Featured Application
Sustainable solutions for prospecting and exploration of rare earth elements (REEs). Technological applications of REEs: catalysts, ceramics, and metallurgy. Robust analytical techniques for analyzing REE in multi-element geological matrices.
Abstract
Minerals bearing rare earth elements (REEs) are formed through long geological processes, among which monazite, bastnasite, xenotime, and ionic adsorption clays are the most economically exploited. Although Brazil has one of the largest reserves of REEs on the planet, its production is still not significant on the world stage. China remains dominant, with the largest reserves of REEs and controlling more than half of world production. Due to their important application in advanced clean and low-carbon energy technologies, REEs have become fundamental to the energy transition process. Technological applications related to catalyst synthesis, ceramics production, and metallurgy have been explored. Furthermore, the use of REEs in devices of great demand today, such as computer memory, rechargeable batteries, and mobile phones, has been cited. With the growing demand for these critical minerals, large mining companies are seeking to implement cleaner production policies in their processes and save natural resources to minimize the environmental impacts of the exploration. Robust analytical techniques have made it possible to characterize these elements in multi-element geological matrices, with the increasing exploration and identification of new REE mineral reserves.
1. Introduction
The rare earth elements (REEs) are a group of 17 elements, including 15 internal transition elements belonging to the lanthanide series [Lanthanum (La), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb), and Lutetium (Lu)], beyond Yttrium (Y) and Scandium (Sc), which have similar chemical and physical characteristics to the lanthanides due to their electronic distribution and because they occur in the same ore deposits as them [1,2]. REEs have a total abundance of approximately 183 ppm in the Earth’s crust [3].
The series of REEs presents a very particular phenomenon of lanthanide contraction. Both the atomic radius and the trivalent ionic radius (REE3+) decrease with increasing atomic number due to weak shielding by the 4f electrons of the nuclear charge on the outermost-shell electrons, which thus causes an increase in the effective nuclear charge with an increase in the atomic number. The decrease in the ionic radius is continuous and discrete, ranging from 1.061 Å (La3+) to 0.848 Å (Lu3+). As lanthanide contraction keeps these ions in the series with approximately the same size and the same oxidation state, this makes it so that the ions have very similar chemical properties and can substitute each other in the crystal lattice of their minerals [2,4].
REEs have relatively large atomic radii, which restricts the process of mineral replacement with other groups of chemical elements. As most REE ions are trivalent, they can eventually replace Ca2+, Zr4+, Mn2+, Th4+, and U4+ cations in minerals. Eu2+, Sm2+, or Yb2+ cations can replace Pb2+, Ca2+, Sr2+, and Na+ cations, while Ce4+, Pr4+, or Tb4+ cations can replace U4+ and Th4+. Differences in charge initiate the process of double substitution or vacancy generation, but still, the substitution process occurs due to similarity in the sizes of the elements’ atomic radii [5].
REEs have been given this name due to the difficulty of finding mineral reserves with a degree of purity and concentration that makes their exploitation viable. Furthermore, the similarity in chemical properties among REEs disfavors the processing stages of minerals containing these elements. Previously, the processes of separation and recovery of REEs were carried out through successive recrystallization and precipitation. More recently, extraction methods using solvents have been studied and used to increase the efficiency of the separation process [6]. Solvent extraction has been the most commonly used technique in the separation of REEs, especially in industries, due to its rapid extraction process, large separation capacity, and high extraction capacity. This procedure involves the selective migration of a metal ion within two solutions. During the extraction process, REE in an aqueous medium is attracted and captured by the extractant (in the organic phase) due to its chelating properties [7,8,9,10].
In this sense, three classic routes can be used for the extraction of REEs from mineral sources, such as acid leaching, alkaline conversion, and acid baking. Many monazite processing activities for REE extraction involve baking with sulfuric acid or alkaline digestion. Alkaline digestion is used for monazite concentrates, while lower-grade monazites tend to be processed using sulfuric acid baking [11].
Concentrates containing rare earths or calcined residues are typically treated with inorganic acids. Individual or mixed rare earths are then extracted from the impregnated leach solutions using solvent extraction. Various commercial extractants belonging to different classes are used for extraction [12]. Thus, the choice of lixiviant depends on the subsequent recovery method of REEs, which can be TBP (tributyl phosphate), P507 (2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester), or amine-based extractants [11]. Additionally, other extraction agents have shown promise in solvent extraction of REEs, such as ionic liquids [13], Cyanex 923 (a mixture of trialkyl phosphine oxides with n-octyl and n-hexyl chains) [14], oxime molecules [15], TODGA (N,N,N′,N′-tetraoctyldiglycolamide) [16], and D2EHPA (di-(2-ethyl hexyl) phosphoric acid) [17], among others [8,18].
In recent years, REEs have attracted attention due to their classification as critical elements for the technological development of a country, being an important ally in the process of energy transition from fossil to renewable sources [19,20]. Therefore, REEs have played a fundamental role in the modern technological landscape and in global sustainable development efforts [21,22].
This work aims to carry out a review of the primary sources and technological applications of REEs, in addition to addressing business investments related to the mineral exploration of REEs. Furthermore, the main techniques for the identification, quantification, and determination of the crystalline phases of REEs in multi-element geological matrices, characterized by high sensitivity, selectivity, and good resolution of possible interferences, are discussed.
2. Rare Earth Elements: Source, Typical Minerals, and Worldwide and Brazilian Reserves
REEs are found mainly in primary geological sources, characterized by the natural formation of the mineral through long and complex geochemical processes. Geochemistry is an area of knowledge that investigates the distribution of chemical elements in the Earth’s crust and distinguishes the processes (magmatic, tectonic, and hydrothermal) and conditions (temperature and pressure) involved in the formation of different mineral deposits. In this sense, geochemical studies have demonstrated that these chemical elements can constitute the main matrix of the ore or be incorporated into minerals in the form of traces during their formation process through a variety of mechanisms of inclusions or substitutions in the crystalline structure [23,24].
Mineral deposits can be divided into three groups: (i) metallic; (ii) non-metallic; and (iii) agrominerals. Basic metallic deposits, made up of the elements Fe, Al, Cu, Cr, and Ni, tend to be the most exploited due to their demand in the production of steel and electronic equipment, followed by other elements of lower utility in production, such as Pt, Au, Ga, In, Li, Mn, Mo, Nb, Ta, Ti, W, and V [25]. However, in recent years, metallic mineral deposits from REEs have attracted the attention of the world market because they contain strategic chemical elements that can be used to produce high-technology metal oxides and alloys, critical inputs in the advanced technological industry associated with wind energy, electric motors, and super magnets [24,25,26].
Mineral deposits of REEs can be found in all the types of rock present on Earth (igneous, metamorphic, and sedimentary host rocks). However, these chemical elements are more likely to be present in rocks associated with alkaline igneous activity, such as carbonatites or agpaitic nepheline syenites. As an example, carbonatite is an igneous rock with a volumetric percentage of 50% carbonate, containing less than 20% SiO2 by weight. Syenite is an igneous rock containing less than 20% quartz and more than 65% alkali feldspar [24]. Additionally, REEs are typically located in the fully weathered and semi-weathered layers of the Earth’s crust [21].
Numerous mineral sources containing REEs have already been identified. Although REEs occur in nature only in trace amounts, they are often concentrated in 250 minerals called the rare earth minerals (REMs), which are classified into carbonates, phosphates, silicates, and oxides. However, only a few have important value for the industry, as economic limitations restrict commercial extraction to a small group of minerals [27,28]. Currently, the main sources of REEs are the minerals monazite, bastnasite, and xenotime and ionic adsorption clays. Monazite is one of the most important REMs, as it is the most widely distributed across the globe and was the first mineral used in industrial application. Monazite also often contains thorium and uranium in varying concentrations. Bastnasite is one of the most important sources of light rare earth elements (LREEs), containing between 67 and 73% rare earth oxides (REOs). Xenotime is an important source of phosphate-based heavy rare earth elements (HREEs), containing proportions greater than 42% of REOs. Element Y, which is responsible for 50 to 70% of this proportion, is the main REE found in xenotime. Ionic adsorption clays are also sources of HREEs, and they are adsorbed on the clay surface in the form of ions. The concentration of REOs in clays can vary from 0.05 to 0.3% [4,11].
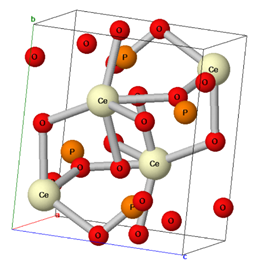
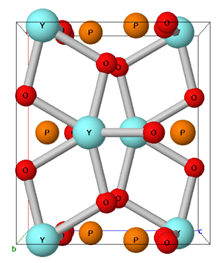
Table 1 presents the general formula and chemical structure of the main minerals containing REEs formed by geochemical processes. The minerals monazite and xenotime have the general formula ABO4, where group B is replaced by P5+—except for in monazite, where some replacements by Si4+ or As5+ are possible—forming mostly phosphate minerals. Group A can be replaced by REE3+, by some actinide elements, such as U4+ and Th4+, or by Y3+, as is the case with monazites forming a monoclinic crystalline system. On the other hand, xenotime is a phosphate mineral in which group A is replaced exclusively by Y3+, and in rare exceptions by Yb3+, in a tetragonal crystalline system. Bastnasite is a carbonate mineral with the general formula AB(CO3), with Fluorine (F−), or, in some cases, hydroxyl (OH−), as a substituent in group B. In this mineral, group A can be replaced by an REE3+, Th4+, or Y3+, forming a hexagonal crystalline system [29,30,31].

Table 1.
The chemical structure of the main minerals containing rare earth elements.
REE contents in soils are governed mainly by the chemical processes of formation of the origin mineral deposits. However, climatic conditions, organic matter, and the concentration of clay minerals also play important roles in determining these levels, which are often controlled by physical phenomena [32]. Among the minerals formed on the Earth’s surface through weathering and sedimentary processes are ionic adsorption clay-type deposits, placer sediments, and marine sediments [33].
Rocks, sediments, and soils rich in clay minerals have the ability to adsorb metal ions in the thin layer of the Earth’s surface and can concentrate REEs in ion adsorption-type deposits (IADs). These clay minerals, from the class of hydrated aluminum and magnesium phyllosilicates, have diverse layered chemical structures that lead to a series of ionic retention mechanisms, such as cation exchange, surface complexation, ligand exchange, structural incorporation, surface precipitation, and precipitation induced by surface redox reactions [34].
Some studies have shown that in shallow soils, clay minerals such as kaolinite (aluminosilicate) weakly adsorb REEs that are easily leachable. In this case, it is likely that the REEs are in the form of spherical hydrated complexes, presenting coordination numbers of 8 to 9 with water molecules. In deep soils, clay minerals have a high adsorption capacity because they are abundant in halloysite (aluminosilicate), which has a large surface area, high porosity, and high availability of metallic adsorption sites such as Fe. Furthermore, they can efficiently retain HREEs leached by progressive lateritic weathering of the original granite or shallow soils and can form economically valuable deposits [35,36].
It is worth mentioning that a search for innovative REE mining techniques has taken place, enabling the green, efficient, and selective recovery of these elements. Compared to conventional REE mining techniques, these new techniques can achieve greater recovery efficiency, a reduction in the use of leaching agents (with a consequent reduction in environmental impacts), and a reduction in metallic impurities in the REE obtained [37].
Another possible method of obtaining REEs is through secondary sources. Currently, there is growing interest in understanding the life cycle impacts of REEs. In China, the processes of mining, smelting, and separation to obtain REEs have had significant environmental impacts, such as erosion, pollution, soil acidification, and serious damage to surface vegetation, in addition to generating high levels of radioactivity due to the presence of Th [38]. Thus, many studies have already explored, through secondary sources, sustainable solutions to the environmental impacts caused by traditional exploration. Some examples of methods of obtaining REEs from secondary sources include the recycling of waste from various electronic components, such as notebooks and cell phones, as well as permanent magnets, batteries, fluorescent lamps, wind turbines, liquid crystal monitors, and phosphorus-based industrial waste [39,40].
Global reserves of REEs are relatively abundant. However, their truly mineable concentrations are less frequent than those for most other mineral elements. It is also known that the largest reserves are not necessarily those with the greatest economic potential. The greatest REE demand is for the element Nd, a light rare earth, as well as for some heavy rare earths, such as Eu, Tb, and Dy [24]. Due to the electro-deficient nature of REE ions in forming stable oxides, the quantity of reserves and commercial extraction is often estimated and calculated based on equivalent rare earth oxides (EqREO) and not on the elemental form of the metals [27].
It is estimated that world reserves contain a total of around 90 million tons of REOs. Of this total, China has the largest reserves, with 44 million tons (49% of world reserves), followed by Brazil (21 million tons), India (6.9 million tons), Australia (5.7 million tons), Russia (3.8 million tons), Vietnam (3.5 million tons), and the United States (1.9 million tons). Other countries are also included in the figure below; however, these have reserves of less than 2 million tons or unknown or unavailable reserves (Figure 1) [41]. Despite the enormous resources of REEs in Brazil, a large part of this volume is related to minerals whose processing has not yet been established or is environmentally or economically unfeasible [42].
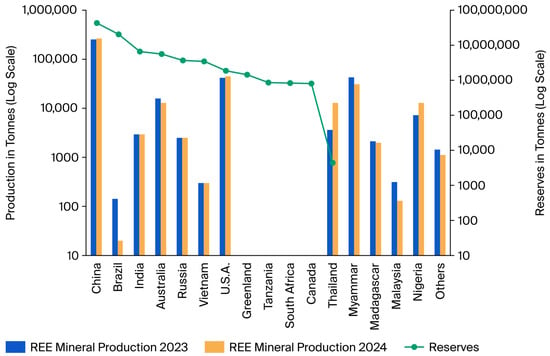
Figure 1.
