1. Introduction
With the transformation of economic structures in emerging economies and developing countries, the continuous consumption of traditional energy sources and shallow-depth mineral resources has increased annually, exerting significant pressure on energy security and stability. Coalbed methane (CBM) is an unconventional natural gas that plays a vital role in optimizing the energy supply stability and facilitating the transition to clean energy. Its development and utilization are inevitable outcomes of the future diversification of the energy industry. The genesis of CBM is generally classified into biogenic, thermogenic, and mixed origins [
1,
2,
3]. Approximately 20% of global natural gas resources may be biogenic [
4], with Permian coals in the Bowen Basin and Jurassic Walloon coal measures in the Surat Basin still actively generating biogenic methane [
5,
6]. Extensive research has been conducted on the factors and mechanisms that influence biogenic methane production from coal [
7,
8,
9,
10].
In recent years, researchers have successively proposed the concept of microbially enhanced coalbed methane (MECBM) [
11,
12]. This technology, based on anaerobic fermentation theory, aims to degrade organic components in coal through specialized metabolic pathways and biological enzyme systems of indigenous coal seam microorganisms, generating methane, CO
2, and light hydrocarbons. MECBM holds considerable potential for improving coal mining and utilization efficiency. However, coal biomethanation is a multistep process in which methane production is influenced by factors such as coal rank, liquid environment, and microbial characteristics [
13,
14,
15,
16]. Accordingly, researchers have optimized anaerobic fermentation processes from multiple perspectives. For instance, supplementing nutrients such as nitrogen, phosphorus, cobalt, nickel, and iron can stimulate methanogen growth and enhance biological enzyme-mediated degradation efficacy [
17,
18,
19]. Concurrently, some studies have utilized coal as the sole nutrient source for microbial screening and directional domestication [
20,
21]. Since individual strains typically fail to achieve efficient coal methanation [
22,
23,
24], constructing synthetic microbial consortia by combining domesticated microbial communities [
25] provides a novel approach for CBM enhancement. Additionally, coal pretreatment strategies—including physical methods (e.g., pulverization, hydraulic fracturing) to increase specific surface area and chemical methods (e.g., alkali treatment, bio-oxidation, etc.) to promote coal molecular depolymerization—offer innovative pathways for advancing microbial coal gasification.
Methanogens, as a group of strictly anaerobic archaea, exhibit highly specialized metabolic capabilities. They are generally restricted to utilizing simple molecules such as acetate, hydrogen, carbon dioxide, formate, and methanol. This gives rise to various methanogenic pathways, including hydrogenotrophic, acetoclastic, and methylotrophic types [
26,
27,
28,
29]. These archaea lack the extensive enzymatic systems found in many other microorganisms; they cannot independently hydrolyze or ferment complex organic compounds like proteins, polysaccharides, or lipids into simpler substrates. Consequently, their survival in natural environments depends on close syntrophic interactions with hydrolytic and fermentative bacteria. This consequently implies that the efficiency of coal biodegradation depends not only on consortium structural parameters (such as richness and diversity) but, more critically, on the functional adaptive capacity of the microbial community. That is, under environmental influences, the population structure undergoes dynamic succession and adjusts its metabolic strategies—for example, the methanogenic pathways shifting from solely acetoclastic to a versatile mix of multiple pathways—to efficiently respond to variations in substrate composition and concentration, thereby maintaining the stability of system function [
30,
31]. Furthermore, in situ coal biodegradation for methane production faces efficiency limitations owing to the availability of water-soluble compounds, aggregation of structures, and nutrient scarcity in geological formations.
Most studies have focused on the conversion of coal into methane in batch reactors [
32,
33]. While batch systems facilitate better control of initial conditions, easier replication, and lower costs, microbial processes may terminate due to substrate depletion or the accumulation of inhibitory byproducts [
34]. Intriguingly, coal remains bioavailable even after methane production ceases [
35], with substrate limitations primarily linked to nitrogen and phosphate availability, as evidenced by methane production reactivation following supplementation of these critical nutrients. However, microbial coal gasification technology has not been widely implemented due to limitations in gasification costs and efficiency. Therefore, developing a low-cost, high-efficiency, and widely distributed catalyst for coal biomethanation has become a critical research priority.
This study employed a fed-batch reactor system to conduct anaerobic fermentation experiments of coal. Following the complete cessation of gas production in all reactors, the control group was supplemented with culture medium while the experimental group received biomass supplementation. The gas production characteristics (mixed gas, CO2, and methane) after nutrient supplementation were systematically analyzed to evaluate the feasibility of biomass-mediated reactivation of anaerobic degradation processes in reactors and their gas generation performance. Through comprehensive characterization of microbial communities within reactors, comparative analysis of microbial population differences under distinct nutrient supplementation conditions was performed to identify key microorganisms governing methane production. These findings establish a theoretical foundation for sustainable biomethane production from coal and provide strategic guidance for enhancing coal-to-methane conversion efficiency.
2. Materials and Methods
2.1. Sample Collection and Preparation
The coal samples (BYH) were collected from the Baiyinhua Coal Mine in Inner Mongolia, China. After drying, the coal samples were passed through a 200-mesh sieve. Industrial analysis was performed using the 5E-MAG6700 fully automatic industrial analyzer, with each sample tested three times. The results of the industrial and elemental analyses of the coal samples are shown in
Table 1 and
Table 2, respectively.
2.2. Enrichment and Cultivation of Methanogens
Microbial consortia for gas generation from coal via fermentation can be mesogenic in coal and mine water and exogenous strains. Native strains are advantageous due to their habitat in coal seams and because they can quickly adapt to the environment when activated. However, there is a limited number of native strains, and they often do not involve the crucial conversion of macromolecules in coal to small molecules. Therefore, exogenous strains are preferred because of the associated high cell density and the abundance of strains. These strains can adapt to coal seam environments after targeted domestication. In this study, multiple strains were extracted from sludge under anaerobic conditions and utilized for enrichment experiments.
The cultures were prepared in 250 mL/L serum bottles, and each liter of the medium [based on a liter of distilled and deionized water (DDW)] contained 985 mL/L of the mineral solution, 5 mL/L of a trace metal solution, and 10 mL/L of vitamins. The mineral solution comprised the following: 0.8, 1, 0.1, 0.1, 0.165, and 0.04 g of NaHCO3, NH4Cl, KH2PO4, KCl, MgCl2·6H2O, and CaCl2·2H2O, respectively. The trace metal nutrient solution contained the following: 2, 0.88, 0.41, 0.2, 0.08, 0.02, 0.02, 0.02, 0.02, 0.02, and 0.02 g of aminotriacetic acid, MnCl2·H2O, FeCl2·6H2O, CoCl2·6H2O, ZnCl2, CuCl2·2H2O, NiCl2·6H2O, Na2MoO4·2H2O, Na2SeO3, NaWO4·6H2O, and NaWO4·6H2O, respectively. The vitamin solution included the following: 10, 5, 5, 5, 5, 5, 5, 5, 5, 2, and 2 mg of pyridoxine HCl, mine HCl, riboflavin, calcium pantothenate 5 mg, thioctic acid, p-aminobenzoic acid, nicotinic acid, vitamin B12, mercaptoethanesulfonic acid (MESA), biotin, and folic acid, respectively. During the anaerobic enrichment, 2 g of yeast extract and 2 g of tryptone were added per liter of culture.
