Development of Novel Wearable Biosensor for Continuous Monitoring of Central Body Motion
Abstract
1. Introduction
1.1. The Problem: Life-Threatening Conditions Requiring Rapid Detection
1.2. Current Monitoring Limitations
1.3. Central Actigraphy as a Solution
2. Device Development and Technical Overview
2.1. Hardware Design and Specifications
2.2. System Architecture Overview
2.3. DCM System Architecture: Data Collection and Management
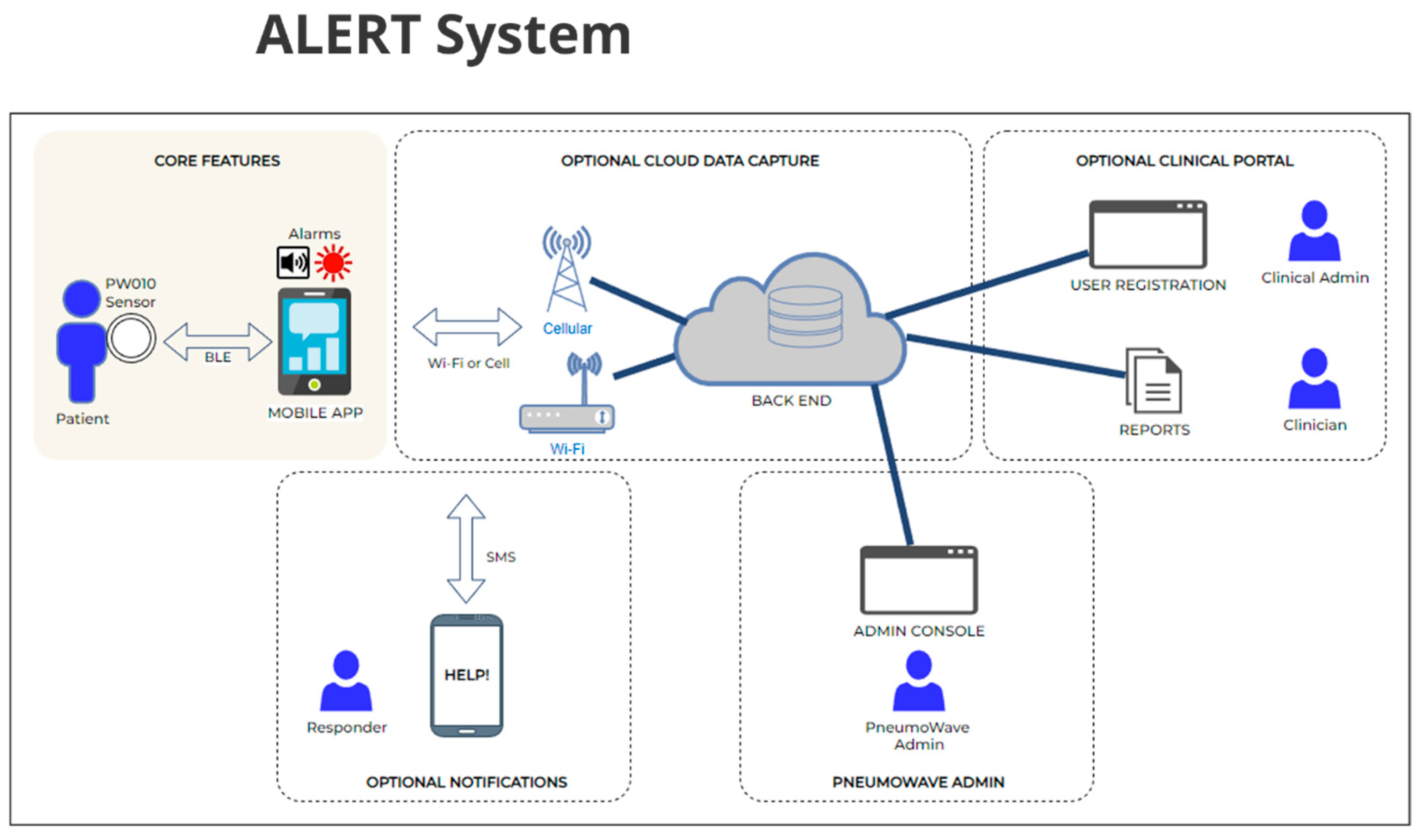
3. ALERT System Architecture: Real-Time Detection and Response
4. Clinical Integration and Applications
4.1. DCM System: Application in Research and Clinical Practice
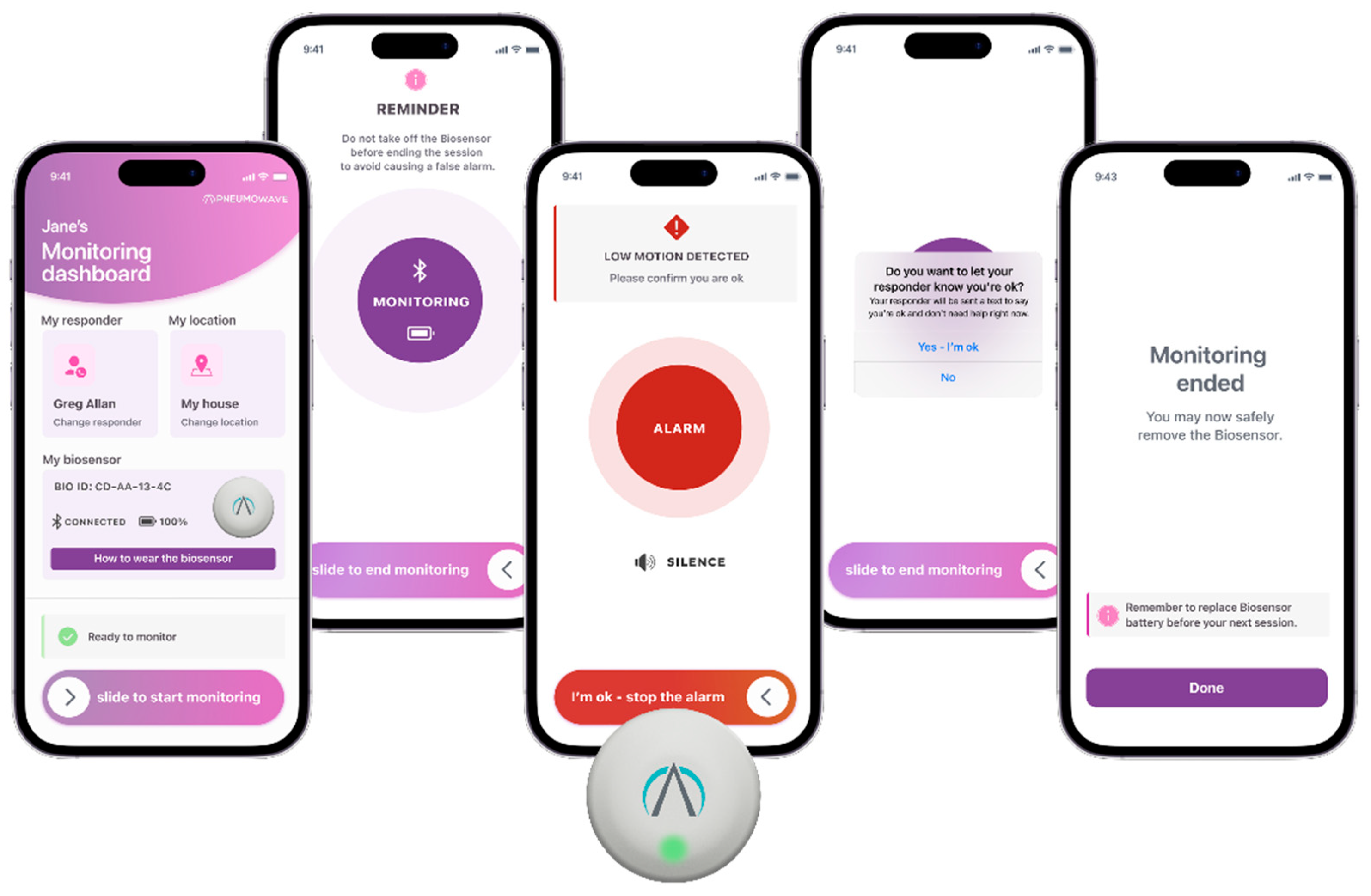
4.2. ALERT System: Application for At-Risk Populations
5. Clinical Testing and Real-World Evidence
User Acceptance and Acceptability
6. Ongoing Device Validation and Performance
6.1. The OD-SEEN Study: Real-World Clinical Data Gathering
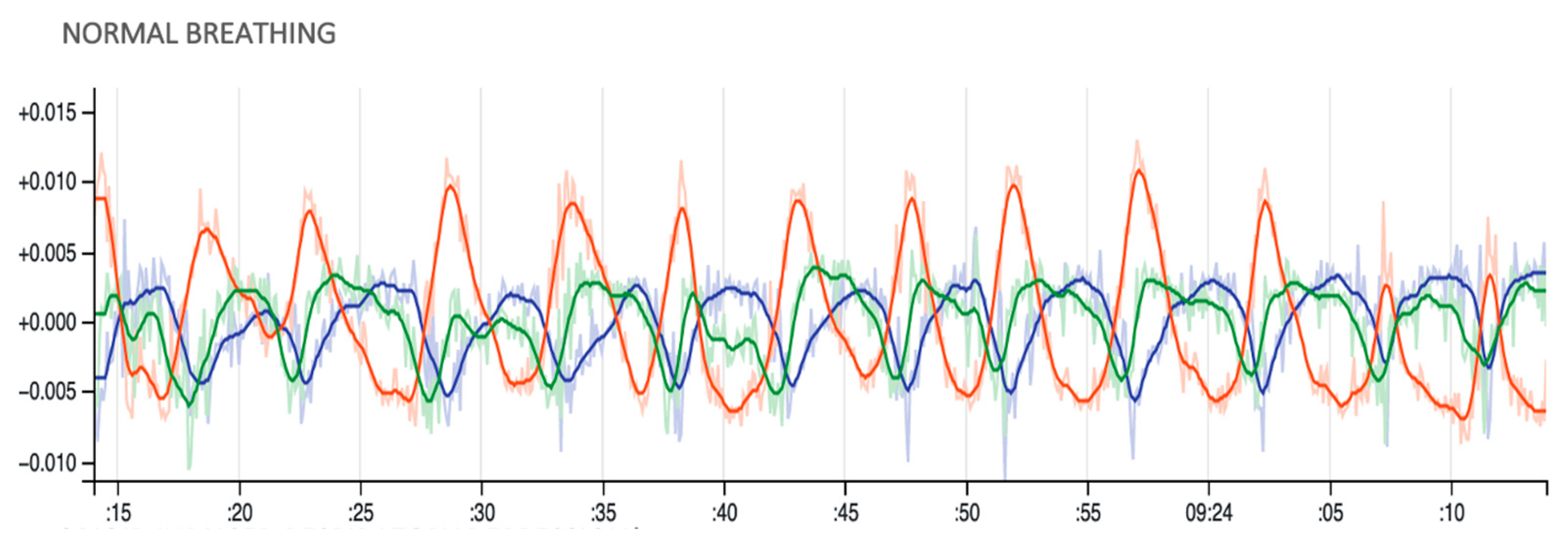
6.2. Preliminary Analysis of Motion Patterns During Drug Use Events
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden Unexpected Death in Epilepsy: Epidemiology, Mechanisms, and Prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.S.; Sampson, L.; Cerdá, M.; Galea, S. Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. Am. J. Public Health 2015, 105, e29–e49. [Google Scholar] [CrossRef] [PubMed]
- Faingold, C.L.; Feng, H.-J. A Unified Hypothesis of SUDEP: Seizure-Induced Respiratory Depression Induced by Adenosine May Lead to SUDEP but Can Be Prevented by Autoresuscitation and Other Restorative Respiratory Response Mechanisms Mediated by the Action of Serotonin on the Periaq. Epilepsia 2023, 64, 779–796. [Google Scholar] [CrossRef]
- Strang, J.; McDonald, R.; Campbell, G.; Degenhardt, L.; Nielsen, S.; Ritter, A.; Dale, O. Take-Home Naloxone for the Emergency Interim Management of Opioid Overdose: The Public Health Application of an Emergency Medicine. Drugs 2019, 79, 1395–1418. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The Spectrum of Epidemiology Underlying Sudden Cardiac Death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef]
