Enzymatic Hydrolysis of Polysaccharide from Houttuynia cordata and Structure Characterization of the Degradation Products and Their α-Glucosidase Inhibitory Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. HCP Preparation
2.3. HCP Enzymatic Hydrolysis
2.3.1. Single-Factor Design
2.3.2. Response Surface Test
2.3.3. Response Surface Model Verification Test
2.3.4. α-Glycosidase Inhibition Assay
2.4. Preparation of Enzymatic Hydrolysates of HCP (EHCP)
2.5. Inhibitory Effects on α-Glucosidase
2.5.1. Inhibition Rate on α-Glucosidase
2.5.2. α-Glucosidase Inhibition Kinetics
2.6. Physicochemical Properties
2.6.1. Chemical Composition Analysis and Solubility
2.6.2. Molecular Weight
2.6.3. Monosaccharide Composition
2.7. Structural Characterization
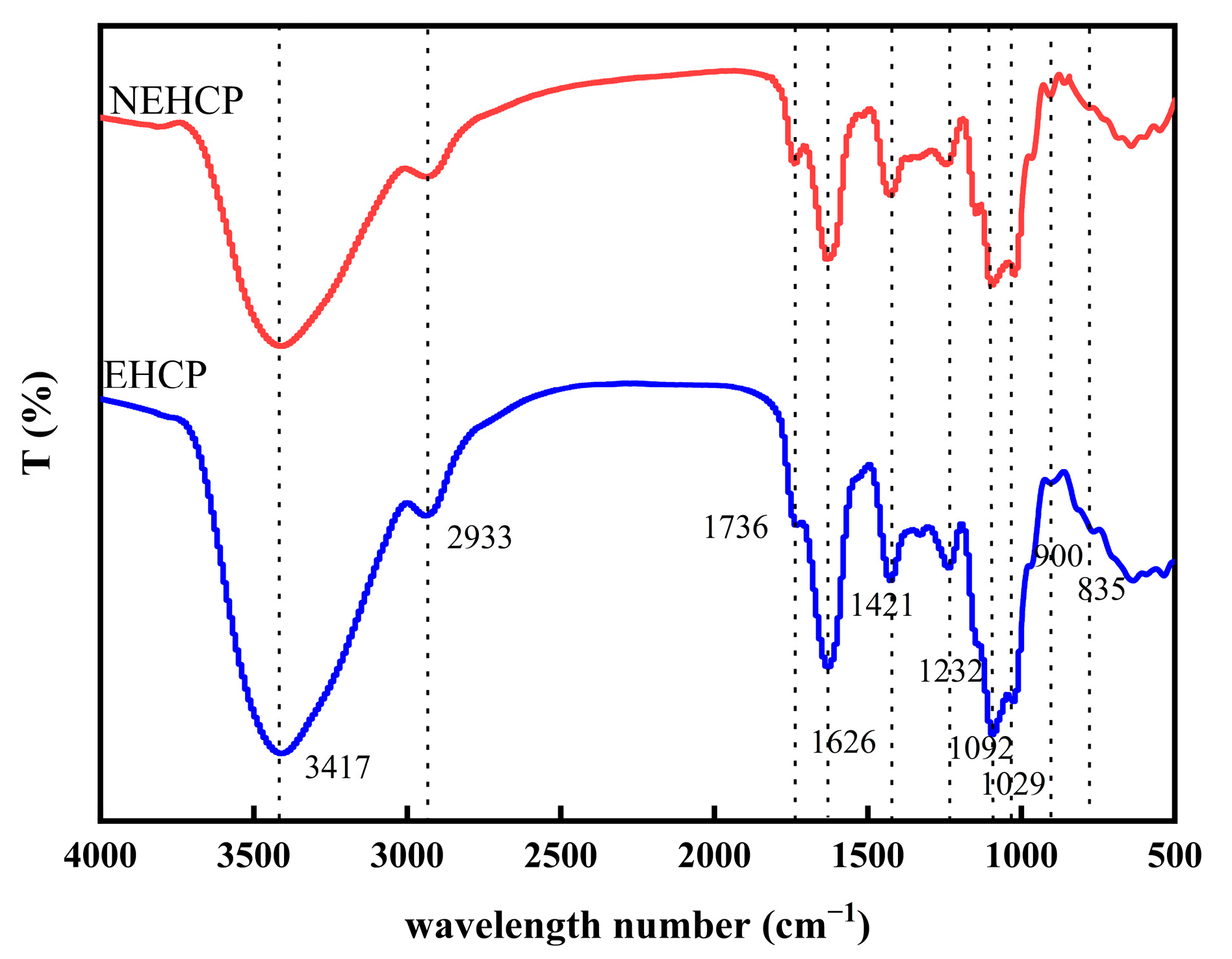
2.7.1. FT-IR Spectrum Analysis
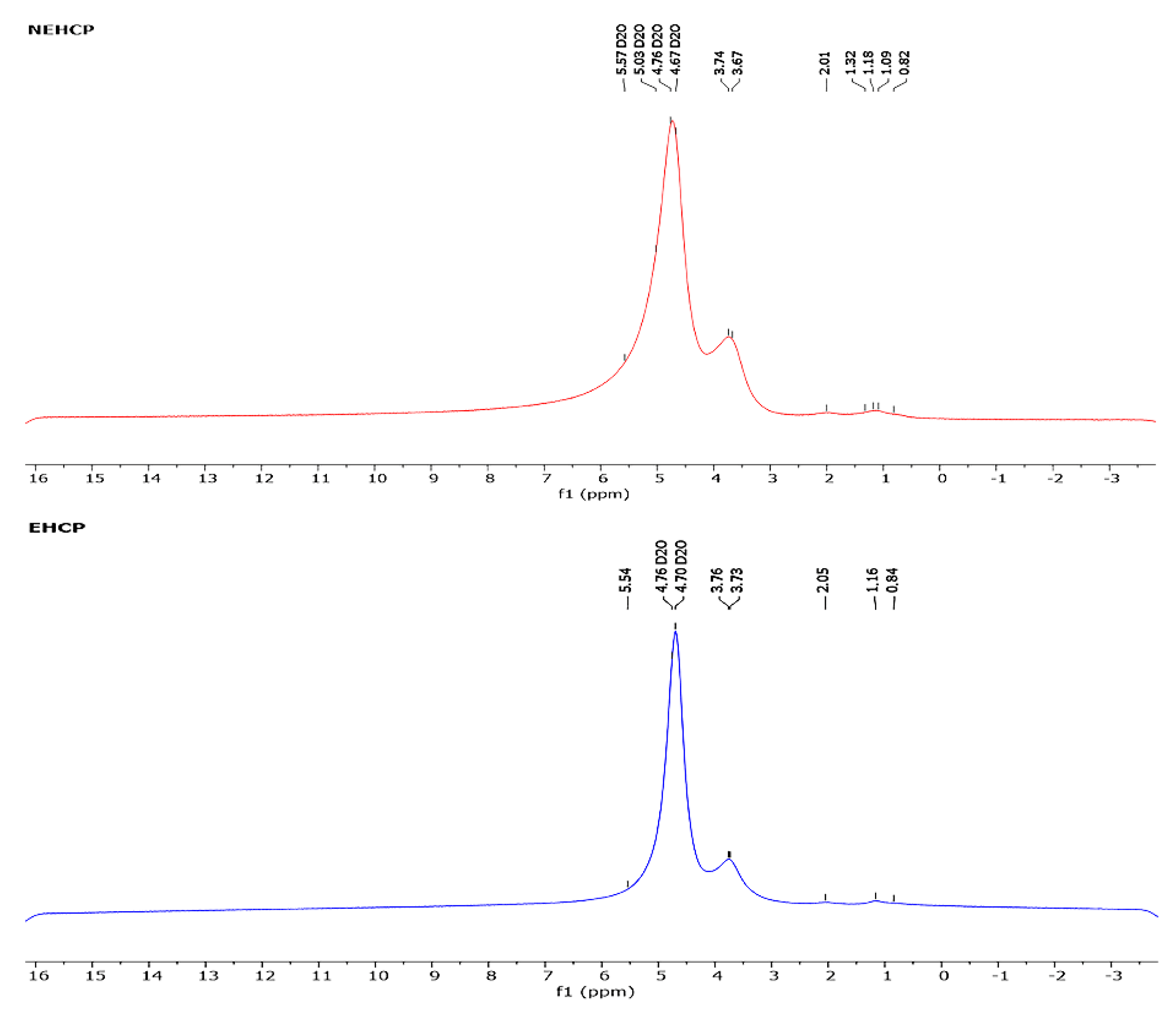
2.7.2. 1H NMR Analysis
2.7.3. Congo Red
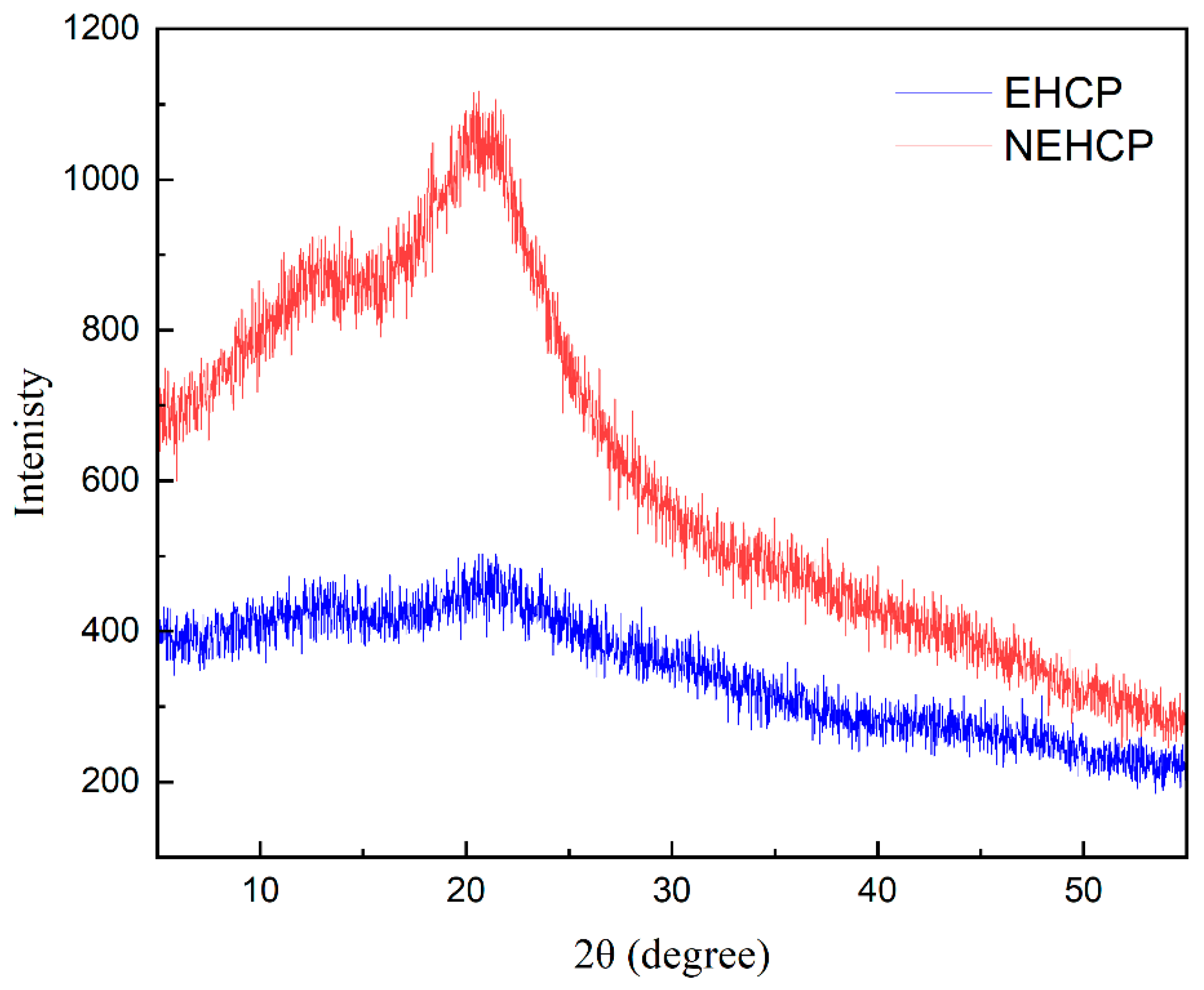
2.7.4. XRD
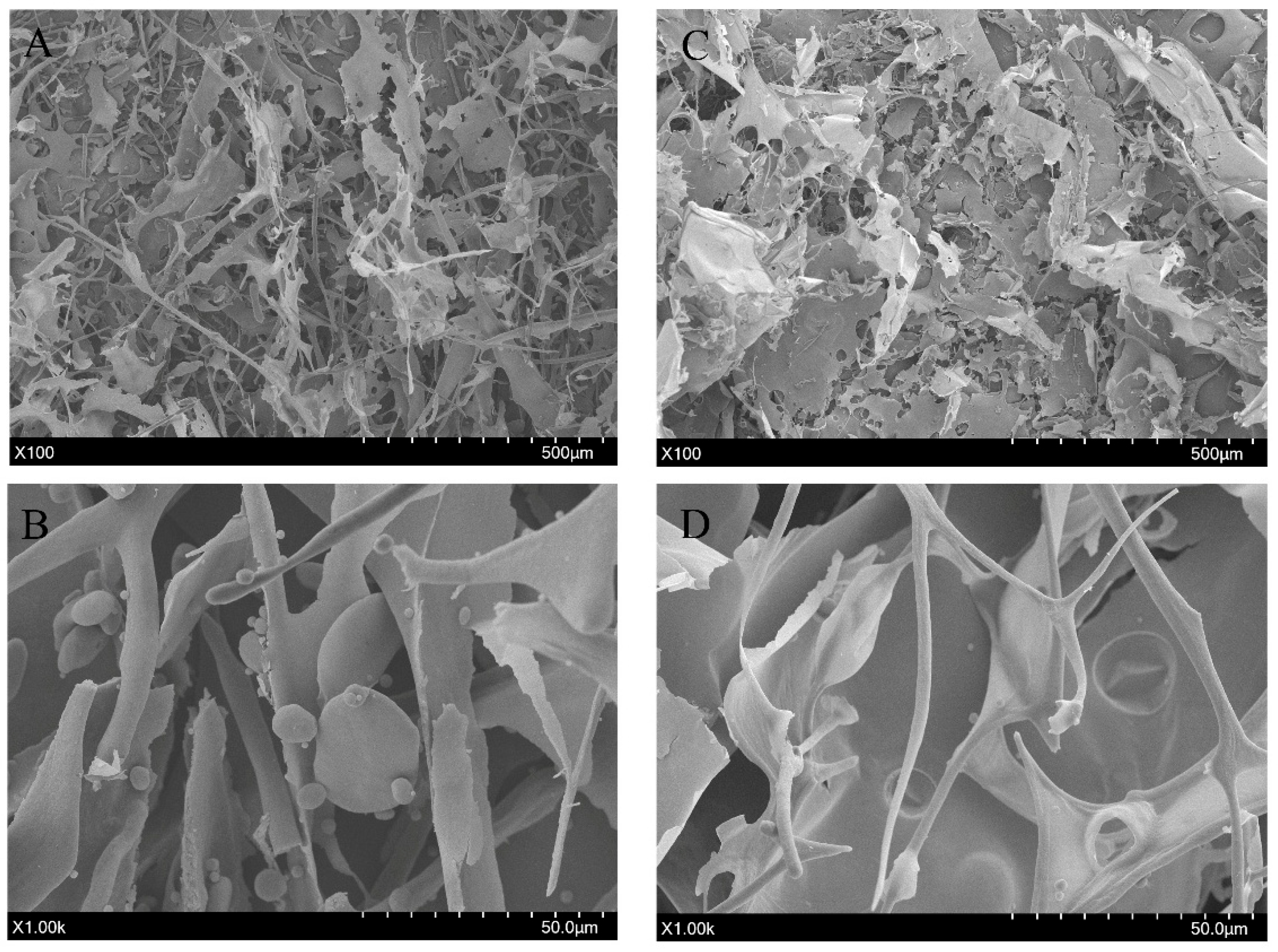
2.7.5. SEM
2.8. Statistical Analysis
3. Results and Discussion
3.1. HCP Enzymatic Hydrolysis
3.1.1. Single-Factor Test Results
3.1.2. Response Surface Test Results and Analysis
3.1.3. Response Surface Test Verification Test
3.2. Inhibitory Effects on α-Glucosidase
3.2.1. Inhibition Rate on α-Glucosidase
3.2.2. α-Glucosidase Inhibition Kinetics
3.3. Physicochemical Properties of the Polysaccharides
3.3.1. Total Polysaccharide, Uronic acid Content, and Solubility
3.3.2. Molecular Weight
3.3.3. Monosaccharide Composition
3.4. Structural Characterization
3.4.1. FT-IR Spectrum Analysis
3.4.2. 1H NMR Analysis
3.4.3. Congo Red
3.4.4. XRD
3.4.5. SEM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCP | Houttuynia cordata polysaccharide |
| EHCP | Enzymatic Hydrolysates of Houttuynia cordata Polysaccharides |
| NEHCP | Non-enzymatically treated Houttuynia cordata polysaccharides |
References
