.; et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J. Cachexia Sarcopenia Muscle 2022, 13, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.J.; Arentson-Lantz, E.J.; Moughan, P.J.; Wolfe, R.R.; Ferrando, A.A.; Church, D.D. Understanding dietary protein quality: Digestible indispensable amino acid scores and beyond. J. Nutr. 2025, 155, 4060–4072. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Church, D.D.; Ferrando, A.A.; Moughan, P.J. Consideration of the role of protein quality in determining dietary protein recommendations. Front. Nutr. 2024, 11, 1389664. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat quality: A review on chemical composition, nutritional attributes, grain anatomy, types, classification, and function of seed storage proteins in bread making quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, essential amino acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK557845/ (accessed on 7 September 2025).
- Iqbal, M.J.; Shams, N.; Fatima, K. Nutritional Quality of Wheat; En IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Prieto-Vázquez del Mercado, P.; Mojica, L.; Morales-Hernández, N. Protein ingredients in bread: Technological, textural and health implications. Foods 2022, 11, 2399. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Mahmud, M.M.C.; Abdi, G.; Wanich, U.; Farooqi, M.Q.U.; Settapramote, N.; Khan, S.; Wani, S.A. New alternatives from sustainable sources to wheat in bakery foods: Science, technology, and challenges. J. Food Biochem. 2022, 46, e14185. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Guillou, L.; Durand, V.; Raymond, M.; Berticat, C. Chronic refined carbohydrate consumption measured by glycemic load and variation in cognitive performance in healthy people. Pers. Individ. Dif. 2023, 206, 112138. [Google Scholar] [CrossRef]
- Bajka, B.H.; Pinto, A.M.; Ahn-Jarvis, J.; Ryden, P.; Perez-Moral, N.; van der Schoot, A.; Stocchi, C.; Bland, C.; Berry, S.E.; Ellis, P.R.; et al. The impact of replacing wheat flour with cellular legume powder on starch bioaccessibility, glycaemic response and bread roll quality: A double-blind randomised controlled trial in healthy participants. Food Hydrocoll. 2021, 114, 106565. [Google Scholar] [CrossRef]
- Pasqualoni, I.; Tolve, R.; Simonato, B.; Bianchi, F. The impact of selected ingredients on the predicted glycemic index and technological properties of bread. Foods 2024, 13, 2488. [Google Scholar] [CrossRef]
- Mulla, M.F.Z.; Mullins, E.; Lynch, R.; Gallagher, E. A mixture design approach to investigate the impact of raw and processed chickpea flour on the techno-functional properties in a bakery application. LWT 2025, 229, 118206. [Google Scholar] [CrossRef]
- Samilyk, M.; Nahornyi, Y.; Marenkova, T.; Bokovets, S. Influence of enriched ingredients on the functional properties and nutritional value of bread. Technol. Audit Prod. Reserv. 2025, 3, 63–68. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- and animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef] [PubMed]
- Ribet, L.; Kassis, A.; Jacquier, E.; Monnet, C.; Durand-Dubief, M.; Bosco, N. The nutritional contribution and relationship with health of bread consumption: A narrative review. Crit. Rev. Food Sci. Nutr. 2024, 1–28. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Ficco, D.B.M. Cereals and cereal-based foods: Nutritional, phytochemical characterization and processing technologies. Foods 2025, 14, 1234. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Bunge, A.C.; Mazac, R.; Clark, M.; Wood, A.; Gordon, L. Sustainability benefits of transitioning from current diets to plant-based alternatives or whole-food diets in Sweden. Nat. Commun. 2024, 15, 951. [Google Scholar] [CrossRef]
- Monnet, A.F.; Laleg, K.; Michon, C.; Micard, V. Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019, 86, 131–143. [Google Scholar] [CrossRef]
- Naveed, H.; Sultan, W.; Awan, K.A.; Imtiaz, A.; Yaqoob, S.; Al-Asmari, F.; Faraz, A.; Qian, J.Y.; Sharma, A.; Mugabi, R.; et al. Glycemic impact of cereal and legume-based bakery products: Implications for chronic disease management. Food Chem. X 2024, 24, 101959. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Naik, H.R.; Zargar, I.A. Influence of hydrocolloids on dough handling and technological properties of gluten-free breads. Trends Food Sci. Technol. 2016, 51, 49–57. [Google Scholar] [CrossRef]
- Imam, Y.T.; Irondi, E.A.; Awoyale, W.; Ajani, E.O.; Alamu, E.O. Application of legumes in the formulation of gluten-free foods: Functional, nutritional and nutraceutical importance. Front. Sustain. Food Syst. 2024, 8, 1251760. [Google Scholar] [CrossRef]
- Anton, A.A.; Ross, K.A.; Lukow, O.M.; Fulcher, R.G.; Arntfield, S.D. Influence of added bean flour (Phaseolus vulgaris L.) on some physical and nutritional properties of wheat flour tortillas. Food Chem. 2008, 109, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- George, E.S.; Daly, R.M.; Tey, S.L.; Brown, R.; Wong, T.H.T.; Tan, S.Y. Perspective: Is it time to expand research on “nuts” to include “seeds”? Justifications and key considerations. Adv. Nutr. 2022, 13, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gaur, S. Edible seeds as pioneers in the preparation of modern bakery products: Challenges and recent applications. Food Chem. Adv. 2024, 4, 100684. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Dodevska, M.; Kukic Markovic, J.; Sofrenic, I.; Tesevic, V.; Jankovic, M.; Djordjevic, B.; Ivanovic, N.D. Similarities and differences in the nutritional composition of nuts and seeds in Serbia. Front. Nutr. 2022, 9, 1003125. [Google Scholar] [CrossRef]
- Guan, Q.H.; Qian, S.; Chen, L.; Feng, X.C. Enhancing the nutritional value of bread by the addition of insect powder: A novel class of food protein additives. J. Insects Food Feed. 2024, 10, 1097–1108. [Google Scholar] [CrossRef]
- Tilami, S.K.; Jurkaninová, L.; Kotíková, Z.; Škvorová, P.; Ferusová, Ž.; Kouřimská, L. Quality and nutritional evaluation of bakery products enriched with cricket meal. Appl. Food Res. 2025, 5, 101303. [Google Scholar] [CrossRef]
- Xie, X.; Yuan, Z.; Fu, K.; An, J.; Deng, L. Effect of partial substitution of flour with mealworm (Tenebrio molitor L.) powder on dough and biscuit properties. Foods 2022, 11, 2156. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Ayu, A.M.; Syiffah, L.; Muthia, H.; Amalina, F.A.; Afifah, D.N.; Nindita, Y.; Maharani, N.; Widyastuti, N.; Anjani, G.; et al. Physicochemical and sensory properties of cookies with cricket powder as an alternative snack to prevent iron deficiency anemia and chronic energy deficiency. Appl. Food Res. 2024, 4, 100485. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Kuang, H.; Tang, C.; Song, J. Edible insects as ingredients in food products: Nutrition, functional properties, allergenicity of insect proteins, and processing modifications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10361–10383. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food safety issues related to uses of insects for feeds and foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Fluerasu, D.; Neagu, C.; Dossa, S.; Negrea, M.; Jianu, C.; Berbecea, A.; Stoin, D.; Lalescu, D.; Brezovan, D.; Cseh, L.; et al. The use of whey powder to improve bread quality: A sustainable solution for utilizing dairy by-products. Foods 2025, 14, 2911. [Google Scholar] [CrossRef]
- Verma, D.K.; Patel, A.R.; Tripathy, S.; Gupta, A.K.; Singh, S.; Shah, N.; Utama, G.L.; Chávez-González, M.L.; Zongo, K.; Banwo, K.; et al. Processing and formulation technology of nutritional and functional food products by utilizing cheese and/or paneer whey: A critical review. J. King Saud. Univ. Sci. 2024, 36, 103508. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Mota, J.; Lameirão, D.; Teixeira, A.F.; Saraiva, C.; Romero-Rodríguez, M.Á.; Vilela, A.; Gonçalves, C. Multidimensional evaluation of local rye bread enriched with whey as a model for food waste valorisation: From recipe development to consumer acceptance. Sustainability 2025, 17, 6710. [Google Scholar] [CrossRef]
- El-Azab, H. Getting the dried whey protein concentrate and its effect on pan bread evaluation. Ann. Agric. Sci. Moshtohor. 2019, 57, 383–394. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- Achalu, B.I.; Tessema, H.A.; Ayele, A.T. Development and characterization of gluten-free bread enriched with whey protein from sorghum-rice composite flours. Appl. Food Res. 2025, 5, 101133. [Google Scholar] [CrossRef]