Rare earth oxide equivalent (EqREO) mineral production in 2023 and 2024 and global reserves. Source: Adapted from [41].
In 2023 and 2024, it is estimated that global REO mineral production increased slightly, from 376 to 394 thousand tons/year, with China (from 255 to 270 thousand tons/year), Nigeria (from 7.2 to 13 thousand tons/year), Thailand (from 3.6 to 13 thousand tons/year), and the United States (from 41.6 to 45 thousand tons/year) being the main countries responsible for this growth. India and Russia maintained constant production at 2.9 and 2.5 thousand tons/year, respectively. In other countries, a significant reduction in the production of these minerals was observed; this is the case for Myanmar (formerly Burma) (43 to 31 thousand tons/year), Australia (from 16 to 13 thousand tons/year), and Malaysia (from 0.31 to 0.13 thousand tons/year). More recent records indicate that Brazilian production of REOs, which amounted to 0.14 thousand tons/year in 2023, decreased to 0.02 thousand tons/year in 2024 (Figure 1). Although Brazil has one of the largest reserves of REOs, its contribution to the global production scenario is quite modest and comes from the remaining reserves from the production of monazite sands at a mining unit in Buena, in the municipality of São Francisco de Itabapoana, in Rio de Janeiro State [43]. Given its large reserve of REOs, Brazil needs to make greater investments in the exploration of these minerals in order to become more competitive in this sector on a worldwide scale.
Although REE-enriched deposits are abundant and diverse in mineralogy, those with current economic value have a limited geographic distribution [44]. Figure 2 shows the global distribution of REE mining projects, including both active mines and advanced projects in Brazil and worldwide.
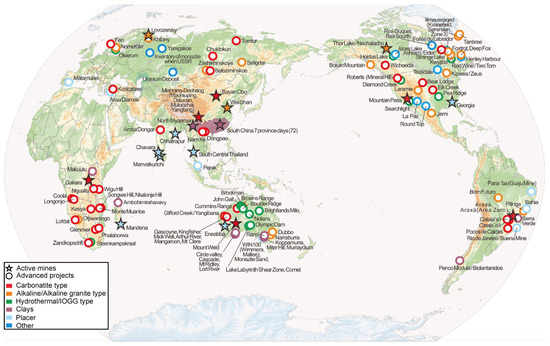
Figure 2.
Global distribution of REE mining projects, including both active mines and advanced projects. Reprinted from Ore Geology Reviews, 157, Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C., Global rare earth elements projects: New developments and supply chains, 105428, Copyright (2025), with permission from Elsevier. Source: [33].
Several locations throughout Brazil have immense potential to produce REEs due to the enormous volume of minerals with a high content of these elements [42]. According to the Ministry of Mines and Energy [43], the main mining feasibility studies and advanced-stage exploration projects in Brazil are located in the Barreiro Complex region, in Araxá (in Minas Gerais State), explored by the Brazilian Metallurgy and Mining Company (CBMM) and the Economic Development Company from Minas Gerais (CODEMIG); in Morro do Ferro in Caldas, in the Alkaline Complex from Poços de Caldas and in Lithium Valley, the latter explored by the mining company Foxfire Metals, all in the Minas Gerais State; in the Serra Dourada Granite explored by Serra Verde Mining in Minaçu (Goiás State); in the Pitinga Polymetallic Complex, explored by Mineração Taboca S.A., in Presidente Figueiredo (Amazonas State); and in the region between Prado and Caravelas, explored by the mining company Energy Fuels (Bahia State).
The Barreiro Complex, in Araxá (Minas Gerais State), is home to one of the most developed REE extraction projects in Brazil. The deposits are associated with alkaline carbonatite rocks and are part of the Alto Paranaíba Igneous Province. These rocks generally form circular structures and develop radial drainage patterns characterized by a thick weathering profile that can reach more than 250 m in depth. With REO levels of 3.0% from 22 million tons (Mt) of mineable reserves, rare earths are obtained as a byproduct of the processing of the mineral pyrochlore for Ni production. The deposits also contain minerals such as monazite, which is abundant in LREEs, as well as others in smaller quantities, such as burbankite, carbocernaite, ancylite, and huanghoite [24,45].
Other deposits of REEs are associated with granitic rocks. As an example, the mineral province of Pitinga (Amazonas State) has approximately 2 million tons (Mt) of xenotime with a content of 1% yttrium oxide, characterized by crystals in the form of millimetric prisms dispersed in the granite matrix. In the Serra Dourada Granite (Goiás State), deposits are associated with the clayey fractions that occur in the saprolite zone of this granite. The mineralizations of these deposits are predominantly ionic adsorption clays with 70% LREEs and 30% HREEs, and a total of approximately 300 million tons (Mt) of reserves is present, with measured contents of REOs + Y of 0.15% [25,45,46].
The 2017 Mineral Summary reported that new measured REE reserves had been approved and some reserves reassessed in Brazil. In Araxá, Minas Gerais State, reserves measuring 44 million tons (Mt) were approved in 2016, with an REE content of 1.41%, amounting to 621 million tons (Mt); in Itapirapuã Paulista, São Paulo State, under the ownership of Vale Fertilizantes S.A., reserves were approved in 2012 with a measured 47 million tons (Mt) of REOs and a content of 4.89%; new reserves were re-evaluated in 2016 in Poços de Caldas, Minas Gerais State, Mineração Terras Raras S.A., and the measurements changed to 158.82 million tons (Mt) with a REOs content of 3.41%; in São Francisco do Itabapoana, Rio de Janeiro State, reserves measured by the Brazilian Nuclear Industries (INB) were re-evaluated in 2016, and a value of 364.8 million tons (Mt) of mineable ore was obtained, with a content of 0.287% monazite found in these reserves, amounting to 1048 tons; and in Vale do Sapucaí, Minas Gerais State, under the ownership of VALE S.A., reserves were approved with a measured 4.5 million tons (Mt) of rare earths containing 60% monazite, equivalent to 2.7 million tons (Mt) [46].
According to information provided in the 2013 Mineral Summary of the former National Department of Mineral Production, unapproved mineable reserves of a deposit in Catalão, Goiás State, previously owned by VALE S/A, Anglo American do Brasil, and now belonging to China Molybdenum (CMOC—Brazil), are estimated to represent 32.8 million tons (Mt), with an average REO content of 8.4% [47].
Although Brazil plays a key role in the global REE scenario, with abundant reserves, one of the biggest challenges the country faces in exploring these critical elements is the lack of investment in research and innovation in companies and universities, as well as the absence of strategic planning [42]. Value addition is also a challenge, as Brazil still primarily exports raw ore. Therefore, to become more competitive in the global market, the country must invest in mineral processing and transformation within its own territory [48].
On the other hand, the extraction of REEs from industrial by-products (e.g., coal combustion products and red mud) and secondary sources (e.g., electronics and lamp waste and permanent magnets) suggests pathways for obtaining new supplies of critical metals, based on sustainability strategies, industrial symbiosis, and circular economy. REEs can be obtained from a variety of secondary products such as (i) coal combustion products (such as fly ash, bottom ash, and incinerator ash); (ii) industrial by-products (such as slag, phosphogypsum, and red mud); and (iii) electronic waste (including nickel-metal hydride batteries, computer hard drives, cell phones, and speakers) [49,50,51,52,53].
Some secondary sources are richer than ores in high-value REEs (such as Sc), and thus the quantities of REEs contained in these sources could meet current global demand even at low extraction yields. Since processes to extract REEs from secondary sources are under development, it is not yet clear which will be profitable at scale and which will generate the least environmental impact. Furthermore, this process requires advanced separation and extraction technologies to achieve high yields of raw materials and high purities of recovered REEs. So, there are many challenges to be overcome before secondary sources become real sources of REEs [52,54]. Despite this, recycling offers potential advantages over primary production, mainly because it can avoid the costly steps of separating low-value REEs (La and Ce) as well as the concomitant radioactive elements [54]. From the point of view of environmental sustainability, it is also worth highlighting the concern regarding the generation of waste from the mining of REEs from primary sources, which often contain radioactive elements (such as uranium and thorium) that harm human health and the natural environment [55,56,57].
3. Industrial Applications of Rare Earth Elements
REEs make up the list of critical minerals relevant to the technological development of a nation. According to [58], critical minerals are those that are essential to the national or economic security of a country, are vulnerable to fluctuations along their supply chain, and perform an essential function in the manufacture of a product whose absence would have significant consequences for economic security. The list of critical minerals is based on a quantitative assessment of threshold criteria for each individual supply risk component, such as disruptive potential, commercial exposure, and economic vulnerability. In the list published by the United States [19], among the REEs, only Pm is not listed as a critical mineral. Despite being part of the lanthanide series, Promethium is an extremely rare element in nature and can be replaced by other REEs with similar properties.
REEs have intrinsic properties that guarantee their use in a diverse range of modern technologies. These include optical, catalytic, and magnetic properties, high thermal stability, luminescence, hydrogen storage capacity, and exceptional electrical conductivity. These properties guarantee technical, structural, and energy benefits such as miniaturization, durability, stability, speed, reduced energy consumption, and greater energy efficiency [28,59,60].
Table 2 presents the main sectors of commercial application of critical REEs and their technological applications: (i) synthesis of catalysts (75% of REEs produced serve this market) for a variety of chemical, petrochemical and environmental control reactions; (ii) production of ceramics and special glasses and polishing (10% of REEs produced), in which REEs are used as an additive to improve the mechanical and thermal properties and polishing of the material’s optical components; (iii) phosphorus and other related uses (10% of REEs produced), such as the use of REEs as activators in phosphorus-based production of energy in the form of light, in the production of lasers for material processing, in the development of optical fibers for telecommunications, in spectroscopy and in medicine for diagnosis and treatment; and (iv) metallurgy and metallic alloys (5% of REEs produced), in the production of lighter magnets with strong magnetic fields that can be used in motors, electrical generators and loudspeakers and the production of special metallic alloys that can be used to manipulate the properties of materials [19,59,61]. Additionally, REEs have been employed as doping elements in high-power fiber lasers, which have many industrial and scientific applications, such as in laser cutting, laser welding, laser cleaning, and precision laser processing, including in material processing areas and the medical and military sectors [62]. Other possible technological applications of REEs include computer memory, rechargeable batteries, super magnets, mobile phones, LED lighting, superconductors, fluorescent materials, phosphate-binding agents, solar panels, and magnetic resonance imaging agents [28,60].

Table 2.
Some sectors of commercial application of rare earth elements (REEs).
More specifically, rare earth luminescence materials have rapidly advanced in areas such as general lighting, backlit display applications, information sensing, photoelectric devices, modern agriculture, optical storage, and anti-counterfeiting [63]. Furthermore, promising applications of REEs related to aerospace structures, biomedical implants, and IoT devices, among others, have also been considered [64].
Therefore, with technological advances in the development of new materials containing REEs, the search for emerging and more refined applications of these elements has been increasingly explored.
REE luminescence materials have attracted significant attention, experiencing rapid technological advancement. With people’s increasing demand for a better quality of life, the development of new lighting sources emphasizes comprehensive light quality, integrating multifunctional applications. Thus, the applications of REE luminescent materials involve (i) full-spectrum lighting phosphors for general lighting applications; (ii) phosphors for wide color gamut LCD backlights; (iii) phosphor-converted LED (pc-LED) NIR phosphors utilized in agricultural lighting, security monitoring, food/medical testing, and related fields; (iv) up-conversion luminescence materials employed in bioimaging, anti-counterfeiting, and information security; (v) bulk luminescence materials applied in lasers, deep-sea and deep-space exploration, as well as national defense and security; (vi) temperature-sensitive luminescence materials for detection; (vii) persistent luminescence (PersL) material used in industrial testing and non-destructive evaluation, biological imaging and detection, optical information storage, and high-end anti-counterfeiting technologies; and (viii) anticounterfeiting materials deployed in intelligent anti-counterfeiting systems and food/drug safety monitoring. However, information regarding the industrial development status of these materials and their application trends remains insufficient. This is mainly due to the lack of more detailed information on advances in mechanistic research and technical progress in the development of REE luminescence materials, coupled with a lack of collaborative innovation between industry, academia, and research [64,65,66].
Over time, the search for innovative solutions in energy conversion and storage for a more sustainable world has driven interdisciplinary research into REE-containing materials (primarily related to REE-based nanomaterials), exploring numerous applications, from active doping and co-doping to tri-doping and innovative composites [67]. In that regard, recent advances in the application of REOs in energy conversion have shown remarkable progress. With increasing energy demand and environmental concerns, the search for clean energy sources (such as solar, biomass, wind, hydroelectric, geothermal, and nuclear) has been increasingly explored. However, given the spectral sensitivity limitations of conventional photovoltaic systems (which convert sunlight into electricity), applications of unconventional solar cells using REE ions have been increasingly considered. These unconventional cells can harness lower-intensity infrared (IR) and ultraviolet (UV) light, converting it into visible wavelengths of the solar spectrum. Despite significant technological advancements, the physicochemical mechanisms of unconventional cells must be rigorously researched (including REE luminescence mechanisms, quantum aspects, and the concepts of upconversion and downconversion) in order to increase the energy conversion efficiency of these materials [68,69,70,71].