- Xue, H.; Hao, Z.; Gao, Y.; Cai, X.; Tang, J.; Liao, X.; Tan, J. Research progress on the hypoglycemic activity and mechanisms of natural polysaccharides. Int. J. Biol. Macromol. 2023, 252, 126199. [Google Scholar] [CrossRef]
- Ma, W.; Xiao, L.; Liu, H.; Hao, X. Hypoglycemic natural products with in vivo activities and their mechanisms: A review. Food Sci. Hum. Wellness 2022, 11, 1087–1100. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci. Hum. Wellness 2023, 12, 986–1007. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Li, D.; Yang, Y.; Xu, Y.; Wang, L. Characterization and inhibitory activities on α-amylase and α-glucosidase of the polysaccharide from blue honeysuckle berries. Int. J. Biol. Macromol. 2020, 163, 414–422. [Google Scholar] [CrossRef]
- Li, J.-J.; Chen, G.-D.; Fan, H.-X.; Hu, D.; Zhou, Z.-Q.; Lan, K.-H.; Zhang, H.-P.; Maeda, H.; Yao, X.-S.; Gao, H. Houttuynoid M, an Anti-HSV Active Houttuynoid from Houttuynia cordata Featuring a Bis-houttuynin Chain Tethered to a Flavonoid Core. J. Nat. Prod. 2017, 80, 3010–3013. [Google Scholar] [CrossRef]
- Liu, J.; Zou, J.; Wang, J.; Wang, R.; Zhai, S.; Chang, X.; Zhang, X.; Sun, J.; Luan, F.; Shi, Y. Extraction, purification, structural features, and pharmacological properties of polysaccharides from Houttuynia cordata: A review. Int. J. Biol. Macromol. 2024, 279, 135230. [Google Scholar] [CrossRef]
- Wei, P.; Luo, Q.; Hou, Y.; Zhao, F.; Li, F.; Meng, Q. Houttuynia Cordata Thunb.: A comprehensive review of traditional applications, phytochemistry, pharmacology and safety. Phytomedicine 2024, 123, 155195. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dong, Z.; Zhang, L.; Wu, T.; Pan, S.; Xu, X. Structure characterization and biological activity of polysaccharides from different parts of Houttuynia cordata Thunb. J. Chin. Inst. Food Sci. Technol. 2024, 24, 276–286. [Google Scholar]