2.3. Batch Gas Production Experiment
In this experiment, 250 mL saline bottles were used as reactors, and Xilingol lignite was used as the coal sample. Culture medium (CM), sawdust (SD), and blue-green algae (BA) were used as supplementary nutrient sources. The difference in the inoculated strains was that the inoculation was carried out during the gas production stop stage in the process of enriching the composite strains. At this stage, the nutrients in the reactor were completely consumed, ensuring that no other carbon sources were present in the inoculation solution. Eighteen fermentation bottles were prepared. Among them, nine fermentation bottles, containing coal powder, formed the experimental group and were labeled Exp-1 to Exp-9. The remaining nine fermentation bottles, without any added carbon source, served as the control group and were labeled Ctr-1 to Ctr-9. T-shaped rubber stoppers were used to seal the anaerobic operation box, and after sealing, aluminum caps were crimped onto the bottles. The fermentation bottles were shaken evenly and placed in a water bath at 35 °C. The gas generated in the reactor was discharged through a syringe needle, a one-way valve, and a hose to maintain atmospheric pressure inside the reactor at the same level as that on the outside. The experimental setup is shown in
Figure 1. The experiment was conducted in three stages. In the first stage, 20 g of coal powder was added to the experimental group, while no coal powder was supplemented in the control group; both groups were fermented separately. Upon stabilization of the gas production curves, the second stage was initiated, in which different nutrient sources were added to monitor the resumption of gas production. After the gas production curves stabilized again, the third stage was carried out. To account for the consumption of available substances in coal and to evaluate the effect of nutrient reduction on methane production, nutritional supplementation in the third stage was reduced fivefold. The feeding strategies for the fermentation bottles at different periods are summarized in
Table 3.
2.4. Instruments and Methods
2.4.1. Determination of Gas Production Components
The main components of the gas produced by fermentation were CH4, CO2, and H2. The composition of the gas was determined using a Zhejiang Fuli 9790plus gas chromatograph. Both CH4 and CO2 were detected using a Thermal Conductivity Detector (TCD), and CO2 and N2 were also detected with a TCD. Argon (99.999%) was used as the carrier gas. The flow rates of hydrogen and air were 30 and 300 mL/min, respectively. The temperatures of the syringe injection port, column oven, first TCD, and second TCD were set to 120 °C, 100 °C, 200 °C, and 130 °C, respectively. The standard gas mixture consisted of 1% each of CH4, CO2, and H2, with 97% N2. A 1 mL gas sample was drawn from the reactor using a 1 mL sampler and injected into the gas chromatograph for analysis.
2.4.2. Molecular Biology Tests
Microorganisms in the fermentation broth were enriched using a 0.22-micron filter and a vacuum filtration device. Polymerase chain reaction (PCR) was used to detect microbial communities in the fermentation broth before and after the reaction. For bacterial amplification, 338F and 806R primers were used, producing an amplicon of 468 bp. For archaea, 524F and 958R primers were used, producing an amplicon of 434 bp. PCR products from the same sample were pooled and analyzed using 2% agarose gel electrophoresis. Based on the electrophoresis results, the TruSeq DNA Sample Prep Kit was used to construct the MiSeq library.
