Usefulness of Flavonoids and Phenolic Acids in Differentiating Honeys Based on Geographical Origin: The Case of Dominican Republic and Spanish Honeys
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Analysis of Phenolic Compounds by Liquid Chromatography (HPLC)
2.2.1. Standards and Reagents
2.2.2. Extraction
2.2.3. Chromatographic Conditions
2.3. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Phenolic Compounds
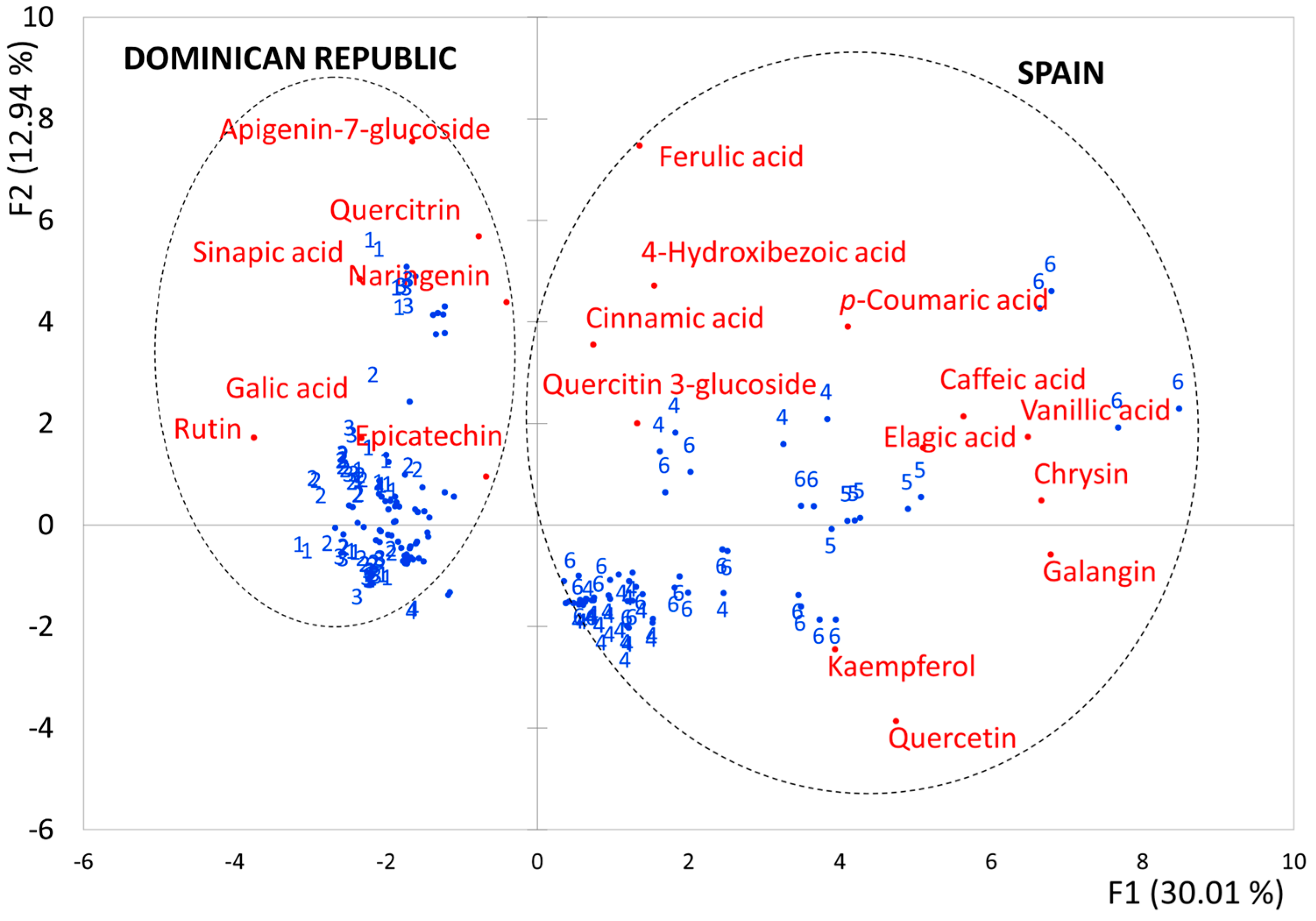
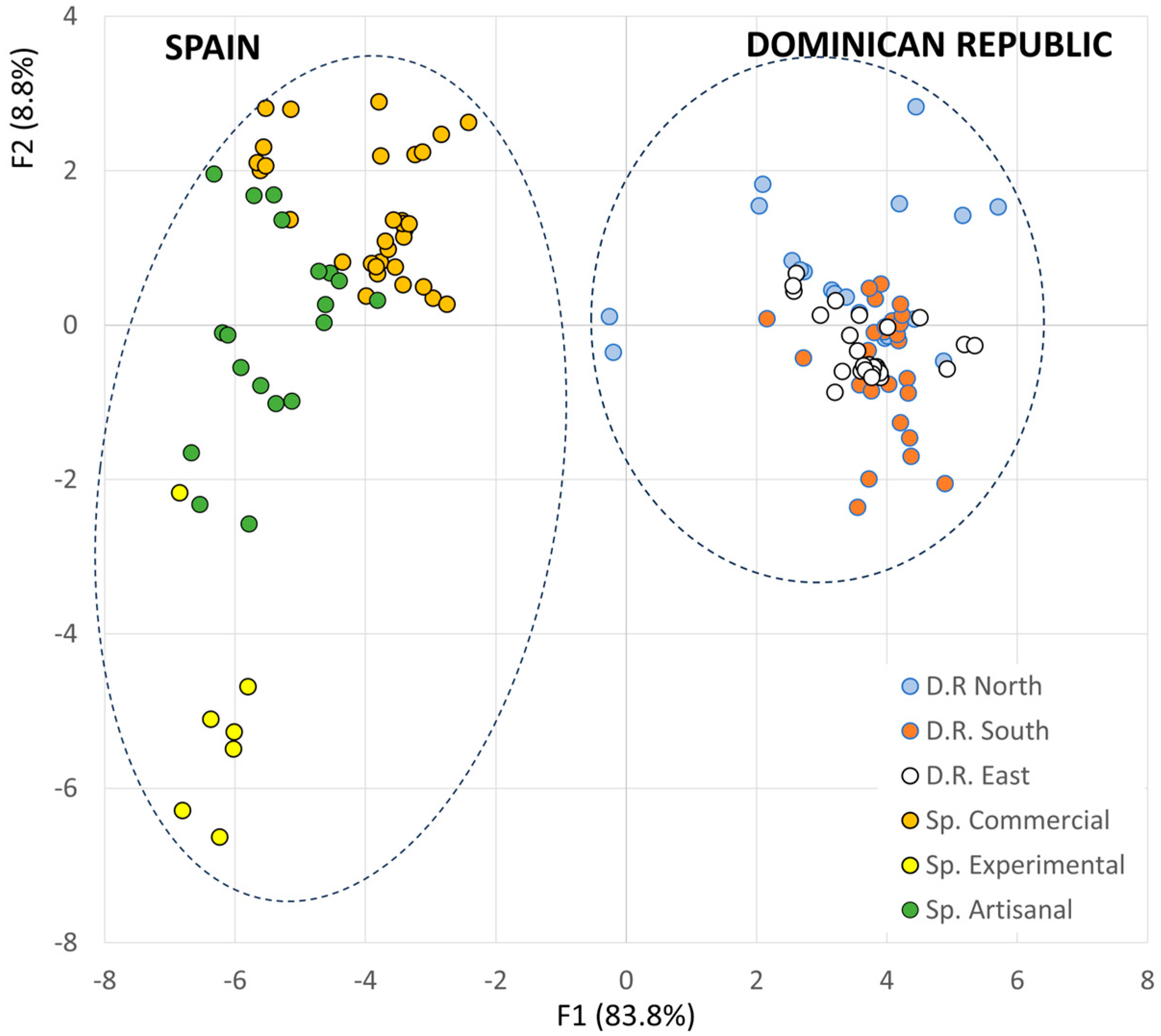
3.2. Identification of the Variables with the Highest Discriminant Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| D.R. | Dominican Republic |
| FLV | Flavonoids |
| PHA | Phenolic Acids |
References
- CXS 12-1981; Standard for Honey. Codex Alimentarius Commission: Rome, Italy, 2022. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 5 September 2025).