- Liu, X.; Tian, J.; Pan, Y.; Li, Z.; Zhou, Z.; Pan, Z.; Tai, H.; Xing, Y. Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata. Foods 2022, 11, 3622. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Tian, H.; Wang, Q.; Gao, M.; Xu, G.; Sun, Y.; Song, D.; Li, W.; Ji, C. Advance in Hippophae rhamnoides polysaccharides: Extraction, structural characteristics, pharmacological activity, structure-activity relationship and application. Int. J. Biol. Macromol. 2024, 270, 132420. [Google Scholar] [CrossRef]
- Jia, Y.; Lu, Y.; Wang, Y.; Zhang, M.; He, C.; Chen, H. Spheroidization of ultrasonic degraded corn silk polysaccharide to enhance bioactivity by the anti-solvent precipitation method. J. Sci. Food Agric. 2022, 102, 53–61. [Google Scholar] [CrossRef]
- Ma, F.; Wang, D.; Zhang, Y.; Li, M.; Qing, W.; Tikkanen-Kaukanen, C.; Liu, X.; Bell, A.E. Characterisation of the mucilage polysaccharides from Dioscorea opposita Thunb. with enzymatic hydrolysis. Food Chem. 2018, 245, 13–21. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, D.; Cao, H.; Bai, Y. Enzymatic hydrolysis of polysaccharide from Auricularia auricula and characterization of the degradation product. Int. J. Biol. Macromol. 2020, 162, 127–135. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, M.; Chen, Y.; Lou, Y.; Luo, R.; Chen, J.; Zhang, Y.; Li, J.; Wang, W. Optimization of the polysaccharide hydrolysate from Auricularia auricula with antioxidant activity by response surface methodology. Int. J. Biol. Macromol. 2018, 113, 543–549. [Google Scholar] [CrossRef]
- Hu, T.-G.; Zou, Y.-X.; Li, E.-N.; Liao, S.-T.; Wu, H.; Wen, P. Effects of enzymatic hydrolysis on the structural, rheological, and functional properties of mulberry leaf polysaccharide. Food Chem. 2021, 355, 129608. [Google Scholar] [CrossRef]
- He, M.; Tang, S.; Xu, T.; Yuan, Y.; Wu, T.; Pan, S.; Xu, X. Acetylation of the polysaccharide from Houttuynia cordata rhizome and their α-glucosidase inhibition mechanism. J. Food Sci. 2024, 89, 2672–2683. