Abstract
Elevated plasma homocysteine (tHcy) is a modifiable risk factor for stroke and cardiovascular disease, influenced by genetic and lifestyle factors. Resistance-based training may reduce tHcy, but the impact of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism remains unclear. This study examined the effects of a 26-week combined aerobic and resistance training program on stroke-related risk factors and tHcy levels in patients with chronic ischemic stroke, stratified by MTHFR genotype. Forty-six patients (mean age: 57.7 ± 8.9 years) completed supervised training three times per week. Assessments before and after the intervention included anthropometry, cardiorespiratory fitness, biochemical markers, and tHcy. Dietary intake was monitored and remained stable. Significant improvements were observed in body weight, fat mass, waist-to-hip ratio, VO2max, and 6 min walk distance. tHcy decreased significantly overall (p < 0.01), with reductions confined to the CC genotype group (p < 0.01). BMI declined in CC and CT groups (p < 0.01 and p < 0.05), while fat-free mass, triglycerides, fasting glucose, and blood pressure showed no changes, likely due to pharmacological control. These findings suggest that combined training improves body composition and functional capacity, while genotype-specific reductions in tHcy highlight the potential of personalized rehabilitation strategies integrating genetic and nutritional considerations in stroke care.
1. Introduction
Stroke is a major cerebrovascular disorder characterized by acute neurological deficits resulting from interrupted cerebral blood flow or intracranial hemorrhage [1]. Globally, approximately 12 million new strokes occur annually, with more than 7 million deaths, while tens of millions of survivors are left with long-term disabilities and sequelae [1].
A large proportion of stroke survivors experience multi-domain impairments across motor, sensory, cognitive, perceptual, and language functions. Notably, upper-limb (arm/hand) impairments are particularly prevalent, observed in over half of acute-phase patients, with many exhibiting persistent functional limitations. As these deficits significantly hinder activities of daily living (ADL) and societal reintegration, identifying and managing modifiable risk factors is of critical importance [2].
Total plasma homocysteine (tHcy) is recognized as an independent risk marker for cardiovascular diseases, including stroke. Systematic reviews and meta-analyses report that higher tHcy is associated with a greater risk of ischemic stroke [3,4] and with an approximately 25–35% increase in all-cause mortality in a linear manner [5].
tHcy, a sulfur-containing amino acid produced during methionine–one-carbon metabolism, is significantly influenced by dietary folate and vitamins B12 and B6, as well as by the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism. The TT homozygous variant reduces MTHFR enzyme activity by approximately 70–75%, leading to higher circulating tHcy levels [6].
Physical activity and exercise improve multiple stroke risk factors, including blood pressure, lipids, and inflammation. Meta-analytic evidence indicates that an acute (single) bout of exercise transiently elevates tHcy levels. Both moderate-intensity, long-duration acute exercise and high-intensity, short-duration acute exercise increase homocysteine concentrations, with prolonged low-to moderate-intensity exercise showing the greatest rise. However, resistance training decreases tHcy over the longer term, whereas aerobic training alone shows no clear effect [7]. In contrast, in 6-month interventions among patients with stroke, a randomized controlled trial reported significant reductions in tHcy with both high-intensity aerobic training and low-intensity balance/flexibility programs [8], and a pre–post study reported reductions after combined aerobic-plus-resistance training [9], suggesting that changes in dietary folate/B-vitamin intake and differences in genotype should be considered concurrently.
Regarding nutritional or pharmacologic interventions, in large secondary-prevention RCTs (e.g., VITATOPS), lowering tHcy with B-vitamins did not produce a clear overall reduction in recurrent vascular events [10], whereas in a large primary-prevention RCT conducted in a population without folic-acid fortification (CSPPT), adjunct folic acid significantly reduced first-ever stroke risk [11]. Thus, while B-vitamins lower tHcy by roughly 25% and on average modestly (≈10%) reduce the relative risk of stroke, the magnitude of benefit depends on baseline folate status and population characteristics [12].
Accordingly, stroke rehabilitation should be grounded in combined aerobic-plus-resistance training; when the goal is tHcy management, an adequate emphasis on resistance components is advisable. Interpretation of tHcy should consider folate and B-vitamin intake and MTHFR genotype, which are directly involved in homocysteine metabolism [7,8,9,10,11,12].
Therefore, this study aimed to assess the effects of a 6-month combined aerobic and resistance training program structured to emphasize resistance exercise on stroke-related risk factors and plasma homocysteine levels, stratified by MTHFR C677T genotype, in patients with chronic ischemic stroke. Dietary intake was held constant throughout the intervention to eliminate nutritional confounding.
