1. Introduction
Vanadium is a strategically important metal due to its unique physicochemical properties, such as high strength, corrosion resistance, and the ability to enhance the characteristics of alloys.
The main application of vanadium is in the production of high-strength steels, as well as its use in catalysts and the chemical industry. In recent years, its role in the development of energy storage technologies has attracted significant attention, particularly in the production of vanadium redox flow batteries (VRFBs). These batteries, known for their long service life and fast charging capabilities, are considered a promising solution for large-scale energy storage, which is driving increased demand for the metal in the energy sector [
1].
According to the International Energy Agency (IEA, 2023), global vanadium consumption continues to grow steadily, with China remaining the leading consumer, accounting for more than half of the world’s vanadium use—primarily for steel production. Significant consumption is also observed in the United States, Europe, Japan, and developing Asian countries such as India and South Korea. Vanadium mining and processing play a key role in the global market and are primarily concentrated in China, Russia, South Africa, and Brazil. In addition to primary extraction, the processing of secondary resources and industrial waste is gaining increasing importance, helping to ensure supply stability amid geopolitical risks and demand fluctuations. According to several studies [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13], further growth in vanadium consumption will be driven by the development of the metallurgical, construction, and automotive industries, as well as the adoption of innovative technologies for metal extraction and application in energy and environmental protection.
Against the backdrop of a steady increase in global vanadium demand and expanding areas of application, the strategic importance of this metal for the economy of the Republic of Kazakhstan is also growing. This importance is due to vanadium’s unique physicochemical properties—high mechanical strength, corrosion resistance, and the ability to modify the structure and properties of metal alloys. These characteristics determine its wide range of applications, from metallurgy and the chemical industry to modern energy storage technologies [
3].
The main vanadium reserves in the Republic of Kazakhstan are concentrated in the Karatau vanadium basin, which includes the Balasauskandyk and Kurumsak deposits. The Balasauskandyk deposit, which accounts for about 65% of Kazakhstan’s total vanadium reserves, is among the largest in the world in terms of mineral resource volume [
14]. However, the industrial development of these deposits is significantly constrained by the lack of efficient processing and extraction technologies, which results in a large portion of the resources being classified as subeconomic ore (subeconomic ore refers to ore whose metal content or mining conditions do not currently permit economically viable extraction) [
15]. The future development of Kazakhstan’s vanadium industry directly depends on the implementation of innovative ore beneficiation methods, as well as investment attraction to enhance the industrial exploitation of this strategically important raw material [
16,
17,
18,
19].
The aim of this study is to determine the optimal conditions for the electrochemical oxidation of vanadium-bearing ore after preliminary oxidative roasting with different charge compositions, as well as to establish the kinetic patterns and mechanisms of vanadium leaching in a sulfuric acid medium, in order to increase the efficiency of its extraction during hydrometallurgical processing.
A review of the literature [
20,
21,
22] shows that traditional methods of processing vanadium-bearing ores—including acid leaching without roasting or with low-temperature roasting (up to 700 °C)—are insufficiently effective for extracting vanadium from the Balasauskandyk and Kurumsak deposits. This is due to the complex mineralogical form in which vanadium occurs in the ore—as sulfides, hydroxides, as part of micas (in the form of isomorphic substitution), and in anionic complexes. Despite the implementation of pyrometallurgical, hydrochemical, and sorption technologies at the industrial pilot site of Balausa LLP, they do not ensure complete oxidation of vanadium to V
5+, which significantly reduces the efficiency of its extraction. In addition, there is no integrated processing scheme in place that would allow for the recovery of other valuable components, particularly uranium and rare earth elements (REEs).
In this context, the application of the electrochemical processing method appears to be a promising direction. This approach combines three processes in the anode region: vanadium oxidation, its leaching, and solution activation under the influence of direct electric current. The use of electrochemical oxidation can significantly improve vanadium recovery rates and ensure comprehensive processing of vanadium-bearing ores from the Greater Karatau region, including the extraction of accompanying elements. Conducting such research is of high scientific and practical relevance, as it contributes to the creation of a national production base for vanadium and associated rare metals.
Currently, there is no available literature on the electrochemical oxidation of vanadium directly from natural mineral raw materials, which emphasizes that the presented experimental studies on the electrochemical oxidation of vanadium from ore materials have been conducted for the first time [
23,
24,
25,
26,
27,
28,
29].
2. Materials and Methods
The original composition of vanadium-bearing ores from the Balasauskandyk and Kurumsak deposits was studied using chemical analysis methods in the laboratory of the K.I. Satpayev Institute of Geological Sciences.
To carry out the oxidative roasting of powdered vanadium-bearing ore, three charge compositions were used. In the first variant, the charge consisted of a mixture of vanadium-bearing ore and calcined soda (GOST 83-79) in a mass ratio of 1:1. In the second variant, sodium chloride (GOST 4233-77) was used as the oxidizing agent, also in a 1:1 ratio with the ore. The third variant of the charge included a mixture of calcined soda and sodium chloride in a mass ratio of 9:1. The specified sodium salts and their mixture were introduced with a 20% excess relative to the stoichiometrically required amount [
30]. Oxidative roasting was carried out at a temperature of 700–850 °C. The possible reactions occurring during the roasting process were determined based on thermodynamic calculations performed using the HSC Chemistry 7.1 software package:
Thermodynamic calculations using HSC Chemistry 7.1 were performed to predict the stability of various forms of vanadium during electrochemical oxidation. These calculations allowed us to confirm the possibility of the formation of target compounds and compare theoretical data with experimental results.