- Kowalski, S.; Ciesarová, Z.; Kukurová, K.; Tobolková, B.; Polovka, M.; Skoczylas, Ł.; Tabaszewska, M.; Mikulec, K.; Mikulec, A.; Buksa, K. Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys. Appl. Sci. 2025, 15, 5810. [Google Scholar] [CrossRef]
- Barrera, O.I.C.; Llanos, G.A.H. Factores que determinan las propiedades fisicoquímicas de la miel de abejas: Revisión Sistemática de Literatura. Rev. Mutis 2023, 13, 1–28. [Google Scholar] [CrossRef]
- Derewiaka, D.; Majewska, E.; Pruszkowska, P. The Effects of Bee Additives on the Physico-Chemical and Antioxidant Properties of Rapeseed Honey. Appl. Sci. 2024, 14, 1292. [Google Scholar] [CrossRef]
- Straumite, E.; Bartule, M.; Valdovska, A.; Kruma, Z.; Galoburda, R. Physical and Microbiological Characteristics and Antioxidant Activity of Honey Bee Pollen. Appl. Sci. 2022, 12, 3039. [Google Scholar] [CrossRef]
- Escriche, I.; Conchado, A.; Peral, A.M.; Juan-Borrás, M. Volatile markers as a reliable alternative for the correct classification of citrus monofloral honey. Food Res. Int. 2023, 168, 112699. [Google Scholar] [CrossRef]
- Jiang, W.; Battesti, M.J.; Yang, Y.; Jean-Marie, É.; Costa, J.; Béreau, D.; Paolini, J.; Robinson, J.C. Melissopalynological Analysis of Honey from French Guiana. Foods 2024, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Juan-Borrás, M.D.S. Herramientas Analíticas en la Clasificación de Mieles en Base a Criterios de Calidad e Inocuidad. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2016. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; D’Arcy, B.; Singanusong, R.; Datta, N.; Caffin, N.; Raymont, K. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J. Agric. Food Chem. 2004, 52, 210–214. [Google Scholar] [CrossRef]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the presence and contents of phytochemicals in honey samples: Phenolic compounds as Indicators to Identify their botanical origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef]
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001, 75, 237–240. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Al Juhaimi, F. Honey as source of natural antioxidants. J. Apic. Res. 2015, 54, 145–154. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Nighat, S.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Bodor, Z.; Benedek, C.; Kovacs, Z.; Zinia Zaukuu, J.L. Identification of Botanical and Geographical Origins of Honey-Based on Polyphenols. Plant-Based Functional Foods and Phytochemicals, 1st ed.; Apple Academic Press: New York, NY, USA, 2021; pp. 125–161. [Google Scholar]
- Tananaki, C.; Rodopoulou, M.A.; Dimou, M.; Kanelis, D.; Liolios, V. The Total Phenolic Content and Antioxidant Activity of Nine Monofloral Honey Types. Appl. Sci. 2024, 14, 4329. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, J.A.; Mondragon Cortez, P.; Rodríguez Rodríguez, R.; Reséndiz Vázquez, J.A.; Rosas Ulloa, P. La miel de abeja y su importancia. Rev. Fuente 2010, 2, 4. [Google Scholar]
- Ahmed, S.; Othman, N.H. Honey as a potential natural anticancer agent: A review of its mechanisms. Evid.-Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef]
- Angarita, L.X.; Cobos, D.M. Estudio Cromatográfico por HPLC-UV, Cuantificación de Fenoles, Flavonoides y Evaluación de la Capacidad Antioxidante en Miel de Abejas. Bachelor’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2017. [Google Scholar]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant characteristics of honey from Mozambique based on specific flavonoids and phenolic acid compounds. J. Food Compos. Anal. 2020, 86, 103377. [Google Scholar] [CrossRef]
- Llinares Grau, M. Propiedades Antioxidantes de Mieles de Mozambique en Base a su Composición en Flavonoides y Ácidos Fenólicos. Bachelor’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2022. [Google Scholar]
- Peñarrieta, J.M.; Tejeda, L.; Mollinedo, P.; Vila, J.L.; Bravo, J.A. Phenolic compounds in food. Rev. Bol. Quim. 2014, 31, 68–81. [Google Scholar] [CrossRef]
- Ntakoulas, D.D.; Pasias, I.N.; Raptopoulou, K.G.; Proestos, C. Authenticity of Greek honey based on phenolic compounds and physicochemical characteristics. Food Chem. 2025, 476, 143465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Z.; Zhong, S.; Zeng, Z. (−)-Gallocatechin Gallate: A Novel Chemical Marker to Distinguish Triadica cochinchinensis Honey. Foods 2024, 13, 1879. [Google Scholar] [CrossRef]