4. Discussion
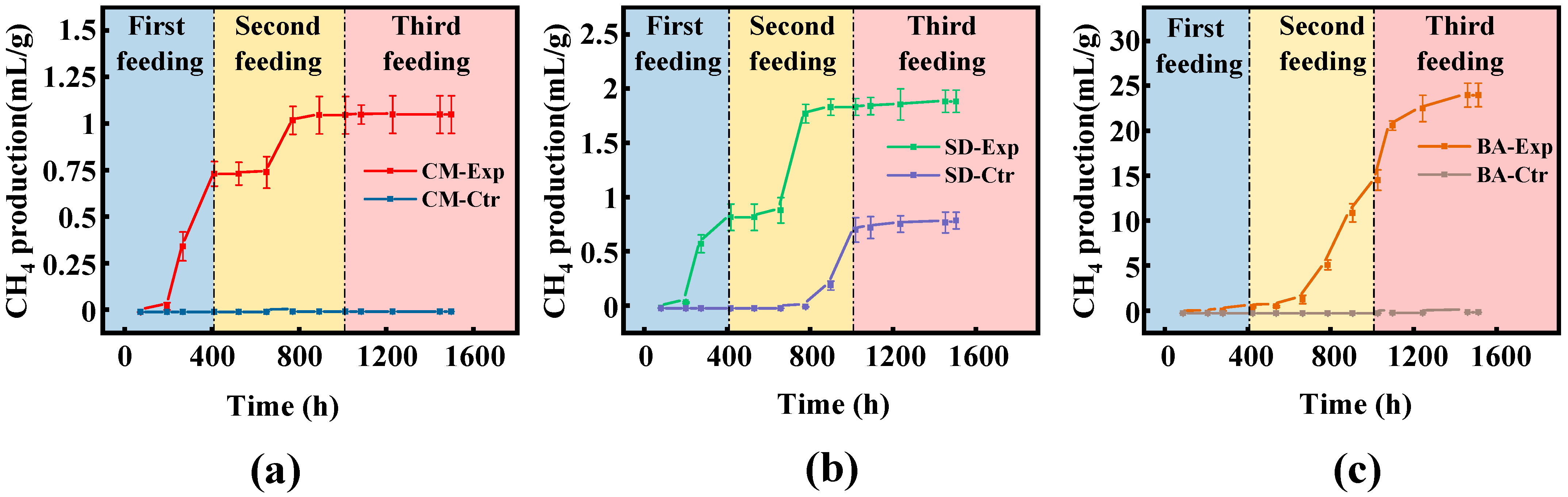
4.1. Differences in Gas Production Performance of Different Supplements
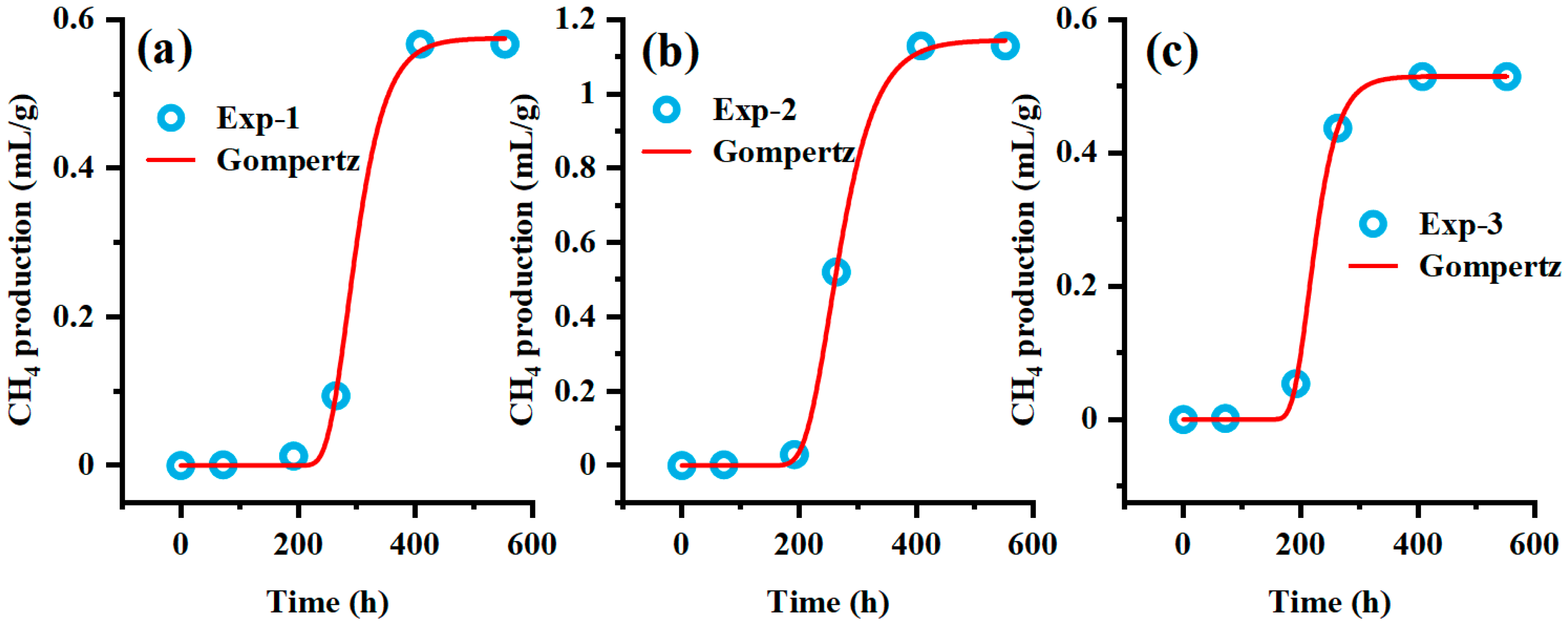
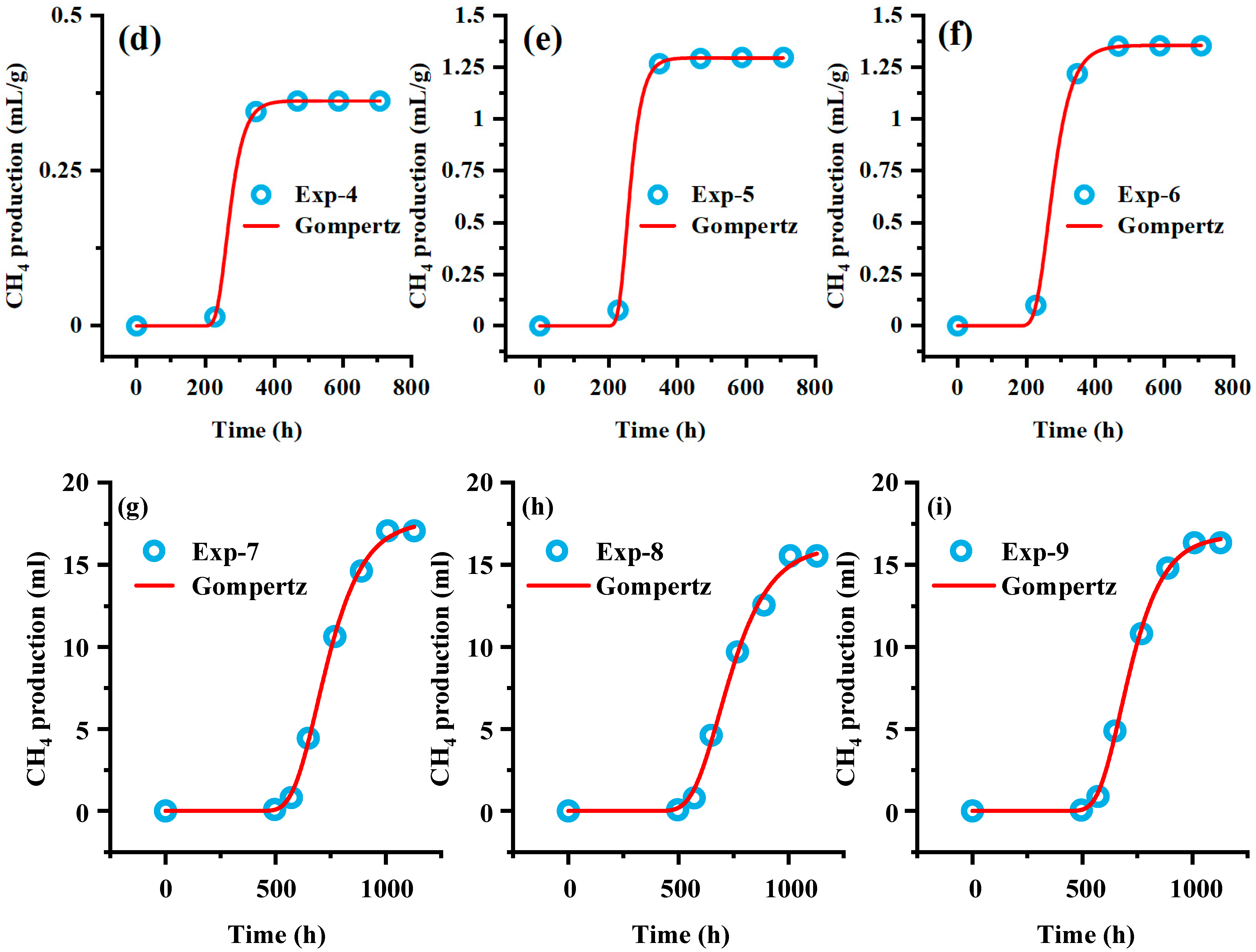
Supplementation with key nutrients can restart the anaerobic digestion (AD) of coal in the fed-batch reactor, and the supplementation of BA can greatly promote methane production. The Gompertz model was applied to fit the methane production curve after the second nutrient supplementation, describing the exponential gas production rate in the curve and the relationship between methane production and time during the lag phase (
Figure 7). The regression fit shows strong and statistically sound results, with a 95% confidence level and coefficients of determination (R
2) all greater than 0.99. The fitting parameters are presented in
Table 4. A
E represents cumulative methane production, while μ
m represents the instantaneous methane production rate at the inflection point of the methane production kinetic curve, corresponding to the theoretical maximum methane production potential. This parameter directly reflects the metabolic activity intensity of methanogenic archaeal communities. Since methanogens lack the ability to directly degrade macromolecular organic matter and must rely on syntrophic metabolic relationships with hydrolytic and fermentative bacteria, μ
m also indirectly indicates the structural complexity of the substrate composition, the efficiency of collaborative operation between microbial communities, as well as the extent to which environmental factors influence microbial methane generation. The BA group showed the highest methane production, likely because BA enhances microbial activity by providing essential nutrients for hydrolytic and fermentative bacteria, thereby stimulating methane production [
36,
37]. Compared with BA, wood chips are mainly composed of lignocellulose, an aromatic polymer with a three-dimensional network structure, making it considerably resistant to degradation [
38,
39]. λ represents the lag phase. During this period, the response of microorganisms to environmental changes is delayed, and the substrate is mainly used for vital activities of the microorganisms themselves. The lag phase reflects the adaptability of microorganisms to the environment. After feed supplementation, the λ values of the CM group and SD group were relatively close, whereas the BA group had λ value approximately 2.5 times higher than that of the other experimental groups. Thus, after supplementation with BA, the microbial adaptability to the environment declined. Based on the fitting results, we speculate that after supplementing feed with citations, the carbon-to-nitrogen ratio (C/N) in the liquid environment decreased, resulting in a decrease in the proportion of organic matter and a short-term ammonia inhibition effect [
40,
41,
42]. After selection, dominant bacterial species are retained and participate in the anaerobic degradation of the substrate.
