1. Introduction
Drinking water disinfection by chlorination or chloramination is indispensable for protecting public health against microbial pathogens. However, these processes inevitably lead to the formation of disinfection by-products (DBPs). Among the DBPs currently regulated, such as trihalomethanes (THMs) and haloacetic acids (HAAs), both toxicity and long-term health effects have been thoroughly reported [
1,
2,
3]. In recent years, an additional group of aromatic DBPs—halobenzoquinones (HBQs)—has received growing attention. Despite their high toxicity, HBQs remain unregulated in drinking water standards [
1,
2,
3]. Their recognition as emerging contaminants of concern stems from strong evidence of cytotoxic, genotoxic, and potentially carcinogenic properties, often at higher levels than many regulated DBPs [
3,
4].
Among HBQs, 2,6-dichloro-1,4-benzoquinone (DCBQ) is the most frequently detected compound in drinking water. Global surveys have reported concentrations ranging from below detection limits to as high as 275 ng/L [
5]. For example, levels up to 165.1 ng/L were observed in chlorinated tap water [
6]. In North America, analyses from nine water purification plants (WPPs) revealed concentrations between 4.5 and 274.5 ng/L [
1], while in Japan, DCBQ was detected in drinking water from 12 WPPs at levels of 8–51 ng/L [
7]. These findings demonstrate that DCBQ is widely distributed and represents a contaminant of international concern.
The formation of HBQs has been linked to both natural organic matter (NOM) and anthropogenic compounds. Experimental chlorination studies confirmed that natural waters contain substances capable of generating HBQs [
8,
9]. Although the specific chemical identity of these precursors has not been fully established, several candidates have been suggested: phenol, chlorophenols, para-substituted phenolic compounds, para-substituted alkyl- and carboxyl-substituted aromatics, and aromatic amines such as aniline and N-methylaniline [
10]. These results indicate that HBQs, and specifically DCBQ, can be formed from NOM containing para-substituted aromatic structures, even in the absence of phenol itself [
10]. In addition to natural sources, anthropogenic contributions are significant. For instance, personal care products (PCPs) like body lotions and sunscreens have been shown to yield high DCBQ levels after chlorination, with reported concentrations up to 5420 ng/L [
11]. This dual origin highlights the persistence and ubiquity of DCBQ in drinking water systems.
From a toxicological perspective, HBQs exhibit markedly higher chronic cytotoxicity compared to regulated DBPs. Their inhibitory concentration (IC
50) values demonstrate significantly greater potency than THMs and HAAs [
3,
4]. Moreover, experimental evidence supports their potential genotoxic and carcinogenic effects, raising critical concerns for human health. Given their frequent detection and toxicity, the need for effective treatment strategies to minimize HBQs, and particularly DCBQ, in drinking water is evident. To this end, several treatment technologies have been explored. Processes such as pre-ozonation, granular activated carbon (GAC), and quartz sand filtration have shown varying degrees of success in mitigating DCBQ levels [
12]. Ozonation has been reported to be effective for the removal of DCBQ precursors [
13], while integrated processes such as O
3/GAC achieved removal efficiencies up to 85.37% under optimized conditions [
14]. However, treatment by ozone or GAC alone resulted in much lower removal rates (29.64% and 32.81%, respectively) [
14]. Similarly, advanced oxidation processes (AOPs) employing UV, H
2O
2, or O
3 individually achieved limited removal (approximately 36.1%), whereas the combined UV/H
2O
2/O
3 system demonstrated much higher efficiencies, reaching up to 94.3% [
15,
16]. These results highlight that while DCBQ can be partially controlled through conventional and advanced treatment processes, removal remains challenging and is highly dependent on treatment configuration and operational conditions.
Despite these advances, current understanding of DCBQ degradation during AOPs remains incomplete. In particular, the UV/H
2O
2 process has been recognized as a promising technology for the degradation of recalcitrant organic contaminants due to its ability to generate hydroxyl radicals, highly reactive and non-selective oxidants capable of breaking down aromatic structures [
17,
18]. However, most studies to date have focused on removal efficiencies under specific conditions, without providing a comprehensive kinetic framework for DCBQ degradation. Furthermore, the effects of UV and UV/H
2O
2 treatments on water quality indicators such as color, aromaticity, dissolved oxygen, and turbidity have not been systematically assessed. This lack of integrated evaluation hinders the ability to predict treatment performance and to optimize operational conditions for full-scale applications [
19,
20,
21].