In recent years, there has been talk of targets for reducing greenhouse gas emissions and, consequently, the development of low-carbon energy sources. The search for critical minerals, such as rare earths, has already led to major geopolitical changes that could replace countries’ dependence on fossil fuel resources with their possible dependence on mineral resources, defining a path for the so-called energy transition. Among the low-carbon energy and transport sectors, there are many advanced technologies, such as wind turbine generators, solar panels, electric vehicle engines, and super magnets, that are only made possible through the use of the critical elements Pr, Nd, Dy, and Tb. It is expected that, as the world progresses through this energy transition, demand for these minerals will grow substantially and further increase their criticality [72].
Regarding the energy transition, hydrogen (H2) has been prioritized as a clean or environmentally friendly, cost-effective, and high-density (~120 MJ/kg) energy source [73,74]. So, the search for economical, scalable, efficient, and rapid approaches for photoelectrochemical (PEC) water splitting for hydrogen generation is essential, as it contributes to the transition toward a green and low-carbon global economy. In this sense, LREE has emerged as a promising option for use in photocatalysts due to its distinct electronic properties, abundant primary source availability, and thermal and physicochemical stability. However, the practical application of LREE-based electrodes and devices presents significant challenges associated with the fabrication of LREE thin films and, therefore, is still in its early stages. Additionally, the challenges of scaling these technologies for industrial use and ensuring long-term device stability must be considered. It is also worth mentioning that among the five LREE elements (La, Ce, Nd, Sm, and Pr), Ce and La have been extensively studied, and greater attention should be given to the potential use of Pr, Sm, and Nd in PEC water splitting and other applications [75].
Similarly, HREEs play a crucial role in clean energy technologies, being essential for the manufacturing of technologies such as batteries, electric motors, and wind turbines. However, as with LREE, the practical use of HREE-based electrodes and devices designed is still in its infancy due to challenges in manufacturing methods, particularly in the development of thin films for hydrogen production. As the world transitions from fossil fuels to sustainable and low-carbon energy sources, market demand for REEs is expected to increase considerably in the coming decades. Furthermore, life-cycle analysis (LCA) and economic analysis can be essential strategies to meet industry demands and ensure the commercial viability of these materials [76].
Refrigeration technology has played an increasingly important role in many fields, such as industrial production, national defense, and the military industry. Currently, despite its low refrigeration efficiency (~40% of the ideal Carnot cycle), the main technology used is gas compression refrigeration. In the search for materials with greater refrigeration efficiency, magnetic refrigeration technology has attracted widespread research attention. In this regard, REE oxides have been promising candidates for low-temperature magnetic refrigeration materials due to their large magnetocaloric effect, strong mechanical and chemical stability, and low cost. It is worth noting that magnetic refrigeration-based materials have important advantages, such as (i) they do not produce harmful gases and do not cause damage to the environment; (ii) they are more efficient (up to ~60% of the ideal Carnot cycle) than conventional gas compression refrigerators; and (iii) they take up less space and produce less noise, due to the use of solid materials as refrigerants, facilitating the miniaturization of devices [77,78]. Additionally, researchers have shown considerable interest in magnetorefrigerants based on rare earth metal–organic frameworks (RE-MOFs) for future cryogenic technologies. Due to its unique electronic structure and magnetic properties, the Gd3+ ion is an excellent candidate for creating molecular magnetorefrigerants with substantial magnetocaloric effects [79,80,81].
Ceramics based on zirconia (ZrO2) doped with REEs have been fundamental in the evolution of materials in several applications due to their greater thermal stability, mechanical strength, and ionic conductivity. Emerging applications of these materials, such as solid oxide fuel cells, thermal barrier coatings, bioceramics, and optical devices, require a comprehensive understanding of the fundamental properties of the solid state to ensure their effective operation. In the biomedical field, REE-doped ZrO2 has been employed as implants for advanced dental, orthopedic, and load-bearing applications due to its superior mechanical strength, fracture toughness, and biocompatibility [82,83,84].
The study of the properties of rare earth dopants for quantum technologies is relatively new and has advanced rapidly in recent years. Leveraging the rise of cutting-edge photonic technologies, on-chip REE quantum devices have emerged that function as quantum memories, single-photon sources, and transducers, often with potential performances unmatched by other solid-state quantum technologies. The emerging field of rare earth nanophotonics inherits the rich history of REE spectroscopy. New platforms based on REE-doped nanocrystals, polycrystalline ceramics, and films adapted for quantum technologies are gaining momentum [85,86,87,88]. Some characteristics of REE-doped nanoparticles are narrow optical and spin linewidths at low temperatures, making them attractive for quantum technologies based on optically addressable spins, such as quantum memories and computers [89]. However, until recently, the detection and utilization of isolated rare earth ions in quantum technologies was hampered by their inherently weak optical transitions [90,91,92].
Given the wide variety of technological applications of REEs, in short, Figure 3 illustrates the main current applications.
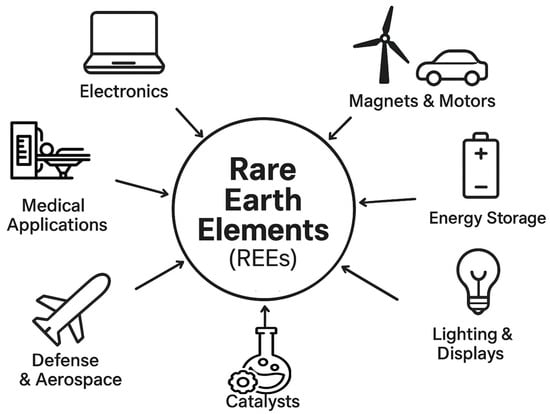
Figure 3.
Main recent technological applications of REEs.
4. Business Investment and Technological Applications of Rare Earth Elements
In this section, the countries and companies that stand out most on the global stage in terms of reserve identification and exploration and processing of REEs are mentioned. As already shown, China practically has a monopoly on this market. However, countries such as the United States, Australia, and Myanmar, among others, have been forced to reinforce their own supply chains, with greater incentives for mining and production of critical minerals with high added value.
It is known that the useful life and exploration of a mine are supported mainly by estimation of the concentration or occurrence of the solid materials of economic interest contained therein (mineral resources) and by the reserves of each ore that can in fact be explored economically. Therefore, for some companies mentioned here, reserves of REE ores will be considered as a subset of their respective mineral resources [93].
Primary deposits dominate REE exploration projects around the world. In general, REE resources are primarily contained in five deposit types: carbonatite; alkali rock/alkaline granite; ionic clays; iron oxide copper-gold (IOCG)/hydrothermal; and placer. Currently, four types (carbonatite, alkali rock, ionic clays, and placer) are under commercial development, with carbonatite-type projects/deposits dominating active production [33].
It is worth noting that there are some uncertainties in the current scenario, mainly related to the heterogeneity of REE deposits, making the estimation of resources and individual REO quite challenging [33].
Challenges related to environmental degradation and pollution are important issues that must be considered in REE exploration projects (including acid mine drainage, tailings, and social issues) at facilities in different regions of the world. Therefore, the inclusion of practical environmental protection and restoration activities must be essential for the success of construction and exploration on new projects [33,94]. Therefore, if industrial projects develop more comprehensive, environmentally friendly, and efficient metallurgical technologies, they may have greater economic potential [95,96].
4.1. Rare Earth Mining Activities in China
China Northern Rare Earth (Group) High-Tech Co., Ltd. (Baotou, Inner Mongolia, China), is a supplier of rare earth products that was founded in 1961 and is a subsidiary of the holding company Baotou Steel Group, the world’s first REE industry, based in China. It is mainly engaged in the production and sales of rare earth raw materials, rare earth functional materials, and rare earth application products. Northern Rare Earth has about 50 branches, with more than 9000 employees, distributed in 11 provinces across China, achieving coordinated development among all regions. The company explores the Bayan Obo mine in western Inner Mongolia (China), a rare symbiotic and polymetallic mineral deposit with a total area of 48 km2 that contains more than 160 types of minerals, mainly bastnasite and monazite in the ratio of 6:4, and 70 additional types of elements, including Fe, Ni, Cu, and rare earths, as well as other minerals. The Bayan Obo mine is considered the largest REE mine in the world, both in terms of recoverable reserves and production. It contains Fe ore reserves totaling around 950 million tons, Ni reserves amounting to 5.19 million tons, and REE ore reserves totaling 36 million tons. Northern Rare Earth’s main products include REE concentrates, carbonates, oxides, and salts, with applications in magnetic materials, polishing, hydrogen storage materials, catalysts, luminescents, nickel–hydrogen batteries, electron spin resonance, and element-based permanent magnets. The company reports a smelting and separation capacity of around 120,000 tons/year, comprising 16,000 tons/year of REEs, 41,000 tons/year of magnetic materials, 32,000 tons/year of polishing materials, 8300 tons/year of hydrogen storage materials, 12,000 tons/year of catalytic materials, and 1 million pieces per year of nickel–metal hybrid batteries, as well as other magnetic and energy-saving products serving the automobile industry [97].
China Rare Earth Resources and Technology Co., Ltd. (Ganzhou, Jiangxi, China), the official parent company since 2022 and formerly known as China Minmetals Corporation, is a China-based company primarily engaged in the processing of medium and HREE ores and in the production and operation of high-purity REOs and other commodities. In addition, it carries out research and development of rare earth technologies, technical consultancy services, and geological analysis. The company carries out separation and processing through the outsourcing of rare earth raw materials and other methods, and its main member companies are Ganxian Hongjin Rare Earth Co., Ltd. (Hongjin, Jiangxi, China); Dingnan Dahua New Material Resources Co., Ltd. (Dingnan, Jiangxi, China); Minmetals (Beijing) Rare Earth Research Institute Co., Ltd. (Beijing, China); and Guangzhou Jianfeng Rare Earth Co., Ltd. (Ganzhou, Jiangxi, China). Its main commercialized products are oxides of Sm, Er, Gd, Ho, Nd, Ce, Tb, La, Y, Eu, Pr, Lu, and Dy, which are widely used in the areas of magnetic materials, luminescent materials, catalytic materials, crystalline elements, high-quality electronic components, and other fields. The member companies have always focused on REEs industrial operations and have established competitive advantages in production technology, quality control of their products, energy conservation and environmental protection, commercial operations, team management and operations compliance, as well as compliance with the ISO9000 [98] andISO14000 [99] certification systems [100].
4.2. Rare Earth Mining Activities in the United States
MP Materials Corp is the largest REE mining and processing company in the Western Hemisphere and the largest outside of China. The company operates the REEs mine and processing facility in Mountain Pass (California) and produces approximately 15% of the rare earth volume consumed annually from bastnasite ore. In 2023, integrated processing facilities were reactivated to produce separate high-purity neodymium and praseodymium oxides (NdPr), the main components in the manufacture of higher-strength permanent magnets, as well as La and Ce oxides and carbonates. Additionally, the company produces a heavy concentrate called SEG+, a combination of the elements Sm, Eu, Gd, Tb, and Dy. The processing of bastnasite ore occurs by separating the REEs from their residue through the processes of crushing, grinding, conditioning, and flotation. The mixed concentrate of REEs is chemically treated, and the process has the function of purifying and precipitating individual REEs. As a result, pure forms of REOs are produced, treated, and packaged to meet customer specifications. The company follows the concept of cleaner production, and its facilities are equipped with state-of-the-art systems for recovering dry waste and saving environmental resources, recycling enough water to meet approximately 95% of the process needs [101].
Energy Fuels is a leading American company in the production of critical minerals, being the main miner and producer in the United States of natural uranium concentrates, in the form of triuranium octoxide (U3O8), which are sold to produce nuclear energy. Currently, conventional uranium production is performed at a single operating plant in Utah (White Mesa Mill) with a licensed capacity of over 8 million pounds of U3O8/year. However, the company also has another production center on standby in Wyoming (Nichols Ranch) called the in situ recovery project (ISR), with a licensed capacity of 2 million pounds of U3O8/year. The company can also produce V and REEs from uranium-bearing minerals based on market needs and conditions. Energy Fuels has invested in the production of REEs, such as mixed-carbonate REEs, and intends to produce elementary commercial quantities of REOs by building infrastructure for separating REEs at White Mesa Mill, with an initial capacity of 2500 to 5000 metric tons of REO/year, including 500 to 1000 metric tons of NdPr oxide or oxalate/year. The objective is to reach a total processing capacity of 15,000 metric tons of REO/year. In early 2023, Energy Fuels announced the acquisition of a new heavy mineral sands project in Brazil (in Southern Bahia State) (Project Bahia) that has the potential to produce between 3000 and 10,000 metric tons of monazite, containing between 300 and 1000 metric tons of NdPr oxide/year, in addition to significant quantities of Ti (ilmenite and rutile) and Zr (zircon) minerals. Currently, the company is in the process of performing sonic drilling in each of the 17 exploration areas in the region, with the aim of better defining its mineral resources and informing the future licensing and mining plan for the end of 2025 or beginning of 2026. Energy Fuels intends to control its own supply of REEs and other critical minerals using low-cost raw materials [102].
NioCorp Developments Ltd. (Denver, CO, USA) is a mineral exploration company in the US that is focused on developing various metal superalloys. The new critical minerals project at Elk Creek, a large underground deposit in southeastern Nebraska, plans to produce Ni, Ti, and Sc as primary products and magnetic rare earths (Nd, Pr, Tb, and Dy) as byproducts. According to the company, a feasibility study was carried out that confirmed mineral resources of 970.3 kilotons (kt) of niobium oxide, 11,337 tons of scandium oxide, 4221 kt of titanium oxide, and 632.9 kt of total REOs, including 26.9 kt of Pr, 98.9 kt of Nd, 2.3 kt of Tb, and 9.1 kt of Dy, which places the project as the second-largest indicated REEs resource in North America, second only to MP Materials’ Mountain Pass deposit. With the aim of facilitating its processing and technical and economic production, metallurgical tests for the recovery of individual rare earths from primary products are already being conducted in a pilot plant located in Quebec, Canada [103].