Methodology for Conducting Experiments on Roasting Vanadium-Bearing Ores
During roasting, a crucial condition was the regular stirring of the charge to prevent its sintering. The air flow rate supplied as the oxidizer was approximately 0.107 m3 per 100 g of ore. The roasting temperature was maintained within the range of 700–850 °C, with a total process duration of 2 h. Upon completion of the roasting, the resulting calcines were cooled, ground, and subjected to semi-quantitative X-ray phase analysis at the laboratory of the K.I. Satpayev Institute of Geological Sciences.
Methodology for experiments on electrochemical oxidation and leaching of vanadium
During the experimental studies, the optimal type of electrochemical cell (electrolysis bath) was first selected, after which the main parameters affecting the degree of vanadium oxidation and its transition into solution were determined, including the type and concentration of the electrolyte, anode material, and process temperature. The ore was ground to a particle size of 10 microns. Sulfuric acid at concentrations of 5%, 10%, and 15% was chosen as the most suitable electrolyte. The ore sample was mixed with the electrolyte in solid-to-liquid ratios (S:L) of 1:2, 1:3, 1:4, and 1:5, at temperatures of 25, 45, 65, and 85 °C, durations of 0.5, 1, 1.5, 2, 2.5, and 3 h, and current densities from 100 to 1000 A/m2, and then subjected to electrochemical oxidation in an electrolyzer equipped with an MK-40 membrane separating the anode and cathode chambers.
Before starting the process, a mixture of ore and electrolyte was introduced into the anode compartment of the electrolyzer and stirred using a mixer at 120–150 rpm, while only acid was poured into the cathode compartment. During electrolysis, vanadium oxidation reactions occur in the anode chamber, while water decomposition with hydrogen evolution takes place at the cathode according to the following reaction:
It should be noted that gaseous oxygen, produced at the anode as a result of the discharge of water molecules or hydroxyl ions, can further participate in the vanadium oxidation process.
In such electrolyzers, where the anode and cathode chambers are separated by an MK-40 membrane, redox reactions occur, accompanied by changes in the oxidation states of individual ions:
In the conducted studies, a lead plate was used as the anode, and titanium served as the cathode. It is known that increasing the current density at the electrodes leads to an increase in their electrode potential, which in turn enhances the reducing or oxidizing properties of the respective electrode. This enables the realization of redox processes that are otherwise difficult to achieve. However, excessive current density reduces current efficiency, as a significant portion of the electrical energy is consumed by side reactions such as oxygen or hydrogen evolution.
On the other hand, the use of electrolyzers with a separating partition (membrane) permeable to ions allows for the production of alkali in the cathode compartment of the electrolyzer.
At the cathode, the water reduction reaction takes place:
In traditional sorption technology for processing productive solutions, alkali can be used in the neutralization processes of commercial regenerates prior to vanadium precipitation.
For electrochemical oxidation, the use of a special electrolyzer has been proposed, in which a strongly oxidizing environment is created in the anode chamber due to oxygen generated from a component of the electrolyte.
All experiments were carried out three times. The values presented are averages.
3. Results
The results of the chemical analysis of the original ore samples are presented in
Table 1 and
Table 2.
Oxidative roasting was carried out in a muffle furnace SNOL-1.4/2.5/1.2/12.5—I1 (
Figure 1) with the supply of an oxidizing gas (air) passing through the filtering layer of the powdered charge.
A special electrolyzer was designed for conducting the electrochemical oxidation process (
Figure 2 and
Figure 3).
The material flows of the roasting process of vanadium-containing ore from the Balasauskandyk and Kurumsak deposits in the presence of a mixture of sodium salts are presented below (
Table 3 and
Table 4).