Therefore, the present study aims to address these knowledge gaps by (i) investigating the degradation behavior of DCBQ under UV and UV/H2O2 treatments, (ii) developing a generalized kinetic model capable of describing DCBQ oxidation under varying oxidant concentrations, and (iii) evaluating the implications of these processes on key water quality parameters. By providing both kinetic insights and practical evaluation, this work advances the understanding of HBQ control strategies and demonstrates the potential of UV/H2O2 as an effective advanced oxidation process for mitigating chlorinated benzoquinones in drinking water treatment.
2. Materials and Methods
Experiments were carried out in a photocatalytic reactor (
Figure 1) equipped with a 150 W medium-pressure mercury lamp (Heraeus, Hanau, Germany) and a magnetic stirrer. The reactor was filled with 1.0 L of an aqueous solution of 2,6-dichloro-1,4-benzoquinone (DCBQ, 98%, Sigma-Aldrich, Darmstadt, Germany; C
6H
2Cl
2O
2) at an initial concentration of 50.0 mg/L. This concentration was selected to provide sufficient analytical resolution and reproducibility in the kinetic measurements, ensuring that the reaction could be monitored with minimal signal-to-noise interference. Although it is higher than typical environmental levels, the purpose of this study was not to replicate a specific real-life scenario but to generate fundamental insights into the mechanistic and operational aspects of the process under controlled laboratory conditions. Next, the pH of the solution was adjusted to 3.0 using 0.1 M hydrochloric acid (Panreac, Castellar del Vallès, Spain; HCl, 37%
v/
v) and monitored with a Kent EIL9142 pH meter (Cambridge, UK). All experiments were conducted at pH 3.0, which was selected to ensure experimental stability and reproducibility and to minimize interferences from side reactions. Acidic conditions are commonly used in laboratory kinetic studies of UV/H
2O
2 systems, as they allow clearer interpretation of the degradation mechanism [
22]. Continuous stirring at 500 rpm was applied, and the reactor temperature was maintained at 25 °C by means of a recirculating cooling bath (Frigiterm-P, Selecta, Barcelona, Spain). Before irradiation, baseline measurements of DCBQ concentration, color, turbidity, and aromaticity were taken.
The required dose of hydrogen peroxide ([H2O2]0 = 0–10.0 mM; Panreac, Castellar del Vallès, Spain; H2O2, 30% w/v) was then introduced, and the UV lamp was turned on at the same time; this point was designated as t = 0. Photocatalytic runs were conducted for 120 min, during which sequential water quality parameters were monitored in real time. Aromaticity and color were determined at 254 nm and 455 nm, respectively, with a UV/Vis spectrophotometer (V-630, Jasco, Madrid, Spain). Dissolved oxygen (DO, mg/L) was measured using a dissolved oxygen meter (HI 9142, Hanna Instruments S.L., Eibar, Spain).
DCBQ concentrations were quantified by HPLC at 254 nm and 25 °C by high-performance liquid chromatography (Waters 2695) equipped with a dual wavelength absorbance detector (Model 2487, Waters Cromatografía S.A., Sardañola del Vallés, Spain). Separation was achieved using a flow rate of 0.8 mL·min−1 with a Zorbax Eclipse PAH column (150 mm) and a corresponding guard column of 12.5 mm (Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of water (A) and acetonitrile (B) by gradient: 95%A/5%B (0 min), 90%A/10%B (3 min), 10%A/90%B (5 min), 100%B (10 min), returning to 95%A/5%B at 12 min.