- Sovik, O.; Thordarson, H. Dead-in-Bed Syndrome in Young Diabetic Patients. Diabetes Care 1999, 22 (Suppl. 2), B40–B42. [Google Scholar]
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden Cardiac Death: Epidemiology and Risk Factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef]
- Fraile-Martinez, O.; García-Montero, C.; Díez, S.C.; Bravo, C.; Quintana-Coronado, M.d.G.; Lopez-Gonzalez, L.; Barrena-Blázquez, S.; García-Honduvilla, N.; De León-Luis, J.A.; Rodriguez-Martín, S.; et al. Sudden Infant Death Syndrome (SIDS): State of the Art and Future Directions. Int. J. Med. Sci. 2024, 21, 848–861. [Google Scholar] [CrossRef]
- Ramesh, S.; Zalucky, A.; Hemmelgarn, B.R.; Roberts, D.J.; Ahmed, S.B.; Wilton, S.B.; Jun, M. Incidence of Sudden Cardiac Death in Adults with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. BMC Nephrol. 2016, 17, 78. [Google Scholar] [CrossRef]
- Tu, E.; Twigg, S.M.; Semsarian, C. Sudden Death in Type 1 Diabetes: The Mystery of the “dead in Bed” Syndrome. Int. J. Cardiol. 2010, 138, 91–93. [Google Scholar] [CrossRef]
- Goldwater, P.N. Current SIDS Research: Time to Resolve Conflicting Research Hypotheses and Collaborate. Pediatr. Res. 2023, 94, 1273–1277. [Google Scholar] [CrossRef]
- Marijon, E.; Narayanan, K.; Smith, K.; Barra, S.; Blom, M.T.; Crotti, L.; Avila, A.D.; Deo, R.; Dumas, F.; Dzudie, A.; et al. The Lancet Commission to Reduce the Global Burden of Sudden Cardiac Death: A Call for Multidisciplinary Action. Lancet 2023, 402, 883–936. [Google Scholar] [CrossRef]
- NIDA. Drug Overdose Deaths: Facts and Figures; NIDA: North Bethesda, MD, USA, 2024. [Google Scholar]
- Tas, B.; Jolley, C.J.; Kalk, N.J.; van der Waal, R.; Bell, J.; Strang, J. Heroin-Induced Respiratory Depression and the Influence of Dose Variation: Within-Subject between-Session Changes Following Dose Reduction. Addiction 2020, 115, 1954–1959. [Google Scholar] [CrossRef]