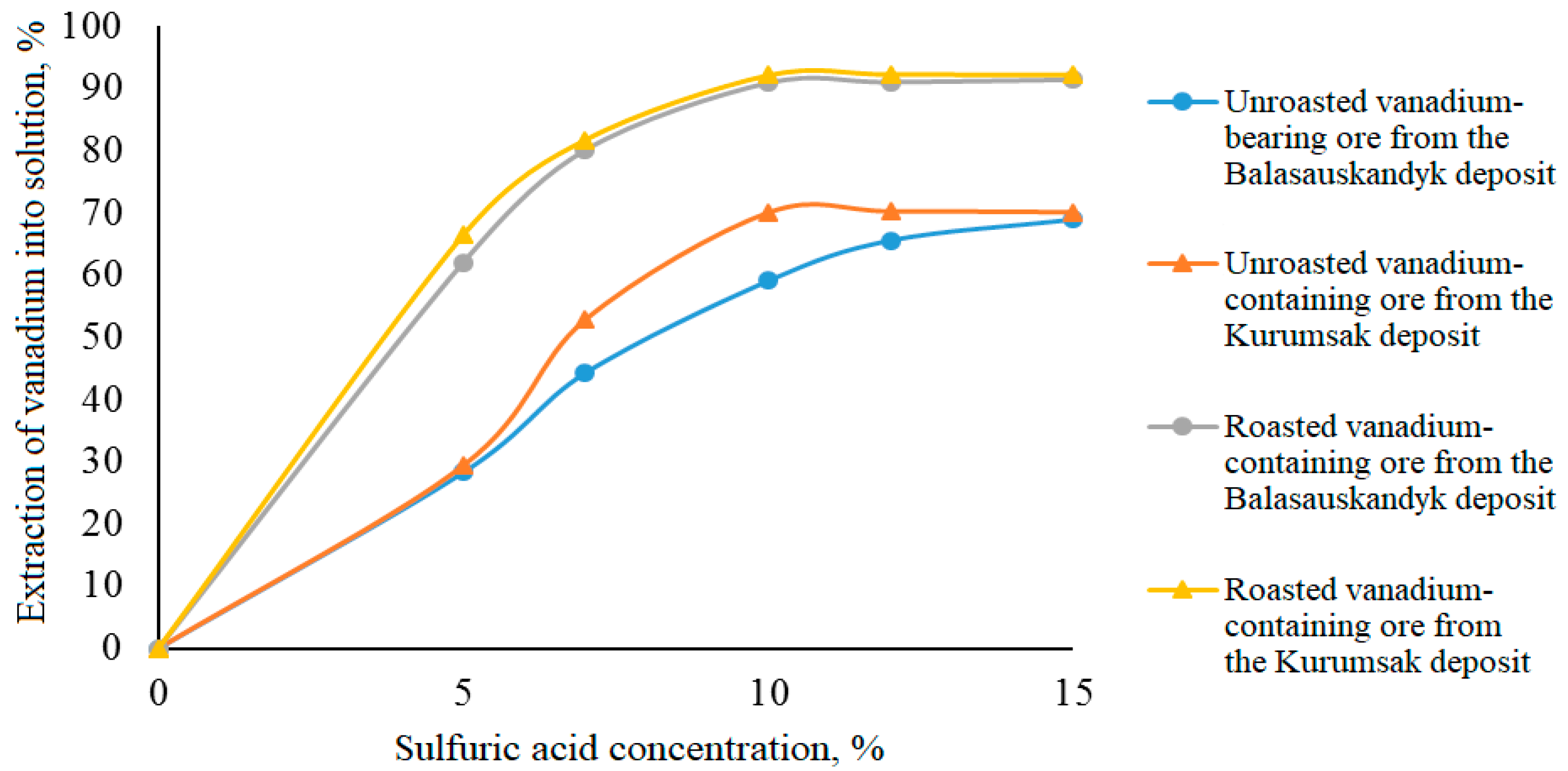
Effect of sulfuric acid concentration on vanadium extraction into solution
As the concentration of sulfuric acid increases from 5% to 15%, the vanadium extraction rate rises from 68.96–70.14% to 90.91–92.19%, respectively, during the leaching of both unroasted and roasted vanadium-containing ores (
Table 5,
Figure 4).
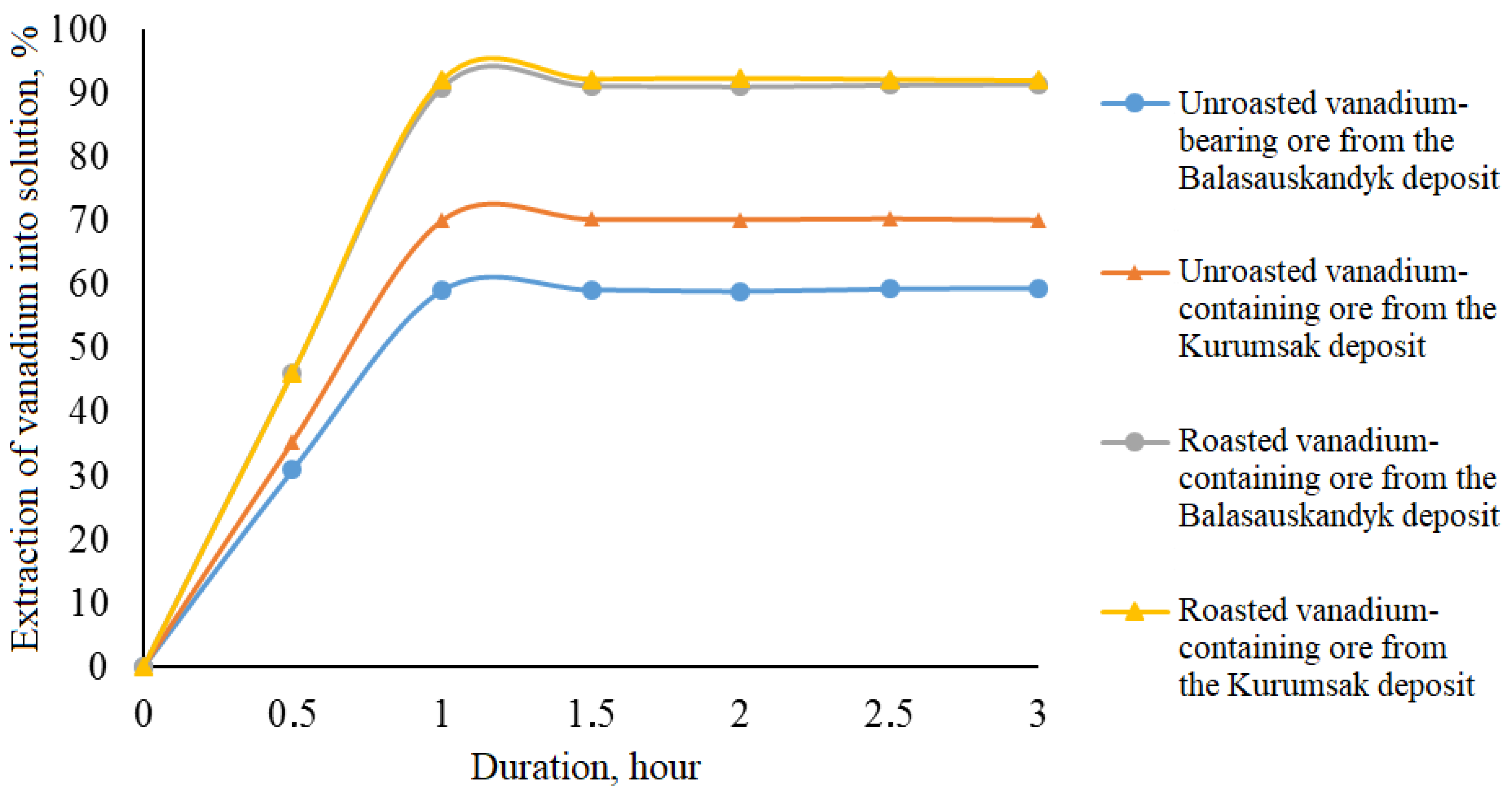
Effect of electrochemical oxidation duration on vanadium extraction into solution