4.3. Rare Earth Mining Activities in Australia
Lynas Rare Earths Ltd. (Perth, WA, Australia) is an Australian company engaged in the extraction and integrated processing of REMs in Australia and Malaysia. It operates the Mount Weld mine (Western Australia) and sends mined material for refining and processing at its separation facility at the Gebeng industrial park in Kuantan (Malaysia), which serves markets in Asia, Europe, and the USA. Its export products are NdPr, La, Ce, and HREEs concentrate (SEG). The company is also commissioning an REEs processing facility in Kalgoorlie (Western Australia) and a construction project for another processing facility in Texas (USA) for 2024. The Mount Weld mine is recognized as one of the main REE deposits in the world and has deposits associated with carbonatite rocks and three mineralogical layers of economic importance: the limonite zone, central zone, and apatite zone. REEs are found predominantly in monazite and in smaller quantities in other minerals, such as ceryanite, crandalite, florencite, and xenotime. According to the 2019 Annual Report, the mineral resource estimate for REE deposits is 55.2 million tons, with an average total REO content of 5.4%, amounting to 3.0 million tons of REOs. Ore reserves are estimated to amount to 19.5 million tons, with an average REO content of 8.5%, totaling 1.648 million tons of REOs [104].
Iluka Resources is a global critical mineral production company headquartered in Perth, Western Australia, with experience in exploration, development, mining, processing, marketing, and rehabilitation of mines and processing facilities in Australia and the United States. It is one of the main producers of zircon, raw materials to produce high-quality titanium dioxide such as natural rutile and synthetic rutile from ilmenite, activated carbon, and iron concentrate, in addition to holding a significant position in rare earth production. REEs are routinely produced through the processing of monazite- and xenotime-rich mineral sands stored at a former mine at Eneabba (Western Australia) and in additional deposits at the Wimmera project (in Victoria State) and the Balranald project (in New South Wales State). According to a 2022 report, the total mineral resources at Eneabba are estimated to amount to 526 million tons, with an average total REO content of 1.2%. The ore reserves are estimated to amount to 0.96 million tons of ore, with an average total REO content of 19.2%. At the Wimmera project, the total mineral resource estimate is 1.384 billion tons, with an average total REO content of 2.4%, and at the Balranald project, the total mineral resource estimate is 54 million tons, with an average total REO content of 0.9%. The company is building a state-of-the-art refining facility near Eneabba that will be capable of processing up to 23,000 tons of REOs per year. The unit will provide facilities for the processing of a variety of raw materials from its own stock or from third-party producers and will allow water and reagents to be recycled and reused, in accordance with sustainable mining strategies [105].
Arafura Rare Earths Limited is an Australian mineral exploration company for REEs, based in Perth (Western Australia). The company has been developing the Nolans project in the Northern Territory of Australia. With low-risk mineral resources, the project aims to meet the global need for NdPr for the production of permanent magnets, as well as other rare earths in varying concentrations. The Nolans Bore mine is a highly strategic deposit of apatite, monazite, and allanite minerals and combined rare earth–phosphate–uranium–thorium (REEs-P-U-Th), which can produce other medium-weight mixed rare earths such as (SEG/REE) oxides, as well as NdPr oxides and phosphoric acid for the fertilizer market. The Nolans project must be integrated into a processing plant with all the required infrastructure for the processing, extraction, and separation of ore products. In 2017, mineral resources were in the order of 56 million tons (Mt), with an average total REO content of 2.6% and total phosphate (P2O5) content of 11%. On the other hand, in 2020, ore reserves amounted to 29.5 million tons (Mt), with an average total REO content of 2.9% and total phosphate (P2O5) content of 13%. According to the company, the mineral inventory has the potential to operate for 38 years, with a projected production capacity of 340,000 tons/year of concentrate [106].
4.4. Rare Earth Mining Activities in Myanmar (Formerly Burma)
Myanmar is one of the main countries exporting rare earths to China. In 2021, it accounted for 70% of the total value of imports of REEs in China. There is little information available about mining projects in the country. However, its mining sites are distributed in the city of Pangwa (in the municipality of Chipwi, in Kachin State), which borders the Chinese province of Yunnan, and are mostly deposits of ionic adsorption clays enriched with 17 types of REEs, including HREEs such as Dy and Tb. The country has been experiencing a civil war for a long time, and, in 2021, it suffered a military coup. In the Kachin State region, frequent clashes between Myanmar’s authoritarian military and the Kachin Independence Army are reported. This troubled political and military context fosters conditions that encourage illegal mining activities and is reportedly impacting the country’s REE production [107,108]. According to recent reports, as the demand for clean technologies increases, concern about the traceability of raw materials, which is important for their manufacture and a company’s environmental, social, and governance (ESG) practices, is increasing. Therefore, the Kachin regions affected by conflicts and illegal mining could impact the supply of rare earths, as the country represents a significant and growing portion of imports of these minerals to China [72].
4.5. Other Featured Rare Earth Mining Activities
Aclara Resources is an HREEs company based in Chile and listed on the Toronto Stock Exchange (Canada), which owns one of the few DyTb deposits outside of China and Myanmar, accounting for more than 95% of the supply of these elements. The company is conducting an HREE development project in the south of Chile (Penco Module) using ionic clays and has announced the discovery of a new HREE deposit in Brazil (in Goiás State), called the Carina Module exploration project, also based on ionic clays. The Penco Module has an NdPr/DyTb ratio of 2.5:1 and an estimated annual production of 50 tons of DyTb and 125 tons of NdPr contained in high-purity mixed carbonate. According to data from 2022, the estimated total mineral resources are in the order of 29.2 million tons, with an average total REO content of 0.23%. Recently, the company’s pilot plant processed 120 tons of ionic clays and produced 107 kg of high-purity rare earth mixed carbonates. In the Carina Module, an NdPr/DyTb ratio of 6:1 is expected, and the plan is to process 25 tons of ionic clays at the pilot plant in Chile and produce 20 kg of high-purity mixed rare earth carbonates. The company developed an innovative extraction method, in partnership with the University of Toronto (Canada) and Chilean academia, called “Circular Mineral Harvesting”. The method uses 100% water from recycled sources, does not use explosives or energy to grind the minerals, does not require a dam for liquid or solid waste since the washed clays are returned to the wells, and recovers 99% of its main exploration reagent (a common fertilizer). The process and product are free from radioactive materials, and 100% of the impacted area must be replanted with native species [109].
5. Characterization Techniques for Minerals Bearing Rare Earth Elements
Analytical techniques play an important role in all aspects of REE research and production activities, such as exploration, mining, extraction, and metallurgy. At each stage of these activities, rocks, ores, minerals, and other related materials need to be analyzed for their REE content in terms of elemental, isotopic, and mineralogical concentrations using different analytical techniques [110].
Because they are present in low concentrations and have very similar physical and chemical properties, the determination of REEs in a geological matrix faces certain challenges, especially if they are mixed with other elements from the same period of the periodic table. Accurate determination of REEs used to be extremely difficult and time-consuming. Recently, with the availability of sophisticated and robust instrumental analytical techniques, this task has become increasingly simplified. Among the currently available techniques, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has been widely applied for the precise determination of REEs in different types of matrices due to its multi-element capacity, wide linear dynamic range, high sensitivity, low interference, ease of operation, and accuracy, followed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and Instrumental Neutron Activation Analysis (INAA) [59]. Therefore, from the point of view of Analytical Chemistry, techniques that employ inductively coupled plasma spectrometry (ICP-OES, ICP-MS, and its variations) have been widely used in the qualitative and quantitative determination of REEs in geological matrices, considering their important application advantages.
Spectrometric techniques, such as Atomic Absorption Spectrometry (AAS), Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), or X-ray Fluorescence (XRF), may have relatively low sensitivity and require extraction/pre-concentration of different elements prior to their determination in geological matrices to avoid spectral interference [111]. In general, for elemental analysis, different ICP-MS techniques are more popular than other analytical techniques due to their excellent performance characteristics. Among these techniques, ICP-MS/MS with solution nebulization or laser ablation sampling is the best and most economical laboratory analytical technique currently available, especially when compared to the more sophisticated HR-ICP-MS (High-Resolution-ICP-MS) and MC-ICP-MS (Multi-Collector-ICP-MS) [110].
The criteria for selecting the best analytical technique for detecting chemical elements are extremely important characteristics, especially when identifying elements present at trace levels, such as REEs. In this context, the limits of detection of an analytical technique represent one of the main factors determining the possibility of identifying chemical species at trace levels. In the field of analytical techniques for metal determination, the best limits of detection (LODs) are obtained through ICP-MS, with values in the order of 1.0 to 100 ng L−1. The ICP-OES technique presents lower performance, with LOD values in the order of 1.0 to 100 µg L−1. Another factor to be considered is that the technique has a wide analytical working range, at concentrations as low as ng L−1 and as high as mg L−1, allowing samples with different concentrations to be analyzed together [112].
Several studies have used techniques for identifying and quantifying REEs that are characterized by low limits of detection and a wide working range [113,114,115,116]. However, for some techniques, the accurate determination of REEs in mineral matrices presents analytical challenges from both a measurement perspective and a sample preparation perspective. With regard to sample preparation, complete digestion of samples containing REEs is quite complex, since lanthanide oxides are known to be refractory materials, requiring aggressive dissolution conditions to be representative of the mineral content [115]. Table 3 presents the main analytical techniques used in studies to determine REEs and other elements in various geological and reference matrices.

Table 3.
Some main analytical techniques used to determine rare earth elements and other elements in geological and reference matrices.
Conventional methods for converting geological samples into solutions for further analysis rely on wet acid decomposition or alkaline fusion. Some examples are presented in Table 2. Methods using wet acid decomposition take a long time to complete (several hours), require high temperatures (conventional or microwave heating) and high pressure, and predominantly employ concentrated mineral acids or mixtures thereof. Alternatively, alkaline fusion is widely used for the total dissolution of samples containing refractory components, such as rocks. This method is commonly used for samples that do not dissolve in mineral acids upon heating. However, some problems with ICP-MS analysis hinder its use, such as the high solids content in the solutions, which can cause interference and lead to the deposition of these solids at the ICP-MS interface. The long time required for these procedures and the high risk of contamination are other inherent problems [111,122,123].
Research was conducted to evaluate the concentration of REEs in Brazilian reference soils using soil samples taken from the coastal zone to the semi-arid region of Pernambuco State (Brazil). A total of 35 topsoil samples were taken from areas covered by native vegetation with minimal anthropic influence. The soil samples were air-dried and passed through a 2 mm nylon sieve, then ground in an agate mortar and, finally, passed through a stainless-steel sieve with a 0.3 mm mesh. After sampling and preparation, approximately 1.0 g of each soil sample was digested in a microwave oven in Teflon containers with high-purity acids in 9 mL of HNO3 and 3 mL of HCl (USEPA 3051A Method). The digested extracts were transferred to certified 25 mL Falcon bottles (NBR ISO/IEC) filled with ultrapure water and slowly filtered through filter paper. For the characterization of REEs and iron (Fe), five REE standard solutions were prepared from the 1000 mg/L standard (Titrisol®, Merck; Darmstadt, Hesse, Germany), together with a blank sample. The concentrations of the standard solutions used for constructing analytical curves ranged from 1.0 to 25 μg L−1. The analyses were carried out using an ICP-OES/Optima 7000 (Perkin Elmer; Shelton, CT, USA), with a cyclonic spray chamber employed as a nebulization system. The average concentrations of REEs in reference soils in Brazil ranged from 0.6 to 43.48 mg kg−1 for LREEs and from 0.05 to 4.45 mg kg−1 for HREEs and presented the following order of concentration: Ce > La > Nd > Pr > Y > Sm > Gd > Sc > Dy > Yb > Eu > Er > Tb > Ho > Lu > Tm. REE levels in soils are mainly governed by the origin of mineral deposits, but climatic conditions, organic matter, and clay concentrations also play important roles in determining these levels. The lowest concentrations of REEs were found in sandy sediments (silicates), while the highest concentrations were observed in basalt, biotite gneiss, and clayey sediments. The USEPA 3051A method is not capable of extracting REEs from silicates. In general, the lower the recovery, the greater the association of the element with these silicate minerals in the soil. It was verified that the concentrations of organic carbon and Fe could adequately predict the concentrations of REEs in the studied soils, helping to estimate the concentrations of these elements in soils from Brazil and similar soils under tropical conditions [32].
Some studies have evaluated a new instrument to adequately quantify atomic composition in a set of composite matrices with minimal sample preparation. In this sense, the compatibility of three sample opening techniques (acid digestion in an open vessel, digestion assisted by microwave radiation, and alkaline fusion with borate—LiBO2) was evaluated for the quantification of REEs in mineral matrices of reference (britolite, granite and bauxite, syenite, and lujavrite), using ICP-MS/MS (Inductively Coupled Plasma Tandem Mass Spectrometry). In addition, the analytical performance of the MIP-AES (Microwave Induced Plasma Atomic Emission Spectrometry), ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) and ICP-MS (Inductively Coupled Plasma Mass Spectrometry), techniques was evaluated in relation to that of other plasma-based instruments. The results showed that the acidic treatment did not completely digest the REMs. Microwave-assisted digestion and alkaline borate fusion were acceptable for dissolving the minerals, and fusion was considered the fastest and most effective method among those tested. The comparative study showed that the concentrations obtained through ICP-MS/MS were in agreement with the values of reference materials; this technique was much more suitable than the other analytical techniques tested for the quantification of REEs, which exhibited low detection and/or interference in spectra for some elements/isotopes [115].