2. Materials and Methods
2.1. Study Design
This study was conducted as a 26-week randomized trial using a cluster sampling approach to investigate the effects of combined aerobic and resistance training on tHcy and stroke-related risk factors in patients with chronic ischemic stroke. Participants were clustered by ischemic stroke and subsequently assigned according to MTHFR C677T genotyping by PCR, with groups classified into homozygous wild-type Cytosine+Cytosine (CC), heterozygous Cytosine+Thymine (CT), and homozygous mutant Thymine+Thymine (TT) genotypes. The intervention consisted of supervised training three times per week for 26 weeks, with each session lasting approximately 70 min, including a 10 min warm-up, 50 min of structured main exercise, and a 10 min cool-down. The aerobic component consisted of cycling at 60% VO2 reserve, while the resistance component included 13 exercises performed at 50–80% of one-repetition maximum (1RM). Resistance intensity was reassessed every four weeks using the rating of perceived exertion (RPE), and loads were increased by 5% when the reported RPE fell below 18 to maintain progressive overload. Functional capacity was monitored by conducting the 6 min walk test (6MWT) every four weeks to adjust aerobic intensity. Assessments were conducted before and after the intervention. Pre-test assessments included body composition, blood analysis, anthropometry, graded exercise test (GXT), 6MWT, energy intake analysis, and estimated 1RM, while post-test assessments included body composition, blood analysis, anthropometry, GXT, 6MWT, and energy intake analysis. The outcome variables of this study were plasma tHcy levels, cardiorespiratory fitness, body composition, and blood biomarkers [Figure 1]. The study protocol was approved by the Institutional Ethics Committee of the College of Physical Education, Kyung Hee University (approval number: KHSIRB-18-010, date of approval: 26 September 2019), and written informed consent was obtained from all participants prior to study enrollment.
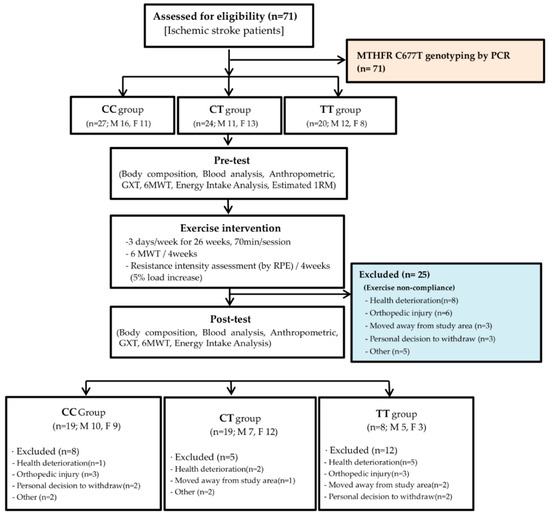
Figure 1.
Consort flow chart. (CC: Cytosine+Cytosine, CT: Cytosine+Thymine, TT: Thymine+Thymine, GXT: graded exercise test, 6MWT: 6 min walk test, RPE: Rating of Perceived Exertion, 1RM: 1-repetition maximum.).
2.2. Participants
A total of 71 patients with ischemic stroke were recruited via flyers posted on the Dankook University campus and in the Chungnam area. Written informed consent was obtained from all participants before enrollment. Estrogen is known to influence homocysteine metabolism, and postmenopausal women typically have higher circulating homocysteine levels compared with premenopausal women [13]. The average age of natural menopause among Korean women is approximately 48–52 years [14], and epidemiological data indicate that the prevalence of stroke in Koreans begins to increase after the age of 45. Based on these considerations, participants aged ≥48 years were included to ensure clinical relevance regarding estrogen-related metabolic changes and stroke risk [15]. The inclusion criteria were as follows: (1) age ≥ 48 years; (2) postmenopausal status for women, confirmed using structured questionnaires; (3) history of a single ischemic stroke occurring at least one year prior to study participation; and (4) ability to ambulate independently, with or without an assistive device. Among the initially enrolled 71 participants, 25 (17 men and 8 women) were excluded due to non-compliance with the prescribed exercise program. Consequently, 46 patients (mean age: 57.74 ± 8.90 years; male: n = 22, 57.55 ± 8.52 years; female: n = 24, 57.92 ± 9.41 years) completed the 26-week intervention and post-intervention assessments [Table 1]. These participants were further stratified into three groups based on MTHFR C677T genotype: CC, CT, and TT. This classification was incorporated into subsequent analyses.

Table 1.
Sex-specific distribution of MTHFR C677T genotypes.
2.3. Measurement
2.3.1. Blood Collection and Assessment of Biomarkers
Serum aliquots were stored at −80 °C until they were assayed. Triglyceride (TG) levels were measured by enzymatic colorimetry and were analyzed using the Cobas integra® 800 software version 4.1 (Roche, Basel, Switzerland). Plasma glucose levels were measured with a commercially available kit (glucose hexokinase kit; ADVIA 1650, Bayer, Tokyo, Japan). tHcy level was determined by fast protein liquid chromatography using a radiometric tyrosine assay. Systolic and diastolic blood pressures were measured on the right arm with the patients in a sitting position, twice, after a 5 min rest, using an automatic sphygmomanometer (Bpbio320, InBody, Seoul, Republic of Korea).