Experiments to determine vanadium extraction into solution depending on the duration of electrochemical oxidation were conducted at the following time intervals: 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 h. The obtained results are presented in
Table 6 and
Figure 5.
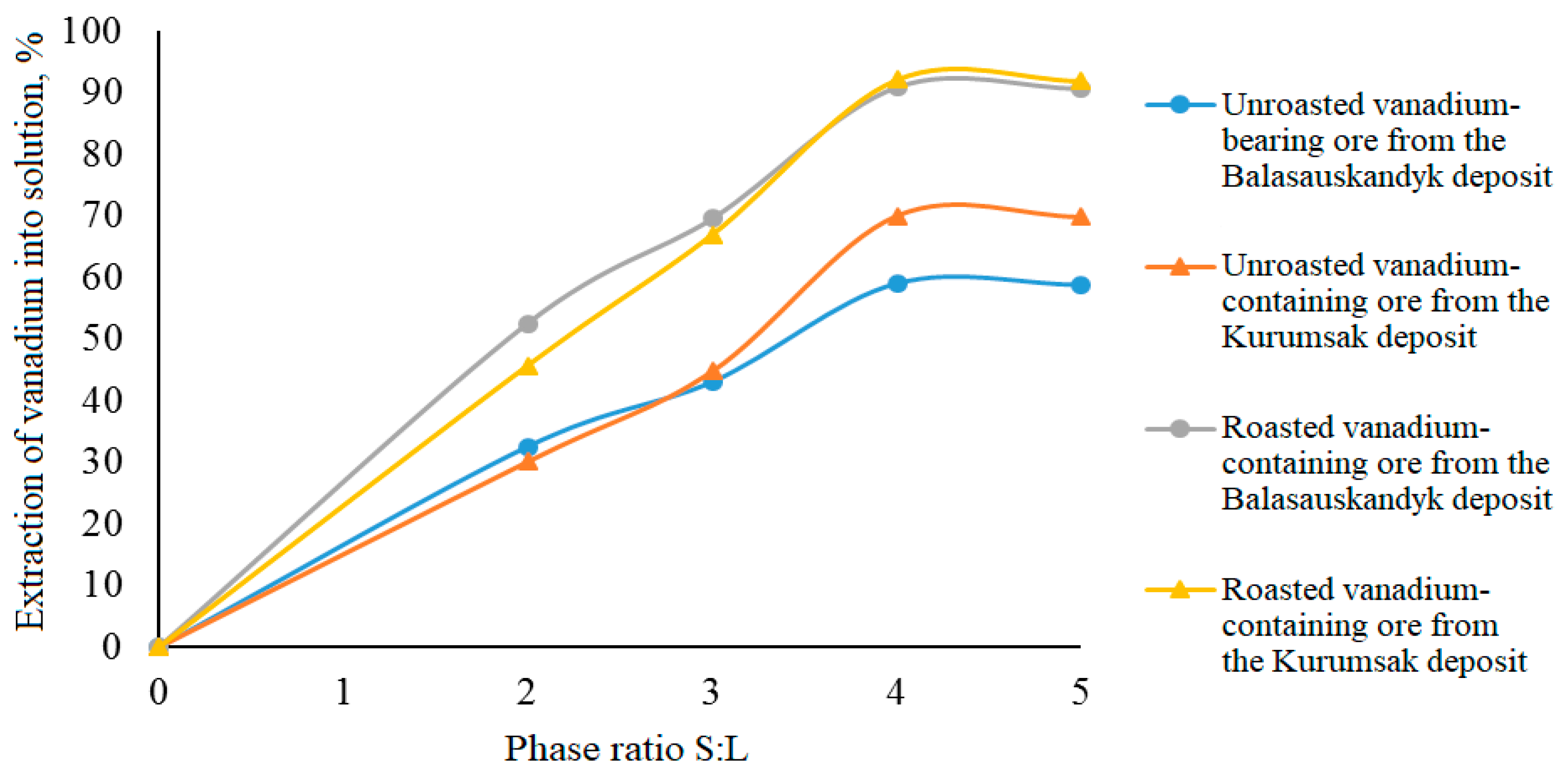
Effect of solid-to-liquid phase ratio on vanadium extraction into solution
In this series of experiments, the solid-to-liquid (S:L) ratio varied from 1:2 to 1:5 (
Table 7,