- Castro, E.; Quicazán, M.; Mojica, A.; Zuluaga-Domínguez, C. Bioactive and physicochemical profile of honey collected from Colombian organic and conventional coffee growing areas. J. Apic. Res. 2021, 62, 518–529. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Maciel, L.G.; Nunes, D.S. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. TrAC Trends Anal. Chem. 2016, 80, 266–279. [Google Scholar] [CrossRef]
- Bonet Galarza, M. Desarrollo de un Método Analítico Para la Determinación de la Actividad Antioxidante Mediante HPLC. Bachelor’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2020. [Google Scholar]
- Tahir, H.E.; Arslan, M.; Mahunu, G.K.; Shi, J.; Zou, X.; Gasmalla, M.A.A.; Mariod, A.A. Data fusion approach improves the prediction of single phenolic compounds in honey: A study of NIR and Raman spectroscopies. eFood 2020, 1, 173–180. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borrás, M. Standardizing the analysis of phenolic profile in propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Ministerio de Medio Ambiente y Recursos Naturales (MMARN). Atlas de Biodiversidad y Recursos Naturales de la República Dominicana, 2nd ed.; Ministerio de Medio Ambiente y Recursos Naturales (MMARN): Santo Domingo, Dominican Republic, 2012; Available online: https://ambiente.gob.do/app/uploads/2016/10/ATLAS-2012.pdf (accessed on 5 September 2025).
- Dirección General de Ganadería (DIGEGA), Gobierno de la República Dominicana, Informe Producción Pecuaria. Available online: https://ganaderia.gob.do/transparencia/index.php/estadisticas/2024/category/1251-exportaciones-de-productos-pecuarios (accessed on 16 September 2025).
- Clúster Apícola Dominicano (CLUSAPIDOM). Diagnóstico del Sector Apícola de la República Dominicana. Oxfam Intermón. Santo Domingo, República Dominicana. Programa de Cooperación Binacional Haití-República Dominicana—Componente de Desarrollo del Comercio Informal. Number CRIS: FED/2017/040-148. 2020. [Google Scholar]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.L.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Lianda, R.L.; Sant’Ana, L.D.; Echevarria, A.; Castro, R.N. Antioxidant activity and phenolic composition of Brazilian honeys and their extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; Ribeiro, F.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- da Silva, I.A.; da Silva, T.M.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Bastos, L.E.; de Oliveira, E.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.; Hacke, A.C.M.; Mazer Etto, R.; Boligon, A.A.; Takeda, I.; Marques, J.A.; Pereira, R.P. Evaluation of the antioxidant activity and phenolic composition of different monofloral and polyfloral Brazilian honey extracts. Chem. Biodivers. 2024, 21, e202400971. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; González-Paramás, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant Characterization of Native Monofloral Cuban Honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Santos-Buelgae, C.; Morroni, G.; Simoni, S.; Forbes-Hernandez, T.Y. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Caja, G.; Nieto, A.; Elhadi, A.; Salama, A.A.K.; Piedrafita, J.; Albanell, E.; Riba, C.; De Linares, C.; Cardellach, P.; Belmonte, J. Seguimiento de un colmenar periurbano en el Vallés Occidental. In Proceedings of the XIX Jornadas de Producción Animal AIDA, Zaragoza, Spain, 1–2 June 2021; Asociación Interprofesional para el Desarrollo Agrario: Zaragoza, Spain, 2021; p. 35. Available online: https://www.aida-itea.org/aida-itea/files/jornadas/2021/comunicaciones/2021_SGEG_34.pdf (accessed on 9 September 2025).
- Caja, G.; Elhadi, A.; Nieto, A.; Hernández, J.; González-González, S.; Blanch, J.; Rojas, E.; González-Luna, S.; Sancho, G.; Salama, A. Evolución de la producción y composición de miel en el colmenar experimental UABee desde su creación: 2019–2021. In Proceedings of the 10º Congreso Nacional de Apicultura, Virtual, 10–12 February 2022; Asociación para el Fomento de los Congresos Apícolas, Ed.; Don Folio: Córdoba, Spain, 2022; pp. 64–65. Available online: https://www.pajueloapicultura.com/wp-content/uploads/2022/12/Libro_resumenes.pdf (accessed on 9 September 2025).