4.2. Microbial Community Features
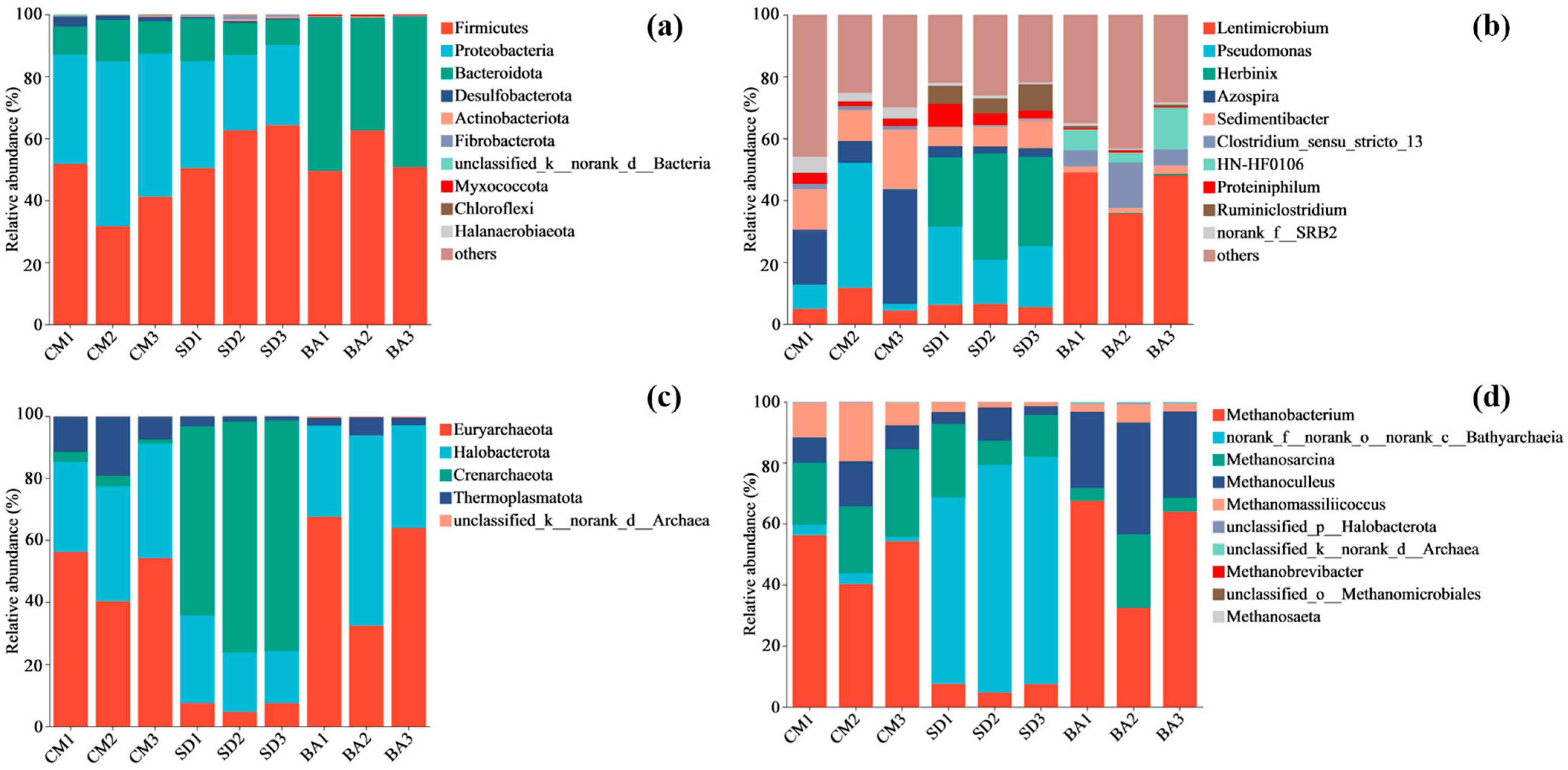
The Venn analysis results demonstrated significant divergence in microbial composition within reactors under different nutrient supplementation conditions, suggesting that nutrient supplementation induced habitat modification and consequently led to distinct differentiation in microbial community structures. Notably, microbial community alterations following nutrient addition have been extensively observed in various Microbially Enhanced Coal Bed Methane (MECBM) approaches in recent years. For instance, during anaerobic coal degradation, substances such as algae and lignocellulose are added to supply nutrients for biodegradation and stimulate microbial activity [
43,
44,
45,
46]. In the CM group (coal-only group),
Azospira, a denitrifying bacterium widely used for wastewater nitrogen removal [
47], and
Pseudomonas, which mediates heterotrophic nitrification-aerobic denitrification to convert NH
4+-N compounds into bioavailable nitrate [
48] dominated the microbial community. In contrast, the SD and BA groups exhibited higher abundances of coal-degrading genera. This discrepancy likely arose because microorganisms in the CM group, lacking external nutrients, prioritized survival by extracting limited bioavailable elements directly from lignite, restricting functional diversity in anaerobic degradation. The bacterial community composition revealed that competitive dominance of
Azospira and
Pseudomonas in the CM group inhibited other degradative microorganisms, thereby restricting the breakdown of complex organic compounds in coal and limiting anaerobic degradation processes, which collectively explains its minimal methane production. However, since anaerobic degradation constitutes a multi-stage process, determining which specific reaction step became rate-limiting due to the reduced degradative microbial population in the CM group requires further investigation.
When SD was added,
Pseudomonas and
Herbinix became dominant.
Herbinix is a cellulose-degrading bacterium. Because SD contains large amounts of cellulose, increased cellulose-degrading bacteria enable the decomposition of cellulose-like components in coal. Consequently, the content of available carbon sources in the reactor increased [
49]. Notably, PCA and Venn analyses indicated higher bacterial community similarity between the CM and SD groups than between the CM and BA groups, suggesting that SD-induced preliminary degradation modified dominant taxa while maintaining partial overlap in final degradation products with CM. The bacterial community structure in the BA group was relatively simple. Blue-green algae, used as a nutrient supplement, contain large amounts of protein, which
Proteiniphilum can utilize as a carbon source, decomposing it into acetic acid, ethanol, and carbon dioxide [
50,
51].
Clostridium sensu stricto 13 is involved in carbon metabolism, producing small molecular compounds such as acetic acid and propionic acid and potentially ethanol [
52,
53]. In this process, the diversity of methane synthesis pathways determines the product diversity. Moreover, life activities and functional implementation of the products of dominant genera are interdependent and closely linked, with a positive feedback effect between the two. Therefore, despite high bacterial community diversity in the BA group, dominant genera were still extremely prominent. Among them, the introduction of nutrients may also be accompanied by other components (such as trace elements, etc.), and the effects of these components on the anaerobic degradation of coal are not clear. Therefore, in the subsequent research process, specific functional genes or metabolic fluxes can be analyzed to further explain the relationship between different metabolic pathways.