2.3.2. MTHFR C677T Genotypes
Total blood DNA was extracted and purified from 200 µL of whole blood treated with EDTA (an anticoagulant) using the Axygen Blood DNA Miniprep Kit according to manufacturer’s instructions (Axygen Biosciences, Seoul, Republic of Korea). Genotyping for C677T variants was performed using the method described by Frosst et al. [16] MTHFR C677T polymorphisms were detected by polymerase chain reaction amplification and restriction digestion with HinfI to distinguish mutant and wild type alleles.
2.3.3. Anthropometric
The height and weight of all the subjects in light clothes were measured before and after their 6-month exercise training. Body weight (kg) and height (m) were measured using an automatic stadiometer and scale (BSM 330, InBody, Republic of Korea) measuring device, and BMI was calculated by dividing body weight by the square of height. Body composition (fat mass, fat free mass) was assessed using bioelectrical impedance analysis (InBody 570, Biospace, Republic of Korea). To standardize measurement conditions, participants were instructed to fast for at least 8 h and to refrain from vigorous physical activity during the preceding 24 h. All measurements were performed in the laboratory at 9:00 a.m. with participants in a fasting state. Waist circumference (WC) and hip circumference (HC) were measured according to standardized anthropometric procedures. The waist-to-hip ratio (WHR) was calculated by dividing WC by HC. WC and HC were measured by two trained exercise specialists on two consecutive days. The coefficients of variation for WC and HC were 0.8% for women and 0.8% for men.
2.3.4. Cardiorespiratory Fitness
Cardiorespiratory fitness was assessed using a graded exercise test and a 6 min walk test [17]. The graded exercise test was conducted on a cycle ergometer to determine maximal oxygen uptake (VO2max) using the RAMP protocol. The RAMP protocol was performed on an electronically braked cycle ergometer (Excalibur Sport ergometer, Lode, The Netherlands) at a constant cadence of 70 rpm, starting at 0 W. The workload was progressively increased by 15 W per minute until the participant reached voluntary exhaustion. During the test, respiratory gas exchange and heart rate were continuously monitored in real time (K4B2, Cosmed, Italy). The 6 min walk test, a field-based method suitable for individuals with lower fitness levels, was performed to evaluate cardiorespiratory fitness by measuring the total distance covered in six minutes. VO2max values obtained prior to the intervention were used to prescribe individualized exercise intensities according to each participant’s fitness level [18].
2.3.5. Energy Intake Analysis
The subjects’ dietary intakes were surveyed using the estimated food record method for a total of 3 days (2 weekdays and 1 weekend) before and after their 6-month exercise training [9]. The auxiliary material, cuisine, recipe, and serving quantities, as well as the food quantities, were recorded, with portion sizes estimated using standardized tools and photographs. The weekly dietary and energy intakes were analyzed using the 3.0-version Computer-aided Nutrient Analysis Program developed by the Nutritional Information Center of the Korean Nutrition Society. Unfortunately, we could not examine vitamin B12 intake, owing to limited data in Can pro professional 3.0. During the exercise-training period, nutrition education was provided to the stroke patients. However, nutritional intervention was not provided in order to verify the effect of exercise training.
2.3.6. Criteria for Stroke Risk Factors
The American Heart Association [19] has suggested the following criteria as stroke risk factors: (1) hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, (2) diabetes was defined as fasting blood glucose level ≥ 110 mg/dL, (3) hyper triglyceride was defined as triglyceride values ≥ 150 mg/dL, (4) obesity was defined as BMI ≥ 25 kg/m2 according to the Korea Society for the Study of Obesity [20], and (5) hyper homocysteinemia was defined as Hcy levels ≥ 16 μmol/L.
2.4. Combined Aerobic and Resistance Exercise Training
Participants engaged in a combined aerobic and resistance training program three times per week for 26 weeks. All sessions were supervised by four certified physical education instructors and included a 10 min warm-up, 50 min of structured main exercise, and a 10 min cool-down involving light walking.
The aerobic component consisted of 20 min of cycling on a stationary ergometer at an intensity corresponding to 60% of each participant’s VO2 reserve. To monitor improvements in cardiorespiratory fitness and ensure appropriate exercise intensity, the 6 min walk test was conducted every four weeks. Based on these results, cycling workloads were individually adjusted [7]. The resistance training component targeted major muscle groups and comprised one set of 13 exercises: abdominal crunch, leg press, leg extension, leg curl, calf press, seated row, chest press, overhead press, biceps curl, seated dip, leg abduction, leg adduction, and lumbar extension. Each exercise was performed at 50–80% of the participant’s one-repetition maximum (1RM), with 8–13 repetitions. Resistance loads were reassessed regularly, and if a participant’s rating of perceived exertion (RPE) for an exercise was reported as below 18, the load was increased by 5% to maintain progressive overload and optimal training intensity [21].