- Bertoncelj, J.; Polak, T.; Kropf, U.; Korošec, M.; Golob, T. LC-DAD-ESI/MS analysis of flavonoids and abscisic acid with chemometric approach for the classification of Slovenian honey. Food Chem. 2011, 127, 296–302. [Google Scholar] [CrossRef]
- Merken, H.M.; Beecher, G.R. Measurement of food flavonoids by high performance liquid chromatography: A review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: New York, NY, USA, 2025; Available online: https://www.xlstat.com (accessed on 9 September 2025).
- Guo, X.; Luo, Z.; Zhang, M.; Huang, L.; Wang, H.; Li, Y.; Qiao, X.; Li, A.; Wu, B. The spatiotemporal regulations of epicatechin biosynthesis under normal flowering and the continuous inflorescence removal treatment in Fagopyrum dibotrys. BMC Plant Biol. 2022, 22, 379. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Balzani, A.; Huertas, J.R.; Mercolini, L.; Rodríguez-Pérez, C. Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada). Biol. Life Sci. Forum 2023, 26, 74. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Los Indicadores Económicos del Sector Apícola. Spain. 2024. Available online: https://www.mapa.gob.es/dam/mapa/contenido/ganaderia/temas/produccion-y-mercados-ganaderos/sectores-ganaderos-2/apicultura/informacion-del-sector/indicadores-economicos/indicadores-2024-final_rev.pdf (accessed on 15 September 2025).
- Marcano Fondeur, E.d.J. Informe Sobre la Flora Apícola Dominicana. Santo Domingo, República Dominicana. Santo Domingo, Dominican Republic, 1973. Available online: https://botanicodesantiago.com/wp-content/uploads/2020/09/Marcano-La-Flora-Ap%C3%ADcola-de-la-Rep%C3%BAblica-Dominicana.pdf (accessed on 6 September 2025).
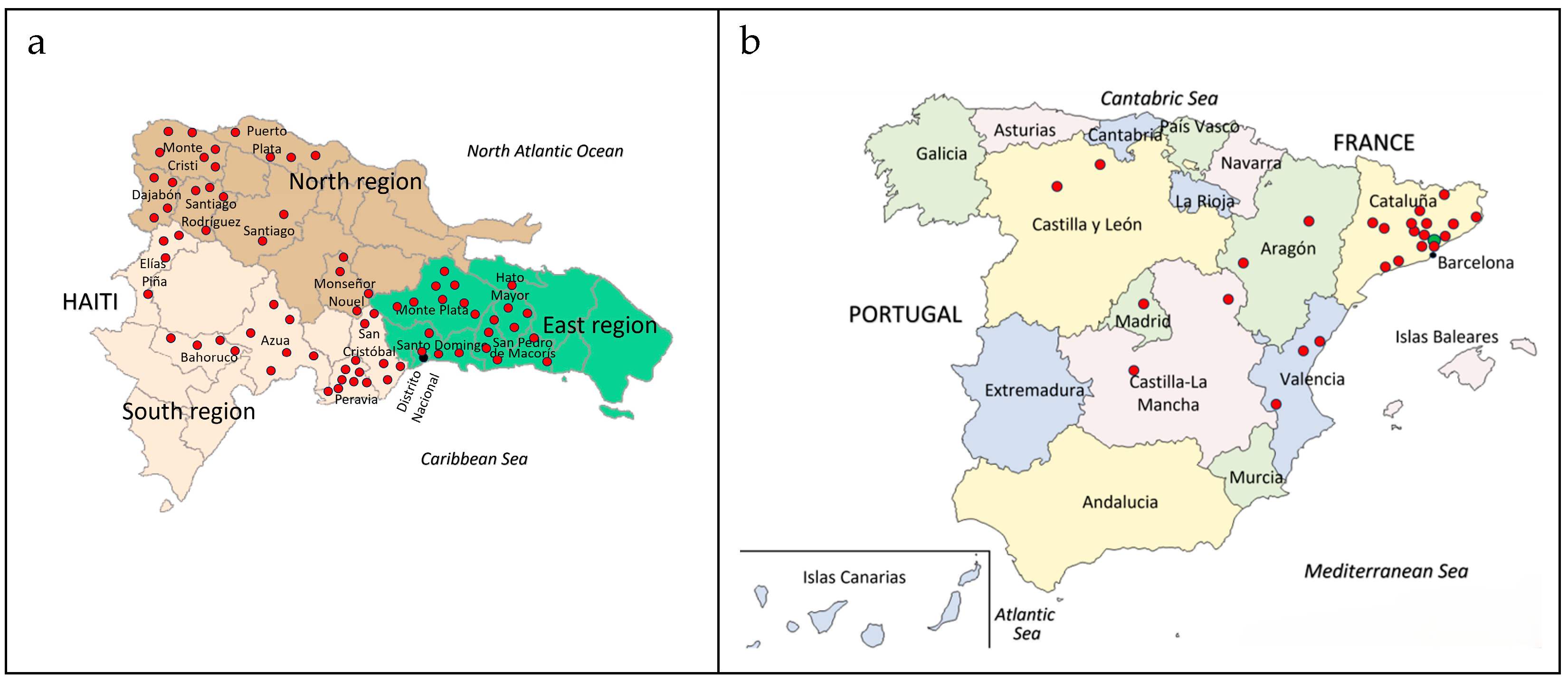
| Phenolic Compounds | RT (min) | λ (nm) | R2 | LOQ (mg/kg) |
|---|---|---|---|---|
| Flavonoids (FLV) | ||||
| Epicatechin | 13.036 | 280 | 0.992 | 0.015 |
| Rutin | 15.400 | 260 | 0.999 | 0.015 |
| Quercitin-3-glucoside | 16.416 | 260 | 0.992 | 0.015 |
| Quercitrin | 18.032 | 260 | 0.992 | 0.015 |
| Apigenin-7-glucoside | 18.192 | 320 | 0.994 | 0.015 |
| Quercetin | 23.429 | 380 | 0.996 | 0.015 |
| Naringenin | 26.147 | 290 | 0.992 | 0.015 |
| Kaempferol | 27.204 | 380 | 0.995 | 0.015 |
| Chrysin | 33.953 | 250 | 0.992 | 0.030 |