Regardless of the type of nutrient supplement used,
Methanosarcina, which participates in multiple methanogenic pathways, as well as hydrogenotrophic methanogens such as
Methanobacterium and
Methanoculleus [
54,
55], were present in the reactors of each group. In the CM group, archaeal communities were predominantly composed of
Methanosarcina and
Methanobacterium, indicating that lignite degradation involves a complex process mediated by diverse methanogenic pathways. Compared with the CM group, the SD group exhibited a higher abundance of an unclassified genus within
Bathyarchaeia, but lower abundances of other methanogenic archaea. Given that
Bathyarchaeia plays a significant role in carbon cycling within methane-rich tropical coastal ecosystems, and considering the higher methane production in the SD group than in the CM group, it is hypothesized that this unclassified genus may adapt to the co-fermentation environment of coal and SD, contributing to methanogenesis. As illustrated by the PCA and Venn diagrams (
Figure 3 and
Figure 4), BA and CM groups exhibited similar archaeal community structures, with the distinction in the dominance of hydrogenotrophic
Methanobacterium and
Methanoculleus in the BA group. This distinction may be attributed to the higher abundance of
Proteiniphilum, which facilitated more efficient protein degradation from BA, resulting in reduced acetate production and increased CO
2 and H
2 levels. These conditions provided sufficient substrates for hydrogenotrophic methanogenesis, consistent with the findings of Hahnke et al. [
56].
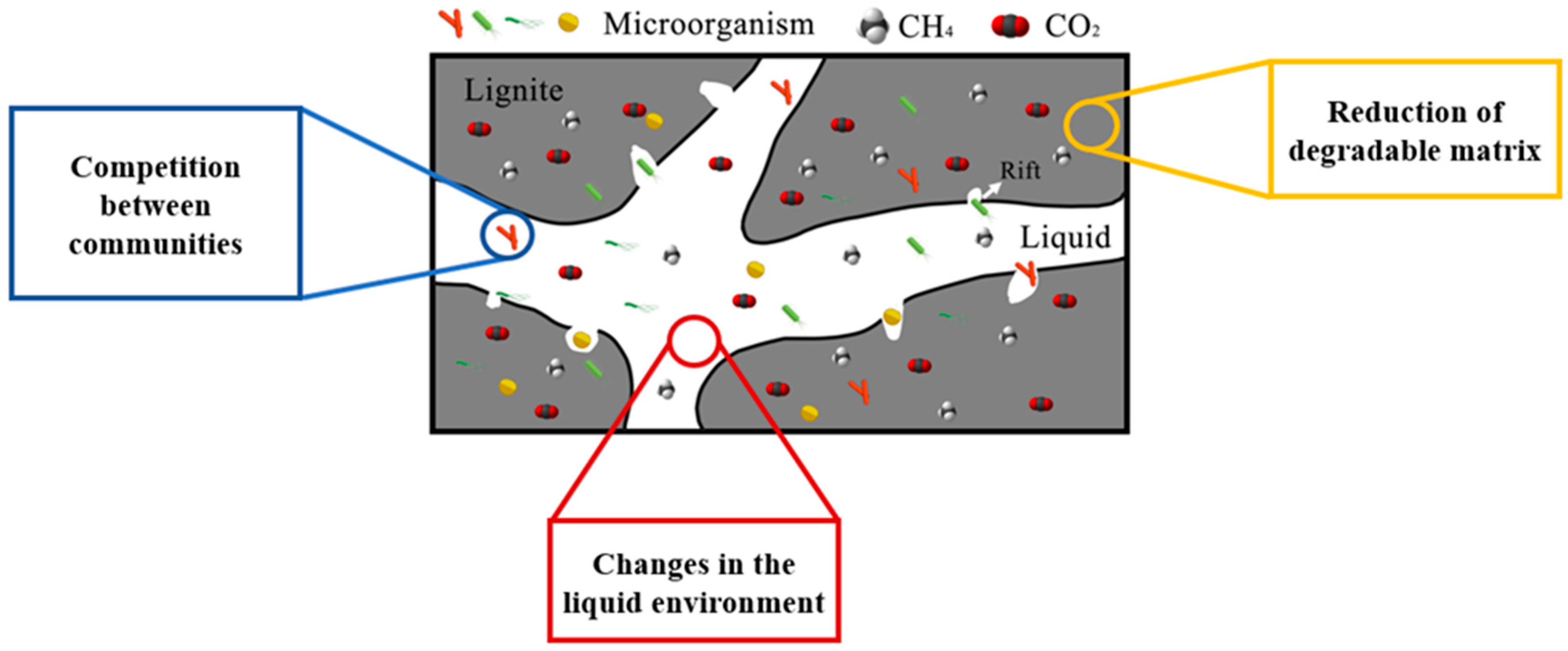
4.3. Factors Contributing to the Cessation of Gas Production
The cessation of coal biomethanation could be attributed to the coupling effects of multiple factors (
Figure 8). In reactors, utilizable components within the degrading matrix are non-renewable. Although bacterial catabolic systems are highly efficient [
57], when available substrate content falls below the metabolic threshold, substrate limitation effects hinder enzymatic reactions, leading to a near-zero biocatalytic efficiency [
58,
59]. Anaerobic degradation systems inherently contain self-inhibitory factors, such as free ammonia and volatile fatty acids. When any inhibitory factor exceeds the minimum inhibitory concentration [
60,
61], microbial stress response mechanisms are triggered, resulting in altered cell membrane permeability, inactivation of key metabolic enzymes, and prolonged population lag phases [
59]. Notably, when pH drops below 4.0 or exceeds 10.0, methane production inhibition reaches 100% compared to neutral conditions [
62]. Metagenomic analyses revealed that the micro-ecosystem within the reactors comprised multi-trophic functional microbial communities, including hydrolytic bacteria, oxidizing bacteria, and methanogenic archaea. Environmental stressors that reduce microbial abundance or inactivate the metabolism of these key functional microbes disrupt interspecies electron transfer chains, causing abrupt declines in reaction rates. Importantly, substrate competition between sulfate-reducing bacteria and methanogens may induce preferential substrate metabolism, while sulfide (HS−) generated by this process irreversibly inhibits methanogenic archaea by suppressing critical enzyme systems [
63,
64]. Additionally, nutrient metabolism dysregulation is a critical factor in process termination. The C/N exerts a significant regulatory effect on microbial metabolism. Previous studies indicate a positive coupling relationship between the carbon and nitrogen cycles during AD [
65]. The optimal C/N for anaerobic degradation ranges from 20 to 30, under which methanogens and denitrifying bacteria synergistically promote organic carbon generation, enhance system acid tolerance, and enable the expression of methane-critical genes (e.g., mcrA) [
66,
67]. Deviations from this range (either excessively high or low C/N) may induce acidification or ammonia inhibition, thereby restricting methane production.
