Abstract
Two essential oils: cinnamon and clove were used to aromatize rapeseed honey for the first time. For comparison self-distilled and commercially purchased essential oils were used. All essential oils and obtained flavored honeys were assessed for antioxidant activity, volatile fraction composition (GC-MS) and polyphenolic profiles (HPTLC). The results indicated that while in the case of clove oils the differences in the enrichment of the honey composition were not dependent on the origin of essential oil, the effect of the self-distilled cinnamon oil addition was completely different than for its market equivalent. The commercial cinnamon oil contained a larger amount (59.58%) of eugenol than the self-produced oil, probably as a result of the admixture of cinnamon leaf oil or different raw material used for commercial essential oil production. The described studies may constitute a basis for introducing to the market a new type of product based on honey and phytoadditives. The essential oil flavored honey is characterized not only by new desirable organoleptic features but also by highly enhanced antioxidant and probably antibacterial properties, which, however, requires further research.
1. Introduction
The number of consumers looking for natural products with a beneficial effect on health and well-being is constantly growing. Food products with a beneficial health effect, especially those based on natural ingredients, are becoming increasingly popular. Undoubtedly, honey, herbs, medicinal plant extracts as well as essential oils are among such products. New concepts are emerging, which aim to increase the benefits of honey by combining it with various additives: other bee products, fruits, herbs and spices [1,2,3,4,5]. Such combinations are attractive to consumers, expand the product range and allow for innovative introduction of bee products and medicinal plants into the diet. It has been proven that the addition of herbs and fruits in dried or freeze-dried form has a positive effect on the antioxidant, antimicrobial and antiviral potential of such products [2,3,4,5,6], making them an interesting category of functional foods. The latest trends in enriching honey with phytochemicals include the introduction of plant extracts, which can facilitate product standardization. Known and studied examples include the addition of extracts from Sophora flowers [7], pine buds [8], chokeberry fruit [9] and a mixture of multiple extracts and essential oils [10]. The migration of elements and polyphenolic compounds from herbs and spices into the matrix of infused honey was also studied, confirming the significant influence of additives on the nutritional potential of infused honeys [11].
Essential oils (EOs), which are mixtures of volatile substances obtained from plants, have numerous well-known biological properties. They have long been used in medicine, cosmetics and food industries. Their main components belong to the groups of terpenes and terpenoids, aromatic compounds, phenols, aliphatic alcohols and ketones, and esters [12]. They exhibit antibacterial, antiviral, antioxidant, anti-inflammatory, insecticidal, anthelminthic, antiparasitic and cytotoxic effects [12,13]. One of the particularly well-known and described properties of essential oils is their antimicrobial effect. The complex composition of each oil is reflected not only in its characteristic mode of action but above all in its fragrance properties, guarantying their use to impart fragrance to cosmetics or food products. The method of obtaining oils depends on the physicochemical properties of the plant from which they are obtained, the diversified quality of the raw material and the place and time of collection. The main methods of obtaining essential oils include hydrodistillation, steam distillation, solvent extraction, enfleurage, cold pressing and maceration; various modern methods are also being sought [12].
Mixing honey with essential oils is a completely new idea, which not only creates new specific sensory properties but can also shape the bioactivity of obtained enriched honeys. The term ‘flavored honey’ is sometimes used to describe honey with the addition of fruit, juices or plant extracts, which also change the sensory characteristics of the honey [14]. The only known report of infusing honey with essential oils is the study of Mateescu et al. [15], who mixed mint, sage, basil and fennel oils with honey, obtaining products of good quality and at the same time an enriched profile of volatile aroma compounds. In turn, Paduraru et al. [10] used essential oils from Matricaria chamomilla, Salvia sclarea and Salvia officinalis together with royal jelly and numerous plant additives to enrich honey. Attempts have already been made to assess bioactivity of honey combined with essential oils (from eucalyptus, oregano and lavender) in in vitro and in vivo studies, achieving synergistic antioxidant, antibacterial and anti-inflammatory effects [16,17,18]. This gives rise to the assumption that the novel product in the form of honey enriched with essential oils also has enhanced biological potential while achieving a convenient form to incorporate into the diet. The aim of this study was to prepare and evaluate the effect of the addition of various essential oils (cinnamon and clove) on the antioxidant properties and consumer acceptability of rapeseed honey. Additionally, for the first time the impact of the type of oil (commercially available and self-distilled) was compared.
2. Materials and Methods
2.1. Material and Chemicals
Rapeseed honey was obtained from a local apiary (Sieklówka, Poland). Essential oils (clove and cinnamon) were purchased from Etja (Elblag, Poland). Cloves and cinnamon bark (unspecified botanical origin) (Prymat, Jastrzębie-Zdrój, Poland) were purchased at a local grocery store. Analytical standards (eugenol, cinnamaldehyde and kaempferol) were obtained from Sigma Aldrich (St. Louis, MO, USA). All used chemicals were of at least analytical grade.