Figure 6). The other parameters under investigation remained unchanged and corresponded to the conditions of the previous experimental series.
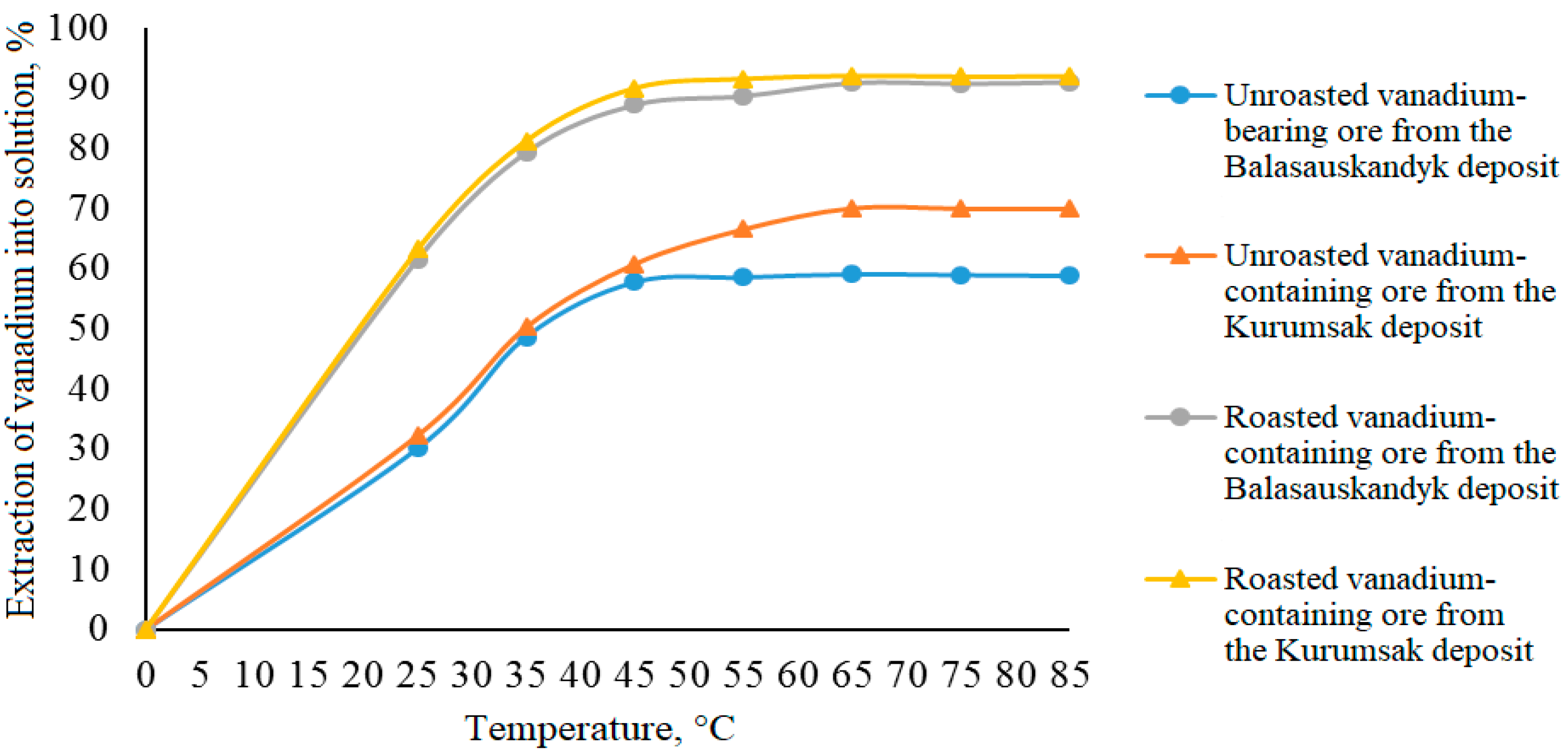
Effect of electrochemical oxidation temperature on vanadium extraction into solution
In these experiments, the temperature varied from 25 to 85 °C while keeping other parameters constant (sulfuric acid concentration, duration, and solid-to-liquid ratio). The results are presented in
Table 8 and
Figure 7.