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- van Wilgenburg, M.G.M.; Werkman, E.M.A.; van Gorkom, W.H.; Soons, J.B.J. Criticism of the Use of Coomassie Brilliant Blue G-250 for the Quantitative Determination of Proteins. Cclm 1981, 19, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Liu, Y.; Wu, X.-L.; Liu, L.-Z.; Fu, W.; Song, D.-D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Yang, N.; Li, Y.; Xing, F.; Wang, X.; Li, X.; Li, L.; Yang, J.; Wang, Y.; Zhang, M. Composition and structural characterization of pectin in micropropagated and conventional plants of Premma puberula Pamp. Carbohydr. Polym. 2021, 260, 117711. [Google Scholar] [CrossRef]
- Zheng, Q.; Jia, R.-B.; Ou, Z.-R.; Li, Z.-R.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and α-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wang, B.; Zhang, W.; Ren, X.; Zhang, Y.; Jiang, L.; Dong, C.; Zhao, G. Optimization of Black Garlic Protein Extraction Process and Exploration of Its Properties and Functions with Enzymatic Hydrolysis Products. Molecules 2025, 30, 125. [Google Scholar] [CrossRef]
- Acosta, O.; Víquez, F.; Cubero, E. Optimisation of low calorie mixed fruit jelly by response surface methodology. Food Qual. Prefer. 2008, 19, 79–85. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, Y.; Yang, F.; Zheng, L.; Ma, Y.; Liu, Q.; Cai, L.; Gong, W.; Wang, B. Preparation and structure-activity relationship of highly active black garlic polysaccharides. Int. J. Biol. Macromol. 2022, 220, 601–612. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Wei, Z.-J. Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, D.; Zeng, Q.; He, J.; Zhao, M.; Tang, Y. Optimization of enzymatic hydrolysis process, structural characterization and antioxidant activity analysis of Gastrodia elata polysaccharide. Sci. Technol. Food Ind. 2025, 46, 205–214. [Google Scholar]