2.5. Statistical Analysis
All statistical analyses were performed using SPSS software (version 23.0 for Windows; IBM Corp., NY, USA). The sample size was calculated using G*Power version 3.1. A two-time-point, three-group repeated-measures ANOVA (group × time interaction) was assumed with a medium effect size (f = 0.25), a significance level (α) of 0.05, a statistical power of 0.80, a correlation among repeated measures of 0.50, and ε (epsilon) = 1.0. The analysis indicated that approximately 45 participants were required; considering a 20% dropout rate, we planned to recruit at least 54 participants. Ultimately, a total of 71 participants were enrolled. Descriptive statistics, including the mean and standard deviation (SD), were computed for each variable. Normality of the data distribution was assessed using the Shapiro–Wilk test prior to conducting parametric analyses. To assess changes over time and between groups following the 26-week aerobic exercise intervention, a two-way repeated measures analysis of variance (RM two-way ANOVA) was conducted. For ANOVA, effect sizes were reported as partial eta-squared (η2), with large effects defined as η2 ≥ 0.13 [22]. Where significant time main effects were detected, within-group differences over time were further examined using paired t-tests (large effect sizes; Cohen’s d = 0.7) [23], and p-values were adjusted for multiple comparisons using the Bonferroni correction. Statistical significance was set at p < 0.05 for all analyses.
3. Results
3.1. Dietary Intake
No significant time-by-group interaction effects or main effects of time and group were observed for any dietary intake variables over the 26-week intervention [Table 2].

Table 2.
Effects of 26-week combined exercise training on dietary intake according to MTHFR C677T genotypes in patients with ischemic stroke (Mean ± SD).
3.2. Body Composition and Cardiorespiratory Fitness
Body weight showed a significant main effect for time (p < 0.001, partial η2 = 0.574). Post hoc comparisons indicated significant reductions in the CC, CT, and TT groups (p < 0.001, p < 0.001, and p < 0.01, respectively).
Fat mass exhibited a significant main effect for time (p < 0.01, partial η2 = 0.216), with post hoc analysis demonstrating significant decreases in the CC and CT groups (p < 0.05, p < 0.01, respectively).
WHR presented a significant main effect for time (p < 0.001, partial η2 = 0.348). Subsequent pairwise comparisons revealed significant reductions in the CC, CT, and TT groups (p < 0.01, p < 0.01, and p < 0.05, respectively).
VO2max demonstrated a significant main effect for time (p < 0.001, partial η2 = 0.250), with both the CT and TT groups showing significant improvements compared with baseline (all p < 0.05).
The 6 min walk test also revealed a significant main effect for time (p < 0.001, partial η2 = 0.250), with significant gains observed in the CT and TT groups relative to baseline (all p < 0.05).
There was no significant time-by-group interaction or main effects of time and group for fat free mass [Table 3].

Table 3.
Effects of 26-week combined exercise training on body composition and cardiorespiratory fitness according to MTHFR C677T genotypes in patients with ischemic stroke (Mean ± SD).
3.3. Stroke Risk Factors
Homocysteine levels showed a significant main effect for time (p < 0.01, partial η2 = 0.228), with post hoc comparisons indicating a significant reduction in the CC group (p < 0.01).
BMI demonstrated a significant main effect for time (p < 0.01, partial η2 = 0.215), and post hoc analysis revealed significant reductions in BMI in the CC and CT groups (p < 0.01 and p < 0.05, respectively).
There were no significant time-by-group interactions or main effects of time and group for TG, FBG, SBP, or DBP [Table 4].

Table 4.
Effects of 26-week combined exercise training on stroke risk factors according to MTHFR C677T genotypes in patients with ischemic stroke (Mean ± SD).
4. Discussion
In this study, a 26-week combined training program integrating aerobic exercise with resistance training produced significant main effects of time on body weight, fat mass, WHR, and 6MWT performance, despite stable dietary intake throughout the intervention. Total plasma tHcy also declined over time, with a distinct post hoc reduction in the MTHFR CC group, indicating a genotype-dependent response. By contrast, traditional stroke risk markers (TG, FBG, SBP, and DBP) did not change significantly.
The concurrent reductions in body weight (CC, CT, and TT) and fat mass (CC, CT) in the absence of changes in fat-free mass indicate a preferential loss of adiposity without compromise of lean tissue, consistent with the healthy weight-management effects of a resistance-based combined program over six months [24,25]. Waist circumference serves not only as a measure of abdominal obesity but also as an effective predictor of hypertension and dyslipidemia, and is associated with recurrent stroke risk [26,27]. In this context, the decrease in WHR across all MTHFR genotypes (CC/CT/TT) observed after six months likely reflects reductions in central adiposity, which via attenuated inflammation and improved endothelial function may lower cardiovascular risk and the likelihood of stroke recurrence [28].