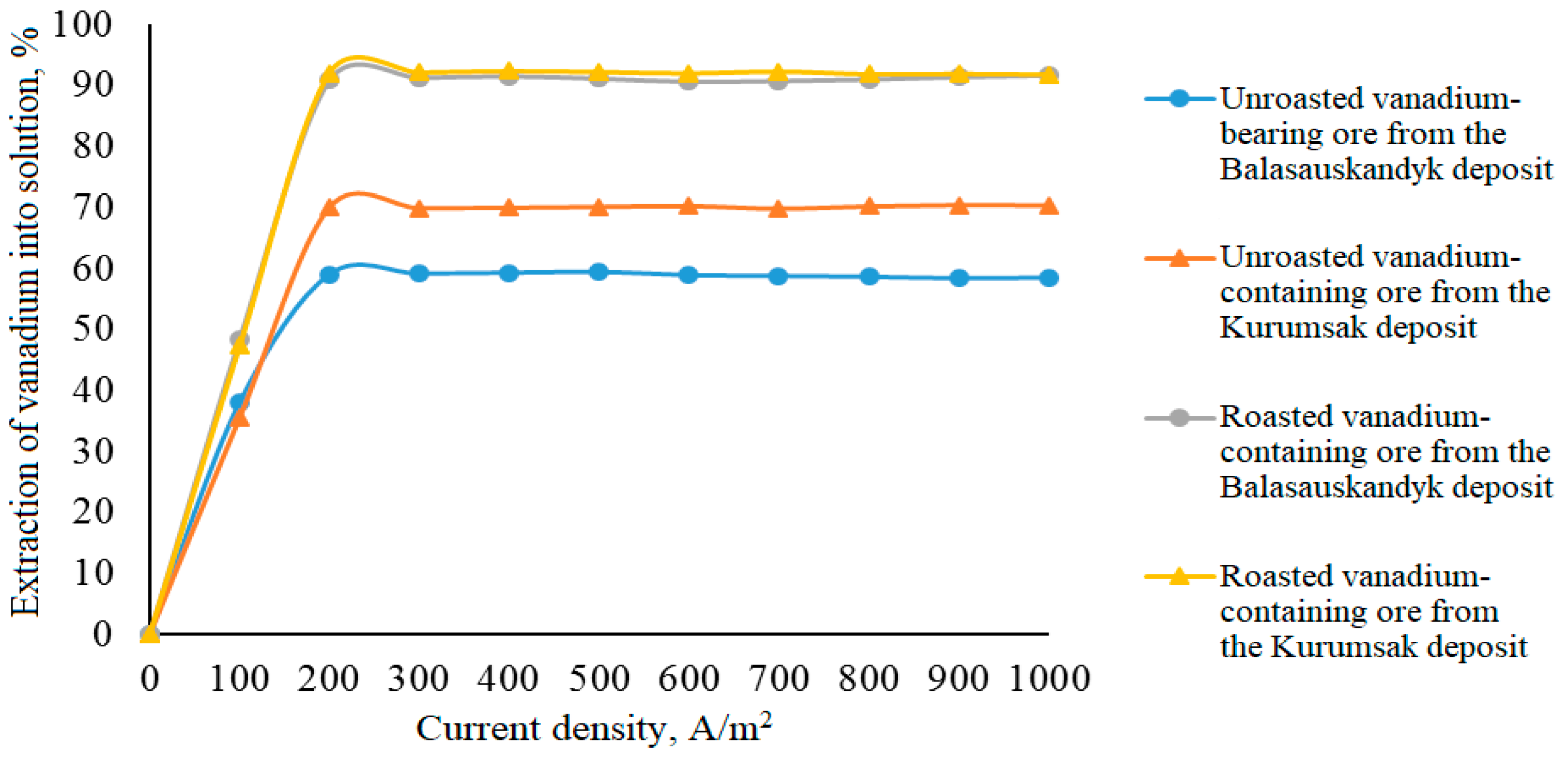
Effect of current density on vanadium leaching efficiency
Experiments to determine the dependence of vanadium leaching efficiency on current density were conducted in the range of 100–1000 A/m
2. All other studied parameters were kept constant and were similar to those in the previous series. The obtained results are presented in
Table 9 and
Figure 8.