2.2. Essential Oils Distillation
The essential oils were obtained by hydrodistillation using a Deryng apparatus (Lab-Szkło, Kraków, Poland). In a round-bottomed flask, 50 g of crushed raw material was weighed and then 500 mL of distilled water was poured. The flask was connected to the Deryng apparatus, the receiver was filled with water, and the setting and tightness of the tap were checked. The cooling water flow was turned on and heated for 3 h counted from the moment the contents of the flask began to boil and the first drops of oil appeared. The process was repeated as needed to obtain the appropriate volume of oils; 2.5 mL of clove oil and 1.5 mL of cinnamon oil were obtained in total.
2.3. Preparation of Flavored Honeys
One hundred grams of previously liquefied rapeseed honey was weighed into glass jars. Each sample was then subjected to a creaming process, which consisted of mixing them with a hand mixer for 3 min. Appropriate volumes of tested EOs were added to the honey samples prepared in this way, obtaining samples flavored with 0.1 and 0.3% (v/w) of each EO. The honey samples combined with the oils were further creamed for 2 more days in the same manner as before. After the creaming process, the honey was placed in the refrigerator (5 °C) for 2 weeks stabilization and then stored in dark conditions at room temperature (20 ± 2 °C). Honey analyses were performed after 1 and 6 months of storage.
2.4. Total Phenolic Content
Total phenolic content was determined for EOs (diluted with methanol) and honeys (20% aqueous solutions, further diluted if necessary) using the method described by Miłek et al. [3]. Total phenolic content was expressed in gallic acid equivalents based on the standard curve y = 0.336x (r2 = 0.9914).
2.5. Antioxidant Capacity
Antioxidant capacity was assessed by DPPH and FRAP (only for flavored honeys) methods, as described by Miłek et al. [3]. The same sample dilutions were used as in the case of the analysis of phenolic compounds. Results were expressed in Trolox equivalents based on standard curves (y = 15.553x; r2 = 0.9975 for DPPH and y = 0.1523x; r2 = 0.9995 for FRAP).
2.6. HPTLC Analysis
To prepare samples for HPTLC analysis, honeys were subjected to solid-phase extraction, removing sugars and concentrating polyphenols, according to a slightly modified method of Grabek-Lejko et al. [4], using Sep-Pak Plus Short C18 columns (Waters, Milford, MA, USA). The extraction procedure was carried out by passing through the columns sequentially: 10 mL of methanol, 10 mL of water acidified with HCl (pH 2), 100 mL of the sample (100 mL of 20% solution in acidified water), 100 mL of water at pH 2, and 5 mL of methanol. The purified fraction was subjected to chromatographic analyses. Chromatographic separation was performed using the HPTLC set (CAMAG, Muttenz, Switzerland), as in the work of Miłek et al. [3]. To determine polyphenolic compounds, HPTLC SilicaGel 60 F254 plates (Merck, Darmstadt, Germany) were used, on which 3 μL of appropriate extract or essential oil was applied as 8 mm wide bands from the lower edge of the plate. The standards used were kaempferol, eugenol and cinnamaldehyde (250 μg/mL). The separation was carried out in a chromatography chamber saturated with the mobile phase (chloroform, ethyl acetate and formic acid, 5:4:1). The obtained results were visualized using an HPTLC imager (TLC Visualizer, CAMAG, Muttenz, Switzerland) at wavelengths of 254 and 366 nm. Additionally, the plate was derivatized with p-anisaldehyde using an automatic TLC derivatizer (CAMAG, Derivatizer, Muttenz, Switzerland). The results were again documented in visible light and UV 366 nm.
2.7. GC-MS Analysis
Chromatographic analysis was performed to identify volatile organic compounds (VOCs) in essential oils (EOs) and selected honey samples spiked with EOs. The isolation of VOCs from a honey sample using headspace solid-phase microextraction (HS-SPME) and their chromatographic analysis (GC-MS) was carried out according to the method described by Matłok et al. [19]. Twenty grams of each honey sample were weighed into glass beakers and sealed with aluminum foil. A polydimethylsiloxane (PDMS) fiber (100 µm, Supelco, Bellefonte, PA, USA) was introduced into the control honey sample for 30 min and into the EO-enriched honey for 2 min. The fiber was then transferred to a Varian 450-GC gas chromatograph (Palo Alto, CA, USA) coupled with a Varian 250-MS mass spectrometer (Palo Alto, CA, USA) for analysis. A capillary column (30 m × 0.23 mm × 0.25 µm) with a moderately polar HP-5 stationary phase (Agilent Technologies, Santa Clara, CA, USA) and helium (purity >99.99%) as the mobile phase (flow rate: 1 mL/min) were used. During the analysis, the oven temperature was initially held at 50 °C for 5 min (isothermal) then increased at a rate of 10 °C/min to 300 °C and held at this final temperature for 5 min (isothermal). The essential oil was analyzed using the same chromatographic separation conditions as above, with the difference that 1 µL of essential oil was injected directly into the chromatograph dispenser (split 1:100). The total analysis time was 35 min. The identification of compounds was verified based on the comparison of their mass spectra with data available in the NIST 08 library, supported by Kovats retention indices. The Kovats retention indices were determined in accordance with the procedure described by Matłok et al. [19], by prior injection of a mixture of n-alkanes (C7–C30) into the gas chromatograph.