Gait impairment is a common post-stroke motor deficit [29] that diminishes activities of daily living and increases fall risk [30]; enhancing gait is therefore an important goal of rehabilitation [31]. In the present study, 6MWT performance improved significantly after 26 weeks (p < 0.001, partial η2 = 0.250), indicating gains in submaximal functional capacity and ambulatory endurance. The 6MWT is widely recognized as a valid indicator of submaximal aerobic capacity and functional endurance in populations with neurological disorders. Enhanced 6MWT performance following intervention reflects improved cardiovascular efficiency, muscle endurance, and neuromotor coordination, which are essential for recovery of mobility after stroke [17]. Clinically, a greater 6MWT distance is associated with improved ability to perform activities of daily living, increased community ambulation, and reduced risk of secondary complications such as cardiovascular events or recurrent stroke. Furthermore, previous studies have reported that even modest gains in 6MWT distance are correlated with better quality of life, social participation, and long-term survival [32]. Therefore, improvements in 6MWT distance observed in this study suggest that the intervention not only promoted physical capacity but also contributed to functional independence and overall prognosis in stroke patients [33]. By genotype, significant improvements were evident in CT and TT, whereas CC did not reach statistical significance. This pattern likely reflects heterogeneity in lesion severity, baseline functional status, and training responsiveness, rather than a true absence of effect in CC. Prior work suggests that early increases in gait speed may be achieved through compensation of the less-affected limb, whereas recovery of the paretic side is relatively delayed [34], implying different adaptation timelines for compensation versus recovery in post-stroke exercise responses [35].
Prior evidence indicates that aerobic training after stroke can reduce fasting glucose and systolic blood pressure relative to non-aerobic controls [36], whereas resistance training improves insulin sensitivity and lipid fractions in chronically disabled stroke survivors [37]. Moreover, a 6-month RCT reported concomitant improvements in lipid profile, glucose, and homocysteine following either high-intensity aerobic training or low-intensity balance/flexibility programs [8]. In contrast to these reports, despite the duration of training, TG, FBG, SBP, and DBP did not change significantly over 26 weeks. Although some epidemiologic and clinical studies report that each 1% reduction in body fat is associated with a 3–8% lower risk of developing hypertension, hypercholesterolemia, and other cardiovascular risk factors [24], the present study found no significant changes in these markers despite decreases in fat mass of −1.70, −2.36, and −2.01 kg in the CC, CT, and TT groups, respectively. A plausible explanation for the lack of significant changes in TG, FBG, SBP, and DBP over the 26-week intervention is the concurrent use of antihypertensive and lipid-lowering therapy in our participants, all of whom were stroke patients. These pharmacological treatments likely maintained baseline values within or close to the normal range, thereby reducing the potential for further improvement through exercise training. Indeed, mean TG levels were ≤150 mg/dL, FBG was ≤120 mg/dL, and resting blood pressure averaged approximately 125/85 mmHg at baseline, indicating a constrained headroom for change. In addition to this ceiling effect, the pharmacological actions of antihypertensive and lipid-lowering drugs themselves may have attenuated the incremental effects of exercise. For example, antihypertensive agents (e.g., ACE inhibitors, beta-blockers, or calcium-channel blockers) reduce vascular resistance and blood pressure through mechanisms that overlap with exercise-induced hemodynamic adaptations [38,39]. Similarly, statins and other lipid-lowering drugs decrease LDL-C and TG while modestly improving endothelial function, potentially masking further lipid improvements attributable to exercise [40]. Thus, the favorable metabolic profile at baseline, shaped in part by pharmacotherapy, may have limited the observable additive benefit of training. In addition to this ceiling effect, the pharmacological actions of antihypertensive and lipid-lowering drugs themselves may have attenuated the incremental effects of exercise. For example, antihypertensive agents (e.g., ACE inhibitors, beta-blockers, or calcium-channel blockers) reduce vascular resistance and blood pressure through mechanisms that overlap with exercise-induced hemodynamic adaptations. Similarly, statins and other lipid-lowering drugs decrease LDL-C and TG while modestly improving endothelial function, potentially masking further lipid improvements attributable to exercise. Thus, the favorable metabolic profile at baseline, shaped in part by pharmacotherapy, may have limited the observable additive benefit of training.
Consistent with prior studies, fasting glucose often shows no significant change in non-diabetic samples [41], and exercise effects can be attenuated in normotensive or normoglycemic individuals [42]. In contrast, BMI declined over time in the CC and CT groups, mirroring the changes in body weight and fat mass and supporting the body-composition benefits of the intervention [24].