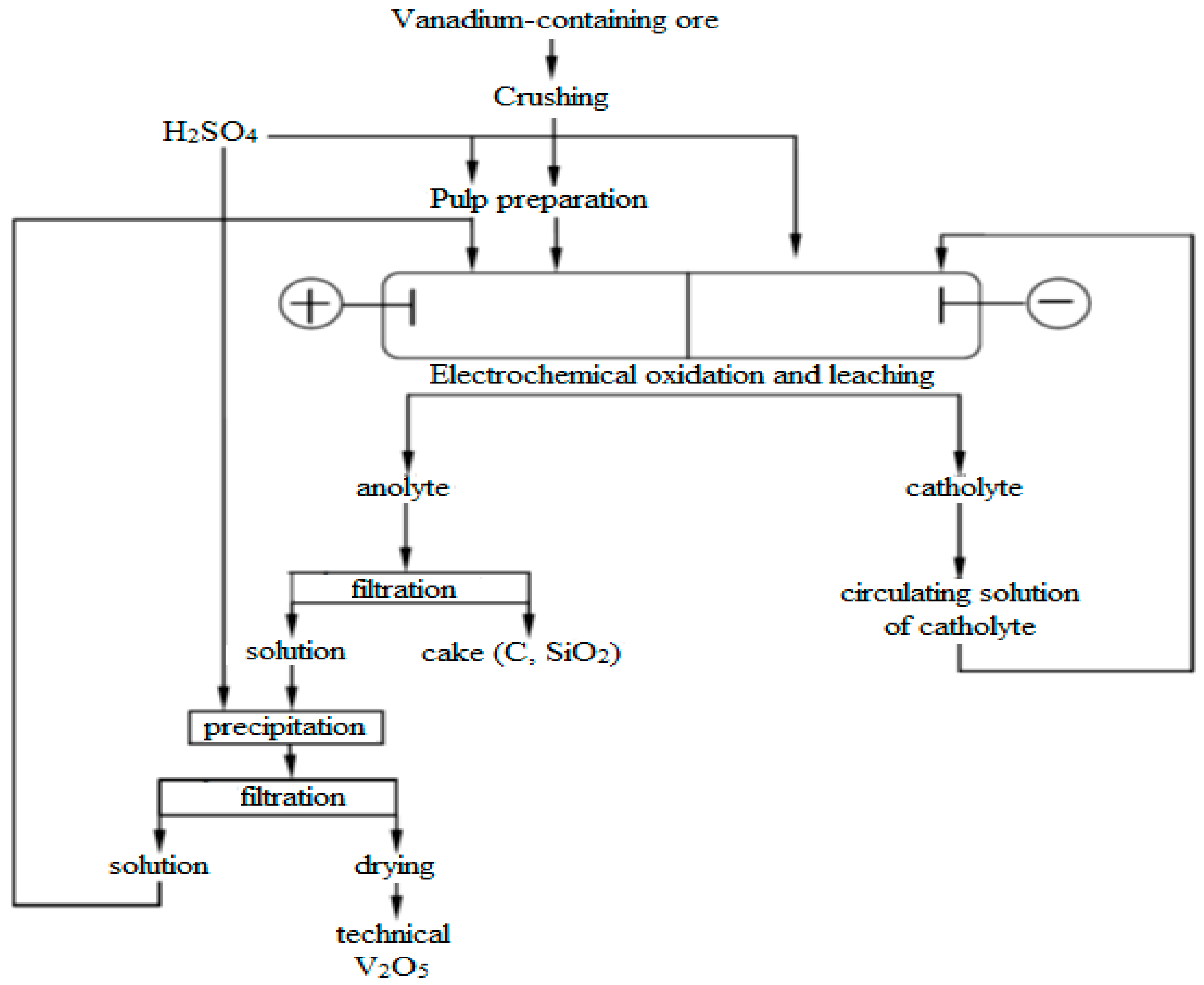
Based on the conducted comprehensive studies, a conceptual technological flowsheet for the processing of hard-to-treat vanadium-bearing ores has been developed, as shown in
Figure 9.
4. Discussion
The analysis of the data presented in
Table 1 and
Table 2 shows an uneven distribution of vanadium in the ores from the Balasauskandyk and Kurumsak deposits. At the same time, in some ore samples from the Kurumsak deposit, the vanadium content exceeds its concentration in the ores of Balasauskandyk.
Oxidative roasting of vanadium-containing ore in the presence of alkali metal salts at temperatures ranging from 700 to 850 °C, with a holding time of 2 h at the given temperature, promotes the conversion of vanadium to a higher oxidation state (
Table 3 and
Table 4).
From the data in
Table 5 and
Figure 4, it follows that during electrochemical oxidation with sulfuric acid of both unroasted and roasted vanadium-containing ores, the maximum degree of vanadium extraction into the solution is achieved at an acid concentration of 10 g/dm
3. The highest vanadium extraction efficiency is observed during the electrochemical oxidation of pre-roasted ore (up to 92.12%) compared to the leaching of unroasted ore (only about 70.1%).
From
Table 6 and
Figure 5, it follows that increasing the duration of electrochemical oxidation of both unroasted and roasted vanadium-containing ores positively affects the dissolution of vanadium into the solution. During the first 0.5 h, vanadium extraction increases from 30.91–35.40% to 46.20–46.23%, and in the following 0.5 h, the extraction rates further increase from 59.09–70.07% to 90.91–92.12%. Extending the process duration from 1.0 to 3.0 h does not have a significant impact on the degree of vanadium extraction into the solution.