- Cappelli, R.; Ottomano Palmisano, G.; Roma, R.; Caponio, F.; Difonzo, G.; De Boni, A. Functional foods acceptability: A consumers’ survey on bread enriched with oenological by-products. Foods 2023, 12, 2014. [Google Scholar] [CrossRef]
- Cappelli, A.; Lupori, L.; Cini, E. Baking technology: A systematic review of machines and plants and their effect on final products, including improvement strategies. Trends Food Sci. Technol. 2021, 115, 275–284. [Google Scholar] [CrossRef]
- Venturi, M.; Cappelli, A.; Pini, N.; Galli, V.; Lupori, L.; Granchi, L.; Cini, E. Effects of kneading machine type and total element revolutions on dough rheology and bread characteristics: A focus on straight dough and indirect (biga) methods. LWT 2022, 153, 112500. [Google Scholar] [CrossRef]
- Abedi, E.; Pourmohammadi, K. Physical modifications of wheat gluten protein: An extensive review. J. Food Process Eng. 2021, 44, e13619. [Google Scholar] [CrossRef]
- Dufour, M.; Chaunier, L.; Lourdin, D.; Réguerre, A.L.; Hugon, F.; Dugué, A.; Kansou, K.; Saulnier, L.; Della Valle, G. Unravelling the relationships between wheat dough extensional properties, gluten network and water distribution. Food Hydrocoll. 2024, 146, 109214. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zhang, Z.; Meng, X.; Yang, T.; Shi, W.; He, R.; Ma, H. Insights into ultrasonic treatment of cereal protein characteristics: Functionality, conformational, and physicochemical properties. Foods 2023, 12, 971. [Google Scholar] [CrossRef]
- Avelar, Z.; Pereira, R.N.; Vicente, A.A.; Rodrigues, R.M. Protein quality of cereals: Technological and functional perspectives. J. Cereal Sci. 2024, 117, 103922. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S. Food proteins: Solubility & thermal stability improvement techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar] [CrossRef]
- Scott, G.; Awika, J.M. Effect of protein-starch interactions on starch retrogradation and implications for food product quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef]
- Gasparre, N.; Rosell, C.M. Wheat gluten: A functional protein still challenging to replace in gluten-free cereal-based foods. Cereal Chem. 2022, 99, 1021–1031. [Google Scholar] [CrossRef]
- Chen, Y.; Gavaliatsis, T.; Kuster, S.; Städeli, C.; Fischer, P.; Windhab, E.J. Crust treatments to reduce bread staling. Curr. Res. Food Sci. 2021, 4, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Karboune, S. A review of bread qualities and current strategies for bread bioprotection: Flavor, sensory, rheological, and textural attributes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1937–1981. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal. Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of physical and chemical factors on the structure of gluten, gliadins, and glutenins studied using spectroscopic methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef]
- Zang, P.; Gao, Y.; Chen, P.; Lv, C.; Zhao, G. Recent advances in the study of wheat protein and other food components affecting gluten network and noodle properties. Foods 2022, 11, 3824. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, R.; Ma, M.; Yang, T.; Sun, Q.; Li, M. Versatile wheat gluten: Functional properties and application in the food-related industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 10444–10460. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Gałka, B.; Kamińska, J.; Jama-Rodzeńska, A.; Król, Z.; Jarki, D.; Fuchs, B. Diversity of chemical composition and nutritional value in grain from selected winter wheat cultivars grown in south-western Poland. Sci. Rep. 2024, 14, 2630. [Google Scholar] [CrossRef]
- Singh, K.; Anjali, N.; Rajput, R. Nutritional enhancement of baked goods using alternative flours: A review. Eur. J. Nutr. Food Saf. 2025, 17, 240–252. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Nigro, G.; Gasparre, N.; Vurro, F.; Pasqualone, A.; Rosell, C.M. Lupin flour as a substitute for wheat in conventional and sourdough bread making: Impact on the physicochemical properties and volatile profile of bread. Eur Food Res Technol. 2025, 251, 1145–1156. [Google Scholar] [CrossRef]
- Rafique, H.; Dong, R.; Wang, X.; Alim, A.; Aadil, R.M.; Li, L.; Zou, L.; Hu, X. Dietary-nutraceutical properties of oat protein and peptides. Front. Nutr. 2022, 9, 950400. [Google Scholar] [CrossRef] [PubMed]
- Holopainen-Mantila, U.; Vanhatalo, S.; Lehtinen, P.; Sozer, N. Oats as a source of nutritious alternative protein. J. Cereal Sci. 2024, 116, 103862. [Google Scholar] [CrossRef]
- Tomičić, Z.M.; Pezo, L.L.; Spasevski, N.J.; Lazarević, J.M.; Čabarkapa, I.S.; Tomičić, R.M. Diversity of amino acids composition in cereals. Food Feed Res. 2022, 49, 12. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M. Seed composition and amino acid profiles for quinoa grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef]
- Escuredo, O.; González Martín, M.I.; Wells Moncada, G.; Fischer, S.; Hernández Hierro, J.M. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J. Cereal Sci. 2014, 60, 67–74. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Effects of processing on the protein quality and functional properties of cold-pressed pumpkin seed meal. Food Res. Int. 2023, 169, 112876. [Google Scholar] [CrossRef]
- Añazco, C.; Ojeda, P.G.; Guerrero-Wyss, M. Common beans as a source of amino acids and cofactors for collagen biosynthesis. Nutrients 2023, 15, 4561. [Google Scholar] [CrossRef]
- Yegrem, L. Nutritional composition, antinutritional factors, and utilization trends of Ethiopian chickpea (Cicer arietinum L.). Int J. Food Sci. 2021, 2021, 5570753. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; McClements, D.J.; Pignitter, M.; Hadidi, M. A comprehensive review of processing, functionality, and potential applications of lentil proteins in the food industry. Adv. Colloid Interface Sci. 2024, 333, 103280. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Luparelli, A.; Trisciuzzi, D.; Schirinzi, W.M.; Caputo, L.; Smiriglia, L.; Quintieri, L.; Nicolotti, O.; Monaci, L. Whey proteins and bioactive peptides: Advances in production, selection and bioactivity profiling. Biomedicines 2025, 13, 1311. [Google Scholar] [CrossRef]
- Almeida, C.C.; Alvares, T.S.; Costa, M.P.; Conte-Junior, C.A. Protein and amino acid profiles of different whey protein supplements. J. Diet Suppl. 2016, 13, 313–323. [Google Scholar] [CrossRef]
- Nachtigall, L.; Grune, T.; Weber, D. Proteins and amino acids from edible insects for the human diet—A narrative review considering environmental sustainability and regulatory challenges. Nutrients 2025, 17, 1245. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.A.; Pereira, S.M.S.; Dias, K.A.; Paes Sda, S.; Grancieri, M.; Jimenez, L.G.S.; De Carvalho, C.W.P.; De Oliveira, E.E.; Martino, H.S.D.; Della Lucia, C.M. Nutritional content, amino acid profile, and protein properties of edible insects (Tenebrio molitor and Gryllus assimilis) powders at different stages of development. J. Food Compost Anal. 2024, 125, 105804. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible insects as a food source: A review. Food Prod. Process Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Malla, N.; Nørgaard, J.V.; Roos, N. Protein quality of edible insects in the view of current assessment methods. Anim. Front. 2023, 13, 50–63. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, C. Sustainable plant-based ingredients as wheat flour substitutes in bread making. NPJ Sci. Food. 2022, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Mel, R.; Malalgoda, M. Oat protein as a novel protein ingredient: Structure, functionality, and factors impacting utilization. Cereal Chem. 2022, 99, 21–36. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat proteins: A perspective on functional properties. LWT 2021, 152, 112307. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Noor, R.A.; Imran, A.; Afzaal, M.; Rasheed, A.; Islam, F.; Iqbal, A.; Zahoor, T.; Naz, S.; et al. A comprehensive review on biochemical and technological properties of rye (Secale cereale L.). Int. J. Food Prop. 2023, 26, 2212–2228. [Google Scholar] [CrossRef]
- Eriksen, A.K.; Brunius, C.; Mazidi, M.; Hellström, P.M.; Risérus, U.; Iversen, K.N.; Fristedt, R.; Sun, L.; Huang, Y.; Nørskov, N.P.; et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: A randomized crossover trial. Am. J. Clin. Nutr. 2020, 111, 864–876. [Google Scholar] [CrossRef]