4.4. Reasons for Differences in Gas Production
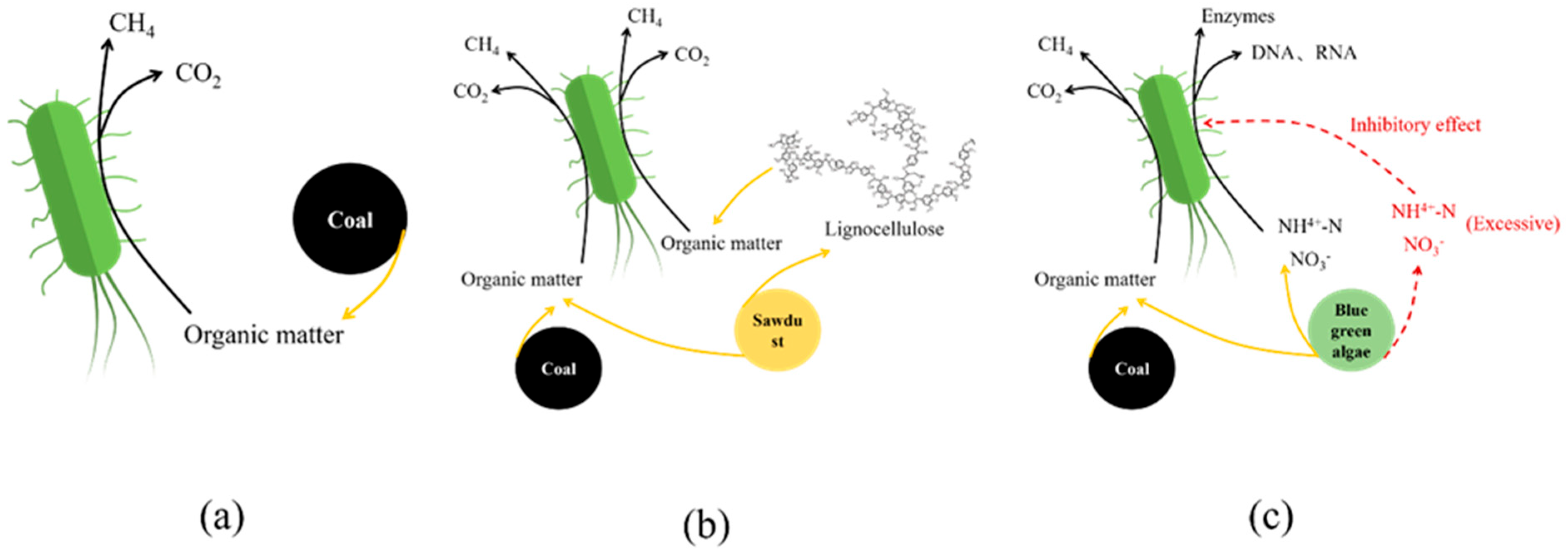
The feeding material significantly influences the reaction termination time.
Figure 9 illustrates the metabolic pathways of substrate degradation in reactors with different feeding supplements. When BA served as a supplement, the reaction duration substantially exceeded the stipulated time while achieving the highest methane production. As a potential nutrient source, BA exhibits a high nitrogen content. Nitrogen, a critical element for cellular protein and nucleic acid synthesis, is essential for microbial growth. Elevated nitrogen levels enhance the abundance of specific methanogens in anaerobic degradation [
68,
69]. This suggests that nitrogen enrichment in the BA group promotes microbial activity and population growth related to anaerobic degradation, thereby sustaining and optimizing lignite decomposition.
Clostridium sensu stricto 13, the dominant species associated with carbon metabolism in the BA group, supports this hypothesis. During nitrogen-rich substrate degradation, amino groups from proteins and nitrogenous bases from nucleic acids may release ammonia nitrogen [
70,
71], a process corroborated by the presence and functional mechanisms of
Proteiniphilum. The acetate degradation pathway is highly sensitive to ammonia during AD [
72]. Excessive ammonia inhibits methyl-CoM reductase expression in
Methanosaeta, suppressing acetate metabolism, while shifting microbial communities toward
Methanosarcina dominance. Concurrently, methane production transitions to the syntrophic acetate oxidation–hydrogenotrophic methanogenesis pathway [
73], which is consistent with our microbial community analysis results.
When wood chips (SD group) served as the supplement, methane production, initial reaction time, and reaction duration after the first feeding were comparable to those of the CM group but remained significantly lower than those of the BA group. The reaction time after the second feeding was relatively prolonged, approaching that of the BA group, demonstrating that the SD group exhibited a higher methanogenic potential than the CM group. The presence of
Herbinix indicated the existence of a certain amount of utilizable lignocellulosic biomass in the wood chips. Lignocellulosic biomass is primarily composed of cellulose (38–50%), hemicellulose (23–32%), and lignin (10–25%) [
74]. Cellulose consists of linear glucose polymers interconnected via β-1,4-glycosidic bonds. The glucose units form crystalline structures through hydrogen bonding. Coupled with the recalcitrant nature of β-1,4-glycosidic bonds, cellulose exhibits high stability and mechanical strength [
74,
75]. Hemicellulose, a heteropolysaccharide, has more branched and intricate structures than cellulose. This complexity necessitates the use of diverse enzymatic systems that act on distinct glycosidic bonds and monosaccharide residues [
76]. Lignin is typically a three-dimensional network polymer formed by phenylpropane units linked via ether and carbon–carbon bonds, characterized by high complexity and irregularity. Owing to its complex structure, high chemical bond energy, and tight association with carbohydrates, lignin creates physicochemical barriers that drastically reduce enzymatic hydrolysis rates [
77,
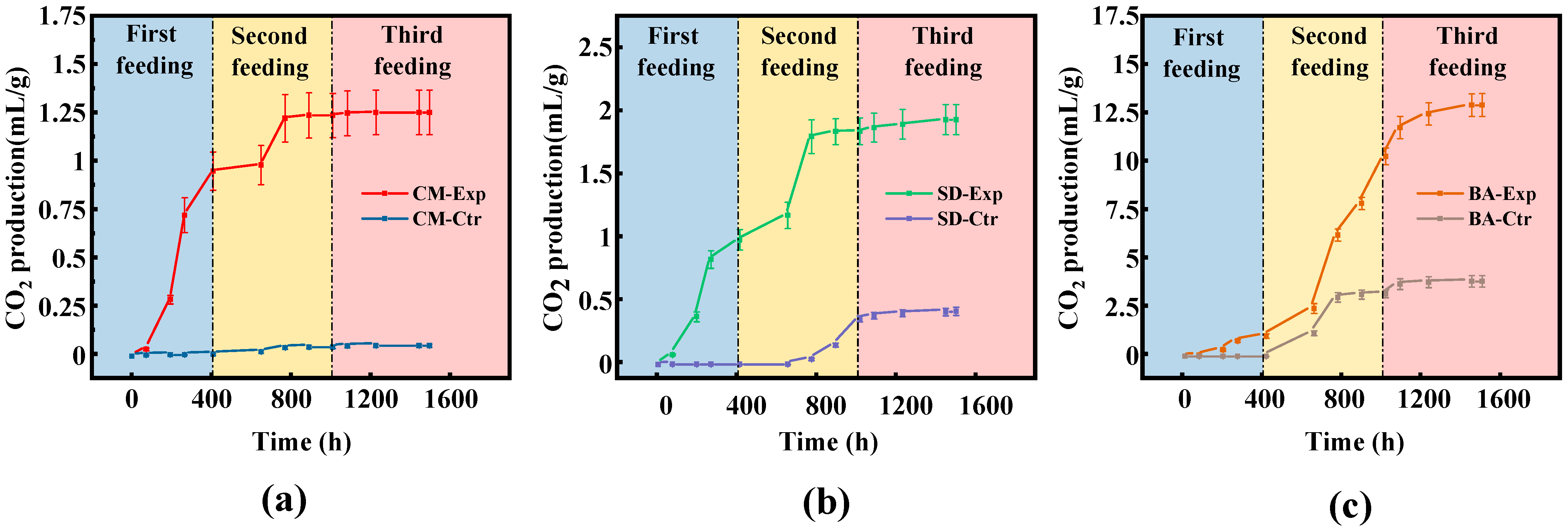
78]. Consequently, the limited availability of effective substrates from wood chips during microbial lignite degradation effectively explains why methane production in the SD group was comparable to that in the CM group but significantly lower than that in the BA group.