2.8. Organoleptic Evaluation
In order to analyze acceptance of the honey samples enriched with essential oils, the 10-point hedonic scale was used. The organoleptic evaluation included color, smell, consistency and taste as well as the overall note. Intensity of perception was evaluated from 1 (dislike extremely) to 10 (like extremely) by a 10-person sensory panel. Approximately 50 g of honey samples were given, with approximately 2 g taken for taste testing. Mineral water was used as a neutralizer between individual samples.
2.9. Statistical Analysis
The studies were performed in triplicate, and the obtained results were presented as the mean value with standard deviation (±SD). Statistical analysis of the significance of differences was performed using a t-test and Šidák’s multiple comparison test, preceded by a two-way ANOVA analysis, using GraphPad Prism 10.4.1 software (GraphPad Software Inc., San Diego, CA, USA).
3. Results and Discussion
3.1. Essential Oils Evaluation
In the first step, the essential oils used to enrich rapeseed honey were assessed for their composition and properties. For both types of oil, clove and cinnamon, two variants were compared: self-obtained by hydrodistillation and commercial equivalents purchased. In all four EO samples, the total content of phenolic compounds and antioxidant activity were assessed using the DPPH method (Table 1).

Table 1.
Total phenolic content and antiradical activity of used essential oils.
The values of the antioxidant potential of essential oils given in the literature vary greatly and are presented in different ways, which makes it difficult for comparison. While both tested clove oils were approximately similar in terms of both analyzed parameters (TPC and DPPH), the commercial cinnamon oil contained about 200 times more compounds, reducing the Folin-Ciocalteu reagent, than the self-distilled one. Similarly, the antiradical activity was many times higher for this EO (p < 0.001). For clove oils, significant differences were also shown, but the values were comparable. The total phenol content was also similar to the data reported in the scientific literature. Sarrami et al. [20] found a phenol content of 345.95 mg GAE/g for clove EO, while Zengin and Baysal [21] found 635.32 mg GAE/g. The data in the literature are often converted to mass rather than volume of the oil, but considering its density, reported in the range of 1.019–1.070 g/cm3 [22,23], it can be assumed that our results are in line with the literature data. In turn, for cinnamon oil, the density is, according to various data, 1.053–1.104 g/cm3 [24,25]. However, the content of phenolic compounds is strongly depending on the type of cinnamon or the addition or contamination of the essential oil from the leaves of this plant. The content of polyphenols, especially flavonoids, is higher in the leaves than in the bark of cinnamon [26]. In the study by Dev et al. [27], TPC values obtained for EO from Indian cinnamon bark of unspecified species ranged from 0.17 to 1.39 mg GAE/mL, depending on the roasting conditions of the bark. These values are similar to ours obtained for the oil distilled under laboratory conditions (1.98 mg GAE/mL). Moreover, a higher result (5.7 mg/g of oil) was obtained by Kamaliroosta et al. [28] for the essential oil from C. zeylanicum bark. In turn, Aydin et al. [29] report a value of 268.2 mg/mL for commercially purchased C. zeylanicum oil, but it is not precisely specified whether this was pure oil from cinnamon bark. High phenolic contents may suggest the presence of cinnamon leaf essential oil. For clove oils, IC50 values for the DPPH method range from 15.8 to 108.85 μg/mL according to Alfikri et al. [23], depending on the degree of maturity of the clove buds. In turn, for the FRAP method, based on a different mechanism, the value of the reducing power was 4357.45 mmol TE/mL [21]. For cinnamon essential oils the data scatter is even greater: IC50 values range from 147 μg/L [30] to 0.41 mg/mL [31].
The profile of volatile compounds present in the analyzed essential oils was also determined using GC-MS (Table 2).

Table 2.
Volatile composition of essential oils tested.
Chromatographic analysis showed that the compound found in the largest amount in distilled cinnamon EO was cinnamaldehyde (89.62%), while in commercial oil it was eugenol (59.58%). Eugenol is known to be the main component of cinnamon leaf essential oil, ranging up to 75% [32]; therefore, its dominance in commercial oil suggests the admixture of leaf oil. Cinnamaldehyde was also found in a significant amount in commercial oil (34.62%); however, due to the dominance of eugenol in this case it constituted a smaller percentage of the total composition. Similarly, in the study by Gotmare and Tambe [33], the main component of cinnamon EO obtained by distillation was cinnamaldehyde in the amount of 91.5%. Moreover, gas chromatography (GC-MS) of the EO obtained from the bark of Cinnamomum zeylanicum identified 71.5% of cinnamaldehyde, which was the main component of this oil [34]. Compounds such as linalool or eugenol acetate, which also only occurred in commercial oil, accounted for more than 1%. However, less than 1% were observed for caryophyllene, cinnamyl acetate or benzyl benzoate content. Other compounds identified in commercial oil such as camphene, D-limonene, 3-carene or copaene and others presented in the table occurred in trace amounts. In turn, in the distilled oil, apart from cinnamaldehyde, 1,6-diphenyl-1,5-hexadiene was detected, constituting 4.45%. Less than 1% was copaene or (Z)-3-phenylacrylaldehyde, which also occur in the commercial oil. Others identified included benzopropanal (0.73%), cineole (0.42%) and (Z)-2-methoxycinnamaldehyde (0.49%). Trace amounts also included styrene and fenchol. A total of 20 compounds were identified in the commercial oil and 27 in the distilled oil. In the case of cinnamon EOs, the plant species is strongly important. Studies have shown that raw materials of unspecified geographical origin usually contain large amounts of coumarin, a compound with hepatotoxic properties, while in good quality Ceylon cinnamon (Cinamonum verum), the content of this compound was trace, whereas other bioactive components were dominated [35].