- Detchewa, P.; Prasajak, P.; Phungamngoen, C.; Sriwichai, W.; Naivikul, O.; Moongngarm, A. Substitution of rice flour with rice protein improved quality of gluten-free rice spaghetti processed using single screw extrusion. LWT 2022, 153, 112512. [Google Scholar] [CrossRef]
- El-Sohaimy, A.S.; Shehata, G.M.; Djapparovec, T.A.; Mehany, T.; Zeitoun, A.M.; Zeitoun, M.A. Development and characterization of functional pan bread supplemented with quinoa flour. J. Food Process Preserv. 2021, 45, e15180. [Google Scholar] [CrossRef]
- Coţovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of quinoa seed fractions and their application in wheat bread production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef]
- Martínez, E.; Álvarez-Ortí, M.; Rabadán, A.; Millán, C.; Pardo, J.E. Elaboration of cookies using oils and flours from seeds and nuts: Effects on technological, nutritional and consumer aspects. Foods 2022, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of nuts and their potential health benefits—An overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- Ozón, B.; Cotabarren, J.; Geier, F.R.; Kise, M.P.; García-Pardo, J.; Parisi, M.G.; Obregón, W.D. Development of fortified breads enriched with plant-based bioactive peptides derived from the chia (Salvia hispanica L.) expeller. Foods 2023, 12, 3382. [Google Scholar] [CrossRef]
- Olombrada, E.; Mesias, M.; Morales, F.J. Risk/benefits of the use of chia, quinoa, sesame and flax seeds in bakery products: An update review. Food Rev. Int. 2024, 40, 1047–1068. [Google Scholar] [CrossRef]
- Sandri, L.T.B.; Santos, F.G.; Fratelli, C.; Capriles, V.D. Development of gluten-free bread formulations containing whole chia flour with acceptable sensory properties. Food Sci. Nutr. 2017, 5, 1021–1028. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; et al. A comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar] [CrossRef]
- Zorzi, C.Z.; Garske, R.P.; Flôres, S.H.; Thys, R.C.S. Sunflower protein concentrate: A possible and beneficial ingredient for gluten-free bread. Innov. Food Sci. Emerg. Technol. 2020, 66, 102539. [Google Scholar] [CrossRef]
- Papagianni, E.; Kotsiou, K.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Development of “clean label” gluten-free breads fortified with flaxseed slurry and sesame cake: Implications on batter rheology, bread quality and shelf life. Food Hydrocoll. 2024, 150, 109734. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Canale, M.; Dugo, G.; Oliveri, C.; Scarangella, M.C.; Amenta, M.; Crupi, A.; Spina, A. Effects of partial replacement of durum wheat re-milled semolina with bean flour on physico-chemical and technological features of doughs and breads during storage. Plants 2023, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Sparvoli, F.; Laureati, M.; Pilu, R.; Pagliarini, E.; Toschi, I.; Giuberti, G.; Fortunati, P.; Daminati, M.G.; Cominelli, E.; Bollini, R. Exploitation of common bean flours with low antinutrient content for making nutritionally enhanced biscuits. Front. Plant Sci. 2016, 7, 928. [Google Scholar] [CrossRef]
- Shrivastava, C.; Chakraborty, S. Bread from wheat flour partially replaced by fermented chickpea flour: Optimizing the formulation and fuzzy analysis of sensory data. LWT 2018, 90, 215–223. [Google Scholar] [CrossRef]
- Xing, Q.; Kyriakopoulou, K.; Zhang, L.; Boom, R.M.; Schutyser, M.A.I. Protein fortification of wheat bread using dry fractionated chickpea protein-enriched fraction or its sourdough. LWT 2021, 142, 110931. [Google Scholar] [CrossRef]
- Starkute, V.; Bartkiene, E.; Mockus, E.; Rocha, J.M.; Cernauskas, D.; Mozuriene, E.; Ruibys, R.; Orhun, G.E.; Klupsaite, D. Green lentil fortification of wheat bread: A strategy for quality improvement and acrylamide reduction. Food Chem. X 2025, 30, 102900. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, K.; Palassaros, G.; Matsakidou, A.; Mouzakitis, C.; Biliaderis, C.G.; Lazaridou, A. Roasted-sprouted lentil flour as a novel ingredient for wheat flour substitution in breads: Impact on dough properties and quality attributes. Food Hydrocoll. 2023, 145, 109164. [Google Scholar] [CrossRef]
- Ni, Q.; Ranawana, V.; Hayes, H.E.; Hayward, N.J.; Stead, D.; Raikos, V. Addition of broad bean hull to wheat flour for the development of high-fiber bread: Effects on physical and nutritional properties. Foods 2020, 9, 1192. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, N. Development and sensory evaluation of value-added bakery products developed from germinated soybean (Glycine max) varieties. J. Appl. Nat. Sci. 2019, 11, 211–216. [Google Scholar] [CrossRef]
- Ibadullah, W.Z.W.; Mokhtar, F.; Abedin, N.H.Z.; Rashid, N.-K.M.A.; Ibrahim, N.H.; Mustapha, N.A. Okara-enriched wheat bread: Effect on nutritional, physicochemical, and sensory properties. J. Biochem. Microbiol. Biotechnol. 2024, 12, 110–113. [Google Scholar] [CrossRef]
- Paul, S.; Kulkarni, S.; Chauhan, R.N. Utilization of whey in bakery products—A review. Indian J. Dairy Sci. 2022, 75. Available online: https://epubs.icar.org.in/index.php/IJDS/article/view/101108 (accessed on 14 May 2025). [CrossRef]
- Carter, B.G.; Cheng, N.; Kapoor, R.; Meletharayil, G.H.; Drake, M.A. Invited review: Microfiltration-derived casein and whey proteins from milk. J. Dairy Sci. 2021, 104, 2465–2479. [Google Scholar] [CrossRef]
- Komeroski, M.R.; Homem, R.V.; Schmidt, H.O.; Rockett, F.C.; de Lira, L.; Vitória da Farias, D.; Kist, T.L.; Doneda, D.; De o Rios, A.; De Oliveira, V.R. Effect of whey protein and mixed flours on the quality parameters of gluten-free breads. Int. J. Gastron. Food Sci. 2021, 24, 100361. [Google Scholar] [CrossRef]
- Balivo, A.; d’Errico, G.; Genovese, A. Sensory properties of foods functionalised with milk proteins. Food Hydrocoll. 2024, 147, 109301. [Google Scholar] [CrossRef]
- Borges, M.M.; da Costa, D.V.; Trombete, F.M.; Câmara, A.K.F.I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci. 2022, 46, 100864. [Google Scholar] [CrossRef]
- Amoah, I.; Cobbinah, J.C.; Yeboah, J.A.; Essiam, F.A.; Lim, J.J.; Tandoh, M.A.; Rush, E. Edible insect powder for enrichment of bakery products—A review of nutritional, physical characteristics and acceptability of bakery products to consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Tarahi, M.; Hedayati, S.; Niakousari, M. Supplementation of cereal products with edible insects: Nutritional, techno-functional, and sensory properties. Food Rev. Int. 2024, 40, 3398–3423. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Haber, M.; Mishyna, M.; Martinez, J.J.I.; Benjamin, O. The influence of grasshopper (Schistocerca gregaria) powder enrichment on bread nutritional and sensorial properties. LWT 2019, 115, 108395. [Google Scholar] [CrossRef]
- Tanga, C.M.; Nzomo, A.M.; Ndegwa, P.N.; Ekesi, S.; Khamis, F.M.; Akutse, K.S.; Ong’amo, G.; Ochieng, B.O.; Kababu, M.; Beesigamukama, D.; et al. Desert locust (Schistocerca gregaria) flour as an emerging functional ingredient for baking flavorful and nutritious whole wheat bread. Appl. Food Res. 2025, 5, 100802. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten-free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef] [PubMed]
- Gantner, M.; Sadowska, A.; Piotrowska, A.; Kulik, K.; Sionek, B.; Kostyra, E. Wheat bread enriched with house cricket powder (Acheta domesticus L.) as an alternative protein source. Molecules 2024, 29, 711. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Absi, Y.; Hernández-Jiménez, M.; Revilla, I. Nutritional value, mineral composition, fatty acid profile and bioactive compounds of commercial plant-based gluten-free flours. Appl. Sci. 2023, 13, 2309. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Pasqualone, A.; Caponio, F. Potential use of plant-based by-products and waste to improve the quality of gluten-free foods. J. Sci. Food Agric. 2022, 102, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Mironeasa, S. Current approaches in using plant ingredients to diversify range of bakery and pasta products. Appl. Sci. 2022, 12, 2794. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Bao, Y.; Liu, F.; Wang, H.; Wang, Y. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Technol. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- Kai, K.; Ma, G.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional performance of plant proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Wirkijowska, A.; Gasiński, A.; Średnicka-Tober, D.; Trafiałek, J.; Kazimierczak, R. Oat and oat processed products—Technology, composition, nutritional value, and health. Appl. Sci. 2023, 13, 11267. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A review of health-beneficial properties of oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Fakayejo, D.A.; Oluwajuyitan, T.D. Nutritional characteristics, glycaemic index and blood glucose lowering property of gluten-free composite flour from wheat (Triticum aestivum), soybean (Glycine max), oat-bran (Avena sativa) and rice-bran (Oryza sativa). Appl. Food Res. 2021, 1, 100022. [Google Scholar] [CrossRef]