- Xi, L.; Weibing, X.; Shuyong, F.; Sheng-Hua, L.; Xiong, F.; Chin-Ping, T.; Ping-Ping, W.; Zu-Man, D.; Chun, C. The effect of the molecular weight of blackberry polysaccharides on gut microbiota modulation and hypoglycemic effect in vivo. Food Funct. 2024, 15, 8586–8603. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Al-Wraikat, M.; Niu, L.; Zhou, F.; Zhang, Y.; Wang, M.; Ren, J.; Fan, J.; Zhang, B.; Wang, L. Degradation enhances the anticoagulant and antiplatelet activities of polysaccharides from Lycium barbarum L. leaves. Int. J. Biol. Macromol. 2019, 133, 674–682. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Chan, J.Y.-W.; Chan, B.C.-L.; Lin, H.-Q.; Han, X.-Q.; Zhou, X.; Wan, D.C.-C.; Wang, Y.-F.; Leung, P.-C.; Fung, K.-P.; et al. Structural characterization and immunomodulatory effect of a polysaccharide HCP-2 from Houttuynia cordata. Carbohydr. Polym. 2014, 103, 244–249. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Zhong, Q.-W.; Zhou, T.-S.; Qiu, W.-H.; Wang, Y.-K.; Xu, Q.-L.; Ke, S.-Z.; Wang, S.-J.; Jin, W.-H.; Chen, J.-W.; Zhang, H.-W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Jia, X.; Hu, J.; He, M.; Zhang, Q.; Li, P.; Wan, J.; He, C. α-Glucosidase inhibitory activity and structural characterization of polysaccharide fraction from Rhynchosia minima root. J. Funct. Foods 2017, 28, 76–82. [Google Scholar] [CrossRef]
- Bu, Y.; Yin, B.; Qiu, Z.; Li, L.; Zhang, B.; Zheng, Z.; Li, M. Structural characterization and antioxidant activities of polysaccharides extracted from Polygonati rhizoma pomace. Food Chem. X 2024, 23, 101778. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; He, Y.; Li, J.; Ma, K.; Zhang, Y.; Li, H.; Yin, C.; Zhang, Y. Stability evaluation of gardenia yellow pigment in presence of different phenolic compounds. Food Chem. 2022, 373, 131441. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Wang, Y.; Deng, Y.; Zhang, J. Structural characterization and in vitro hepatoprotective activity of an acidic polysaccharide from Dendrobium nobile Lindl. flower. Int. J. Biol. Macromol. 2025, 284, 138100. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Hu, X.; Wang, Y.; Wang, J.; Tang, F.; Liu, Y. Structural characterization and anti-inflammatory activity of a pectin polysaccharide HBHP-3 from Houttuynia cordata. Int. J. Biol. Macromol. 