The significant reduction in tHcy after 26 weeks aligns with meta-analytic evidence that resistance training lowers chronic tHcy, whereas aerobic training alone exerts no clear chronic effect [7]. The finding that post hoc reductions emerged only in the CC group is biologically plausible, given that the MTHFR 677C>T TT genotype reduces enzyme activity by ≈70–75% and predisposes to higher tHcy [6]; thus, without optimization of one-carbon nutrient status (folate, vitamins B6 and B12), T-allele carriers may exhibit a blunted training-induced decline in tHcy [43].
Because tHcy is closely diet-sensitive, folic-acid therapy has been shown to reduce first-ever stroke risk [11], and, although effects vary by population, B-vitamin supplementation lowers tHcy by roughly 25% and on average reduces stroke risk by ≈10% [12]. In the present study, dietary intake did not change, indicating that the tHcy reduction in CC likely reflects training-induced metabolic adaptations including reductions in central adiposity, dampened inflammation, and improved endothelial function rather than increased folate/B-vitamin intake [43,44]. Given that lower tHcy is associated with reduced cardiovascular risk and lower risk of stroke recurrence [3], these findings suggest that the exercise intervention conferred clinically meaningful benefit. Nevertheless, because responses differed by genotype, future programs in similar cohorts should consider a personalized approach that integrates genetic factors with targeted nutritional strategies.
Taken together, these findings indicate that the combined aerobic and resistance training intervention produced clinically meaningful improvements in body composition, functional capacity, and homocysteine regulation in stroke patients, even under stable dietary intake and pharmacological treatment. To provide a balanced interpretation of our results, we also considered the strengths, limitations, and future perspectives of this study.
A notable strength of this study is the relatively long intervention duration (26 weeks) and the use of a combined aerobic-plus-resistance training program, which allowed us to evaluate both body-composition and functional outcomes in stroke survivors under real-world pharmacological treatment. The inclusion of MTHFR genotyping further extends the clinical relevance by demonstrating that genetic variation may modulate homocysteine responses to exercise, highlighting the value of personalized rehabilitation approaches [6,45].
This study has several limitations. First, the modest sample size limited the ability to fully examine genotype–exercise interactions with sufficient statistical power. Second, participants were receiving antihypertensive and glucose-or lipid-lowering medications, which may have contributed to ceiling effects and masked additional benefits of training on conventional cardiometabolic markers [39,40]. As discontinuation of prescribed therapy was not feasible, future studies should be stratified by medication class or incorporate drug use as a covariate. Third, dietary intake was assessed but not controlled, and vitamin B12 could not be evaluated because the CAN-PRO database used in this study does not include B12 content. Given that vitamin B12 status is a major determinant of homocysteine levels [46,47], its absence constrains interpretation of genotype-dependent changes. Future trials should directly assess serum B12 or validated dietary intake to clarify this relationship [48,49]. Finally, functional outcomes were restricted to the 6MWT, and additional measures of gait quality, balance, and strength would provide a more comprehensive view of rehabilitation effects [29,30,31].
Finally, functional outcomes were restricted to the 6MWT, and other measures of gait quality, balance, or muscle strength were not included [29,30,31]. Future research should aim to replicate these findings in larger multicenter trials with stratification by genotype and pharmacotherapy, while also integrating comprehensive nutritional assessment. Moreover, interventions that vary training intensity, volume, and modality should be tested to determine the optimal prescription for reducing both conventional cardiometabolic risk markers and homocysteine in stroke populations [7,41,42]. Personalized rehabilitation programs that combine exercise with genotype-informed nutritional strategies and careful monitoring of concomitant medications may provide the most effective approach to reducing recurrence risk and improving long-term outcomes in stroke survivors.
5. Conclusions
This study demonstrated that a 26-week combined aerobic and resistance training program produced significant improvements in body composition specifically reductions in body weight, fat mass, and waist-to-hip ratio and enhanced functional capacity, as measured by the 6MWT, in patients with chronic ischemic stroke, despite stable dietary intake. Importantly, plasma tHcy declined significantly in the MTHFR CC genotype group, indicating a genotype-dependent metabolic adaptation to exercise. By contrast, traditional stroke risk markers, including triglycerides, fasting blood glucose, and blood pressure, did not change significantly, likely reflecting pharmacological management and ceiling effects.
From a clinical perspective, these findings underscore the potential of structured combined training to promote healthy body composition, improve functional independence, and lower tHcy levels, thereby contributing to reduced cardiovascular risk and stroke recurrence. At the same time, the heterogeneity in tHcy response across genotypes highlights the importance of personalized rehabilitation strategies that integrate genetic screening with targeted nutritional support. Future programs should consider tailoring exercise prescriptions to individual genetic and pharmacological profiles to maximize therapeutic benefit and long-term outcomes in stroke survivors.