- Ivanišová, E.; Čech, M.; Hozlár, P.; Zaguła, G.; Gumul, D.; Grygorieva, O.; Makowska, A.; Kowalczewski, P.Ł. Nutritional, antioxidant and sensory characteristics of bread enriched with wholemeal flour of Slovak black oat varieties. Appl. Sci. 2023, 13, 4485. [Google Scholar] [CrossRef]
- Timm, M.; Offringa, L.C.; Van Klinken, B.J.W.; Slavin, J. Beyond insoluble dietary fiber: Bioactive compounds in plant foods. Nutrients 2023, 15, 4138. [Google Scholar] [CrossRef]
- Stępniewska, S.; Słowik, E.; Cacak-Pietrzak, G.; Romankiewicz, D.; Szafrańska, A.; Dziki, D. Prediction of rye flour baking quality based on parameters of swelling curve. Eur. Food Res. Technol. 2018, 244, 989–997. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic profiling of sourdough fermented wheat and rye bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, P.; Michniewicz, J.; Buchowski, M.S.; Paschke, H. Effects of fibre-rich rye milling fraction on the functional properties and nutritional quality of wholemeal rye bread. J. Food Sci. Technol. 2020, 57, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Bukhovets, V.A.; Davydova, L.V.; Korotkova, L.A.; Tochilkin, A.S.; Saidullayeva, Y.T. Research of bakery properties of varieties of selection rye. IOP Conf. Ser. Earth Environ Sci. 2021, 640, 022013. [Google Scholar] [CrossRef]
- Bieniek, A.; Buksa, K. The influence of arabinoxylan of different molar masses on the properties of rye bread baked by the postponed baking method. Foods 2024, 13, 2482. [Google Scholar] [CrossRef]
- Jonsson, K.; Andersson, R.; Bach Knudsen, K.E.; Hallmans, G.; Hanhineva, K.; Katina, K.; Kolehmainen, M.; Kyrø, C.; Langton, M.; Nordlund, E.; et al. Rye and health—Where do we stand and where do we go? Trends Food Sci. Technol. 2018, 79, 78–87. [Google Scholar] [CrossRef]
- Lu, J.; Hansson, H.; Johansson, D.P.; Landberg, R.; Langton, M. Microstructure and viscosity of in vitro-digested rye and wheat food products. Food Hydrocoll. 2024, 154, 109990. [Google Scholar] [CrossRef]
- Carcea, M. Value of wholegrain rice in a healthy human nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Muchlisyiyah, J.; Shamsudin, R.; Basha, R.K.; Shukri, R.; How, S.; Niranjan, K.; Onwude, D. Parboiled rice processing method, rice quality, health benefits, environment, and future perspectives: A review. Agriculture 2023, 13, 1390. [Google Scholar] [CrossRef]
- Soltani, A.; Golmakani, M.; Fazaeli, M.; Niakousari, M.; Hosseini, S.M.H. Evaluating the effect of different physical pretreatments and cooking methods on nutritional (starch digestibility) and physicochemical properties of white rice grains (Fajr cultivar). LWT 2023, 184, 115101. [Google Scholar] [CrossRef]
- Bresciani, A.; Pagani, M.A.; Marti, A. Rice: A versatile food at the heart of the Mediterranean diet. In The Mediterranean Diet: An Evidence-Based Approach; Galanakis, C.M., Ed.; Springer: Cham, Switzerland, 2021; pp. 193–229. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.S. Rice-based gluten-free foods and technologies: A review. Foods 2023, 12, 4110. [Google Scholar] [CrossRef]
- Qin, W.; Lin, Z.; Wang, A.; Chen, Z.; He, Y.; Wang, L.; Liu, L.; Wang, F.; Tong, L. Influence of particle size on the properties of rice flour and quality of gluten-free rice bread. LWT 2021, 151, 112236. [Google Scholar] [CrossRef]
- Ding, X.L.; Wang, L.J.; Li, T.T.; Wang, F.; Quan, Z.Y.; Zhou, M.; Huo, Z.; Qian, J. Pre-gelatinisation of rice flour and its effect on the properties of gluten-free rice bread and its batter. Foods 2021, 10, 2648. [Google Scholar] [CrossRef]
- Ito, S.; Arai, E. Improvement of gluten-free steamed bread quality by partial substitution of rice flour with powder of Apios americana tuber. Food Chem. 2021, 337, 127977. [Google Scholar] [CrossRef] [PubMed]
- Franco, W.; Evert, K.; Van Nieuwenhove, C. Quinoa flour, the germinated grain flour, and sourdough as alternative sources for gluten-free bread formulation: Impact on chemical, textural and sensorial characteristics. Fermentation 2021, 7, 115. [Google Scholar] [CrossRef]
- Azizi, S.; Azizi, M.H.; Moogouei, R.; Rajaei, P. The effect of quinoa flour and enzymes on the quality of gluten-free bread. Food Sci. Nutr. 2020, 8, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Coţovanu, I.; Mironeasa, C.; Mironeasa, S. Nutritionally improved wheat bread supplemented with quinoa flour of large, medium and small particle sizes at typical doses. Plants 2023, 12, 698. [Google Scholar] [CrossRef]
- Gil, J.V.; Esteban-Muñoz, A.; Fernández-Espinar, M.T. Changes in the polyphenolic profile and antioxidant activity of wheat bread after incorporating quinoa flour. Antioxidants 2021, 11, 33. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Sreechithra Ray, A.; Sakhare, S.D. Effect of a novel high-fibre component from quinoa on the properties of bread-making. Int. J. Food Sci. Technol. 2024, 59, 6421–6430. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Assessing the performance of different grains in gluten-free bread applications. Appl. Sci. 2020, 10, 8772. [Google Scholar] [CrossRef]
- Boukid, F.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for bread fortification: A necessity or a choice? Trends Food Sci Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Onyango, E. Legume protein: Properties and extraction for food applications. In Research on Legumes; IntechOpen eBooks: London, UK, 2022; Volume 2. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Karathanos, V.T. Physical properties, sensory acceptance, postprandial glycemic response, and satiety of cereal-based foods enriched with legume flours: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2722–2740. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of legumes in extrusion cooking: A review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Proserpio, C.; Bresciani, A.; Marti, A.; Pagliarini, E. Legume flour or bran: Sustainable, fiber-rich ingredients for extruded snacks? Foods 2020, 9, 1680. [Google Scholar] [CrossRef]
- Nartea, A.; Kuhalskaya, A.; Fanesi, B.; Orhotohwo, O.L.; Susek, K.; Rocchetti, L.; Di Vittori, V.; Bitocchi, E.; Pacetti, D.; Papa, R. Legume byproducts as ingredients for food applications: Preparation, nutrition, bioactivity, and techno-functional properties. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1953–1985. [Google Scholar] [CrossRef]
- Olakanmi, S.J.; Jayas, D.S.; Paliwal, J. Implications of blending pulse and wheat flours on rheology and quality characteristics of baked goods: A review. Foods 2022, 11, 3287. [Google Scholar] [CrossRef]
- Orozco-Angelino, X.; Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Extrusion as a tool to enhance the nutritional and bioactive potential of cereal and legume by-products. Food Res. Int. 2023, 169, 112889. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Sujka, K.; Księżak, J.; Bojarszczuk, J.; Ziarno, M.; Studnicki, M.; Krajewska, A.; Dziki, D. Assessment of physicochemical properties and quality of the breads made from organically grown wheat and legumes. Foods 2024, 13, 1244. [Google Scholar] [CrossRef]
- Guan, C.; Long, X.; Long, Z.; Lin, Q.; Liu, C. Legumes flour: A review of the nutritional properties, physiological functions and application in extruded rice products. Int. J. Food Sci. Technol. 2022, 58, 300–314. [Google Scholar] [CrossRef]
- Tebben, L.; Chen, G.; Tilley, M.; Li, Y. Improvement of whole wheat dough and bread properties by emulsifiers. Grain Oil Sci. Technol. 2022, 5, 59–69. [Google Scholar] [CrossRef]
- Bresciani, A.; Marti, A. Using pulses in baked products: Lights, shadows, and potential solutions. Foods 2019, 8, 451. [Google Scholar] [CrossRef]
- Atudorei, D.; Atudorei, O.; Codină, G.G. The impact of germinated chickpea flour addition on dough rheology and bread quality. Plants 2022, 11, 1225. [Google Scholar] [CrossRef]
- Ungureanu-Iuga, M.; Atudorei, D.; Codină, G.G.; Mironeasa, S. Rheological approaches of wheat flour dough enriched with germinated soybean and lentil. Appl. Sci. 2021, 11, 11706. [Google Scholar] [CrossRef]