- Baird, A.; White, S.A.; Das, R.; Tatum, N.; Bisgaard, E.K. Whole Body Physiology Model to Simulate Respiratory Depression of Fentanyl and Associated Naloxone Reversal. Commun. Med. 2024, 4, 114. [Google Scholar] [CrossRef]
- Boom, M.; Niesters, M.; Sarton, E.; Aarts, L.; Smith, T.W.; Dahan, A. Non-Analgesic Effects of Opioids: Opioid-Induced Respiratory Depression. Curr. Pharm. Des. 2012, 18, 5994–6004. [Google Scholar] [CrossRef]
- Yu, Y.; Jain, B.; Anand, G.; Heidarian, M.; Lowe, A.; Kalra, A. Technologies for Non-Invasive Physiological Sensing: Status, Challenges, and Future Horizons. Biosens. Bioelectron. X 2024, 16, 100420. [Google Scholar] [CrossRef]
- Marcolino, M.S.; Oliveira, J.A.Q.; D’Agostino, M.; Ribeiro, A.L.; Alkmim, M.B.M.; Novillo-Ortiz, D. The Impact of MHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth 2018, 6, e8873. [Google Scholar] [CrossRef]
- Cho, S.; Ensari, I.; Weng, C.; Kahn, M.G.; Natarajan, K. Factors Affecting the Quality of Person-Generated Wearable Device Data and Associated Challenges: Rapid Systematic Review. JMIR Mhealth Uhealth 2021, 9, e20738. [Google Scholar] [CrossRef] [PubMed]
- Jarque-Bou, N.J.; Sancho-Bru, J.L.; Vergara, M. A Systematic Review of EMG Applications for the Characterization of Forearm and Hand Muscle Activity during Activities of Daily Living: Results, Challenges, and Open Issues. Sensors 2021, 21, 3035. [Google Scholar] [CrossRef] [PubMed]
- Kline, C. Actigraphy (Wrist, for Measuring Rest/Activity Patterns and Sleep) BT. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 17–21. ISBN 978-1-4419-1005-9. [Google Scholar]
- Marshall, B.D.L.; Milloy, M.-J.; Wood, E.; Montaner, J.S.G.; Kerr, T. Reduction in Overdose Mortality after the Opening of North America’s First Medically Supervised Safer Injecting Facility: A Retrospective Population-Based Study. Lancet 2011, 377, 1429–1437. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Karamouzian, M.; Marshall, B.D.L. The North American Opioid Crisis: How Effective Are Supervised Consumption Sites? Lancet 2022, 400, 1403–1404. [Google Scholar] [CrossRef]