Author Contributions
K.-H.L. and Y.-A.S. drafted the manuscript and critically revised the manuscript. Y.-A.S. designed the study and collected the data. K.-H.L. analyzed and interpreted the data and performed the statistical analysis. K.-H.L. and Y.-A.S. discussed the results and contributed to the final version of the article. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support or the research, authorship, and publication of this article.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kyung Hee University (approval number: KHSIRB-18-010, date of approval: 26 September 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to ethical and legal restrictions related to genetic information. However, anonymized data may be available from the corresponding author upon reasonable request and with prior approval from the Institutional Review Board of Kyung Hee University.
Acknowledgments
The authors would like to express their sincere gratitude to Jun-Hee Lee for his valuable assistance in preparing the documentation required for IRB approval during the research process and for his critical analysis and academic review of the manuscript, which greatly contributed to enhancing the completeness and quality of this study.
Conflicts of Interest
The authors have no potential conflict of interest to declare with respect to the research, authorship, and publication of this article.
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke Off. J. Int. Stroke Soc. 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Pinzon, R.T.; Wijaya, V.O.; Veronica, V. The role of homocysteine levels as a risk factor of ischemic stroke events: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1144584. [Google Scholar] [CrossRef]
- Holmen, M.; Hvas, A.M.; Arendt, J.F.H. Hyperhomocysteinemia and Ischemic Stroke: A Potential Dose-Response Association-A Systematic Review and Meta-analysis. TH Open Companion J. Thromb. Haemost. 2021, 5, e420–e437. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef] [PubMed]
- Zarembska, E.; Ślusarczyk, K.; Wrzosek, M. The Implication of a Polymorphism in the Methylenetetrahydrofolate Reductase Gene in Homocysteine Metabolism and Related Civilisation Diseases. Int. J. Mol. Sci. 2023, 25, 193. [Google Scholar] [CrossRef]
- Deminice, R.; Ribeiro, D.F.; Frajacomo, F.T. The Effects of Acute Exercise and Exercise Training on Plasma Homocysteine: A Meta-Analysis. PLoS ONE 2016, 11, e0151653. [Google Scholar] [CrossRef]
- Tang, A.; Eng, J.J.; Krassioukov, A.V.; Madden, K.M.; Mohammadi, A.; Tsang, M.Y.C.; Tsang, T.S.M. Exercise-induced changes in cardiovascular function after stroke: A randomized controlled trial. Int. J. Stroke 2014, 9, 883–889. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, S.M.; Shin, Y.A. Effects of exercise training on stroke risk factors, homocysteine concentration, and cognitive function according the APOE genotype in stroke patients. J. Exerc. Rehabil. 2018, 14, 267–274. [Google Scholar] [CrossRef]
- VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: A randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010, 9, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef]
- Hankey, G.J. B vitamins for stroke prevention. Stroke Vasc. Neurol. 2018, 3, 51–58. [Google Scholar] [CrossRef]
- Wouters, M.G.; Moorrees, M.T.; van der Mooren, M.J.; Blom, H.J.; Boers, G.H.; Schellekens, L.A.; Thomas, C.M.; Eskes, T.K. Plasma homocysteine and menopausal status. Eur. J. Clin. Investig. 1995, 25, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lim, J.Y.; Park, H.Y. Age at natural menopause in Koreans: Secular trends and influences thereon. Menopause 2018, 111, 104–110. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, K.; Kang, J.; Koo, J.; Kim, D.H.; Kim, B.J.; Kim, W.J.; Kim, E.G.; Kim, J.G.; Kim, J.M.; et al. Executive summary of stroke statistics in Korea 2018: A report from the Korean Stroke Society and Clinical Research Center for Stroke. J. Stroke 2019, 21, 42–59. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.D.; Martins, C.P.; Ribeiro, T.S.; de Sousa, A.V.; Costa, M.F.P.; Lindquist, A.R.R.; Lindquist, A.R.R. Comparison between the six-minute walk test and the six-minute step test in post-stroke patients. Int. Arch. Med. 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M.; Housh, T.J.; Camic, C.L.; Bergstrom, H.C.; Traylor, D.A.; Schmidt, R.J.; Johnson, G.O. Metabolic parameters for ramp versus step incremental cycle ergometer tests. Appl. Physiol. Nutr. Metab. 2012, 37, 1110–1117. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Adams, R.; Becker, K.; Furberg, C.D.; Gorelick, P.B.; Hademenos, G.; Hill, M.; Howard, G.; Howard, V.J.; Jacobs, B.; et al. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke 2001, 32, 280–299. [Google Scholar] [CrossRef]