In [117], the development and validation of a unique method for determining 42 elements in marine sediments, including REEs at trace levels, was carried out. The simultaneous determination of REEs, within a wide range of concentrations, and possible interferences in complex matrices such as sediments is challenging and requires the development of a specific digestion method to enable complete multi-element analysis. In this study, the EPA 3051A method was used with modifications, which involved the samples being digested using microwave-assisted digestion, with temperature and pressure monitoring conducted, followed by extraction on a hot plate. During the procedure, approximately 200 mg of marine sediment samples were weighed out in PFA (Perfluoroalkoxy Alkane) digestion vessels. To the vessels, 9 mL of 65% HNO3 and 3 mL of 40% HF were added instead of HCl, since HF can dissolve silicates and make the REEs of this matrix available. The microwave oven was programmed for 5 min at a power of 1600 W and 165 °C, and another 5 min at 190 °C. The PFA vessels were transferred to a hot plate to evaporate the HF. After redissolving with 5 mL of HCl (1:1 v/v), evaporation was repeated. The residue was dissolved in 10 mL of HNO3 (1:5 v/v), and the solutions were heated for 30 min at 150 °C. After cooling to room temperature, the solutions were quantitatively transferred to volumetric flasks, which were then filled with ultrapure water to a final volume of 100 mL. One of the containers was filled with only the reagents and subjected to the entire digestion procedure to be used as an analytical blank. The analytical curves of REEs were prepared using a standard solution with a concentration of 10 mg L−1 and at least five solutions of different concentrations, plus the blank. Samples were analyzed using ICP-MS and ICP-OES. According to the authors, the method validation parameters (such as the applicability range, linearity, limit of detection, limit of quantification, selectivity, repeatability, intermediate precision, and accuracy) indicated precise determination of the 42 elements. Subsequently, the method was applied to real sediment samples, revealing concentrations of REEs that varied from 0.26 to 1.1 mg kg−1 (Lu, Tm, Ho, and Eu), from 1.6 to 7.3 mg kg−1 (Yb, Er, Dy, Gd, Sm, and Pr), and from 21.0 to 59.0 mg kg−1 (La, Ce, and Nd).
In another study, the concentrations of REEs in the soil were established based on natural and anthropic antecedents. Aspects of contamination due to the application of phosphate fertilizers were evaluated, in addition to prediction models being developed to better understand the most relevant environmental factors that could explain the variation in analyte concentrations. A total of 175 topsoil samples (0 to 20 cm) georeferenced from a database from the Brazilian Agricultural Research Corporation (Embrapa; Brasília, DF, Brazil) were analyzed. The soil was air-dried, homogenized, ground, and sieved through a 0.05 mm mesh. Finally, approximately 0.5 g of each sample was digested with 10 mL of aqua regia (HNO3:HCl; 3:1, v/v) solution in a programmable microwave digester for 20 to 25 min at 180 °C (USEPA 3051A Method). Subsequently, the extracts were placed in centrifuge tubes, filtered, and diluted in ultrapure water to 20 mL. The In element (1 μg g−1) was added as an internal standard in the final dilution. Analytical curves (0.1, 0.5, 2, 10, and 20 μg kg−1) were constructed from dilutions of standard solutions of REEs (1000 μg kg−1) in a 2% nitric acid solution, based on analyses performed using ICP-MS. According to the results, the median concentrations of REEs in soils cultivated with anthropic action were higher than those in natural reference soils. The concentrations of REEs found for the types of rock in the cultivated soils were as follows: acidic igneous rocks (407.4 mg kg−1) > metamorphic rocks (351.2 mg kg−1) > basic igneous rocks (302 mg kg−1) > alkaline rocks (295 mg kg−1) > unconsolidated sediments (277 mg kg−1) > sedimentary rocks (214.5 mg kg−1). The authors suggest that differences in REE contents in agricultural soils can be attributed to land use (such as prolonged application of phosphate inputs from intense agricultural activity in Brazil), while differences in non-agricultural soils can be attributed to other factors, such as origin materials, biomes, and pedogenic processes [118].
The economic potential of critical trace elements in geological samples is currently underestimated, but they may represent a promising future mineral resource with advances in technologies used in mineral extraction processes. To this end, increasingly advanced instrumental techniques have been studied, with detailed mineralogical and chemical characterization at the micro- and nanoscale [23].
As an example, Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) has been used, which consists of firing a high-power laser beam at a solid mineral sample to convert a quantity of that sample into vapor. Instantly, the vapor is transferred to an ICP-MS-type detection chamber for analysis. The main advantage of this technique is that any solid sample, in low quantities, can be vaporized, and there is no need for digestion with acids, avoiding the expense of inputs and exposure to dangerous products. In some studies, the LA-ICP-MS technique has proven capable of evaluating critical elements that make up minerals such as pyrite and apatite and refining genetic models of multi-stage mineral formation in complex mineral systems and has helped to reconstruct fluid sources and ore formation processes in some environments [119]. The authors also cite other studies in which the distribution of REEs in hematite and magnetite deposits has been studied at the microscale using a combination of two techniques: LA-ICP-MS and High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM).
Another technique that has been widely used in micro- and nanoscale geochemical studies is High-Resolution Transmission Electron Microscopy (HRTEM) coupled with Selected Area Electron Diffraction (SAED) and Energy-Dispersive X-ray Spectroscopy (EDS). As reported by [120], traditional methods for detecting nanoscale mineral elements only estimate elemental concentrations and cannot detect their morphological characteristics. Compared with other analysis methods, HRTEM has the advantage of measuring geological anomalies, due to its high sensitivity, and detecting the morphology and nanoscale structure of a single particle type (in this case, nanoparticles), due to its high resolution. For analyses using HRTEM, the nanoparticles are previously supported on circular metal grids coated in amorphous carbon, called TEM grids, which is a necessary step for analysis using this technique.
In a polymetallic pyrite deposit located in the province of Inner Mongolia (Northern China), 42 samples of nanoparticles captured by a device called a geogas collector were evaluated. This device has the function of directing the upward flow of gas that emanates from the Earth’s mantle through a tube and collecting, using TEM grids placed at the end of this tube, the mineral nanoparticles that are carried along with the gas. These samples were also compared with 42 corresponding soil samples from the quaternary sediments of this deposit. The HRTEM technique was used to analyze characteristics such as the size, shape, chemical composition, and structure of these particles. The results showed that the particles had either subcircular, elliptical, or irregular shapes, with sizes ranging from 5 nm to 400 nm, and their primary components were dominated by the elements Fe, Cu, Zn, and Pb, with percentages of 80.2%, 82.5%, 49.1%, and 59.6%, respectively. It was verified that the particles transported by the upward gas flow and the soil particles had a common origin, with good correlation between the hidden ore bodies and the rock wall revealed through the identification of many sulfides and metallic oxides/hydroxides, native metallic alloys, non-metallic oxides, chlorides, non-metallic hydroxides, and sulfate particles, as well as precious metals containing REE particles. Various particles have been found in elementary associations such as S-Cu, S-Zn, S-Pb, S-Mo, S-Fe-Zn, S-Fe-Cu, S-Fe-Cu-Mo, S-Fe-Zn-Pb, and Cr-Mn-Fe. The authors inferred that geogas prospecting could be used to explore various types of deep mineral deposits, since different particles transported by ascending gas flows come from different types of deposits [120].
In recent years, the technique of bottom-up nanoparticle capture of geogas associated with HRTEM analysis has contributed to the characterization not only of REEs in mineral and geological matrices but also of a series of denser elements, including Au and Pb. To this end, a study was carried out at a gold deposit in Chaihulanzi (China) to conduct prospecting for natural nanoparticles originating from deep faults, ascending gas flows, soil, shallow groundwater, and deep groundwater. Analyses were carried out using HRTEM to determine the class, shape, lattice parameters, chemical components, and associations of the minerals present. The results showed that the natural nanoparticles from the five environments studied contained deposit-forming elements, including Au, Ag, Fe, Cu, Zn, Pb, Sn, As, Mo, and Bi, among others, which are transported mainly by groundwater and the upward gas flow and migrate to the surface due to temperature, pressure gradients, topography, or pressure between aquifers. In different types of samples, associated elements, such as Au-Cu, Fe-Cu, Pb-Zn, Cu-Sn, and Fe-As, were observed. The particles had an average size below 300 nm and presented varying shapes, including spheres, hexagons, rhombuses, and irregular shapes. Minerals bearing metallic elements exist mainly in the form of oxides and sulfates, suggesting that faulting and oxidation are the main formation mechanisms. The characteristics of these nanoparticles can directly reflect information from mineral bodies and can thus be used to guide prospecting [121].
Additionally, conceptual advancement in Green Analytical Chemistry has encouraged researchers to incorporate new analytical tools that allow noninvasive (or minimal sample treatment) analysis of solid samples. In this sense, solid-state techniques such as XRF have been used to determine REEs in geological matrices. However, the quality of the results obtained depends on the choice of sample preparation method, measurement conditions, calibration strategy, and also the appropriate selection of analytical lines, given the serious problems of overlapping the characteristic X-ray lines of REEs with those of other important elements present in geological materials [122,123,124]. Although less sensitive, XRF is still popular for samples with high concentrations of REEs due to its ease of operation [110].
With the introduction of high-resolution detectors, many trace elements, including REEs, can be determined simultaneously, without any chemical separation, in a wide variety of rocks and minerals using Instrumental Neutron Activation Analysis (INAA). The advantages of nuclear analytical techniques include their wide range of applicability to various types of matrices and their exceptional sensitivity to many elements. However, the accuracy and limit of detection of REE data using INAA strongly depend on the type of material analyzed and the content of various interfering elements, which provide a high background or general signal and therefore may impede reliable REE analysis, especially in geological matrices [125].
Adeti et al. [126] compared the use of four analytical techniques for the detection of REEs in volcanic rocks from Ghana. The techniques evaluated were ICP-MS, Silver-anode X-ray tube XRF, Am-241 excitation-based XRF, and INAA. The accuracy of data obtained by Am-241 excitation-based XRF was comparable to ICP-MS (R2 = 0.9559) and INAA (R2 = 0.9451) and quite different from those obtained by Ag-anode X-ray tube XRF (R2 = 0.6129). The authors attribute these results to the limitations of the tube-based XRF excitation system for REE analysis, caused primarily by interference between the K-series X-ray emission of transition metals and the relatively low intensities of the L-series lines of REEs. Therefore, for analyses that require minimal sample preparation and shorter analysis time compared to other techniques, the Am-241 excitation-based system offers a reliable and accurate alternative for the analysis of REEs in environmental samples.
Another technique used in the characterization of REEs in geological matrices and which requires little or no sample preparation is Laser-Induced Breakdown Spectroscopy (LIBS). However, the determination of REEs in geological samples by LIBS presents great challenges due to higher detection limits and the strong interference of other elements in the rock matrix [110,127,128]. In addition to this, other technologies that have been used in the qualitative and quantitative identification of different REE minerals in situ include X-ray Diffractometry (XRD) [129,130], Electron Probe Micro Analyzer (EPMA) [131,132], Secondary Ion Mass Spectrometry or Ion Microprobe (SIMS) [133,134], and Scanning Electron Microprobe (SEM-EDS) [135,136,137,138].
Based on spectral calibration adjustments, LIBS can be a viable alternative for detecting low-concentration LREEs in geological matrices. Thus, Jin et al. [139] proposed a new method for detecting LREE in natural rock samples using double-pulse LIBS (DP-LIBS) integrating plasma imaging information. The authors demonstrated that this spectral calibration method overcame the limitations of traditional LIBS in the ultra-precise quantitative analysis of trace REEs (which include spectral effects) and effectively suppressed matrix effects. Limits of detection ranging from 0.57 to 9.56 mg kg−1 were obtained, and recovery values for six LREEs (La, Ce, Pr, Nd, Eu, and Sm) were in the range of 92.04 to 119.18%.
Alternatively, hyperspectral remote sensing techniques have the potential to identify REMs based on their sharp and distinct absorption characteristics in visible-near-infrared (VNIR) and shortwave-infrared (SWIR) electromagnetic spectral profiles. The advantage of these techniques is that they can cover larger areas in a limited time. It is worth noting that the accuracy of the results often requires verification using laboratory techniques such as XRD and ICP-MS [110]. Nd is one of the most abundant LREEs, which can provide distinct absorption characteristics in the VNIR region of the electromagnetic spectrum [140,141,142,143]. However, hyperspectral remote sensing techniques have shown promise in detecting other REEs (such as La, Ce, Pr, Nd, Sm, Eu, Er, and Yb) in soils in different countries such as Brazil, Canada, and China [144,145,146].
6. Conclusions
The primary sources of REEs are mineral deposits formed through long geological processes, but REEs can also be obtained from secondary sources through sustainable recycling methods. The main minerals bearing REEs that can be economically exploited are monazite, bastnasite, xenotime, and ionic adsorption clays.
China is the dominant figure both in terms of reserves and production of REEs. Other countries, such as the United States, Australia, and Myanmar, despite having smaller declared reserves than Vietnam, Brazil, Russia, and India, occupy the second, third, and fourth positions in the world production of these elements, respectively. Although Brazil has one of the world’s largest reserves of rare earths, its contribution to the global production scenario is still very small. Currently, mining projects in Brazil are concentrated in the states of Minas Gerais, Goiás, Amazonas, and Bahia.