Also, GC-MS analysis of clove EOs, including both distilled and commercial variants, revealed significant differences in their chemical composition, which may affect their properties and potential applications. In both samples, eugenol was the dominant compound, accounting for 78.826% and 78.551% of the self-distilled and commercial EOs, respectively. Caryophyllene was the second most abundant compound, accounting for 8.965% of the distilled oil and 7.125% of the commercial oil. It is a sesquiterpene known for its anti-inflammatory properties. Humulene (1.244%) was also identified in the distilled oil, which was not present in the commercial oil. Eugenol acetate was present in both samples but at a higher level in the commercial oil (12.186%) than in the distilled oil (6.345%). This compound adds a sweet, balsamic note to the scent of clove oil.
Although the strong differences were found for commercial and self-distilled EOs, both were used to spike rapeseed honey.
3.2. Enriched Honeys Evaluation
The total content of phenolic compounds and antioxidant capacity (DPPH and FRAP methods) were checked in honeys enriched with essential oils in comparison to the control rapeseed honey (Table 3).

Table 3.
Total phenolic content and antioxidant capacity of flavored honeys.
In all cases, except for the addition of laboratory-distilled cinnamon oil, a multiple enhancement of the antioxidant activity of honey and the content of total phenolic compounds was noted. Moreover, the enrichment was dose dependent, the higher proportion of EO added to the honey, the higher enrichment was observed. The lack of significant enrichment with the addition of distilled cinnamon oil correlates with its weaker properties as such. The strongest effect of enhancing the properties of honey was achieved in the case of antioxidant potential measured by the FRAP method, which reached above 50 times the initial value for pure honey. A significant concentration of active compounds or synergistic action of the oil components affects its antioxidant activity. According to Nurzyńska-Wierdak [36], the presence of eugenol most likely influenced the strong antioxidant properties of basil or clove oil. Our own studies showed that eugenol was the compound found in the largest amount in commercial cinnamon EO, while in distilled EO a small amount was determined. In commercial oil, larger amounts of compounds with antioxidant activity were identified, while in distilled oil, the same compounds were found in trace amounts or not at all. Hence, enriching honey with commercial oil could have translated into strengthening its antioxidant activity compared to honey with the addition of distilled oil, which did not show this strengthening.
Rapeseed honey is known to be one of the weakest varieties of honey in terms of antioxidant potential, which is why it is justified to choose this variety for enrichment [5]. Similarly, the enhancement of its health-promoting properties has been observed many times before with the introduction of various plant additives [3,4,5,11]. This method of increasing the health-promoting potential of honey can be considered biofortification and can also be achieved by adding extracts isolated from plants [8]. Essential oils were previously occasionally used to flavor honey, but it has been shown that this does not deteriorate the quality parameters of honey [15]. The use of essential oils instead of powdered herbs or spices may have the advantage of reducing the risk of microbiological contamination of such a product. Introducing plant additives may carry such a risk [14]. From the point of view of the bioactivity of such a new product, the combination of honey with essential oils may promote better dissolution of the oils and their absorption in the intestines [17]. It is worth emphasizing that there were no changes in honey features such as color or consistency with the low amount of the additive introduced. Moreover, there was no separation observed during storage, which sometimes happens when herbs or fruits are added.
The prepared flavored honeys were also assessed for storage stability for a period of 6 months. In most cases, no significant changes nor significant increases in the tested properties were observed. A statistically significant reduction in the antiradical potential of DPPH was observed only in the case of honeys with a higher admixture of clove oil and commercial cinnamon oil. Previous studies indicate different behavior of honeys with additives during storage. Both significant decreases in antioxidant properties and increases, even up to 150% compared to the product before storage, were observed [5]. However, when the additive is introduced in the form of solid particles, there is variability caused by the degree of fragmentation of the additive and the ability to migrate bioactive substances into the honey matrix.
Honeys enriched with a higher content of each essential oil as well as the control rapeseed honey were also subjected to analysis of the volatile composition (Table 4).

Table 4.
Volatile composition of control honey and honeys enriched with EOs.
Analysis of the volatile fraction of the control rapeseed honey confirms that its composition is not particularly rich. Its volatile fraction was dominated by two compounds: 2,4-di-tert-butylphenol (31.366%) and 1,3,5,6,7-pentamethylbicyclo [3.2.0] hepta-2,6-diene (15.126%). In addition, benzoic acid, common in Polish varietal honeys [37], and 2-phenylethanol, previously identified in rapeseed honeys [38], were also identified in smaller amounts.