- Mengozzi, A.; Chiavaro, E.; Barbanti, D.; Bot, F. Heat-induced gelation of chickpea and faba bean flour ingredients. Gels 2024, 10, 309. [Google Scholar] [CrossRef]
- Bojňanská, T.; Musilová, J.; Vollmannová, A. Effects of adding legume flours on the rheological and breadmaking properties of dough. Foods 2021, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Rolandelli, G.; Farroni, A.; del Pilar Buera, M. Raw materials: Traditional and non-conventional cereals, pseudo-cereals, oilseeds and legumes. In Designing Gluten Free Bakery and Pasta Products; Springer International Publishing: Cham, Switzerland, 2023; pp. 19–61. [Google Scholar]
- Marpalle, P.; Sonawane, S.K.; Arya, S.S. Effect of flaxseed flour addition on physicochemical and sensory properties of functional bread. LWT 2014, 58, 614–619. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.; Millán-Linares, M.C.; Haros, C.M. Effect of chia as an ingredient in bread making on the nutritional quality, mineral availability, and glycemic index of bread. Foods 2020, 9, 663. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed proteins—Properties and application as a food ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Hernández-López, I.; Ortiz-Solà, J.; Alamprese, C.; Barros, L.; Shelef, O.; Basheer, L.; Rivera, A.; Abadias, M.; Aguiló-Aguayo, I. Valorization of local legumes and nuts as key components of the Mediterranean diet. Foods 2022, 11, 3858. [Google Scholar] [CrossRef]
- Yang, J.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Van Der Linden, E.; Sagis, L.M. Competition of rapeseed proteins and oleosomes for the air-water interface and its effect on the foaming properties of protein-oleosome mixtures. Food Hydrocoll. 2021, 122, 107078. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Dai, Y.; Yu, D.; Cheng, J. Interfacial adsorption properties, rheological properties and oxidation kinetics of oleogel-in-water emulsion stabilized by hemp seed protein. Food Hydrocoll. 2022, 137, 108402. [Google Scholar] [CrossRef]
- Peng, D.; Ye, J.; Jin, W.; Yang, J.; Geng, F.; Deng, Q. A review on the utilization of flaxseed protein as interfacial stabilizers for food applications. J. Am. Oil Chem. Soc. 2022, 99, 723–737. [Google Scholar] [CrossRef]
- Tang, Y.R.; Ghosh, S. A review of the utilization of canola protein as an emulsifier in the development of food emulsions. Molecules 2023, 28, 8086. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours: Influence of chemical composition on nutritional and technological aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Álvarez-Rivera, G.; Viejo, J.L.; Martín-Cabrejas, M. Investigating edible insects as a sustainable food source: Nutritional value and techno-functional and physiological properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, physicochemical, techno-functional and antioxidant properties of three edible insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- D’Antonio, V.; Battista, N.; Sacchetti, G.; Di Mattia, C.; Serafini, M. Functional properties of edible insects: A systematic review. Nutr. Res. Rev. 2021, 36, 98–119. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Silva, N.F.N.; Jessen, F.; Mohammadifar, M.A.; Stephani, R.; De Carvalho, A.F.; Perrone, Í.T.; Casanova, F. Edible insect as an alternative protein source: A review on the chemistry and functionalities of proteins under different processing methods. Heliyon 2023, 9, e14831. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Kamran, F.; Stankov, S.; Rathod, N.B.; Teixeira-Costa, B.E.; Fidan, H.; Bhat, M.I.; Sofi, S.A. Unveiling the diversity of non-conventional proteins—From sources, extraction, technofunctionality, nutraceutical potential to advancement in food applications—A systematic review. Waste Biomass Valor. 2024, 16, 29–51. [Google Scholar] [CrossRef]
- Zielińska, E. Evaluating the functional characteristics of certain insect flours (non-defatted/defatted flour) and their protein preparations. Molecules 2022, 27, 6339. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as valuable sources of protein and peptides: Production, functional properties, and challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent insight on edible insect protein: Extraction, functional properties, allergenicity, bioactivity, and applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Urbina, P.; Marin, C.; Sanz, T.; Rodrigo, D.; Martinez, A. Effect of HHP, enzymes and gelatin on physicochemical factors of gels made by using protein isolated from common cricket (Acheta domesticus). Foods 2021, 10, 858. [Google Scholar] [CrossRef]
- Mishyna, M.; Keppler, J.K.; Chen, J. Techno-functional properties of edible insect proteins and effects of processing. Curr. Opin. Colloid Interface Sci. 2021, 56, 101508. [Google Scholar] [CrossRef]
- Kumar, S.; Queiroz, L.S.; Marie, R.; Nascimento, L.G.L.; Mohammadifar, M.A.; De Carvalho, A.F.; Brouzes, C.M.C.; Fallquist, H.; Fraihi, W.; Casanova, F. Gelling properties of black soldier fly (Hermetia illucens) larvae protein after ultrasound treatment. Food Chem. 2022, 386, 132826. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, M.H.; Yong, H.I.; Kang, M.; Jung, S.; Choi, Y. Porcine myofibrillar protein gel with edible insect protein: Effect of pH-shifting. LWT 2021, 154, 112629. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Santos, C.; Rocha, C.; Marques, C.; Rodrigues, C.; Manita, F.; Sousa, F.; Félix, M.; Silva, S.; Rodrigues, S. Whey-bread, an improved food product: Evaluation of textural characteristics. J. Culin. Sci. Technol. 2020, 18, 40–53. [Google Scholar] [CrossRef]
- Tița, M.A.; Moga, V.-M.; Constantinescu, M.A.; Bătușaru, C.M.; Tița, O. Harnessing the potential of whey in the creation of innovative food products: Contributions to the circular economy. Recycling 2024, 9, 79. [Google Scholar] [CrossRef]
- Madenci, A.B.; Bilgiçli, N. Effect of whey protein concentrate and buttermilk powders on rheological properties of dough and bread quality. J. Food Qual. 2014, 37, 117–124. [Google Scholar] [CrossRef]
- Arab, S.A.; Kaemipoor, M.; Alkhaleel, R.; Mahdian, A. Recent trends in developing whey products by advanced technologies. Scholars Acad. J. Biosci. 2023, 11, 74–79. Available online: https://www.saspublishers.com/media/articles/SAJB_112_74-79_XIuqrSb.pdf (accessed on 14 May 2025). [CrossRef]
- Rasouli Pirouzian, H.; Alakas, E.; Cayir, M.; Yakisik, E.; Toker, O.S.; Kaya, Ş.; Tanyeri, O. Buttermilk as milk powder and whey substitute in compound milk chocolate: Comparative study and optimisation. Int. J. Dairy Technol. 2021, 74, 246–257. [Google Scholar] [CrossRef]
- Rababah, T.; Al-U’Datt, M.; Al-Mahasneh, M.; Alsaad, A.; Gammoh, S.; Mahili, H.; Abdhamead, K.B.; Almajwal, A.; Ajouly, T.; Bartkute-Norkuniene, V. Improving the functional and sensory properties of cookies by ultrasonic treatment of whey proteins. J. Food Qual. 2022, 2022, 6902592. [Google Scholar] [CrossRef]
- Tsanasidou, C.; Kosma, I.; Badeka, A.; Kontominas, M. Quality parameters of wheat bread with the addition of untreated cheese whey. Molecules 2021, 26, 7518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koukoumaki, D.I.; Tsouko, E.; Papanikolaou, S.; Ioannou, Z.; Diamantopoulou, P.; Sarris, D. Recent advances in the production of single cell protein from renewable resources and applications. Carbon Resour. Convers. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein—State-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Hassoun, A.; Cropotova, J.; Trif, M.; Rusu, A.V.; Bobiş, O.; Nayik, G.A.; Jagdale, Y.D.; Saeed, F.; Afzaal, M.; Mostashari, P.; et al. Consumer acceptance of new food trends resulting from the fourth industrial revolution technologies: A narrative review of literature and future perspectives. Front. Nutr. 2022, 9, 972154. [Google Scholar] [CrossRef]
- Mahmoud, N.; Ferreira, J.; Raymundo, A.; Nunes, M.C. Improvement of protein, mineral and bioactivity content of wheat bread using microalgae biomass: Comparative study of Chlorella vulgaris, Phaeodactylum tricornutum and Tetraselmis chuii. Appl. Sci. 2024, 14, 2483. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in wheat flour breads of different alveographic strengths. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Wan, G.; Lu, X.; Xie, L.; Yu, T.; Tang, H. Metabolic engineering for the production of single-cell proteins from renewable feedstocks and their applications. Adv. Biotechnol. 2024, 2, 35. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single-cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.; Bárzana, E. Single cell protein|Yeasts and bacteria. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 431–438. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Spirulina—An invaluable source of macro- and micronutrients with broad biological activity and application potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef]