During anaerobic degradation, partial organic matter in coal is dissolved owing to microbial activity. Bacteria in the fermentation solution secrete hydrolytic enzymes that facilitate the decomposition of organic polymers into long-chain (C
10–C
36) alkanes, long-chain fatty acids, and monocyclic aromatic hydrocarbons. These soluble organic compounds serve as electron donors for degradation, producing CO
2, while H+ acts as an electron acceptor [
79]. In this process, the aromatic and aliphatic regions of lignin in the SD can be depolymerized by removing the corresponding side chain groups. These components subsequently participate in methane production, which is consistent with our finding that partial organic matter utilization in SD moderately enhanced methanogenesis, resulting in a slightly higher methane yield in the SD group than in the CM group. Proteins, lipids, and carbohydrates in BA are depolymerized into small-molecular compounds such as aromatic hydrocarbons, esters, and alcohols. Notably, lignin has been identified in the cell walls of certain algae species [
80,
81,
82], suggesting that BA may partially substitute SD to promote anaerobic degradation. However, the structural complexity of lignocellulosic biomass substantially limits its degradation efficiency when conventional methods are used [
83,
84]. Although bacterial extracellular lytic enzymes can degrade these materials [
85], the lignocellulose content remains a critical factor influencing degradation rates. This mechanism effectively explains the observed differences in CO
2 production among the experimental groups after substrate supplementation: constrained by organic matter composition/structure and microbial abundance/activity, both the SD and CM groups exhibited lower CO
2 production than the BA group.
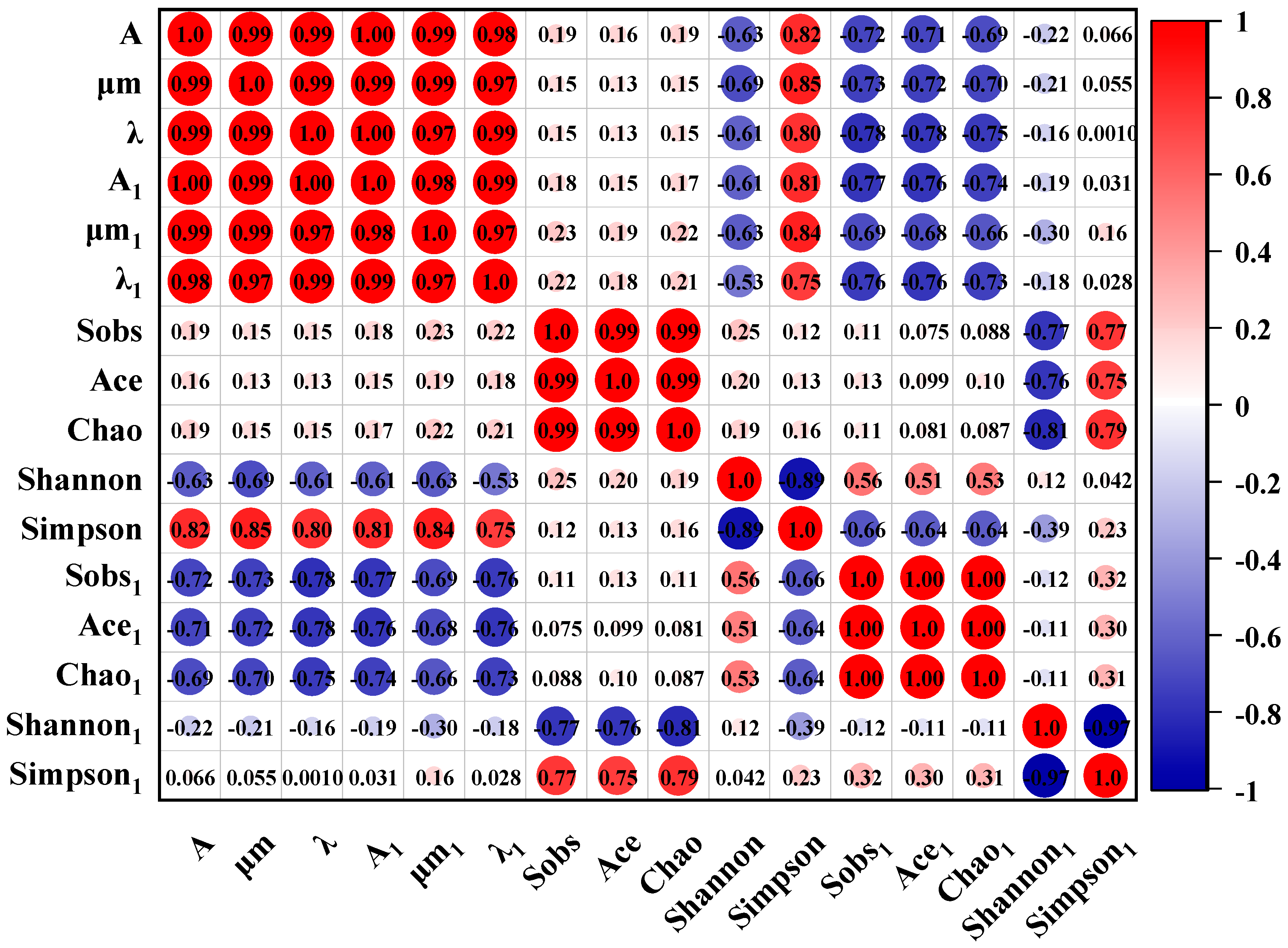
4.5. Correlation Analysis
The improved Gompertz model was employed to investigate the fitting of CO
2 and CH
4 production during anaerobic fermentation before the third substrate supplementation. The Pearson correlation coefficient method was used to calculate the eigenvalues of the correlation matrix between the normalized raw variables to elucidate the intrinsic relationships among the stochastic variables. The results of the correlation analysis are presented in
Figure 10. Quantitative analysis of nine parameters was conducted, including kinetic parameters (A, μm, and λ) (
Table 5) and microbial diversity indices (Sobs, Ace, Chao, Shannon, and Simpson) (
Table 6). The Pearson coefficient is particularly suitable for assessing correlations between continuous variables [
86,
87].
The experimental data revealed significant correlations between the CO
2 and CH
4 kinetic parameters (r > 0.9), consistent with the gas production trends. This phenomenon may be related to the following biochemical reaction:
CO2 is reduced to methane as an electron acceptor, accompanied by a decrease in entropy and changes in methane production parameters. These mechanisms effectively explain the synchronized trends in the CO2 and CH4 production curves. Alpha diversity indices indicated comparable richness and diversity of archaeal communities across the groups. For bacterial communities, the Sobs, Ace, and Chao indices (indicators of species richness) demonstrated the highest species abundance in the SD group and the lowest in the CM group, with similar bacterial evenness across the nutrient supplementation groups. The BA group exhibited the highest species richness. Bacterial richness indices showed negligible correlations with gas production kinetic parameters (r < 0.3), suggesting that CH4 and CO2 production was associated with specific bacterial subpopulations rather than with overall richness. Archaeal richness indices displayed negative correlations with gas production parameters (r < −0.5), potentially due to competitive interactions among different methanogenic pathways. Higher archaeal diversity may introduce functional redundancy, where increased methanogen species compete for nutrients without enhancing gas production efficiency, suppressing CH4 and CO2 yields.