- Haam, J.H.; Kim, B.T.; Kim, E.M.; Kwon, H.; Kang, J.H.; Park, J.H.; Kim, K.K.; Rhee, S.Y.; Kim, Y.H.; Lee, K.Y. Diagnosis of Obesity: 2022 Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity. J. Obes. Metab. Syndr. 2023, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.; Vincent, K.R. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 2006, 14, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabilit. 2025, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Brellenthin, A.G.; Lanningham-Foster, L.M.; Kohut, M.L.; Li, Y. Aerobic, resistance, or combined exercise training and cardiovascular risk profile in overweight or obese adults: The CardioRACE trial. Eur. Heart J. 2024, 45, 1127–1142. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Ostchega, Y.; Hughes, J.P.; Terry, A.; Fakhouri, T.H.; Miller, I. Abdominal obesity, body mass index, and hypertension in US adults: NHANES 2007–2010. Am. J. Hypertens. 2012, 25, 1271–1278. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, W.J.; Khera, A.V.; Kim, J.Y.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Won, H.H. Association between adiposity and cardiovascular outcomes: An umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur. Heart J. 2021, 42, 3388–3403. [Google Scholar] [CrossRef]
- Kolmos, M.; Christoffersen, L.; Kruuse, C. Recurrent ischemic stroke–a systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105935. [Google Scholar] [CrossRef]
- Smulders, K.; van Swigchem, R.; de Swart, B.J.; Geurts, A.C.; Weerdesteyn, V. Community-dwelling people with chronic stroke need disproportionate attention while walking and negotiating obstacles. Gait Posture 2012, 36, 127–132. [Google Scholar] [CrossRef]
- Weerdesteyn, V.; de Niet, M.; van Duijnhoven, H.J.; Geurts, A.C. Falls in individuals with stroke. J. Rehabil. Res. Dev. 2008, 45, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, C.; Roerdink, M.; Meskers, C.G.M.; Beek, P.J.; Janssen, T.W.J. Walking-adaptability therapy after stroke: Results of a randomized controlled trial. Trials 2021, 22, 923. [Google Scholar] [CrossRef]
- Awad, L.N.; Reisman, D.S.; Binder-Macleod, S.A. Distance-induced changes in walking speed after stroke: Relationship to community walking activity. J. Neurol. Phys. Ther. 2019, 33, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Fulk, G.D.; He, Y. Minimal clinically important difference of the 6-minute walk test in people with stroke. J. Neurol. Phys. Ther. 2018, 42, 235–240. [Google Scholar] [CrossRef]
- Buurke, J.H.; Nene, A.V.; Kwakkel, G.; Erren-Wolters, V.; Ijzerman, M.J.; Hermens, H.J. Recovery of gait after stroke: What changes? Neurorehabilit. Neural Repair 2008, 22, 676–683. [Google Scholar] [CrossRef]
- Chi, N.F.; Huang, Y.C.; Chiu, H.Y.; Chang, H.J.; Huang, H.C. Systematic Review and Meta-Analysis of Home-Based Rehabilitation on Improving Physical Function Among Home-Dwelling Patients with a Stroke. Arch. Phys. Med. Rehabil. 2020, 101, 359–373. [Google Scholar] [CrossRef]
- Brouwer, R.; van Dijk, A.; de Greef, B.; van Meeteren, N.; Visser-Meily, J.; Toorenburgh, S. Effect of aerobic training on vascular and metabolic risk factors for recurrent stroke: A meta-analysis. Disabil. Rehabil. 2021, 43, 2084–2091. [Google Scholar] [CrossRef]
- Ivey, F.M.; Ryan, A.S.; Hafer-Macko, C.E.; Goldberg, A.P.; Macko, R.F. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: A preliminary report. Stroke 2007, 38, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Bangalore, S.; Bavishi, C. Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Wiysonge, C.S.; Bradley, H.A.; Volmink, J.; Mayosi, B.M.; Mbewu, A.; Opie, L.H.; Bethel, A. Beta-blockers for hypertension. Cochrane Database Syst. Rev. 2017, 1, CD002003. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Collins, R. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Church, T.S.; Blair, S.N.; Cocreham, S.; Johannsen, N.; Johnson, W.; Kramer, K.; Mikus, C.R.; Myers, V.; Nauta, M.; Rodarte, R.Q.; et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: A randomized controlled trial. JAMA 2010, 304, 2253–2262. [Google Scholar] [CrossRef]
- Corso, L.M.; Macdonald, H.V.; Johnson, B.T.; Farinatti, P.; Livingston, J.; Zaleski, A.L.; Blanchard, A.; Pescatello, L.S. Is Concurrent Training Efficacious Antihypertensive Therapy? A Meta-analysis. Med. Sci. Sports Exerc. 2016, 48, 2398–2406. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Nolan, A.; Sites, C.K.; Ades, P.A.; Poehlman, E.T. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation 2002, 105, 564–569. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Clarke, R.; Sherliker, P.; Hin, H.; Nexo, E.; Hvas, A.M.; Schneede, J.; Birks, J.; Ueland, P.M.; Emmens, K.; Scott, J.M.; et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin. Chem. 2007, 53, 963–970. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch. Ärzteblatt Int. 2008, 105, 680–685. [Google Scholar] [CrossRef]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37,485 individuals. Arch. Intern. Med. 2010, 170, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).