| Galangin | 34.605 | 250 | 0.997 | 0.015 |
| Phenolic acids (PHA) | ||||
| Gallic acid | 06.751 | 280 | 0.996 | 0.015 |
| 4-Hydroxybenzoic acid | 12.849 | 260 | 0.997 | 0.015 |
| Caffeic acid | 13.606 | 320 | 0.992 | 0.015 |
| Vanillic acid | 13.663 | 250 | 0.992 | 0.015 |
| p-Coumaric acid | 16.803 | 320 | 0.991 | 0.015 |
| Ellagic acid | 16.804 | 250 | 0.993 | 0.015 |
| Sinapic acid | 17.419 | 320 | 0.994 | 0.015 |
| Ferulic acid | 17.625 | 320 | 0.992 | 0.015 |
| Cinnamic acid | 24.679 | 280 | 0.991 | 0.015 |
| Country | Dominican Republic (D.R.) Regions | Spain Origins | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. R. Mean (SD) | Spain Mean (SD) | Anova F-Ratio | North | South | East | Anova F-Ratio | Commercial | Artisanal | Experimental | Anova F-Ratio | |||
| FLV | |||||||||||||
| Flavanols | |||||||||||||
| Epicatechin | 3.2 (3.7) | 3.1 (6.1) | ns | 2.3 (2.7) a | 5.3 (4.9) b | 1.6 (0.4) a | 8 *** | 4.2 (7.7) b | 2.6 (4.6) b | 0.63 (0.16) a | 1 ** | ||
| Flavanones | |||||||||||||
| Naringenin | 0.17 (0.05) b | 0.10(0.13) a | 20 *** | 0.20 (0.07) b | 0.16 (0.08) a | 0.16 (0.10) a | 6 ** | 0.07 (0.16) | 0.11 (0.11) | 0.14 (0.03) | ns | ||
| Flavones | |||||||||||||
| Apigenin-7-glucoside | 0.4 (0.8) b | 0.011 (0.034) a | 16 ** | 0.6 (0.9) | 0.15 (0.10) | 0.6 (0.9) | ns | 0.002 (0.005) | 0.002 (0.04) | <LOQ | 2 * | ||
| Chrysin | <LOQ | 2.3 (1.6) | 139 *** | <LOQ | <LOQ | <LOQ | ns | 1.4 (1.0) a | 2.8 (1.6) b | 4.8 (0.33) c | 20 *** | ||
| Flavonols | |||||||||||||
| Galangin | <LOQ | 1.01 (0.51) | ns | <LOQ | <LOQ | <LOQ | ns | 0.8 (0.4) a | 1.1 (0.5) b | 1.8 (0.19) c | 17 *** | ||
| Kaempferol | 0.17 (0.02) a | 0.4 (0.3) b | 48 *** | 0.17 (0.03) | 0.16 (0.01) | 0.17 (0.01) | ns | 0.3 (0.2) a | 0.5 (0.4) b | 0.338 (0.012) a | 3 * | ||
| Quercetin | 0.3 (0.11) a | 0.7 (0.19) b | 216 *** | 0.31 (0.15) b | 0.34 (0.01) b | 0.21 (0.04) a | 10 *** | 0.70 (0.19) b | 0.7 (0.2) b | 0.53(0.06) a | 3 * | ||
| Quercitrin | 0.8 (0.8) b | 0.5 (0.5) a | 5 * | 0.8 (0.7) | 0.8 (0.6) | 0.9 (1.6) | ns | 0.3 (0.5) | 0.6 (0.6) | 0.65 (0.13) | ns | ||
| Quercitin-3-glucoside | 1.1 (4.3) | 1.0 (2.8) | ns | 2.4 (7.3) | 0.3 (0.3) | 0.5 (0.4) | ns | 0.3 (0.2) b | 2 (4) a | 0.10 (0.03) b | 3 * | ||