REEs are included on the list of minerals considered to be critical for the development of a country, especially with the arrival of advanced clean and low-carbon energy technologies such as wind generators, engines for electric cars, solar panels, and super magnets. Among the elements on this list, those predicted to be the most in-demand in the coming years are Pr, Nd, Dy, and Tb.
As they carry out activities with great environmental impact, new companies that invest in the exploration and processing of rare earths have sought to implement cleaner production concepts in their processes, developing more modern facilities with the aim of recovering their waste and saving environmental resources.
A series of robust analytical techniques, along with the development of new sample preparation procedures, have facilitated the identification and quantification of REEs in multi-element geological matrices. These techniques have the main advantages of high sensitivity, selectivity, and interference resolution in the analysis of REEs in different matrices. Among the techniques that stand out most for this purpose are ICP-MS and its variants, such as HR-ICP-MS, LA-ICP-MS, and ICP-MS/MS.
Furthermore, due to the scarcity of surface terrestrial resources, exploration for hidden minerals has intensified, and new analytical techniques such as HRTEM have been explored and implemented. With its advantages in terms of being capable of analyzing nanoscale samples and enabling the determination of the morphology and crystalline phase of particles, HRTEM could certainly become an important analytical tool for characterization in prospecting activities for REEs in the future.
Author Contributions
Conceptualization, L.L.N.G.; methodology, F.F.d.M. and A.P.W.; validation, F.F.d.M. and A.P.W.; data curation, F.F.d.M., A.P.W., B.A.S.C., F.D.R., S.S.A., M.d.G.A.K. and J.B.; writing—original draft preparation, F.F.d.M. and A.P.W.; writing—review and editing, M.d.G.A.K., J.B., J.P.d.A. and L.L.N.G.; visualization, J.P.d.A.; supervision, L.L.N.G.; project administration, L.L.N.G.; funding acquisition, L.L.N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), grant number 405664/2022-2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq) and the Brazilian Company for Industrial Research and Innovation (EMBRAPII) for the scholarships granted. During the preparation of this manuscript, the authors used ChatGPT (GPT-5) for the purpose of sketching the infographic represented in Figure 3.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AAS | Atomic Absorption Spectrometry |
| D2EHPA | di-(2-ethyl hexyl) phosphoric acid |
| DP-LIBS | Double-pulse LIBS |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| EPMA | Electron Probe Micro Analyzer |
| ESG | Company’s Environmental, Social and Governance |
| HAADF-STEM | High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy |
| HREE | Heavy Rare Earth Elements |
| HR-ICP-MS | High-Resolution Inductively Coupled Plasma Mass Spectrometry |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| HRTEM-EDS/SAED | High Resolution Transmission Electron Microscopy coupled with Energy Dispersive X-Ray Spectroscopy and Selective Area Diffraction Pattern |
| IAD | Ion Adsorption-type Deposits |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectroscopy |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| ICP-MS/MS | Inductively Coupled Plasma Tandem Mass Spectrometry |
| INAA | Instrumental Neutron Activation Analysis |
| IOCG | Iron Oxide Copper-Gold |
| IoT | Internet of Things |
| IR | Infrared light |
| LA-ICP-MS | Laser Ablation Inductively Coupled Plasma Mass Spectrometry |
| LCA | Life-Cycle Analysis |
| LIBS | Laser-Induced Breakdown Spectroscopy |
| LOD | Limit of Detection |
| LREE | Light Rare Earth Elements |
| MC-ICP-MS | Multi-Collector Inductively Coupled Plasma Mass Spectrometry |
| MIP-AES | Microwave Induced Plasma Atomic Emission Spectrometry |
| NIR | Near-Infrared |
| P507 | 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester |
| PEC | Photoelectrochemical |
| PersL | Persistent Luminescence |
| REE | Rare Earth Elements |
| REM | Rare Earth Minerals |
| RE-MOFs | Rare Earth Metal–Organic Frameworks |
| REO | Rare Earth Oxides |
| SAED | Selected Area Electron Diffraction |
| SIMS | Secondary Ion Mass Spectrometry or Ion Microprobe |
| SWIR | Shortwave-Infrared |
| TBP | tributyl phosphate |
| TEM | Transmission Electron Microscopy |
| TODGA | N,N,N′,N′-tetraoctyldiglycolamide |
| UV | Ultraviolet light |
| VNIR | Visible-Near-Infrared |
| XRD | X-ray Diffractometry |
| XRF | X-ray Fluorescence |
References
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martín-Ramos, P.; Fiket, Ž.; Bhattacharya, P.; Zhu, Y. Occurrence of Uranium, Thorium and Rare Earth Elements in the Environment: A Review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Shuai, Z.; Zhu, Y.; Gao, P.; Han, Y. Rare Earth Elements Resources and Beneficiation: A Review. Miner. Eng. 2024, 218, 109011. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Geng, Y.; Ye, X.; Ma, S.; Hao, X.; Xin, W.; Chai, Y. A Review of the Main Rare Earth Ore Smelting Processes in the World: From Traditional Methods to New Technologies. J. Rare Earths, 2025; In Press. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Schreiner, B. Separation Hydrometallurgy of Rare Earth Elements; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-28233-6. [Google Scholar]
- Takehara, L.; Shintaku, I.; Rabelo, D.M.; Silveira, F.V. Avaliação do Potencial de Terras Raras no Brasil; CPRM: Brasília, Brazil, 2015.
- de Amorim, J.C.; Ferreira, A.L.O.; Mendonça, A.M.G.D.; Barros Neto, J.; da Silva, A.B.; Santos, T.O.; de Lima, P.T.B.; Nogueira, H.C.N.; de Moraes, T.F.P.; Siqueira, M.L. Mineral monazita, fonte de elementos terras raras, numa jazida importante da Paraíba. In Desvendando a Engenharia: Sua Abrangência e Multidisciplinaridade; Editora Científica Digital: São Paulo, Brazil, 2021; Volume 2, pp. 339–353. [Google Scholar]
- Ismail, N.A.; Aziz, M.A.A.; Yunus, M.Y.M.; Hisyam, A. Selection of Extractant in Rare Earth Solvent Extraction System: A Review. Int. J. Recent Technol. Eng. 2019, 8, 728–743. [Google Scholar]
- Merroune, A.; Brahim, J.A.; Berrada, M.; Essakhraoui, M.; Achiou, B.; Mazouz, H.; Beniazza, R. A Comprehensive Review on Solvent Extraction Technologies of Rare Earth Elements from Different Acidic Media: Current Challenges and Future Perspectives. J. Ind. Eng. Chem. 2024, 139, 1–17. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, H.; Huang, X.; Liu, Z.; Lin, X.; Xu, K. Enhanced Solvent Extraction of Rare Earth Elements in Ultra-High Phase Ratio with Pore-Throat Microchannels. Sep. Purif. Technol. 2025, 353, 128513. [Google Scholar] [CrossRef]
- Udawattha, D.S.; Alam, S. Predicting the Distribution Coefficient in the Solvent Extraction of Rare Earth Elements. Sep. Purif. Technol. 2025, 377, 134382. [Google Scholar] [CrossRef]
- Alves, R.C.; Nascimento, M.; Paulino, J.F.; Afonso, J.C. Selection of a Hydrometallurgical Process for Rare Earths Extraction from a Brazilian Monazite. Hydrometallurgy 2021, 200, 105556. [Google Scholar] [CrossRef]
- Belova, V.V. Development of Solvent Extraction Methods for Recovering Rare Earth Metals. Theor. Found. Chem. Eng. 2017, 51, 599–609. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent Extraction of Rare-Earth Elements with Ionic Liquids: Toward a Selective and Sustainable Extraction of These Valuable Elements. Curr. Opin. Green Sustain. Chem. 2021, 27, 100428. [Google Scholar] [CrossRef]
- Dewulf, B.; Cool, V.; Li, Z.; Binnemans, K. Effect of Polar Molecular Organic Solvents on Non-Aqueous Solvent Extraction of Rare-Earth Elements. Sep. Purif. Technol. 2022, 294, 121197. [Google Scholar] [CrossRef]
- Atanassova, M.; Kukeva, R.; Kurteva, V. New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules. Molecules 2023, 28, 7467. [Google Scholar] [CrossRef] [PubMed]
- Belfqueh, S.; Chapron, S.; Giusti, F.; Pellet-Rostaing, S.; Seron, A.; Menad, N.; Arrachart, G. Selective Recovery of Rare Earth Elements from Acetic Leachate of NdFeB Magnet by Solvent Extraction. Sep. Purif. Technol. 2024, 339, 126701. [Google Scholar] [CrossRef]
- Delić, M.; Ristić, M.; Đolić, M.; Perić-Grujić, A.; Onjia, A. Dispersive Liquid–Liquid Chelate Microextraction of Rare Earth Elements: Optimization and Greenness Evaluation. Metals 2025, 15, 52. [Google Scholar] [CrossRef]
- Salehi, H.; Maroufi, S.; Nekouei, R.K.; Sahajwalla, V. Solvent Extraction Systems for Selective Isolation of Light Rare Earth Elements with High Selectivity for Sm and La. Rare Met. 2025, 44, 2071–2084. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023.
- Zhang, H.; Jiang, J. The Evolving Impact of Renewable Energy Investment on the Rare Earth Trade Network: An Industrial Chain Perspective. Energy 2025, 332, 137195. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, H.; Sun, D.; Yan, F.; Liu, C.; Zhang, X.; Dong, L. Analysis of Slope Stability in Ion-Adsorption Rare Earth Mine Under In Situ Leaching Condition. Appl. Sci. 2025, 15, 6677. [Google Scholar] [CrossRef]
- Behrsing, T.; Blair, V.L.; Jaroschik, F.; Deacon, G.B.; Junk, P.C. Rare Earths—The Answer to Everything. Molecules 2024, 29, 688. [Google Scholar] [CrossRef]
- Keith, M.; Hayes, S.M.; Ciobanu, C.L.; Fougerouse, D.; Reich, M. Editorial: Micro-to Nano-Analytical Challenges towards Trace Element Characterization of Ore Minerals: New Perspectives and Applications for Sustainable Georesources. Front. Earth Sci. 2023, 11, 1227737. [Google Scholar] [CrossRef]
- Smith, M.P.; Moore, K.; Kavecsánszki, D.; Finch, A.A.; Kynicky, J.; Wall, F. From Mantle to Critical Zone: A Review of Large and Giant Sized Deposits of the Rare Earth Elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef]
- Martins, C.; Lima, P.C.R.; Teixeira, L.S.; Teixeira, M.P.; de Queiroz Filho, A.P. Minerais Estratégicos e Terras-Raras; Edições Câmara: Brasília, Brazil, 2014. [Google Scholar]
- Silva, C.M.; Lode, S.; Aasly, K.; Kowalczuk, P.B. Early-Stage Application of Process Mineralogy Methodologies for Mineral Tracking in Flotation of Rare Earth Elements (REE)-Bearing Minerals from a Deposit in Norway. Miner. Eng. 2023, 202, 108268. [Google Scholar] [CrossRef]
- Pathapati, S.V.S.H.; Free, M.L.; Sarswat, P.K. A Comparative Study on Recent Developments for Individual Rare Earth Elements Separation. Processes 2023, 11, 2070. [Google Scholar] [CrossRef]
- Julapong, P.; Numprasanthai, A.; Tangwattananukul, L.; Juntarasakul, O.; Srichonphaisarn, P.; Aikawa, K.; Park, I.; Ito, M.; Tabelin, C.B.; Phengsaart, T. Rare Earth Elements Recovery from Primary and Secondary Resources Using Flotation: A Systematic Review. Appl. Sci. 2023, 13, 8364. [Google Scholar] [CrossRef]
- Ralph, J. Database of Minerals, Rocks, Meteorites and Their Locations. Available online: https://www.mindat.org/ (accessed on 13 September 2023).
- Shivaramaiah, R.; Anderko, A.; Riman, R.E.; Navrotsky, A. Thermodynamics of Bastnaesite: A Major Rare Earth Ore Mineral. Am. Mineral. 2016, 101, 1129–1134. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; Shivaramaiah, R.; Kent, P.R.C.; Stack, A.G.; Navrotsky, A.; Riman, R.; Anderko, A.; Bryantsev, V.S. Crystal Structures, Surface Stability, and Water Adsorption Energies of La-Bastnäsite via Density Functional Theory and Experimental Studies. J. Phys. Chem. C 2016, 120, 16767–16781. [Google Scholar] [CrossRef]
- da Silva, Y.J.A.B.; do Nascimento, C.W.A.; da Silva, Y.J.A.B.; Biondi, C.M.; Silva, C.M.C.A.C. Rare Earth Element Concentrations in Brazilian Benchmark Soils. Rev. Bras. Cienc. Solo 2016, 40, e0150413. [Google Scholar] [CrossRef]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global Rare Earth Elements Projects: New Developments and Supply Chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Liu, X.; Tournassat, C.; Grangeon, S.; Kalinichev, A.G.; Takahashi, Y.; Marques Fernandes, M. Molecular-Level Understanding of Metal Ion Retention in Clay-Rich Materials. Nat. Rev. Earth Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.-F. The Role of Clay Minerals in Formation of the Regolith-Hosted Heavy Rare Earth Element Deposits. Am. Mineral. 2020, 105, 92–108. [Google Scholar] [CrossRef]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of Rare Earth Elements in Regolith-Hosted Clay Deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Ran, L.; Zhu, R.; Ling, B.; Liang, X.; Kang, S.; Wang, Y.; Wei, J.; Ma, L.; et al. A Green and Efficient Technology to Recover Rare Earth Elements from Weathering Crusts. Nat. Sustain. 2022, 6, 81–92. [Google Scholar] [CrossRef]
- Nassar, N.T.; Lederer, G.W.; Padilla, A.J.; Gambogi, J.; Cordier, D.J.; Brainard, J.L.; Lessard, J.D.; Charab, R. Rock-to-Metal Ratios of the Rare Earth Elements. J. Clean. Prod. 2023, 405, 136958. [Google Scholar] [CrossRef]
- Shanthi Bhavan, J.; Joy, J.; Pazhani, A. Identification and Recovery of Rare Earth Elements from Electronic Waste: Material Characterization and Recovery Strategies. Mater. Today Commun. 2023, 36, 106921. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Virolainen, S.; Massima Mouele, E.S.; Laatikainen, M.; Repo, E.; Laatikainen, K. The Recent Progress of Ion Exchange for the Separation of Rare Earths from Secondary Resources—A Review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Sumaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025.