- Ng, J.; Sutherland, C.; Kolber, M.R. Does Evidence Support Supervised Injection Sites? Can. Fam. Physician 2017, 63, 866. [Google Scholar]
- Levengood, T.W.; Yoon, G.H.; Davoust, M.J.; Ogden, S.N.; Marshall, B.D.L.; Cahill, S.R.; Bazzi, A.R. Supervised Injection Facilities as Harm Reduction: A Systematic Review. Am. J. Prev. Med. 2021, 61, 738–749. [Google Scholar] [CrossRef]
- Dow-Fleisner, S.J.; Lomness, A.; Woolgar, L. Impact of Safe Consumption Facilities on Individual and Community Outcomes: A Scoping Review of the Past Decade of Research. Emerg. Trends Drugs Addict. Health 2022, 2, 100046. [Google Scholar] [CrossRef]
- Goodhew, M.; Salmon, A.M.; Marel, C.; Mills, K.L.; Jauncey, M. Mental Health among Clients of the Sydney Medically Supervised Injecting Centre (MSIC). Harm Reduct. J. 2016, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, D.; Wettemann, C.; Fockele, C.E.; Frohe, T.; Williams, W.; Holland, N.; Oliphant-Wells, T.; Meischke, H.; van Draanen, J. “Another Tool in the Toolkit”—Perceptions, Suggestions, and Concerns of Emergency Service Providers about the Implementation of a Supervised Consumption Site. Int. J. Drug Policy 2023, 115, 104005. [Google Scholar] [CrossRef] [PubMed]
- Tas, B.; Walker, H.; Lawn, W.; Matcham, F.; Traykova, E.V.; Evans, R.A.S.; Strang, J. What Impacts the Acceptability of Wearable Devices That Detect Opioid Overdose in People Who Use Opioids? A Qualitative Study. Drug Alcohol. Rev. 2024, 43, 213–225. [Google Scholar] [CrossRef]
- Grap, M.J.; Hamilton, V.A.; McNallen, A.; Ketchum, J.M.; Best, A.M.; Arief, N.Y.I.; Wetzel, P.A. Actigraphy: Analyzing Patient Movement. Heart Lung 2009, 61, 1–7. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Rivolta, M.W.; Karmacharya, R.; Scarabottolo, N.; Pugnetti, L.; Garegnani, M.; Bovi, G.; Scalera, G.; Ferrarin, M.; Sassi, R. Performance Comparison between Wrist and Chest Actigraphy in Combination with Heart Rate Variability for Sleep Classification. Comput. Biol. Med. 2017, 89, 212–221. [Google Scholar] [CrossRef]