- Montevecchi, G.; Santunione, G.; Licciardello, F.; Köker, Ö.; Masino, F.; Antonelli, A. Enrichment of wheat flour with spirulina: Evaluation of thermal damage to essential amino acids during bread preparation. Food Res. Int. 2022, 157, 111357. [Google Scholar] [CrossRef]
- Mora, H.B.; Piñeros, M.; Moreno, D.E.; Restrepo, S.R.; Jaramillo, J.C.; Solano, Ó.Á.; Fernandez-Niño, M.; Barrios, A.G. Multiscale design of a dairy beverage model composed of Candida utilis single cell protein supplemented with oleic acid. J. Dairy Sci. 2019, 102, 9749–9762. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; Barco-Mendoza, G.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ochoa, M.A.V.; González-Aguilar, G.A. The production of single-cell proteins as a strategy for reincorporating food waste and agricultural by-products into the processing chain. Bioengineering 2022, 9, 623. [Google Scholar] [CrossRef]
- Kobayashi, Y.; El-Wali, M.; Guðmundsson, H.; Guðmundsdóttir, E.E.; Friðjónsson, Ó.H.; Karlsson, E.N.; Roitto, M.; Tuomisto, H.L. Life-cycle assessment of yeast-based single-cell protein production with oat processing side-stream. Sci. Total Environ. 2023, 873, 162318. [Google Scholar] [CrossRef]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’Anna, E.S.S. Cultivation of Arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino acid profile. Braz. J Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Oliveira, M.C. Lipid, fatty acid, protein, amino acid and ash contents in four species of Brazilian red algae. Braz. J. Oceanogr. 2010, 58, 177–186. [Google Scholar] [CrossRef]
- Fleurence, J. Perspectives for the use of seaweeds in human nutrition and for marine animal production. Trends Food Sci. Technol. 2012, 27, 56–64. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Tewari, A. Effect of soda ash industrial effluent on the protein content of two green algae. J. Environ. Biol. 2008, 29, 933–937. [Google Scholar]
- Putri, R.I.I.; Arief, D.; Cahyaningsih, A. Single cell protein production by Chlorella sp. using food processing wastewater as culture medium. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 2322–2328. [Google Scholar] [CrossRef]
- Spalvins, A.; Zihare, L.; Blumberga, D. Single cell protein production from residual biomass: Comparison of various industrial by-products. Environ. Clim. Technol. 2018, 22, 52–65. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, D.; Chen, H. Toward an industrially viable process for potato starch processing waste using mixed cultures: Single-cell protein production. Bioresour. Technol. 2013, 137, 322–329. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass Valor. 2016, 9, 57–64. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed culture of Kluyveromyces marxianus and Candida krusei for single cell protein production and organic load removal from whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- García-Garibay, M.; Ramírez, M.; Rodríguez, M.E. Single cell protein: Yeasts and bacteria. Rev. Educ. Bioquím. 2014, 33, 35–45. [Google Scholar]
- Wongputtisin, P.; Phaowphaisal, I.; Intanon, S. Use of Bacillus subtilis isolates from Tua nao to improve the nutritional quality of soybean hulls. J. Microbiol. Biotechnol. 2014, 24, 913–922. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Algur, O.F. Single-cell protein production from ram horn hydrolysate by bacteria. Bioresour. Technol. 2002, 85, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, D.; Chen, H. Production of single cell protein with two-step fermentation for potato starch processing wastewater treatment. Biotechnol. Lett. 2014, 36, 987–993. [Google Scholar] [CrossRef]
- Baldensperger, J.; Mer, J.L.; Hannibal, L.; Quinto, P.J. Solid state fermentation of banana wastes. Biotechnol. Lett. 1985, 7, 743–748. [Google Scholar] [CrossRef]
- De Gregorio, A. SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Bioresour. Technol. 2002, 83, 89–94. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Song, J.; Zhang, L.; Dong, J.; Yang, Q. Production of single-cell protein with two-step fermentation for treatment of potato starch processing waste. Cellulose 2014, 21, 3637–3645. [Google Scholar] [CrossRef]
- Liu, B.; Song, J.; Li, Y.; Niu, J.; Wang, Z.; Yang, Q. Towards industrially feasible treatment of potato starch processing waste by mixed cultures. Appl. Biochem. Biotechnol. 2013, 171, 1001–1010. [Google Scholar] [CrossRef]
- Fernandes Almeida, R.; Borges, L.A.; Anacleto, D.; de Souza, M.S.N.; da Silva, L.B.M.; Monroy, Y.M.; Batista, E.A.C.; Machado, A.P.O. Gluten-free cookies: A comprehensive review of wheat flour substitutes. Food Humanit. 2025, 4, 100549. [Google Scholar] [CrossRef]
- Kotsiou, K.; Sacharidis, D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 2021, 124, 107322. [Google Scholar] [CrossRef]
- Irigoytia, K.; Espósito, N.; de Escalada Pla, M.; Parodi, M.; Genevois, C. Technological and multi-sensory analysis approach to holistically understand the quality and consumer perception of gluten-free breads with alternative flours. Int. J. Food Sci. Technol. 2024, 59, 8606–8614. [Google Scholar] [CrossRef]
- Zarzycki, P.; Wirkijowska, A.; Pankiewicz, U. Functional bakery products: Technological, chemical and nutritional modification. Appl. Sci. 2024, 14, 12023. [Google Scholar] [CrossRef]
- Dean, D.; Rombach, M.; Vriesekoop, F.; Mongondry, P.; Le Viet, H.; Laophetsakunchai, S.; Urbano, B.; Briz, T.; Xhakollari, V.; Atasoy, G.; et al. Against the grain: Consumer’s purchase habits and satisfaction with gluten-free product offerings in European food retail. Foods 2024, 13, 3152. [Google Scholar] [CrossRef]
- Kušar, A.; Pravst, I.; Kupirovič, U.P.; Grunert, K.G.; Kreft, I.; Hristov, H. Consumers’ preferences towards bread characteristics based on food-related lifestyles: Insights from Slovenia. Foods 2023, 12, 3766. [Google Scholar] [CrossRef]
- Scheibenzuber, S.; Pucci, E.; Presenti, O.; Serafini, G.; Nobili, C.; Zoani, C.; Duta, D.E.; Mihai, A.L.; Criveanu-Stamatie, G.D.; Belc, N.; et al. Consumers’ acceptance of new food ingredients from the food industry’s by-products: A focus group study. Front. Nutr. 2025, 12, 1509833. [Google Scholar] [CrossRef]
- Andaregie, A.; Shimura, H.; Chikasada, M.; Sasaki, S.; Mulugeta, M.A.; Worku, A.; Wubalem, A.; Sato, S.; Addisu, S.; Takagi, I. Assessing eating habits and preferences for bakery products among urban dwellers in Ethiopia. Cogent Bus. Manag. 2024, 11, 2375621. [Google Scholar] [CrossRef]
- Benayad, A.; Taghouti, M.; Benali, A.; Aboussaleh, Y.; Benbrahim, N. Nutritional and technological assessment of durum wheat-faba bean enriched flours, and sensory quality of developed composite bread. Saudi J. Biol. Sci. 2020, 28, 635–642. [Google Scholar] [CrossRef]
- De Melo, B.G.; Tagliapietra, B.L.; Clerici, M.T.P.S. Evolution of the technological, sensory, and nutritional quality of gluten-free cookies: A critical review. Food Sci. Technol. 2023, 43, e75822. [Google Scholar] [CrossRef]
- Wang, Y.; Maina, N.H.; Coda, R.; Katina, K. Challenges and opportunities for wheat alternative grains in breadmaking: Ex-situ- versus in-situ-produced dextran. Trends Food Sci. Technol. 2021, 113, 232–244. [Google Scholar] [CrossRef]
- Senarathna, S.; Mel, R.; Malalgoda, M. Utilization of cereal-based protein ingredients in food applications. J. Cereal Sci. 2024, 116, 103867. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers. Off. J. Eur. Union. 2011, L304, 18–63. [Google Scholar]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current challenges of alternative proteins as foods of the future. NPJ Sci Food 2024, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No 1169/2011 and repealing Regulation (EC) No 258/97 and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union. 2015, L327, 1–22. [Google Scholar]
- Grundy, H.; Romero, M.R.; Brown, L.C.; Parker, M. Review of Methods for the Detection of Allergens in Novel Food Alternative Proteins; FSA Research And Evidence: London, UK, 2024. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Standard for Wheat Flour (CXS 152-1985); Revised 1995; FAO/WHO: Rome, Italy, 1985; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/ru/ (accessed on 2 October 2025).