| Rutin | 1.1 (1.0) | <LOQ | 63 *** | 1.04 (1.14) b | 1.9 (0.9) c | 0.35 (0.67) a | 16 *** | <LOQ | <LOQ | <LOQ | |||
| Σ average FLV | 10.8 | 14.2 | 7.8 | 8.8 | 4.5 | 8.1 | 10.4 | 9.0 | |||||
| PHA | |||||||||||||
| Hydroxybenzoic acids | |||||||||||||
| Ellagic acid | <LOQ | 4.0 (4.1) | 69 *** | <LOQ | <LOQ | <LOQ | ns | 3.3 (1.6) | 4.6. (6.0) | 5.1 (1.3) | ns | ||
| Gallic acid | 0.3 (0.3) | 0.04 (0.12) | 27 *** | 0.4 (0.6) b | 0.24(0.15) a,b | 0.18 (0.06) a | 3 * | 0.05 (0.15) | 0.03 (0.12) | <LOQ | ns | ||
| 4-Hydroxybenzoic acid | 2.0 (1.2) | 2.4 (1.7) | ns | 1.9 (1.1) | 2.2 (1.4) | 1.7 (1.2) | ns | 2.5 (2.6) | 2.3 (0.7) | 1.9 (0.4) | ns | ||
| Vanillic acid | 0.6 (0.5) | 12.0 (11.0) | 74 *** | 0.6 (0.3) a,b | 0.8 (0.3) b | 0.4 (0.2) a | 4 * | 6.2 (4.4) a | 14.7 (13.3) b | 27.0 (2.3) c | 15 *** | ||
| Hydroxycinnamic acids | |||||||||||||
| Caffeic acid | 0.4 (0.4) a | 1.8 (1.8) b | 39 *** | 0.4 (0.4) | 0.5(0.5) | 0.4 (0.4) | ns | 0.8 (0.7) a | 2.1 (1.9) b | 4.8 (0.5) c | 24 *** | ||
| Ferulic acid | 0.4 (0.6) | 0.5 (0.3) | ns | 0.5 (0.7) | 0.22 (0.07) | 0.5 (0.7) | ns | 0.39 (0.24) | 0.5 (0.4) | 0.60 (0.04) | ns | ||
| p-Coumaric acid | 0.3 (0.2) | 2.6 (0.45) | 18 *** | 0.30 (0.24) | 0.27 (0.11) | 0.27 (0.02) | ns | 1.5 (1.1) | 3.7 (6.6) | 2.5 (0.3) | ns | ||
| Sinapic acid | 0.20 (0.11) | 0.031 (0.13) | 64 *** | 0.15 (0.01) a | 0.26 (0.13) b | 0.19 (0.11) a | 8 *** | 0.006 (0.021) | 0.07 (0.21) | <LOQ | ns | ||
| Cinnamic acid | 0.3 (1.1) | 0.3 (0.7) | ns | 0.156 (0.014) | 0.5 (1.8) | 0.16 (0.02) | ns | 0.25 (0.75) | 0.4 (0.9) | 0.11 (0.04) | ns | ||
| Σ average PHA | 4.5 | 20.1 | 4.4 | 4.7 | 3.8 | 15.0 | 28.3 | 42.0 | |||||
| Function 1 83.8% | Function 2 8.8% | |
|---|---|---|
| Ferulic acid | −1.623 | 0.494 |
| Apigenin-7-glucoside | 1.428 | −0.027 |
| Chrysin | −1.124 | 0.919 |
| Naringenin | 1.121 | 0.451 |
| Quercetin | −0.697 | 0.725 |
| Caffeic acid | 0.664 | −1.892 |
| Quercitin 3-glucoside | 0.649 | 1.047 |
| Sinapic acid | 0.509 | −0.254 |
| Galangin | −0.469 | −0.570 |
| Galic acid | −0.467 | −0.669 |
| Rutin | 0.163 | 0.054 |
| 4-Hydroxibezoic acid | 0.052 | 0.205 |
| Predicted Group Membership | ||||||||
|---|---|---|---|---|---|---|---|---|