The Simpson and Shannon indices primarily reflect species diversity. For bacterial communities, no significant differences in diversity were observed among groups. The Simpson index showed strong correlations with gas production kinetic parameters (r > 0.7), whereas the Shannon index exhibited inverse correlations (r < −0.5). This suggests that greater species diversity and community evenness enhance the likelihood of competitive exclusion, thereby diminishing the dominance of gas-producing species. For archaeal communities, both Simpson and Shannon indices showed negligible correlations with gas production parameters (r ≈ 0). Combined with the archaeal Venn diagram analysis, this implies that the gas production processes are predominantly governed by a few key species. As long as the number and activity of key species remain stable, the kinetic parameters of gas production do not fluctuate significantly.
4.6. Feasibility of Industrial Application
In this study, a preliminary economic analysis was conducted to evaluate the economic feasibility of anaerobic degradation reactors with nutrient recirculation. The primary objective was to enhance methane production using additives, thereby improving economic returns. At an industrial scale, economies of scale from large-scale equipment would reduce methane production costs [
88]. All economic evaluation parameters were determined based on the experimental data and real-world conditions, using an RMB/USD exchange rate of 0.14 (January 2025). The input–output relationships are illustrated in
Figure 11.
Nevertheless, subsequent evaluations should incorporate capital expenditure (e.g., equipment procurement, installation, and maintenance costs) and operational costs (e.g., labor, energy expenses) into the economic assessment framework. Advanced evaluation methodologies should be adopted to further substantiate the comparative advantages of different supplements during in situ applications [
89]. For instance, the Discounted Cash Flow valuation method [
90], which involves forecasting future cash flows and calculating their present values using predetermined discount rates, can be employed for economic analysis. Furthermore, Life Cycle Assessment [
91] should be implemented to comprehensively evaluate the environmental impacts across all stages, from supplement production, transportation, and storage to utilization in gasification processes and subsequent waste management. By integrating economic analysis with a monetized approach, this methodology quantifies the costs throughout the microbial coal gasification cycle, thereby providing robust evidence for determining the relative merits of different supplements.
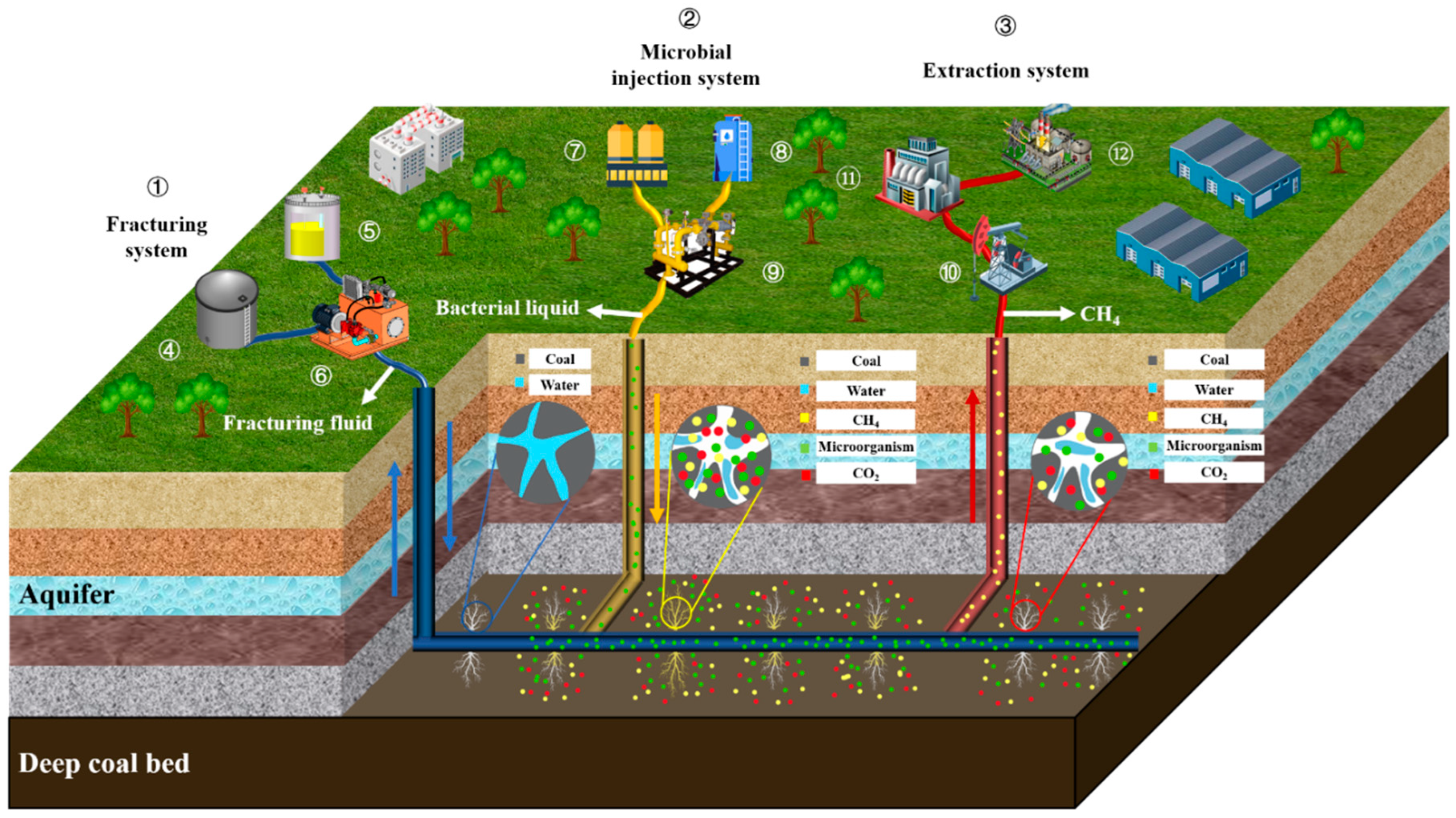
As a novel approach to green coal utilization, in situ microbial coal gasification holds significant potential for deep coal resource exploitation. However, two critical challenges persist: (1) insufficient understanding of multiphase reaction processes in deep coal seam microbial gasification, complicating long-term process stability control, and (2) extreme deep-seam conditions (high-stress, high-salinity, and high-temperature environments) posing substantial barriers to microbial growth and metabolism. Consequently, most proposed solutions remain in preliminary developmental stages. Based on the experimental findings, this study proposes an in situ microbial coal gasification methodology, as illustrated in
Figure 12.
Hydraulic fracturing is performed on coal seams, with bacterial inoculum and nutrients configured in surface systems. The mixture is transported via pipelines to coal seams, where it is combined with the fracturing fluid to execute hydraulic fracturing operations. Following fracturing, microorganisms infiltrate coal seam fractures and participate in microbial coal degradation. The generated methane is extracted through gas recovery pipelines via these fractures, serving as gas migration channels, and is ultimately transported to the surface for collection and utilization.
The development of microbial coal gasification technology has the following significant implications: (1) enhanced utilization of coal resources, (2) CO2 utilization through microbial-mediated carbon cycling, and (3) clean, efficient, and low-carbon energy utilization. However, challenges persist owing to geological variability, and parameters including rock fracture porosity and permeability coefficients substantially influence anaerobic fermentation processes, while maintaining microbial activity in deep geological strata presents technical difficulties. Consequently, critical research priorities involve creating adaptive conditions for diverse microbial communities, improving the physiological stability of in situ reactor systems, and optimizing high-efficiency CH4 production.