From
Table 7 and
Figure 6, it can be seen that increasing the solid-to-liquid ratio (S:L) from 1:2 to 1:4 raises the vanadium extraction into the solution from 30.14–32.60% to 59.09–70.07% and from 45.77–52.60% to 90.91–92.12%, respectively, during electrochemical oxidation of unroasted and roasted vanadium-containing ores. Further increasing the S:L ratio from 1:4 to 1:5 has practically no effect on vanadium extraction efficiency.
As shown by the data in
Table 8 and
Figure 7, the degree of vanadium extraction into the solution increases as the temperature rises from 25 to 65 °C. The maximum vanadium extraction reaches 70.07% from unroasted vanadium-containing ore and 92.12% from roasted ore. Increasing the temperature from 65 to 85 °C has practically no effect on vanadium extraction rates.
Analysis of the results showed that for more complete vanadium extraction into the solution from both unroasted and roasted vanadium-containing ores, it is advisable to conduct the leaching process at a temperature not lower than 65 °C. It was found that increasing the temperature positively affects the electrolyte’s conductivity and promotes the intensification of the electrochemical oxidation process. At the same time, raising the temperature can cause indirect effects, including changes in overpotential during gas evolution (H2, O2) as well as an increase in side reactions.
It was also established that the concentration of the initial solution influences the course of the electrochemical oxidation process. If the solution concentration is low and the process is carried out at high current densities to achieve a high potential, conditions in the near-electrode layer may lead to hydrogen or oxygen evolution at the respective electrodes due to insufficient diffusion of ions undergoing discharge, which reduces the current efficiency of the target product.
From
Table 9 and
Figure 8, it can be seen that increasing the current density during the electrochemical oxidation of unroasted and roasted vanadium-containing ores positively affects the transfer of vanadium into the solution. At a current density of 100 A/m
2, vanadium extraction ranged from 35.55–38.05% to 47.30–48.31%. When the current density was doubled, vanadium extraction from unroasted and roasted ores increased, respectively, from 59.09–70.07% to 90.91–92.12%. Further increasing the current density from 200 to 1000 A/m
2 did not have a significant effect on the degree of vanadium extraction into the solution.
One of the important characteristics of the electrochemically activated solution is the oxidation–reduction potential (ORP). ORP was measured using a platinum reference electrode relative to a silver chloride standard electrode, model ESL-15-11, with a high-impedance millivoltmeter model pH-150, which had a measurement error of 0.1 mV.
The current load was regulated by a B5-70 DC power supply, operating in voltage stabilization and current stabilization modes. Voltage and current were measured using a multimeter.
In the experiments, the optimal sulfuric acid concentration was 10% with an anode current density of 200 A/m2, a temperature of 65 °C, an electrochemical oxidation process duration of 1 h, and a solid-to-liquid ratio of 1:4. At the end of the experiment, the solution was filtered, and then the vanadium content in the solution and residue was determined.
The degree of vanadium extraction into the solution during electrochemical oxidation was 59–70.1% for unroasted vanadium-containing ores from these deposits, and 90.9–92.1% for pre-roasted ores.
A comparative analysis of vanadium extraction methods is given in
Table 10.
A comparative analysis of vanadium extraction methods (
Table 10) shows that the electrochemical method combines high efficiency with a more favorable environmental profile and the potential to reduce energy consumption while optimizing parameters.
5. Conclusions
The conducted studies showed the possibility of effective electrochemical oxidation of vanadium and confirmed the prospects of the proposed method for extracting metal from solutions. The development of effective technologies for processing vanadium-containing ores from the Balasauskandyk and Kurumsak deposits, characterized by a complex mineralogical structure, has acquired special importance. Thermodynamic modeling serves as a justification for the choice of experimental conditions and confirms their correctness. It was shown that preliminary oxidative roasting of ore with the addition of sodium salts at temperatures of 700–850 °C promotes the conversion of vanadium into readily soluble forms with a high degree of oxidation, which significantly increases the efficiency of subsequent electrochemical leaching.
Experimental data demonstrated the high effectiveness of the electrochemical method, especially when processing pre-roasted material, from which the degree of vanadium extraction into the solution reaches 90.9–92.1%, compared to 59–70.1% for unroasted ore. Optimal technological parameters were identified: sulfuric acid concentration of 10%, temperature of 65 °C, duration of 1 h, solid-to-liquid ratio of 1:4, and current density of 200 A/m2. It was established that increasing the temperature and current density up to certain limits contributes to the intensification of the electrochemical process; however, excessive increases lead to a rise in side reactions and a decrease in efficiency.
The obtained results have important scientific and practical significance, as the electrochemical oxidation of vanadium directly from natural mineral raw materials has been realized for the first time. This opens opportunities for creating highly efficient and environmentally safe technologies for the comprehensive processing of vanadium-containing ores in Kazakhstan, involving subeconomic ore reserves in economic turnover and extracting valuable associated components such as uranium and rare earth elements.