- Sousa Filho, P.; Galaço, A.; Serra, O. Rare Earths: Periodic Table, Discovery, Exploration in Brazil and Applications. Quim Nova 2019, 42, 1208–1224. [Google Scholar] [CrossRef]
- Brasil Brasil Pode Se Tornar Um dos Cinco Maiores Produtores de Terras Raras do Mundo Nos Próximos Anos. Available online: https://www.gov.br/mme/pt-br/assuntos/noticias/brasil-pode-se-tornar-um-dos-cinco-maiores-produtores-de-terras-raras-do-mundo-nos-proximos-anos (accessed on 3 September 2023).
- Chen, P.; Ilton, E.S.; Wang, Z.; Rosso, K.M.; Zhang, X. Global Rare Earth Element Resources: A Concise Review. Appl. Geochem. 2024, 175, 106158. [Google Scholar] [CrossRef]
- Takehara, L.; Silveira, F.V.; Santos, R.V. Potentiality of Rare Earth Elements in Brazil. In Rare Earths Industry: Technological, Economic, and Environmental Implications; Elsevier Inc.: New York, NY, USA, 2015; pp. 57–72. ISBN 9780128023280. [Google Scholar] [CrossRef]
- Brasil. Sumário Mineral 2017; ANM: Brasília, DF, Brazil, 2019; 201p.
- Brasil. Sumário Mineral 2013; Departamento Nacional de Produção Mineral: Brasília, Brazil, 2013; Volume 33.
- Santos, J.E.S. Rare Earth Mining in Brazil: Building a Better Future. Rev. DCS 2025, 22, 1–23. [Google Scholar] [CrossRef]
- Zimmermann, T.; Gößling-Reisemann, S. Critical Materials and Dissipative Losses: A Screening Study. Sci. Total Environ. 2013, 461–462, 774–780. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Du, X.; Graedel, T.E. Uncovering the Global Life Cycles of the Rare Earth Elements. Sci. Rep. 2011, 1, 145. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Gupta, A.; Williams, E.; Gaustad, G. Forecasting Revenue from Primary and Secondary Sources of Rare Earth Elements. Resour. Conserv. Recycl. 2024, 207, 107612. [Google Scholar] [CrossRef]
- Fujita, Y.; McCall, S.K.; Ginosar, D. Recycling Rare Earths: Perspectives and Recent Advances. MRS Bull. 2022, 47, 283–288. [Google Scholar] [CrossRef]
- Prados, T.M.; Barroso, T.L.C.T.; Forster-Carneiro, T.; Lovón-Canchumani, G.A.; Colpini, L.M.S. Life Cycle Assessment and Circular Economy in the Production of Rare Earth Magnets: An Updated and Comprehensive Review. Clean Technol. Environ. Policy 2025, 27, 471–494. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. Assessing Rare Earth Element Mineral Deposit Types and Links to Environmental Impacts. Appl. Earth Sci. 2013, 122, 83–96. [Google Scholar] [CrossRef]
- Nassar, N.T.; Fortier, S.M. Methodology and Technical Input for the 2021 Review and Revision of the U.S. Critical Minerals List; U.S. Geological Survey: Reston, VA, USA, 2021.
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Yan, C.; Huang, X. Review on Progress of Rare Earth Science and Technology in 2024. J. Rare Earths 2025, 43, 2029–2052. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A. A Comparative State-of-Technology Review and Future Directions for Rare Earth Element Separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- Nemova, G. Brief Review of Recent Developments in Fiber Lasers. Appl. Sci. 2024, 14, 2323. [Google Scholar] [CrossRef]
- Tongyu, G.; Yongxi, Z.; Zhao, Q.; Chenger, W.; Mobin, D.; Xia, Z.; Xiaoxia, C.; Yuanhong, L.; Ronghui, L. Development Status of Rare Earth Luminescence Material. J. Alloys Compd. 2025, 1037, 182399. [Google Scholar] [CrossRef]
- Zhu, Z.; Ayoub, I.; He, J.; Yang, J.; Zhang, H.; Zhu, Z.; Hao, Q.; Cai, Z.; Ola, O.; Tiwari, S.K. Magnesium Alloys with Rare-Earth Elements: Research Trends Applications, and Future Prospect. J. Magnes. Alloys 2025, 13, 3524–3563. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, H.; Tong, X.; Zhang, J.; Wang, Y.; Xu, S.; Li, X.; Chen, B. Blue Light-Excited Zero-Thermal-Quenching near-Infrared Garnet CaY2Sc2Al2SiO12:Cr3+/Yb3+ Phosphors for Pc-LED Application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 343, 126581. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Q.; Li, K.; Feng, Y.; Fu, Y.; Li, Y.; Zhang, Y.; Qian, X.; Wei, B.; Du, P. Regulating the Broadband Near-Infrared Emission of Eu2+-Doped Ca3Sc2Si3O12 Phosphors through Constructing Defect for Diversified Applications. Chem. Eng. J. 2024, 496, 154360. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Kiong, T.S.; Ismail, A.F. Pioneering Sustainable Energy Solutions with Rare-Earth Nanomaterials: Exploring Pathways for Energy Conversion and Storage. Int. J. Hydrogen Energy 2024, 93, 607–649. [Google Scholar] [CrossRef]
- Rezende, T.K.L.; Carneiro, J.A.; Barbosa, H.P.; Sardela Junior, M.R.; Ferrari, J.L. Recent Advances in Rare Earth Oxides Applied to Energy Conversion: A Short Review. Ceram. Int. 2025, 51, 5583–5596. [Google Scholar] [CrossRef]
- Ansari, A.A.; Nazeeruddin, M.K.; Tavakoli, M.M. Organic-Inorganic Upconversion Nanoparticles Hybrid in Dye-Sensitized Solar Cells. Coord. Chem. Rev. 2021, 436, 213805. [Google Scholar] [CrossRef]
- Peelman, S.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Leaching of Rare Earth Elements: Review of Past and Present Technologies. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–334. [Google Scholar] [CrossRef]
- Shayan, M.E.; Najafi, G.; Ghobadian, B.; Gorjian, S.; Mazlan, M.; Samami, M.; Shabanzadeh, A. Flexible Photovoltaic System on Non-Conventional Surfaces: A Techno-Economic Analysis. Sustainability 2022, 14, 3566. [Google Scholar] [CrossRef]
- Depraiter, L.; Goutte, S. The Role and Challenges of Rare Earths in the Energy Transition. Resour. Policy 2023, 86, 104137. [Google Scholar] [CrossRef]
- Veeramani, K.; Janani, G.; Kim, J.; Surendran, S.; Lim, J.; Jesudass, S.C.; Mahadik, S.; Lee, H.; Kim, T.; Kim, J.K.; et al. Hydrogen and Value-Added Products Yield from Hybrid Water Electrolysis: A Critical Review on Recent Developments. Renew. Sustain. Energy Rev. 2023, 177, 113227. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; Anjos, J.P.; Silva, L.A.; Santos, A.A.; Calixto, E.E.S.; Pessoa, F.L.P.; Almeida, J.L.G.; Andrade Filho, M.; Marinho, F.S.; Rocha, G.O.; et al. Technological Perspectives and Economic Aspects of Green Hydrogen in the Energetic Transition: Challenges for Chemistry. J. Braz. Chem. Soc. 2022, 33, 844–869. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Kiong, T.S.; Ismail, A.F. Revolutionizing Water Splitting: The Role of Light Rare Earth Elements (LREEs) in Photoelectrochemical and Electrochemical Advances. Coord. Chem. Rev. 2025, 543, 216917. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Kiong, T.S.; Ismail, A.F. Leveraging Heavy Rare Earth Elements (H-REEs) in Electrodes to Boost the Efficiency of Photoelectrochemical and Electrochemical Water Splitting. Int. J. Hydrogen Energy 2025, 177, 151569. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Q.; Cheng, H.; Li, X.; Yu, N.; Pan, M.; Yu, Y.; Fang, J.; Hu, X.; Ge, H.; et al. Review of the Research on Oxides in Low-Temperature Magnetic Refrigeration. J. Eur. Ceram. Soc. 2023, 43, 6665–6680. [Google Scholar] [CrossRef]
- Zarkevich, N.A.; Johnson, D.D.; Pecharsky, V.K. High-Throughput Search for Caloric Materials: The CaloriCool Approach. J. Phys. D Appl. Phys. 2018, 51, 024002. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Rabelo, R.; Carrasco-Berlanga, A.; Moliner, N.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G.; Ruiz-García, R.; Mayans, J.; et al. Field-Induced Slow Magnetic Relaxation and Magnetocaloric Effects in an Oxalato-Bridged Gadolinium(iii)-Based 2D MOF. Dalton Trans. 2021, 50, 3801–3805. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, J.; Yao, S.; Zhang, R.; Wang, J.; Cai, D.; Luo, T.; Peng, Y.; Liu, S.; Wen, H. Gd(iii)-Based Inorganic Polymers, Metal–Organic Frameworks and Coordination Polymers for Magnetic Refrigeration. CrystEngComm 2022, 24, 2370–2382. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Cao, C.; Zheng, T.; Wen, H.; Liu, S. Highly Stable Rare Earth Metal-Organic Frameworks for Proton Conduction and Magnetic Refrigeration. J. Mol. Struct. 2025, 1320, 139645. [Google Scholar] [CrossRef]
- Zhang, F.; Batuk, M.; Hadermann, J.; Manfredi, G.; Mariën, A.; Vanmeensel, K.; Inokoshi, M.; Van Meerbeek, B.; Naert, I.; Vleugels, J. Effect of Cation Dopant Radius on the Hydrothermal Stability of Tetragonal Zirconia: Grain Boundary Segregation and Oxygen Vacancy Annihilation. Acta Mater. 2016, 106, 48–58. [Google Scholar] [CrossRef]
- Kalaivani, S.; Ezhilan, M.; Deepa, M.; Kannan, S. Rare Earth Doped Zirconia: Structure, Physicochemical Properties and Recent Advancements in Technological Applications. Prog. Solid State Chem. 2025, 79, 100524. [Google Scholar] [CrossRef]
- Srigurunathan, K.; Meenambal, R.; Guleria, A.; Kumar, D.; Ferreira, J.M.F.; Kannan, S. Unveiling the Effects of Rare-Earth Substitutions on the Structure, Mechanical, Optical, and Imaging Features of ZrO2 for Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 1725–1743. [Google Scholar] [CrossRef]
- Thiel, C.W.; Böttger, T.; Cone, R.L. Rare-Earth-Doped Materials for Applications in Quantum Information Storage and Signal Processing. J. Lumin. 2011, 131, 353–361. [Google Scholar] [CrossRef]
- Macfarlane, R.M. High-Resolution Laser Spectroscopy of Rare-Earth Doped Insulators: A Personal Perspective. J. Lumin. 2002, 100, 1–20. [Google Scholar] [CrossRef]
- Zhong, T.; Goldner, P. Emerging Rare-Earth Doped Material Platforms for Quantum Nanophotonics. Nanophotonics 2019, 8, 2003–2015. [Google Scholar] [CrossRef]
- Kunkel, N.; Goldner, P. Recent Advances in Rare Earth Doped Inorganic Crystalline Materials for Quantum Information Processing. Z Anorg. Allg. Chem. 2018, 644, 66–76. [Google Scholar] [CrossRef]
- Bartholomew, J.G.; Lima, K.O.; Ferrier, A.; Kinos, A.; Karlsson, J.; Rippe, L.; Walther, A.; Scheblykin, I.; Kröll, S.; Goldner, P. High-Resolution Spectroscopic Techniques for Studying Rare-Earth Ions in Nanoparticles. J. Lumin. 2023, 257, 119743. [Google Scholar] [CrossRef]
- Ruskuc, A. Single Rare-Earth Ions in Solid-State Hosts: A Platform for Quantum Networks; California Institute of Technology (Caltech): Pasadena, CA, USA, 2024. [Google Scholar]
- Ruskuc, A.; Wu, C.-J.; Green, E.; Hermans, S.L.N.; Pajak, W.; Choi, J.; Faraon, A. Multiplexed Entanglement of Multi-Emitter Quantum Network Nodes. Nature 2025, 639, 54–59. [Google Scholar] [CrossRef]
- Hermans, S.; Green, E.; Liu, E.; Riera Moral, A.; Ruskuc, A.; Wu, C.J.; Faraon, A. Single Rare-Earth Ions Coupled to a Dense Nuclear Spin Bath: A Challenge and a Resource. In Proceedings of the Quantum Nanophotonic Materials, Devices, and Systems, San Diego, CA, USA, 18–23 August 2024; Aharonovich, I., Soci, C., Sheldon, M.T., Eds.; SPIE: Bellingham, DC, USA, 2024; p. 3. [Google Scholar] [CrossRef]
- CRIRSCO. Exploration Targets, Exploration Results, Mineral Resources and Mineral Reserves; ICMM: London, UK, 2019; 78p, Available online: https://crirsco.com/wp-content/uploads/2023/10/The-CRIRSCO-International-Reporting-Template.pdf (accessed on 1 August 2025).