2022, 210, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.-X.; Wu, Y.-C.; Liu, Y.; Lv, S.-Z.; You, Y.; Zhou, Z.-L.; Chen, X.; Li, H.-J. Structure and hypoglycemic effect of a neutral polysaccharide isolated from sea cucumber Stichopus japonicus. Int. J. Biol. Macromol. 2022, 216, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, P.; Xiao, L.; Gong, Z.; Qin, X.; Nie, J.; Zhu, H.; Zhong, S. Extraction, purification, structural characterization, and anti-inflammatory activity of a polysaccharide from Lespedeza formosa. Int. J. Biol. Macromol. 2025, 300, 140154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, X.; Zhang, L. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effect of temperature. Carbohydr. Polym. 2008, 73, 26–34. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46–52. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Chen, W.; Zhang, W.-M.; Zhang, H. Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: A primary approach. Carbohydr. Polym. 2009, 78, 620–625. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Wei, L.; Mutailifu, P.; Abuduwaili, A.; Paierhati, P.; Lei, C.; Zhiyan, Y.; Yufan, W.; Yili, A. The structural characteristic of acidic-degraded polysaccharides from seeds of Plantago ovata Forssk and its biological activity. Int. J. Biol. Macromol. 2024, 262, 129494. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, T.; Xu, J.; Xi, W.; Shang, E.; Xiao, P.; Duan, J.-A. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef] [PubMed]
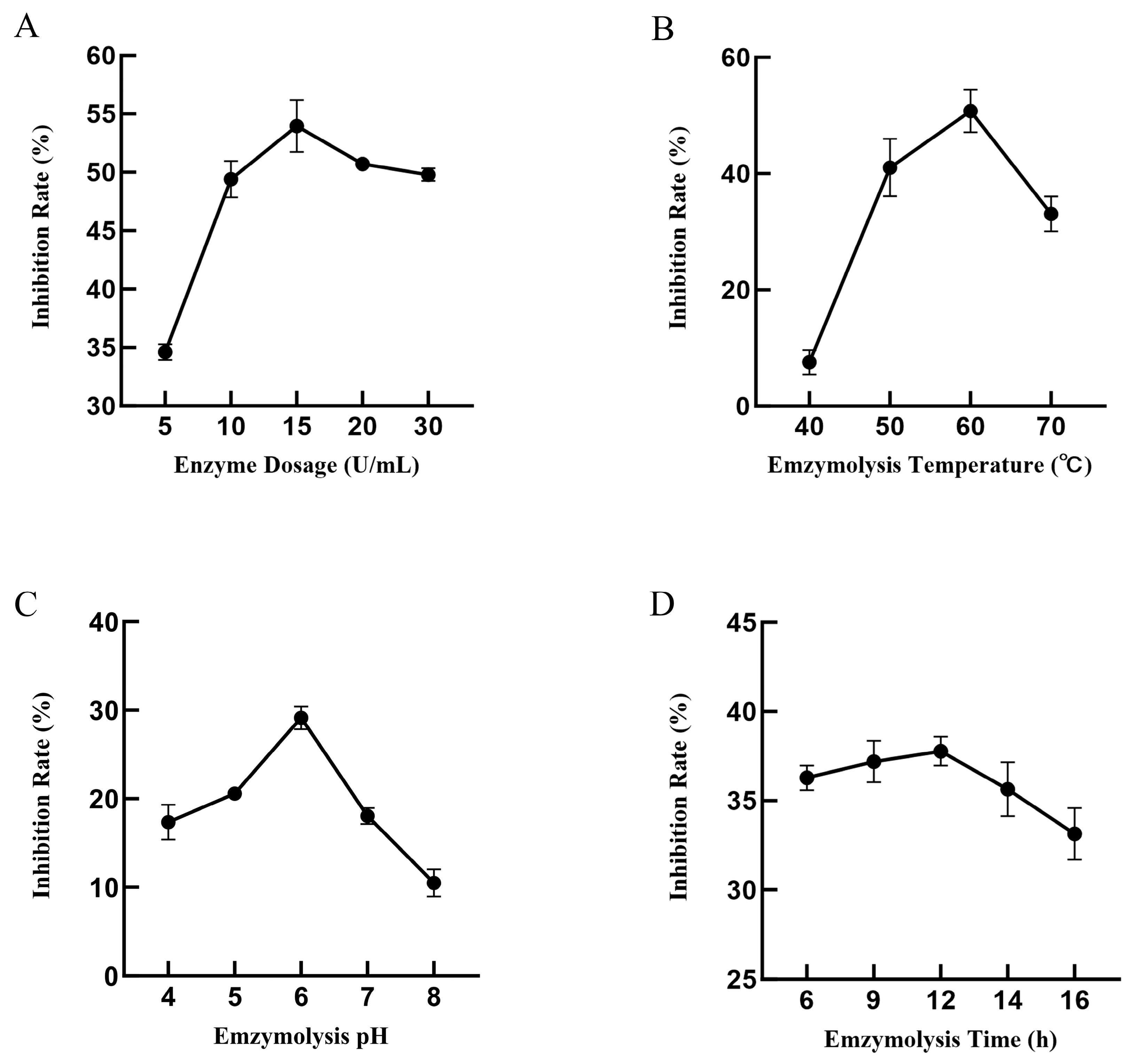
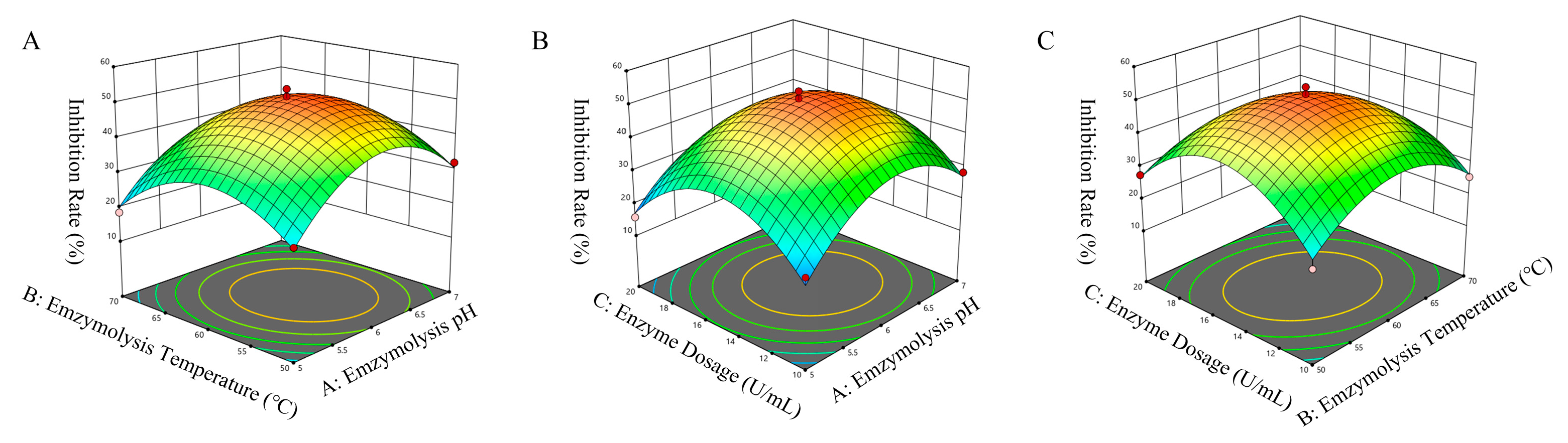
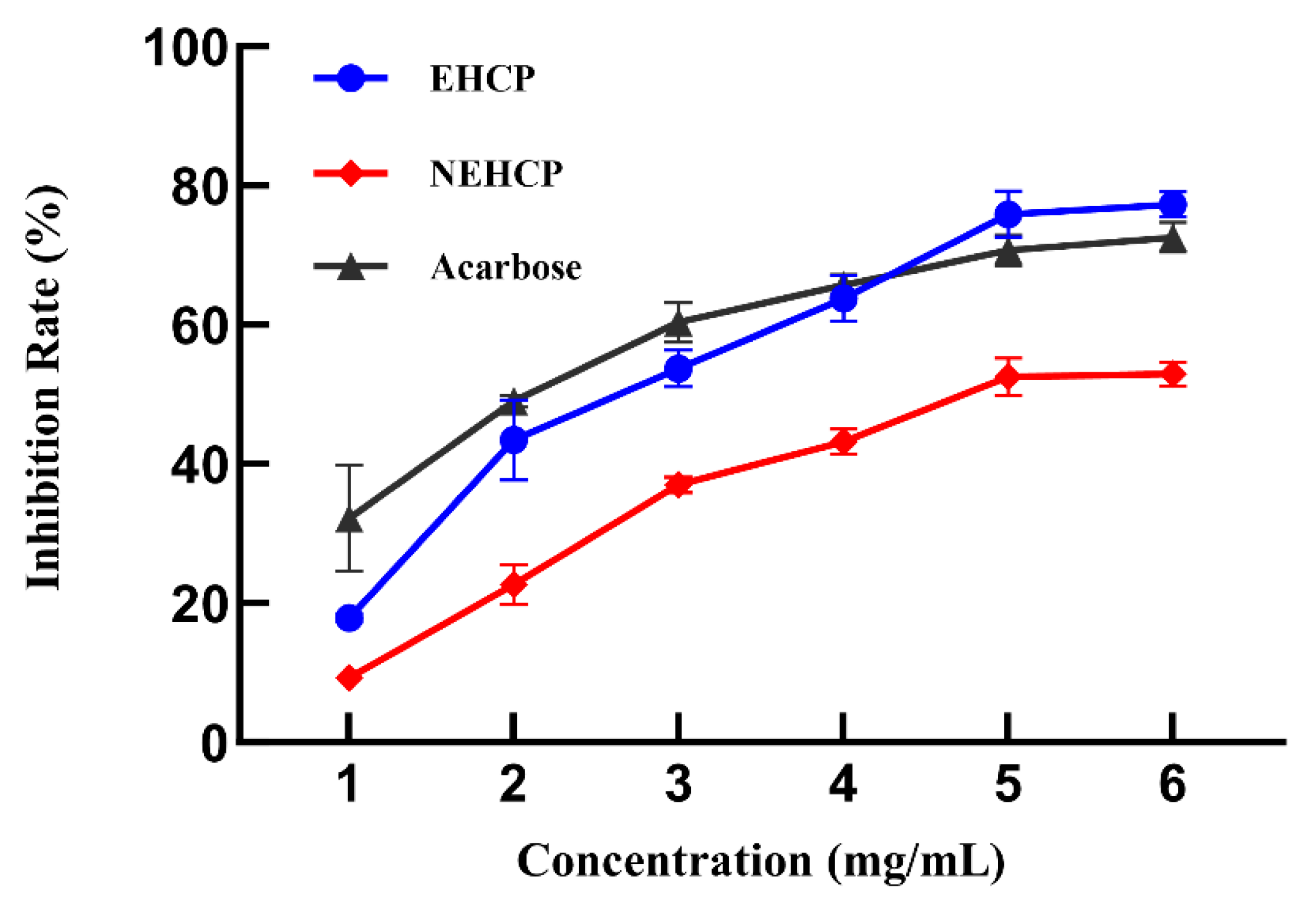
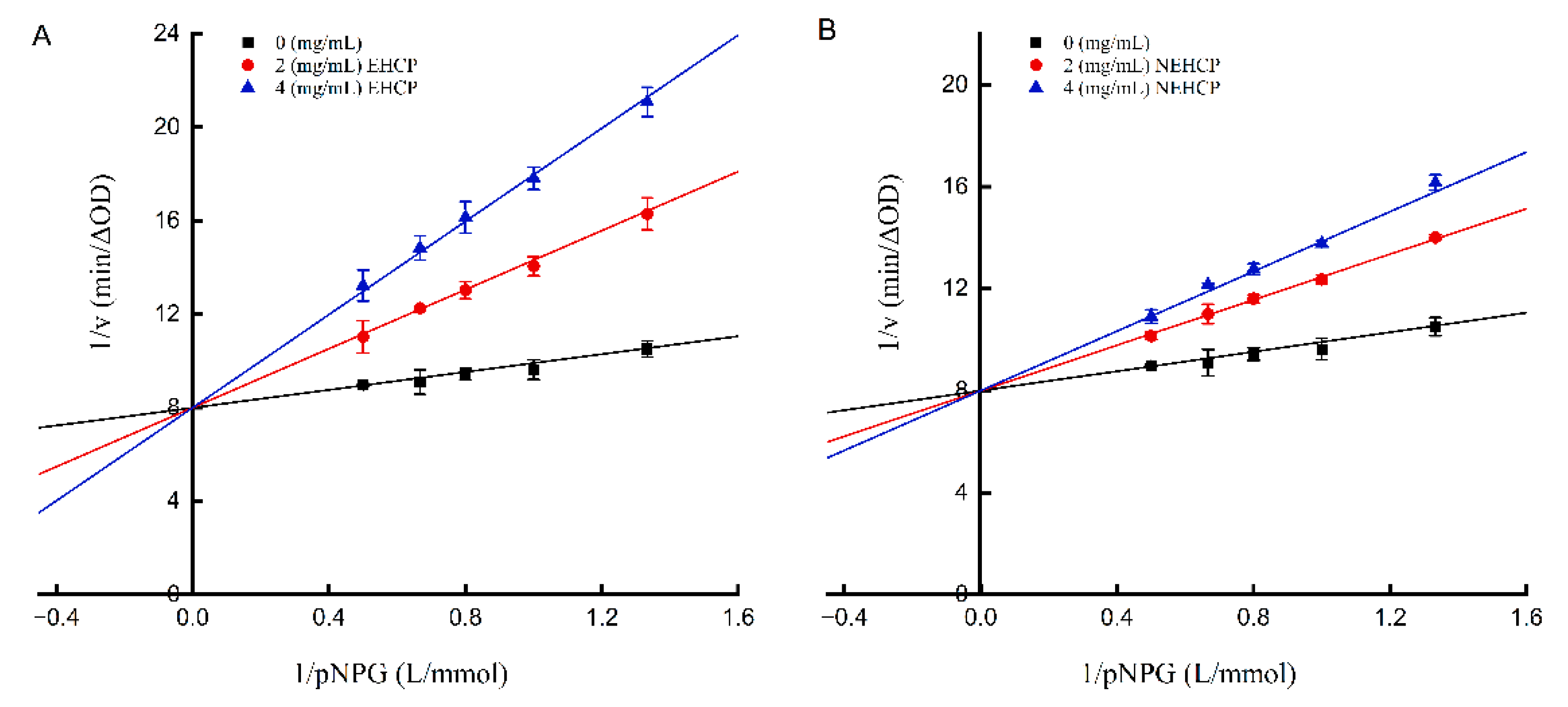


| Levels | Independent Variables | ||
|---|---|---|---|
| A Enzymolysis pH | B Enzymolysis Temperature (°C) | C Enzyme Dosage (U/mL) | |
| −1 | 5 | 50 | 10 |
| 0 | 6 | 60 | 15 |
| 1 | 7 | 70 | 20 |