Cinnamaldehyde, in the case of honey with distilled oil, constituted 90.29% and was the compound present in the largest amount by far. In honey with commercial oil, it constituted 34.54%. The main compound in honey with commercial oil was eugenol (59.46%). The compounds identified for it, which constituted more than 1%, linalool or 2-methoxy-4-(2-propenyl)phenol acetate, did not occur in honey with distilled oil. Less than 1%, in both the case of honey with distilled oil and commercial oil, was (Z)-3-phenylacrylaldehyde. In addition, in honey with commercial oil, one can also distinguish, among others, benzyl benzoate and caryophyllene, which constitute trace amounts in honey with distilled oil. Cineole was detected only for honey with distilled oil in an amount of more than 2%. Other compounds identified exclusively for this sample and constituting less than 1% or trace amounts included copaene, benzofuran, D-limonene, camphene, styrene, bornyl acetate and others. Eugenol appears in high concentrations in honey with the addition of clove oils, dominating the chemical composition (over 94% in both cases with additives). The presence of dominant volatile compounds originating from essential oils, mainly eugenol and cinnamaldehyde, covers the natural fragrance notes of rapeseed honey, which is analytical evidence of the effective flavoring of honey by introducing essential oils into it. The effective transfer of characteristic volatile compounds into honey has been observed previously when honey was enriched with rosemary, sage, oregano, mint, thyme, fennel oil and basil oil [15].
The extracts of honeys flavored with essential oils prepared by SPE as well as the oils themselves were subjected to additional qualitative composition analyses by HPTLC with p-anisaldehyde derivatization. The image of the plates in UV light 254 nm (before derivatization) and white light and UV 366 nm (after derivatization) is shown in Figure 1 and Figure 2 for honeys with cinnamon and clove oil, respectively.
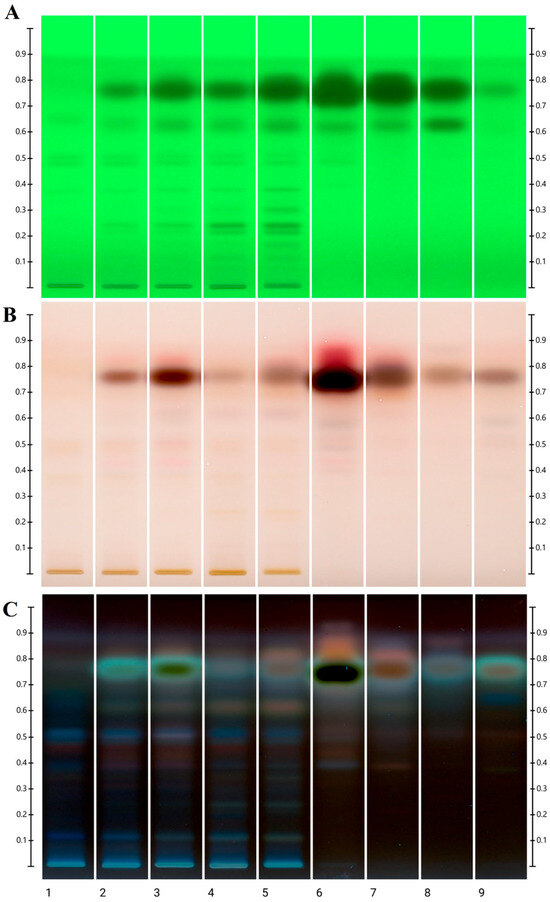
Figure 1.
Image of HPTLC plate for honeys with cinnamon essential oil: (A)—in UV light 254 before derivatization, (B)—in white light after derivatization, (C)—in UV light 366 nm after derivatization. Tracks description: 1—control rapeseed honey, 2—honey with 0.1% commercial oil, 3—honey with 0.3% commercial oil, 4—honey with 0.1% distilled oil, 5—honey with 0.3% distilled oil, 6—commercial essential oil, 7—distilled essential oil, 8—cinnamaldehyde, 9—eugenol.
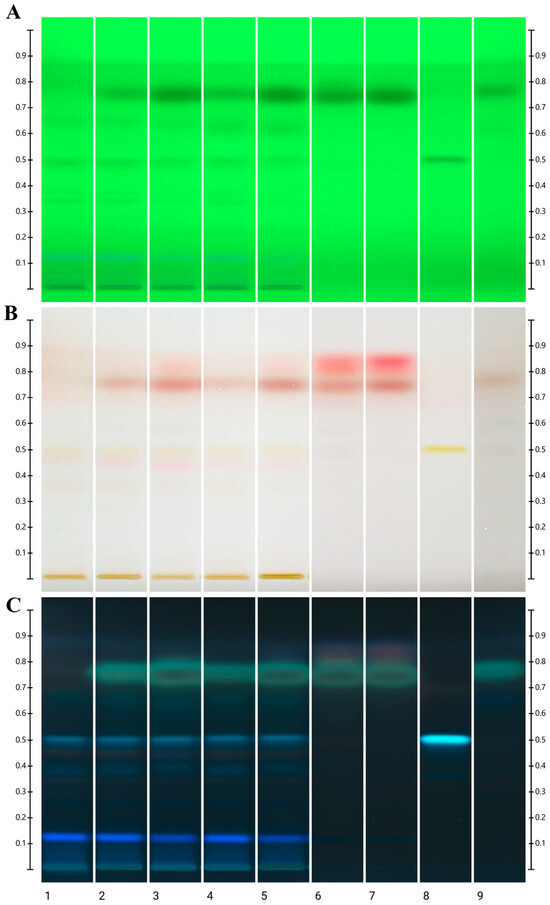
Figure 2.