| Country Group | Subgroup | Dominican Republic (D.R.) | Spain | Total | ||||
| North | South | East | Commercial | Artisanal | Experimental | |||
| Original % | ||||||||
| D.R. | North | 54.2 | 8.3 | 37.5 | 0.0 | 0.0 | 0.0 | 100.0 |
| South | 15.4 | 84.6 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | |
| East | 0.0 | 9.1 | 90.9 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Spain | Commercial brands | 7.1 | 0.0 | 0.0 | 92.9 | 0.0 | 0.0 | 100.0 |
| Artisanal beekeepers | 0.0 | 0.0 | 0.0 | 23.1 | 73.1 | 3.8 | 100.0 | |
| Experimental apiary | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Cross-Validation % | ||||||||
| D.R. | North | 37.5 | 16.7 | 45.8 | 0.0 | 0.0 | 0.0 | 100.0 |
| South | 15.4 | 84.6 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | |
| East | 18.2 | 9.1 | 72.7 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Spain | Commercial brands | 7.1 | 0.0 | 0.0 | 89.3 | 0.0 | 3.6 | 100.0 |
| Artisanal beekeepers | 0.0 | 0.0 | 0.0 | 26.9 | 61.5 | 11.5 | 100.0 | |
| Experimental apiary | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogando-Rivas, P.; Juan-Borrás, M.; Caja, G.; Escriche, I. Usefulness of Flavonoids and Phenolic Acids in Differentiating Honeys Based on Geographical Origin: The Case of Dominican Republic and Spanish Honeys. Appl. Sci. 2025, 15, 11181. https://doi.org/10.3390/app152011181
Ogando-Rivas P, Juan-Borrás M, Caja G, Escriche I. Usefulness of Flavonoids and Phenolic Acids in Differentiating Honeys Based on Geographical Origin: The Case of Dominican Republic and Spanish Honeys. Applied Sciences. 2025; 15(20):11181. https://doi.org/10.3390/app152011181
Chicago/Turabian StyleOgando-Rivas, Paola, Marisol Juan-Borrás, Gerardo Caja, and Isabel Escriche. 2025. "Usefulness of Flavonoids and Phenolic Acids in Differentiating Honeys Based on Geographical Origin: The Case of Dominican Republic and Spanish Honeys" Applied Sciences 15, no. 20: 11181. https://doi.org/10.3390/app152011181
APA StyleOgando-Rivas, P., Juan-Borrás, M., Caja, G., & Escriche, I. (2025). Usefulness of Flavonoids and Phenolic Acids in Differentiating Honeys Based on Geographical Origin: The Case of Dominican Republic and Spanish Honeys. Applied Sciences, 15(20), 11181. https://doi.org/10.3390/app152011181