| Exp No | A-Enzymolysis pH | B-Enzymolysis Temperature (°C) | C-Enzyme Dosage (U/mL) | Y-α-Glucosidase Inhibition Rate (%) |
|---|---|---|---|---|
| 1 | 7 | 60 | 10 | 28.30 ± 0.88 |
| 2 | 6 | 60 | 15 | 49.26 ± 1.54 |
| 3 | 6 | 60 | 15 | 53.47 ± 1.03 |
| 4 | 6 | 60 | 15 | 51.33 ± 1.43 |
| 5 | 5 | 60 | 10 | 20.56 ± 0.72 |
| 6 | 6 | 50 | 10 | 21.58 ± 1.74 |
| 7 | 7 | 50 | 15 | 32.04 ± 1.26 |
| 8 | 6 | 60 | 15 | 49.51 ± 1.84 |
| 9 | 6 | 70 | 20 | 18.34 ± 1.90 |
| 10 | 5 | 70 | 15 | 18.48 ± 1.19 |
| 11 | 7 | 60 | 20 | 17.97 ± 1.57 |
| 12 | 6 | 60 | 15 | 50.67 ± 1.76 |
| 13 | 6 | 50 | 20 | 27.61 ± 0.98 |
| 14 | 7 | 70 | 15 | 24.36 ± 1.33 |
| 15 | 5 | 60 | 20 | 15.80 ± 1.45 |
| 16 | 5 | 50 | 15 | 23.98 ± 1.07 |
| 17 | 6 | 70 | 10 | 25.49 ± 1.52 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2994.61 | 9 | 332.73 | 51.17 | <0.0001 | ** |
| A | 71.1 | 1 | 71.1 | 10.93 | 0.013 | * |
| B | 42.97 | 1 | 42.97 | 6.61 | 0.037 | * |
| C | 32.84 | 1 | 32.84 | 5.05 | 0.0594 | |
| AB | 1.19 | 1 | 1.19 | 0.1827 | 0.6819 | |
| AC | 7.76 | 1 | 7.76 | 1.19 | 0.3108 | |
| BC | 43.43 | 1 | 43.43 | 6.68 | 0.0362 | * |
| A2 | 868.87 | 1 | 868.87 | 133.62 | <0.0001 | ** |
| B2 | 583.09 | 1 | 583.09 | 89.67 | <0.0001 | ** |
| C2 | 1054.46 | 1 | 1054.46 | 162.16 | <0.0001 | ** |
| Residual | 45.52 | 7 | 6.5 | |||
| Lack of Fit | 34.07 | 3 | 11.36 | 3.97 | 0.1082 | |
| Pure Error | 11.45 | 4 | 2.86 | |||
| Cor Total | 3040.13 | 16 | ||||
| RAdj2 = 0.9658 | ||||||
| RPre2 = 0.8148 | ||||||
| Adeq Precision = 18.0148 | ||||||
| C.V.% = 8.20% | ||||||
| Concentration (mg/mL) | Vmax (ΔOD/min) | Km (mg/mL) | Ki (mg/mL) | Inhibition Type | |
|---|---|---|---|---|---|
| EHCP | 0 | 0.13 | 0.24 | 0.92 | competitive |
| 2 | 0.13 | 0.79 | |||
| 4 | 0.13 | 1.25 | |||
| NEHCP | 0 | 0.13 | 0.24 | 1.92 | competitive |
| 2 | 0.13 | 0.52 | |||
| 4 | 0.13 | 0.73 |
| Property | EHCP | NEHCP |
|---|---|---|
| Neutral sugar content (%) | 48.29 ± 1.37 a | 43.50 ± 1.65 b |