- Boukid, F.; Rosene, S. Grain Proteins: Challenges and Solutions in Developing Consumer-Relevant Foods. Cereal Foods World 2020, 65. [Google Scholar] [CrossRef]
- US Food and Drug Administration. 21 CFR Part 136—Bakery Products; FDA: Silver Spring, MD, USA. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-136 (accessed on 2 October 2025).
- US Food and Drug Administration. Guidance for Industry: Food Labeling Guide; FDA: Silver Spring, MD, USA, 2015. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide (accessed on 2 October 2025).
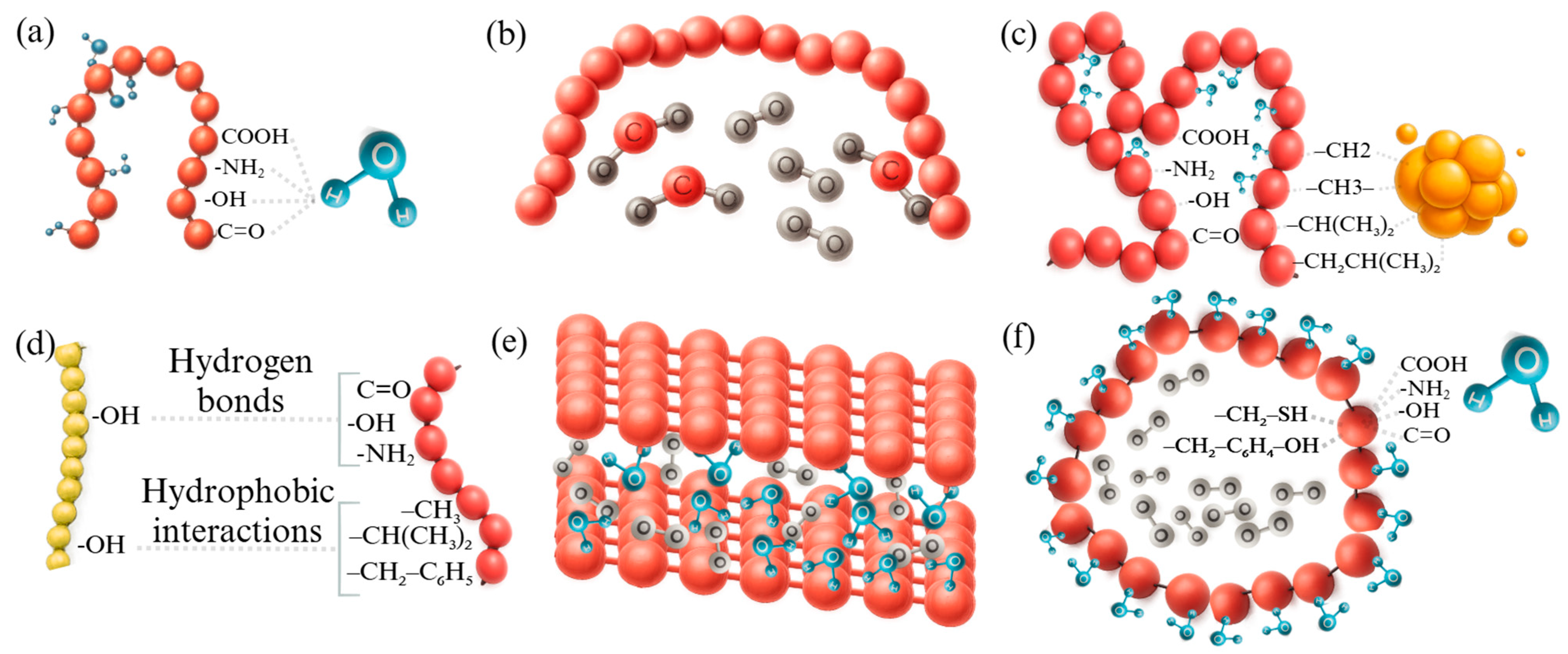
| Group | Source | Abundant Amino Acids | Limiting Amino Acids | Reference |
|---|---|---|---|---|
| Vegetables | Oat | Methionine, cysteine | Lysine | [76,78,79] |
| Rye | Methionine | Lysine, Threonine | [76,80] | |
| Rice | Methionine, cysteine | Lysine | [76,80] | |
| Quinoa | Lysine, Methionine | None clearly limiting (almost complete profile) | [76,81,82] | |
| Lupine | Lysine, Arginine | Valine, cysteine, and methionine | [75,76] | |
| Seeds and nuts | Nut | Arginine, Glutamic acid | Lysine, Methionine | [38] |
| Chia | Lysine, Arginine | Methionine | [83] | |
| Pumpkin seeds | Tryptophan, Arginine | Lysine | [43,84] | |
| Sunflower seeds | Arginine, Glutamic acid | Lysine | [43] | |
| Sesame seeds | Methionine, Cysteine | Lysine | [43] | |
| Legumes | Bean | Lysine | Methionine | [85] |
| Chickpea | Lysine, Tryptophan | Cysteine, methionine, and tryptophan | [86] | |
| Lentils | Lysine, Tryptophan | Methionine | [87] | |
| Broad beans | Lysine, Arginine | Methionine and tyrosine | [40] | |
| Soy | Lysine, Arginine | Cysteine, Methionine (although less limiting than in other legumes) | [75,88] | |
| Sub-products | Liquid cheese whey | Lysine, Leucine, Isoleucine, Valine | No limitations (full profile) | [51,89] |
| Cheese whey powder | Lysine, Cysteine, Leucine | No limitations (full profile) | [51,89] | |
| Cheese whey concentrate (34%) | Lysine, Leucine, Valine | No limitations (full profile) | [51,90] | |
| Cheese whey concentrate (80%) | Lysine, Leucine, Tryptophan | No limitations (full profile) | [51,90] | |
| Cheese whey isolate | Lysine, Leucine, Isoleucine | No limitations (full profile) | [51,90] | |
| Insects | Beetles | Lysine, Methionine | Generally balanced, minor limitations in tryptophan | [91,92] |
| Caterpillars | Lysine, Threonine | Minor limitations in methionine | [91,93] | |
| Bees | Lysine, Arginine | Minor limitations in tryptophan | [93] | |
| Grasshoppers | Lysine, Threonine | Mild limitations in methionine | [91,94] | |
| Locusts | Lysine, Valine | Methionine | [91] | |
| Crickets | Lysine, Threonine, Valine | Slightly low in methionine | [91,94] | |
| Termites | Lysine, Methionine | Almost complete profile, slight deficiencies in tryptophan | [91,94] |
| Protein Origin | Ingredient | Level of Substitution (%) | Protein (g/100 g Dry Matter) | Reference |
|---|---|---|---|---|
| Vegetables | Oat | 10–20 | 10–17 | [97,98] |
| Rye | <30 | 9–15 | [99,100] | |
| Rice | <50 | 6–8 | [101] | |
| Quinoa | 10–15 | 13–15 | [102,103] | |
| Lupine | ≤20 | 35–40 | [77] | |
| Seeds and nuts | Nut | 5–10 | 5–15 | [104,105] |
| Chia | 5–15 | 16–20 | [106,107,108] | |
| Pumpkin seeds | 5–10 | 30–33 | [109] | |
| Sunflower seeds | 5–16 | 20–21 | [110] | |
| Sesame seeds | 5–10 | 17–18 | [107,111] | |
| Legumes | Bean | 10–30 | 21–24 | [112,113] |
| Chickpea | 10–30 | 19–21 | [114,115] | |
| Lentils | ≤20 | 24–26 | [116,117] | |
| Broad beans | 10–20 | 24–26 | [118] | |
| Soy | >20 | 36–40 | [119,120] | |
| Sub-products | Liquid cheese whey | 10–15 | 0.8–1.0 | [121,122] |
| Cheese whey powder | 10–15 | 11–14 | [122,123] | |
| Cheese whey concentrate (WPC 34%) | 10–15 | 34–36 | [124] | |
| Cheese whey concentrate (WPC 80%) | 10–15 | 77–82 | [122,123] | |
| Cheese whey isolate (WPI) | 10–15 | 85–90 | [122] | |
| Insects | Beetles | 5–10 | 40–65 | [44,125] |
| Caterpillars | 5–10 | 45–60 | [126] | |
| Bees | 5–10 | 50–65 | [127,128] | |
| Grasshoppers | 5–20 | 60–70 | [129] | |
| Locusts | 5–20 | 60–70 | [130] | |
| Crickets | 5–30 | 60–70 | [131,132,133] | |
| Termites | 5–10–15 | 35–65 | [126] |
| Microorganism | Protein (%) | Substrate | Production Conditions | Food Applications | Reference |
|---|---|---|---|---|---|
| Algae | |||||
| Arthrospira platensis (“Spirulina”) | 55–70 | Autotrophic media (water, CO2, mineral nutrients) | Phototrophy in open ponds or photobioreactors; alkaline medium (pH ≈ 9–11) with NaHCO3/CO2; 25–35 °C; continuous, aerobic | Fortification of bread, pasta, cookies, yogurt, and other fermented dairy products | [223] |
| Arthrospira (Spirulina) platensis | 63 | Synthetic saline medium | 30–35 °C, 16 h of light, continuous cultivation | Food supplements, functional beverages, nutraceuticals | [228] |
| Gracilaria domingensis | 10.5–18.6 | Natural coastal cultivation | Seasonal harvesting, without induced cultivation | Direct consumption in salads, dried seaweed, agar | [229] |