Image of HPTLC plate for honeys with clove essential oil: (A)—in UV light 254 before derivatization, (B)—in white light after derivatization, (C)—in UV light 366 nm after derivatization. Tracks description: 1—control rapeseed honey, 2—honey with 0.1% commercial oil, 3—honey with 0.3% commercial oil, 4—honey with 0.1% distilled oil, 5—honey with 0.3% distilled oil, 6—commercial essential oil, 7—distilled essential oil, 8—kaempferol, 9—eugenol.
In chromatographical images of all honey extracts tested, a similar arrangement of bands corresponding to compounds originating from the initial rapeseed honey was observed. The identified characteristic marker is kaempferol (Rf = 0.51), a flavonoid abundantly found in Polish honey of this variety [37], as well as another compound, not identified in detail, with a band at Rf = 0.12 with a characteristic navy blue color.
In the case of the admixture of cinnamon oils distilled in laboratory conditions, the revealed pattern of bands in the chromatograms is richer than for the commercial oil. However, there is no clear band originating from eugenol (Rf = 0.78). A very intense, overloaded eugenol band was also present in the commercial oil, which confirms the results from the GC-MS analysis. In a similar Rf range, cinnamaldehyde occurs (present in both oils and honeys with their addition), but the band differs in color from the eugenol band. In addition, in the visualization in UV light 254, an additional band for cinnamaldehyde is revealed, probably from the isomer, which is also visible in all samples. Eugenol was also the dominant band visible in both tested clove oils and honeys with their addition. In fact, next to it, only in honey samples with 0.3% addition of both oils, an additional red band is visible in visible light after derivatization above eugenol (Rf = 0.86). HPTLC analyses confirm that rapeseed honey was significantly enriched by the addition of essential oils, but only the dominant components of the oils were detectable. However, this method allows for quick and relatively easy comparison of individual samples.
The results of the organoleptic evaluation are presented in the form of radar charts (Figure 3).
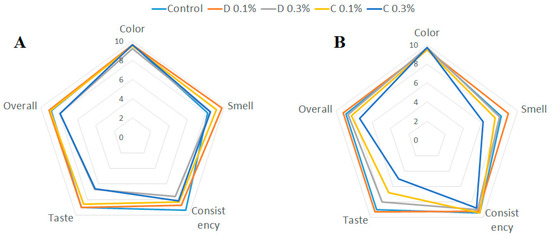
Figure 3.
Results of organoleptic evaluation. (A)—honeys with cinnamon essential oils, (B)—honeys with clove essential oils.
In terms of color, all honeys received high average scores (≥9.2). The smell was rated high for honey enriched with distilled oils at a concentration of 0.1%, and increasing the concentration caused too intense a smell, which was not appreciated by the assessors. The smell of honey with the addition of commercial oils, especially clove, was rated lower, where the smell was described as too sharp. In terms of taste, honeys with distilled oils were rated similarly, and the ratings were higher than honey with the addition of commercial oils. The introduction of essential oils slightly worsened the consistency of honey in the consumer assessment; the honey was described as slightly “runny”. In general, the idea of honey with the addition of essential oils was positively assessed by the evaluators, with a lower share of self-distilled oils being indicated as optimal.
In the case of creamed honeys with plant additives, consumer acceptability is of particular importance. It determines whether the developed product has a chance of success on the market. The attractiveness of honeys with various spice and fruit additives, including cinnamon, was studied by Predanocyova and Šedik [39]. The evaluators indicated features that positively distinguished individual products: color in the case of honey with raspberries, and taste for honey with cinnamon, hazelnuts and cocoa. Honey with cinnamon was generally rated the highest among those examined. Similarly, honey with cinnamon was the most accepted by consumers in another study, ahead of honeys with cardamom and ginger [1]. So far, honeys with the addition of essential oils have not been assessed in this way; there are only reports on the evaluation of honeys enriched with chokeberry extracts, for which it was found that the optimal sensory content of the additive is 0.3–0.4% [9].
4. Conclusions
A new type of product produced by admixing cinnamon or clove EOs to rapeseed honey was proposed. The flavored honeys obtained in this way were positively assessed in terms of organoleptic properties, and a strong enrichment of rapeseed honey in volatile substances from essential oils was also confirmed by GC-MS. Depending on the type of oil (available commercial or self-distilled), the composition of volatilome was strongly dependent on the composition of EOs used. A multiple enhancement of antioxidant properties was obtained in comparison with the control initial honey. To sum up, it seems optimal to add no more than 0.1 v/w of essential oil, and it should be of high quality. Further testing of such products seems justified, especially in terms of expected enhanced antibacterial activity.