- Gupta, P.; Martin, J.L.; Needham, D.M.; Vangala, S.V.; Colantuoni, E.A.; Kamdar, B.B. Use of Actigraphy to Characterize Inactivity and Activity in Patients in a Medical ICU. Heart Lung 2020, 49, 398–406. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef]
- Sadeh, A.; Hauri, P.J.; Kripke, D.F.; Lavie, P. The Role of Actigraphy in the Evaluation of Sleep Disorders. Sleep 1995, 18, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A Review of Wearable Sensors and Systems with Application in Rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Polster, T.; Kjaer, T.W.; Hjalgrim, H. Detection of Generalized Tonic-Clonic Seizures by a Wireless Wrist Accelerometer: A Prospective, Multicenter Study. Epilepsia 2013, 54, e58–e61. [Google Scholar] [CrossRef] [PubMed]
- Van de Vel, A.; Cuppens, K.; Bonroy, B.; Milosevic, M.; Van Huffel, S.; Vanrumste, B.; Lagae, L.; Ceulemans, B. Long-Term Home Monitoring of Hypermotor Seizures by Patient-Worn Accelerometers. Epilepsy Behav. 2013, 26, 118–125. [Google Scholar] [CrossRef]
- Lloyd, C. The Stigmatization of Problem Drug Users: A Narrative Literature Review. Drugs Educ. Prev. Policy 2013, 20, 85–95. [Google Scholar] [CrossRef]
- Somerville, N.J.; O’Donnell, J.; Gladden, R.M.; Zibbell, J.E.; Green, T.C.; Younkin, M.; Ruiz, S.; Babakhanlou-Chase, H.; Chan, M.; Callis, B.P.; et al. Characteristics of Fentanyl Overdose—Massachusetts, 2014–2016. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 382–386. [Google Scholar] [CrossRef]
- Adam, A.; Dillon, J.; Strang, J. Diagnostic Definitions of Overdose and (Opioid-Induced) Respiratory Depression Relevant to Remote Monitoring via Accelerometry. Heroin Addict. Relat. Clin. Probl. 2025, 27, 18. [Google Scholar] [CrossRef]