| Palmaria palmata | ~35 | Marine harvesting | Open sea farming, cold-temperate | Salads, soups, fermented products | [230] |
| Ulva faciata | Up to 35 | Diluted effluent from the sodium carbonate industry | In situ cultivation on contaminated coastline | Direct consumption, effluent purification | [231] |
| Chlorella sp. | ~47.1 | Wastewater from the food industry | 25–30 °C, pH 6.8–7.2, controlled lighting | Protein flours, powdered supplements, green drinks | [232] |
| Chlorella salina | 22–48 | Biodiesel production effluent | Cultivation in photobioreactors | Dietary supplements, food fortification | [233] |
| Yeasts | |||||
| Saccharomyces cerevisiae AXAZ-1 | ~30 | Waste mixture: beer bagasse, molasses, whey, potato peelings, and orange peelings | Solid state fermentation; 30 °C; adjusted humidity; pH 4.5–5.0; 5–7 days | Enrichment of animal feed, enrichment of bakery products | [234] |
| Candida utilis | 46.1 | Potato starch pulp + wastewater | Submerged aerobic fermentation; 30 °C; 300 rpm; aeration 0.1 vvm; 6 days; pH 7.0 | Experimental animal feed (tested on mice), Use for fermented beverages | [235] |
| Candida utilis ATCC 9950 | 40.6 | Potato wastewater (deproteinized) + glycerol (5%) | Submerged culture at 28 °C; 200 rpm; 72 h; pH 5.0 | Feed yeast (food use authorized by GRAS) | [236] |
| Kluyveromyces marxianus CHY1612 | ~38 | Cheese whey + urea | Continuous aerobic fermentation; 40 °C; pH 3.5; HRT 24 h | Animal feed, plant-based yogurts, probiotic drinks, gluten-free breads | [237] |
| Bacteria | |||||
| Methylococcus capsulatus | 60–70 | Methane or methanol as a carbon source | Submerged and aerobic fermentation in loop bioreactors; methane or biogas as sole carbon/energy source; 35–45 °C; moderately neutral; 48-h batches produce ~10 g biomass L−1 | Bacterial flour for aquaculture feed, evaluated in products for human consumption due to its high protein content | [238] |
| Bacillus subtilis | 49.1 | Soybean hull | Solid fermentation, 40 °C, 3 days | Potential in the formulation of fermented foods and nutritional enhancers | [239] |
| Bacillus subtilis | 45.40 | Hydrolyzed ram’s horn | Liquid culture, 30 °C, pH 7.0, 24 h | Feed, not directly applicable to humans; used in SCP studies | [240] |
| Bacillus licheniformis CGMCC 1.813 | 38.21 | Residue and water from potato starch processing | 32.8 °C, pH 6.67, 1.78% inoculum, 2 days | Use in animal feed; GRAS potential | [241] |
| Fungi | |||||
| Fusarium venenatum QuornTMA3/5 | 44 | Glucose derived from starch (syrup), mineral salts, ammonia | Continuous air lift fermenter, glucose as substrate, ~30 °C, pH ≈ 6, highly aerobic; biomass harvested continuously | Meat analog products | [216] |
| A. niger “strain 2” | 6 → 18 | Green banana flour | SSF in reactor 15 kg; humidity 42%; incubation 38 °C→30 °C (hot air 60 °C); air 0.8–2.4 m3 h−1; 43 h fermentation (total 60–68 h) | Protein-rich banana flour for animal feed (livestock, shrimp) | [242] |
| A. niger (wild citrus strain) | 25.6 | Decolorized lemon pulp (≈14% solids) | “Slurry state” (semi-liquid medium); 30 °C; agitation 220 rpm; pH adjusted to 4.0; 5 days | Protein ingredient and source of pectinase for the citrus industry itself | [243] |
| A. niger H3 (UV/EMS mutant) + Bacillus licheniformis (two-step fermentation) | 46.1 | Potato starch pulp and wastewater (15:4 w/w mixture) | Step 1: Solid pre-hydrolysis 4 days (A. niger, 28 °C) → Step 2: Submerged fermentation 6 days; 30 °C; 300 rpm; pH 7.0; 0.1 vvm air | Flour for animal feed (palatability validated in mice) | [244,245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea-Escobar, C.D.; Londoño-Hernández, L.; Benavente-Valdés, J.R.; Balagurusamy, N.; Contreras Esquivel, J.C.; Hernández-Almanza, A.Y. Incorporation of Protein Alternatives in Bakery Products: Biological Value and Techno-Functional Properties. Appl. Sci. 2025, 15, 11279. https://doi.org/10.3390/app152011279
Perea-Escobar CD, Londoño-Hernández L, Benavente-Valdés JR, Balagurusamy N, Contreras Esquivel JC, Hernández-Almanza AY. Incorporation of Protein Alternatives in Bakery Products: Biological Value and Techno-Functional Properties. Applied Sciences. 2025; 15(20):11279. https://doi.org/10.3390/app152011279
Chicago/Turabian StylePerea-Escobar, Carlos Daniel, Liliana Londoño-Hernández, Juan Roberto Benavente-Valdés, Nagamani Balagurusamy, Juan Carlos Contreras Esquivel, and Ayerim Y. Hernández-Almanza. 2025. "Incorporation of Protein Alternatives in Bakery Products: Biological Value and Techno-Functional Properties" Applied Sciences 15, no. 20: 11279. https://doi.org/10.3390/app152011279
APA StylePerea-Escobar, C. D., Londoño-Hernández, L., Benavente-Valdés, J. R., Balagurusamy, N., Contreras Esquivel, J. C., & Hernández-Almanza, A. Y. (2025). Incorporation of Protein Alternatives in Bakery Products: Biological Value and Techno-Functional Properties. Applied Sciences, 15(20), 11279. https://doi.org/10.3390/app152011279