3. Results and Discussion
3.1. Kinetic Model for 2,6-Dichlorobenzoquinone Oxidation
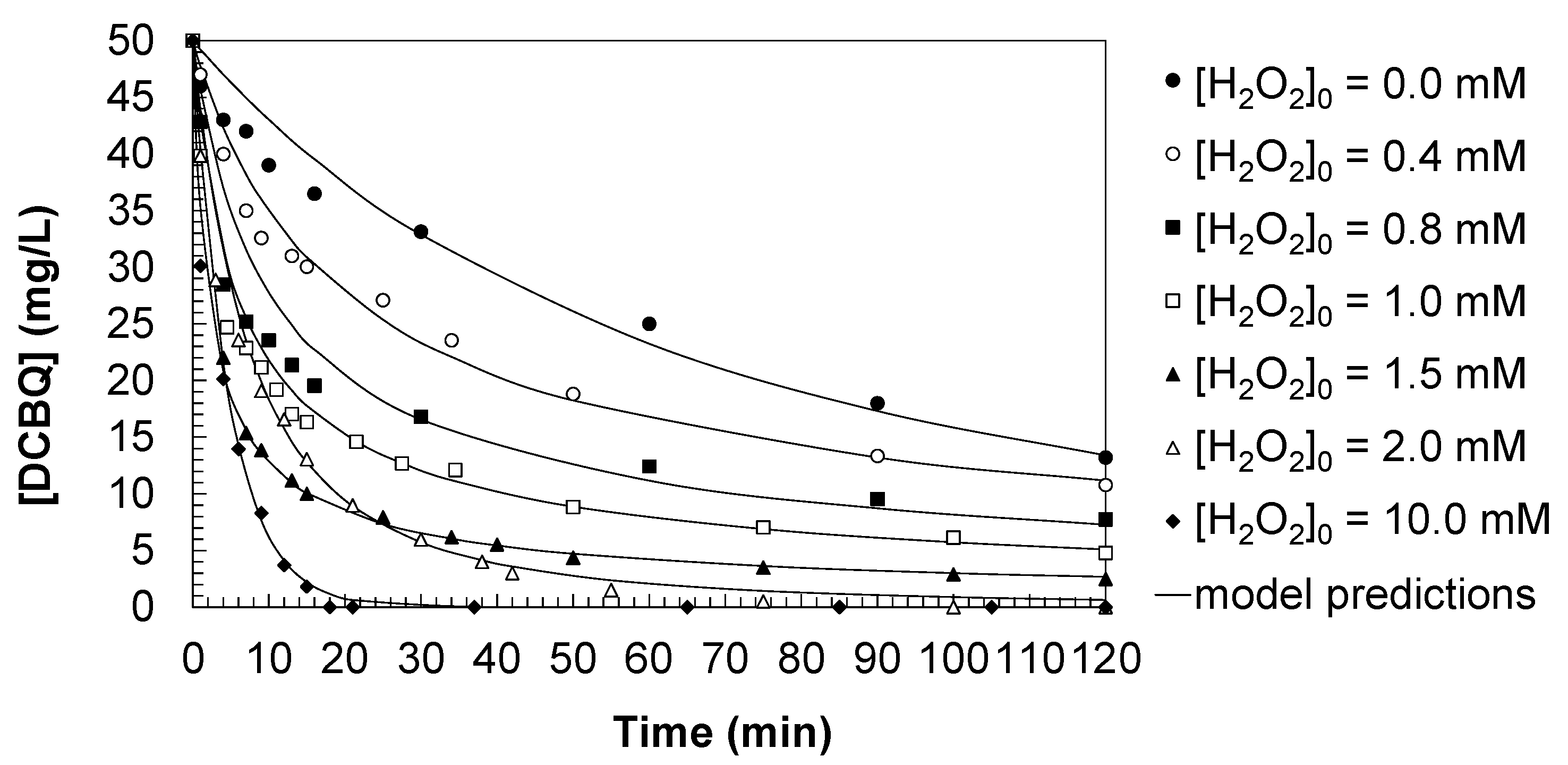
Figure 2 illustrates the kinetic evolution of 2,6-dichlorobenzoquinone (DCBQ) degradation under direct UV photolysis and the UV/H
2O
2 process with different initial oxidant concentrations. In the absence of hydrogen peroxide, direct UV irradiation resulted in a limited decrease in DCBQ concentration, with 12.3 mg/L remaining after 120 min. This confirms that photolysis alone is insufficient for efficient DCBQ removal due to its weak absorption within the emission spectrum of the mercury lamp and the contribution of secondary processes.