- Tas, B.; Lawn, W.; Jauncey, M.; Bartlett, M.; Dietze, P.; O’Keefe, D.; Clark, N.; Henderson, B.; Cowan, C.; Meredith, O.; et al. Overdose Detection Among High-Risk Opioid Users Via a Wearable Chest Sensor in a Supervised Injecting Facility: Protocol for an Observational Study. JMIR Res. Protoc. 2024, 13, e57367. [Google Scholar] [CrossRef]
- Uniting History of Uniting MSIC. Available online: https://www.uniting.org/community-impact/uniting-medically-supervised-injecting-centre--msic/history-of-uniting-msic (accessed on 24 July 2025).





| Device | Accelerometer Only Current Consumption (mA) | Acc + Gyro Current Consumption (mA) |
|---|---|---|
| Bosch BMI330 | 0.15 | 0.79 |
| ST LSMDSOX | 0.17 | 0.55 |
| TDK ICM-45686 | 0.12 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Utrilla, M.; Henderson, B.; Kelly, S.; Meredith, O.; Tas, B.; Lawn, W.; Appiah-Kusi, E.; Dillon, J.F.; Strang, J. Development of Novel Wearable Biosensor for Continuous Monitoring of Central Body Motion. Appl. Sci. 2025, 15, 11027. https://doi.org/10.3390/app152011027
Gonzalez Utrilla M, Henderson B, Kelly S, Meredith O, Tas B, Lawn W, Appiah-Kusi E, Dillon JF, Strang J. Development of Novel Wearable Biosensor for Continuous Monitoring of Central Body Motion. Applied Sciences. 2025; 15(20):11027. https://doi.org/10.3390/app152011027
Chicago/Turabian StyleGonzalez Utrilla, Mariana, Bruce Henderson, Stuart Kelly, Osian Meredith, Basak Tas, Will Lawn, Elizabeth Appiah-Kusi, John F. Dillon, and John Strang. 2025. "Development of Novel Wearable Biosensor for Continuous Monitoring of Central Body Motion" Applied Sciences 15, no. 20: 11027. https://doi.org/10.3390/app152011027
APA StyleGonzalez Utrilla, M., Henderson, B., Kelly, S., Meredith, O., Tas, B., Lawn, W., Appiah-Kusi, E., Dillon, J. F., & Strang, J. (2025). Development of Novel Wearable Biosensor for Continuous Monitoring of Central Body Motion. Applied Sciences, 15(20), 11027. https://doi.org/10.3390/app152011027