Author Contributions
Conceptualization, M.M. and M.D.; methodology, M.M. and T.P.; software, M.T.; validation, M.M., T.P. and M.T.; formal analysis, M.M.; investigation, M.M., M.T., A.S., P.T. and T.P.; resources, M.D.; data curation, M.M. and M.T.; writing—original draft preparation, M.M.; writing—review and editing, M.D.; visualization, M.T.; supervision, M.D.; project administration, M.M.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the Polish Ministry of Science and Higher Education research project within the University of Rzeszów PB/KCHTZ/2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GC-MS | Gas chromatography coupled with mass spectrometry |
| HPTLC | High performance thin layer chromatography |
| EO | Essential oil |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| VOCs | Volatile organic compounds |
| HS-SPME | Headspace solid-phase microextraction |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalents |
| TE | Trolox equivalents |
| Rf | Retardation factor |
References
- Wilczyńska, A.; Newerli-Guz, J.; Szweda, P. Influence of the addition of selected spices on sensory quality and biological activity of honey. J. Food Qual. 2017, 2017, 6963904. [Google Scholar] [CrossRef]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwińczuk, W.; Puchalski, C.; Dżugan, M. The effect of adding the leaves and fruits of Morus alba to rape honey on its antioxidant properties, polyphenolic profile, and amylase activity. Molecules 2020, 25, 84. [Google Scholar] [CrossRef]
- Miłek, M.; Grabek-Lejko, D.; Stȩpień, K.; Sidor, E.; Mołoń, M.; Dżugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dżugan, M. Antiviral and antibacterial effect of honey enriched with Rubus spp. as a functional food with enhanced antioxidant properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Sidor, E.; Hęclik, J.; Lecka-Szlachta, K.; Dżugan, M. The antioxidant, antibacterial and anti-biofilm properties of rapeseed creamed honey enriched with selected plant superfoods. Antibiotics 2023, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Štajner, D.; Popović, B.M.; Čanadanović-Brunet, J.; Dilas, S.; Ćetković, G. Nutritive composition and free radical scavenger activity of honey enriched with of Rosa spp. LWT-Food Sci. Technol. 2014, 55, 408–413. [Google Scholar] [CrossRef]
- Đorđević, S.; Nedić, N.; Pavlović, A.; Milojković-Opsenica, D.; Tešić, Ž.; Gašić, U. Honey with added value—Enriched with rutin and quercetin from Sophora flower. J. Herb. Med. 2022, 34, 100580. [Google Scholar] [CrossRef]
- Szanto, L.G.; Marc, R.A.; Mureşan, A.E.; Mureșan, C.C.; Puşacş, A.; Ranga, F.; Muste, S. Biofortification of acacia and polyflower honey with Pine sylvestris L. bud extracts: Exploring antioxidant variation across developmental stages for enhanced nutritional value. Plant Foods Hum. Nutr. 2025, 80, 47. [Google Scholar] [CrossRef]
- Wang, J.; Hao, J.; Wang, J.; Wang, S.; Fan, Z. Preparation of functional food with enhanced antioxidant properties by adding Aronia melanocarpa polyphenol honey. Foods 2024, 13, 3852. [Google Scholar] [CrossRef]
- Paduraru, E.; Jijie, R.; Simionov, I.A.; Gavrilescu, C.M.; Ilie, T.; Iacob, D.; Lupitu, A.; Moisa, C.; Muresan, C.; Copolovici, L.; et al. Honey Enriched with Additives Alleviates Behavioral, Oxidative Stress, and Brain Alterations Induced by Heavy Metals and Imidacloprid in Zebrafish. Int. J. Mol. Sci. 2024, 25, 11730. [Google Scholar] [CrossRef]
- Czipa, N.; Phillips, C.J.C.; Topa, E.; Kovács, B. Release of elements and phenolic and flavonoid compounds from herbs and spices into acacia honey during infusion. J. Food Sci. Technol. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Żukowska, G.; Durczyńska, Z. Properties and applications of essential oils: A review. J. Ecol. Eng. 2024, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hmar, E.B.L.; Zothantluanga, J.H.; Sharma, H.K. Essential oils: A review on their salient biological activities and major delivery strategies. Sci. Vis. 2020, 20, 54–71. [Google Scholar] [CrossRef]
- Żebracka, A.; Winiarska-Mieczan, A.; Nowakowicz-Dębek, B.; Banach, M.; Drabik, A.; Pulit-Prociak, J.; Chmielowiec-Korzeniowska, A. Assessment of the microbiological quality and bactericidal properties of flavoured honey. Med. Weter. 2022, 78, 556–562. [Google Scholar] [CrossRef]
- Mateescu, C.; Duta, D.; Onisei, T.; Şerbancea, F.; Utoiu, C.; Manolache, F.A.; Dune, A. Flavored cream honey—A healthy food choice for consumers. In Proceedings of the ISB-INMA TEH 2020 International Symposium, Bucharest, Romania, 30 October 2020; pp. 236–245. [Google Scholar]
- Imtara, H.; Al-Waili, N.; Aboulghazi, A.; Abdellaoui, A.; Al-Waili, T.; Lyoussi, B. Chemical composition and antioxidant content of Thymus vulgaris honey and Origanum vulgare essential oil; their effect on carbon tetrachlorideinduced toxicity. Vet. World 2021, 14, 292–301. [Google Scholar] [CrossRef]