| Uronic acid content (%) | 41.18 ± 1.03 a | 39.03 ± 1.48 a |
| Protein content (%) | 1.95 ± 0.10 a | 1.93 ± 0.17 a |
| Reducing sugar content (%) | 7.26 ± 0.18 a | 2.81 ± 0.56 b |
| Solubility | 13.54 ± 0.81 a | 10.74 ± 0.21 b |
| Molecular weight (kDa) | 338.7 | 504.6 |
| Monosaccharide composition (%) | ||
| Rha | 2.12 ± 0.12 a | 1.78 ± 0.03 b |
| Fuc | 2.48 ± 0.13 a | 1.79 ± 0.16 b |
| Xyl | 0.75 ± 0.07 a | 0.79 ± 0.02 a |
| Ara | 0.17 ± 0.09 a | 0.18 ± 0.03 a |
| GlcA | 3.78 ± 0.20 a | 2.98 ± 0.22 b |
| GalA | 77.42 ± 2.75 a | 78.96 ± 3.50 a |
| Man | 3.16 ± 0.23 a | 2.49 ± 0.02 b |
| Glc | 2.08 ± 0.42 a | 5.24 ± 0.50 b |
| Gal | 8.04 ± 0.08 a | 5.77 ± 1.72 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, Z.; Yuan, Y.; Mansour, M.; Wu, T.; Pan, S.; Xu, X. Enzymatic Hydrolysis of Polysaccharide from Houttuynia cordata and Structure Characterization of the Degradation Products and Their α-Glucosidase Inhibitory Activity. Appl. Sci. 2025, 15, 11057. https://doi.org/10.3390/app152011057
Zhang L, Yang Z, Yuan Y, Mansour M, Wu T, Pan S, Xu X. Enzymatic Hydrolysis of Polysaccharide from Houttuynia cordata and Structure Characterization of the Degradation Products and Their α-Glucosidase Inhibitory Activity. Applied Sciences. 2025; 15(20):11057. https://doi.org/10.3390/app152011057
Chicago/Turabian StyleZhang, Lanlan, Zhixuan Yang, Yanan Yuan, Mohammed Mansour, Ting Wu, Siyi Pan, and Xiaoyun Xu. 2025. "Enzymatic Hydrolysis of Polysaccharide from Houttuynia cordata and Structure Characterization of the Degradation Products and Their α-Glucosidase Inhibitory Activity" Applied Sciences 15, no. 20: 11057. https://doi.org/10.3390/app152011057
APA StyleZhang, L., Yang, Z., Yuan, Y., Mansour, M., Wu, T., Pan, S., & Xu, X. (2025). Enzymatic Hydrolysis of Polysaccharide from Houttuynia cordata and Structure Characterization of the Degradation Products and Their α-Glucosidase Inhibitory Activity. Applied Sciences, 15(20), 11057. https://doi.org/10.3390/app152011057