- Zhu, D.; Chen, T.; Zhen, N.; Niu, R. Monitoring the Effects of Open-Pit Mining on the Eco-Environment Using a Moving Window-Based Remote Sensing Ecological Index. Environ. Sci. Pollut. Res. 2020, 27, 15716–15728. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the Rare Earth Elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Yun, Y.; Stopic, S.; Friedrich, B. Valorization of Rare Earth Elements from a Steenstrupine Concentrate Via a Combined Hydrometallurgical and Pyrometallurgical Method. Minerals 2020, 10, 248. [Google Scholar] [CrossRef]
- China Northern Rare Earth Group High-Tech Co., Ltd. About China Northern. Available online: https://www.reht.com/ (accessed on 22 October 2023).
- International Organization for Standardization ISO 9000 Family–Quality Management. Available online: https://www.iso.org/standards/popular/iso-9000-family (accessed on 8 October 2025).
- International Organization for Standardization ISO 14000 Family–Environmental Management. Available online: https://www.iso.org/standards/popular/iso-14000-family (accessed on 8 October 2025).
- China Rare Earth Resources and Technology Co., Ltd. About China Rare Earth. Available online: http://www.cmreltd.com/index.html (accessed on 5 November 2023).
- MP Materials What We Do. Available online: https://mpmaterials.com/ (accessed on 2 September 2023).
- Energy Fuels New Releases. Available online: https://www.energyfuels.com (accessed on 2 September 2023).
- NioCorp Critical Mineral Security New. Available online: https://www.niocorp.com/home/ (accessed on 10 October 2023).
- Lynas Rare Earths About Lynas. Available online: https://lynasrareearths.com/ (accessed on 10 October 2023).
- Iluka Resources Ltd. About Iluka. Available online: https://iluka.com/ (accessed on 12 October 2023).
- Arafura. Available online: https://www.arultd.com/investor/asx-announcements/asx-announcements-2023/ (accessed on 1 December 2024).
- Awng, M.H. Myanmar’s Environment Hit by Rare Earth Mining Boom. Available online: https://earthjournalism.net/stories/myanmars-environment-hit-by-rare-earth-mining-boom (accessed on 7 November 2023).
- Lv, A.; Patton, D. Myanmar Rare Earth Mines Still Awaiting Notice to Restart–Sources. Available online: https://www.reuters.com/article/myanmar-rareearths-mining-idUSKBN30L0MU (accessed on 7 November 2023).
- Aclara | Environmentally Friendly Rare Earths. Available online: https://www.aclara-re.com/ (accessed on 1 December 2024).
- Balaram, V. Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements. Minerals 2023, 13, 1031. [Google Scholar] [CrossRef]
- Gatiboni, T.L.; Iop, G.D.; Diehl, L.O.; Flores, E.M.M.; Muller, E.I.; Mello, P.A. An Ultrasound-assisted Sample Preparation Method of Carbonatite Rock for Determination of Rare Earth Elements by Inductively Coupled Plasma Mass Spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8732. [Google Scholar] [CrossRef]
- DCTech Laboratory Technologies Critérios para Seleção da Melhor Técnica de Absorção Atômica para Detecção de Metais. Available online: https://www.dctech.com.br/criterios-para-selecao-da-melhor-tecnica-de-absorcao-atomica-para-deteccao-de-metais (accessed on 28 August 2023).
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Pinto, F.G.; Junior, R.E.; Saint’Pierre, T.D. Sample Preparation for Determination of Rare Earth Elements in Geological Samples by ICP-MS: A Critical Review. Anal. Lett. 2012, 45, 1537–1556. [Google Scholar] [CrossRef]
- Whitty-Léveillé, L.; Turgeon, K.; Bazin, C.; Larivière, D. A Comparative Study of Sample Dissolution Techniques and Plasma-Based Instruments for the Precise and Accurate Quantification of REEs in Mineral Matrices. Anal. Chim. Acta 2017, 961, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zawisza, B.; Pytlakowska, K.; Feist, B.; Polowniak, M.; Kita, A.; Sitko, R. Determination of Rare Earth Elements by Spectroscopic Techniques: A Review. J. Anal. At. Spectrom. 2011, 26, 2373. [Google Scholar] [CrossRef]
- Carvalho, L.; Reis, A.T.; Soares, E.; Tavares, C.; Monteiro, R.J.R.; Figueira, P.; Henriques, B.; Vale, C.; Pereira, E. A Single Digestion Procedure for Determination of Major, Trace, and Rare Earth Elements in Sediments. Water Air Soil Pollut. 2020, 231, 541. [Google Scholar] [CrossRef]
- Bispo, F.H.A.; de Menezes, M.D.; Fontana, A.; Sarkis, J.E.S.; Gonçalves, C.M.; de Carvalho, T.S.; Curi, N.; Guilherme, L.R.G. Rare Earth Elements (REEs): Geochemical Patterns and Contamination Aspects in Brazilian Benchmark Soils. Environ. Pollut. 2021, 289, 117972. [Google Scholar] [CrossRef]
- Rieger, P.; Magnall, J.M.; Gleeson, S.A.; Oelze, M. Pyrite Chemistry Records a Multistage Ore Forming System at the Proterozoic George Fisher Massive Sulfide Zn-Pb-Ag Deposit, Mount Isa, Australia. Front. Earth Sci. 2023, 11, 892759. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; Yan, H.; Yi, J. TEM Observations of Particles Based on Sampling in Gas and Soil at the Dongshengmiao Polymetallic Pyrite Deposit, Inner Mongolia, Northern China. J. Geochem. Explor. 2015, 158, 95–111. [Google Scholar] [CrossRef]
- Lu, M.; Cao, J.; Wang, Z.; Wang, G. Characteristics of Naturally Formed Nanoparticles in Various Media and Their Prospecting Significance in Chaihulanzi Deposit. Minerals 2022, 12, 1289. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; de Almeida, E. X-Ray Fluorescence Spectrometry for Environmental Analysis: Basic Principles, Instrumentation, Applications and Recent Trends. Chemosphere 2022, 303, 135006. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzhanov, T.F.; Pashkova, G.V.; Chubarov, V.M.; Labutin, T.A.; Popov, A.M. Three Calibration Techniques Combined with Sample-Effective Design of Experiment Based on Latin Hypercube Sampling for Direct Detection of Lanthanides in REE-Rich Ores Using TXRF and WDXRF. J. Anal. At. Spectrom. 2021, 36, 224–232. [Google Scholar] [CrossRef]
- Schramm, R. Use of X-Ray Fluorescence Analysis for the Determination of Rare Earth Elements. Phys. Sci. Rev. 2016, 1, 20160061. [Google Scholar] [CrossRef]
- El-Taher, A. Rare Earth Elements Content in Geological Samples from Eastern Desert, Egypt, Determined by Instrumental Neutron Activation Analysis. Appl. Radiat. Isot. 2010, 68, 1859–1863. [Google Scholar] [CrossRef]
- Adeti, P.J.; Amoako, G.; Tandoh, J.B.; Gyampo, O.; Ahiamadjie, H.; Amable, A.S.K.; Kansaana, C.; Annan, R.A.T.; Bamford, A. Rare-Earth Element Comparative Analysis in Chosen Geological Samples Using Nuclear-Related Analytical Techniques. Nucl. Instrum. Methods Phys. Res. B 2023, 540, 122–128. [Google Scholar] [CrossRef]
- Afgan, M.S.; Hou, Z.; Song, W.; Liu, J.; Song, Y.; Gu, W.; Wang, Z. On the Spectral Identification and Wavelength Dependence of Rare-Earth Ore Emission by Laser-Induced Breakdown Spectroscopy. Chemosensors 2022, 10, 350. [Google Scholar] [CrossRef]
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Ros, V.M. Imaging Rare-Earth Elements in Minerals by Laser-Induced Plasma Spectroscopy: Molecular Emission and Plasma-Induced Luminescence. Spectrochim. Acta Part B Spectrosc. 2019, 151, 12–19. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J.A.; Torró, L.; Aiglsperger, T.; Domènech, C.; Domínguez-Carretero, D.; Llovet, X.; Suñer, P.; Ramírez, A.; Rodríguez, J. REE Ultra-Rich Karst Bauxite Deposits in the Pedernales Peninsula, Dominican Republic: Mineralogy of REE Phosphates and Carbonates. Ore Geol. Rev. 2023, 157, 105422. [Google Scholar] [CrossRef]
- Jo, J.; Shin, D. Geochemical Characteristics of REE-Enriched Weathered Anorthosite Complex in Hadong District, South Korea. Geochem. J. 2023, 57, 13–27. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Hidaka, H.; Nishio, Y.; Amakawa, H.; Nozaki, Y. Ion-Microprobe Analysis of Rare Earth Elements in Oceanic Basalt Glass. Anal. Sci. 1999, 15, 743–748. [Google Scholar] [CrossRef]
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Ling, X.X.; Li, Q.L.; Liu, Y.; Yang, Y.H.; Liu, Y.; Tang, G.Q.; Li, X.H. In Situ SIMS Th–Pb Dating of Bastnaesite: Constraint on the Mineralization Time of the Himalayan Mianning–Dechang Rare Earth Element Deposits. J. Anal. At. Spectrom. 2016, 31, 1680–1687. [Google Scholar] [CrossRef]
- Shi, L.; Sano, Y.; Takahata, N.; Koike, M.; Morita, T.; Koyama, Y.; Kagoshima, T.; Li, Y.; Xu, S.; Liu, C. NanoSIMS Analysis of Rare Earth Elements in Silicate Glass and Zircon: Implications for Partition Coefficients. Front. Chem. 2022, 10, 844953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiao, W.; Chen, D.; Wu, P.; Xie, Y.; Chen, X. Anomalous Concentrations of Rare Earth Elements in Acid Mine Drainage and Implications for Rare Earth Resources from Late Permian Coal Seams in Northern Guizhou. Sci. Total Environ. 2023, 879, 163051. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, L.; Wen, Z.; Nie, T.; Zhang, N.; Zhou, C. The Mechanism Study on the Integrated Process of NaOH Treatment and Citric Acid Leaching for Rare Earth Elements Recovery from Coal Fly Ash. J. Environ. Chem. Eng. 2023, 11, 109921. [Google Scholar] [CrossRef]
- Palozzi, J.; Bailey, J.G.; Tran, Q.A.; Stanger, R. A Characterization of Rare Earth Elements in Coal Ash Generated during the Utilization of Australian Coals. Int. J. Coal Prep. Util. 2023, 43, 2106–2135. [Google Scholar] [CrossRef]
- Sarker, S.K.; Bruckard, W.; Haque, N.; Roychand, R.; Bhuiyan, M.; Pramanik, B.K. Characterization of a Carbonatite-Derived Mining Tailing for the Assessment of Rare Earth Potential. Process Saf. Environ. Prot. 2023, 173, 154–162. [Google Scholar] [CrossRef]
- Jin, X.; Zhan, Y.; Xu, Z.; Fang, J.; Zhou, N.; Qu, D.; Yang, G. Plasma Image-Calibrated Double-Pulse Laser-Induced Breakdown Spectroscopy for High-Precision Quantification of Light Rare Earth Elements in Geological Matrix. Anal. Chem. 2025, 97, 21125–21133. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, S.D. Detection and Relative Quantification of Neodymium in Sillai Patti Carbonatite Using Decision Tree Classification of the Hyperspectral Data. Sensors 2022, 22, 7537. [Google Scholar] [CrossRef]
- Boesche, N.; Rogass, C.; Lubitz, C.; Brell, M.; Herrmann, S.; Mielke, C.; Tonn, S.; Appelt, O.; Altenberger, U.; Kaufmann, H. Hyperspectral REE (Rare Earth Element) Mapping of Outcrops—Applications for Neodymium Detection. Remote. Sens. 2015, 7, 5160–5186. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Tangestani, M.H. Potential of Sentinel-2 MSI Data in Targeting Rare Earth Element (Nd3+) Bearing Minerals in Esfordi Phosphate Deposit, Iran. Egypt. J. Remote Sens. Space Sci. 2022, 25, 697–710. [Google Scholar] [CrossRef]
- Booysen, R.; Jackisch, R.; Lorenz, S.; Zimmermann, R.; Kirsch, M.; Nex, P.A.M.; Gloaguen, R. Detection of REEs with Lightweight UAV-Based Hyperspectral Imaging. Sci. Rep. 2020, 10, 17450. [Google Scholar] [CrossRef]
- Turner, D.J.; Rivard, B.; Groat, L.A. Visible and Short-Wave Infrared Reflectance Spectroscopy of Selected REE-Bearing Silicate Minerals. Am. Mineral. 2018, 103, 927–943. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Pan, X. Potential of Visible and Near-Infrared Reflectance Spectroscopy for the Determination of Rare Earth Elements in Soil. Geoderma 2017, 306, 120–126. [Google Scholar] [CrossRef]
- Maia, A.J.; da Silva, Y.J.A.B.; do Nascimento, C.W.A.; Veras, G.; Escobar, M.E.O.; Cunha, C.S.M.; da Silva, Y.J.A.B.; Nascimento, R.C.; Pereira, L.H.S. Near-Infrared Spectroscopy for the Prediction of Rare Earth Elements in Soils from the Largest Uranium-Phosphate Deposit in Brazil Using PLS, IPLS, and ISPA-PLS Models. Environ. Monit. Assess. 2020, 192, 675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).