- Assaggaf, H.M.; Mrabti, H.N.; Rajab, B.S.; Attar, A.A.; Hamed, M.; Sheikh, R.A.; Bouyahya, A. Singular and combined effects of essential oil and honey of in vivo findings. Molecules 2022, 27, 5121. [Google Scholar] [CrossRef] [PubMed]
- Ángyán, V.D.; Balázs, V.L.; Kocsis, M.; Kocsis, B.; Horváth, G.; Farkas, Á.; Nagy-Radványi, L. Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens. Antibiotics 2025, 14, 146. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Piechowiak, T.; Balawejder, M. Influence of drying temperature on the content of bioactive compounds in Scots pine (Pinus sylvestris L.) shoots as well as yield and composition of essential oils. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 15–24. [Google Scholar] [CrossRef]
- Sarrami, S.; Mohajeri, F.A.; Sadeghizadeh-Yazdi, J.; Jambarsang, S.; Sadrabad, E.K. Chemical composition and antioxidant activity of clove essential oil and its effect on stability of sesame oil under accelerated condition. J. Nutr. Food Secur. 2023, 8, 343–352. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antioxidant and antimicrobial activities of thyme and clove essential oils and application in minced beef. J. Food Process. Preserv. 2015, 39, 1261–1271. [Google Scholar] [CrossRef]
- Caroko, N.; Hartati, I. Assessment of production rate and quality analysis of essential oil of clove oil obtained from hydro-distillation of clove leaves. E3S Web Conf. 2023, 425, 04007. [Google Scholar] [CrossRef]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) bud oil at the different phenological stages in young and mature trees. Scientifica 2020, 2020, 9701701. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.F.S.; Yudice, E.D.C.; Mitra, S.K.; Rosa, D.S. Characterization of rosewood and cinnamon cassia essential oil polymeric capsules: Stability, loading efficiency, release rate and antimicrobial properties. Food Control 2021, 121, 107605. [Google Scholar] [CrossRef]
- Hasibuan, R.; Hidayati, J.; Iqbal, M.; Pratama, M.F.D.; Fazillah, R.; Pramananda, V. Extraction of essential oil from cinnamon (Cinnamomum burmannii) bark using microwave assisted extraction method. IOP Conf. Ser. Earth Environ. Sci. 2024, 1352, 012003. [Google Scholar] [CrossRef]
- Abeysekera, W.P.K.M.; Premakumara, G.A.S.; Ratnasooriya, W.D. In vitro antioxidant properties of leaf and bark extracts of Ceylon cinnamon (Cinnamomum zeylanicum Blume). Trop. Agric. Res. 2013, 24, 128–138. [Google Scholar]
- Dev, M.; Ghosh, M.; Bhattacharyya, D.K. Effects of temperature and time of roasting on the physicochemical and antimicrobial characteristics of cinnamon bark oil. Int. J. Pharm. Sci. Res. 2021, 12, 6692–6695. [Google Scholar] [CrossRef]
- Kamaliroosta, L. Extraction of cinnamon essential oil and identification of its chemical compounds. J. Med. Plants Res. 2012, 6, 609–614. [Google Scholar] [CrossRef]
- Aydin, S. The investigation of total phenolic content and antioxidant properties of some essential oils in Turkey. Biol. Nyssana 2024, 15, 23–27. [Google Scholar] [CrossRef]
- Brodowska, M.K.; Brodowska, J.A.; Śmigielski, K.; Łodyga-Chruścińska, E. Antioxidant profile of essential oils and extracts of cinnamon bark (Cinnamomum cassia). Eur. J. Biol. Res. 2016, 6, 310–316. [Google Scholar] [CrossRef]
- Martiniaková, S.; Ácsová, A.; Hojerová, J.; Krepsová, Z.; Kreps, F. Ceylon cinnamon and clove essential oils as promising free radical scavengers for skin care products. Acta Chim. Slovaca 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Shahina, Z.; Molaeitabari, A.; Sultana, T.; Dahms, T.E.S. Cinnamon leaf and clove essential oils are potent inhibitors of Candida albicans virulence traits. Microorganisms 2022, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Gotmare, S.; Tambe, E. Identification of chemical constituents of cinnamon bark oil by GCMS and comparative study garnered from five different countries. Front. Res. C Biol. Sci. 2019, 19, 33–42. [Google Scholar]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020, 5190603. [Google Scholar] [CrossRef]
- Sowa, P.; Tarapatskyy, M.; Puchalski, C.; Dżugan, M. Quality evaluation of cinnamon marketed in Poland on the basis of determining ratio of cinnamaldehyde-to-coumarin content. Zywnosc. Nauka. Technol. Jakosc 2019, 26, 113–125. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Terapeutyczne właściwości olejków eterycznych. Ann. Univ. Mariae Curie-Sklodowska Sectio EEE Hortic. 2015, 25, 1–19. (In Polish) [Google Scholar]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Ruisinger, B.; Schieberle, P. Characterization of the key aroma compounds in rape honey by means of the molecular sensory science concept. J. Agric. Food Chem. 2012, 60, 4186–4194. [Google Scholar] [CrossRef] [PubMed]
- Predanócyová, K.; Šedík, P. Honey market challenges: Flavored honey as healthy food choice for consumers. J. Microbiol. Biotechnol. Food Sci. 2024, 13, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).