The addition of hydrogen peroxide markedly enhanced the degradation efficiency. At moderate oxidant concentrations ([H2O2]0 = 0.4–1.5 mM), the residual DCBQ concentration decreased progressively with increasing [H2O2], reaching only 2.0 mg/L after 120 min for [H2O2]0 = 1.5 mM. This trend clearly indicates that hydroxyl radicals (HO•), generated from H2O2 photolysis, are the dominant oxidizing agents, and that the overall removal strongly depends on both the pollutant concentration and the availability of reactive species. When operating with excess oxidant ([H2O2]0 ≥ 2.0 mM), complete elimination of DCBQ was achieved. At [H2O2]0 = 2.0 mM, total removal occurred within 120 min, whereas at [H2O2]0 = 10.0 mM, the degradation was significantly faster, with DCBQ fully eliminated well before the end of the irradiation period. This highlights the dual role of hydrogen peroxide: at moderate levels it enhances radical generation, while at excessive levels it may also act as a radical scavenger; however, continuous radical production under high [H2O2] ensures complete pollutant removal.
The experimental data were fitted to a generalized kinetic expression of the form shown in Equation (2), based on the oxidation of DCBQ to degradation products (Equation (1)) by an apparent kinetic constant (k
DCBQ), where
n is the overall reaction order.
Figure 2 compares the experimental data with model predictions, while the kinetic parameters are summarized in
Table 1.
Kinetic model for oxidation by UV light (n = 1.5): under direct UV photolysis, the reaction followed a fractional order of 1.5 with an apparent rate constant of 0.0022 L
0.5 mg
−0.5 min
−0.5. This deviation from first-order kinetics suggests that DCBQ photolysis under mercury lamp irradiation is not solely governed by direct photon absorption, since DCBQ exhibits limited absorption within the emission spectrum of the lamp. Instead, secondary processes such as the formation of excited species and their recombination contribute to the observed fractional order.
Kinetic model for oxidation by UV/H
2O
2 (n = 2.5): when hydrogen peroxide was added at moderate concentrations ([H
2O
2]
0 = 0.4–1.5 mM), the apparent reaction order increased to 2.5. The corresponding rate constants (0.0001–0.0013 L
1.5 mg
−1.5 min
−1) confirm a radical-driven mechanism, where hydroxyl radicals generated from H
2O
2 photolysis dominate the degradation process This higher order indicates that HO
• generated by H
2O
2 photolysis dominate the degradation mechanism. The overall reaction rate thus depends on both the substrate concentration and the availability of HO
•, reflecting the multi-step nature of radical generation and subsequent oxidation of DCBQ.
Kinetic model for oxidation by UV/H
2O
2 operating with excess of oxidant (n = 1.0): under excess oxidant conditions ([H
2O
2]
0 ≥ 2.0 mM), the kinetic behavior shifted. For [H
2O
2]
0 = 2.0 mM, the apparent order decreased to 1.5, suggesting competition between HO
• generation and scavenging by excess H
2O
2. Under excess oxidant conditions ([H
2O
2]
0 = 10.0 mM), the kinetics became pseudo-first order (n = 1.0), with a much higher rate constant of 0.2084 min
−1, leading to complete removal of DCBQ within the irradiation time. This behavior illustrates the dual role of hydrogen peroxide. At moderate concentrations it enhances degradation by supplying hydroxyl radicals. At higher doses, radical scavenging reduces availability, but continuous generation still ensures complete DCBQ removal.
Overall, the proposed kinetic model successfully describes the experimental behavior under all tested conditions, supporting the suitability of UV/H2O2 as an advanced oxidation process for the treatment of chlorinated benzoquinones in water. All experiments were conducted at pH 3.0, which was selected to ensure experimental stability, reproducibility, and to minimize interferences from side reactions. Acidic conditions are commonly used in laboratory kinetic studies of UV/H2O2 systems, as they allow clearer interpretation of the degradation mechanism.
3.2. Kinetic Model for Water Color Changes During the DCBQ Oxidation
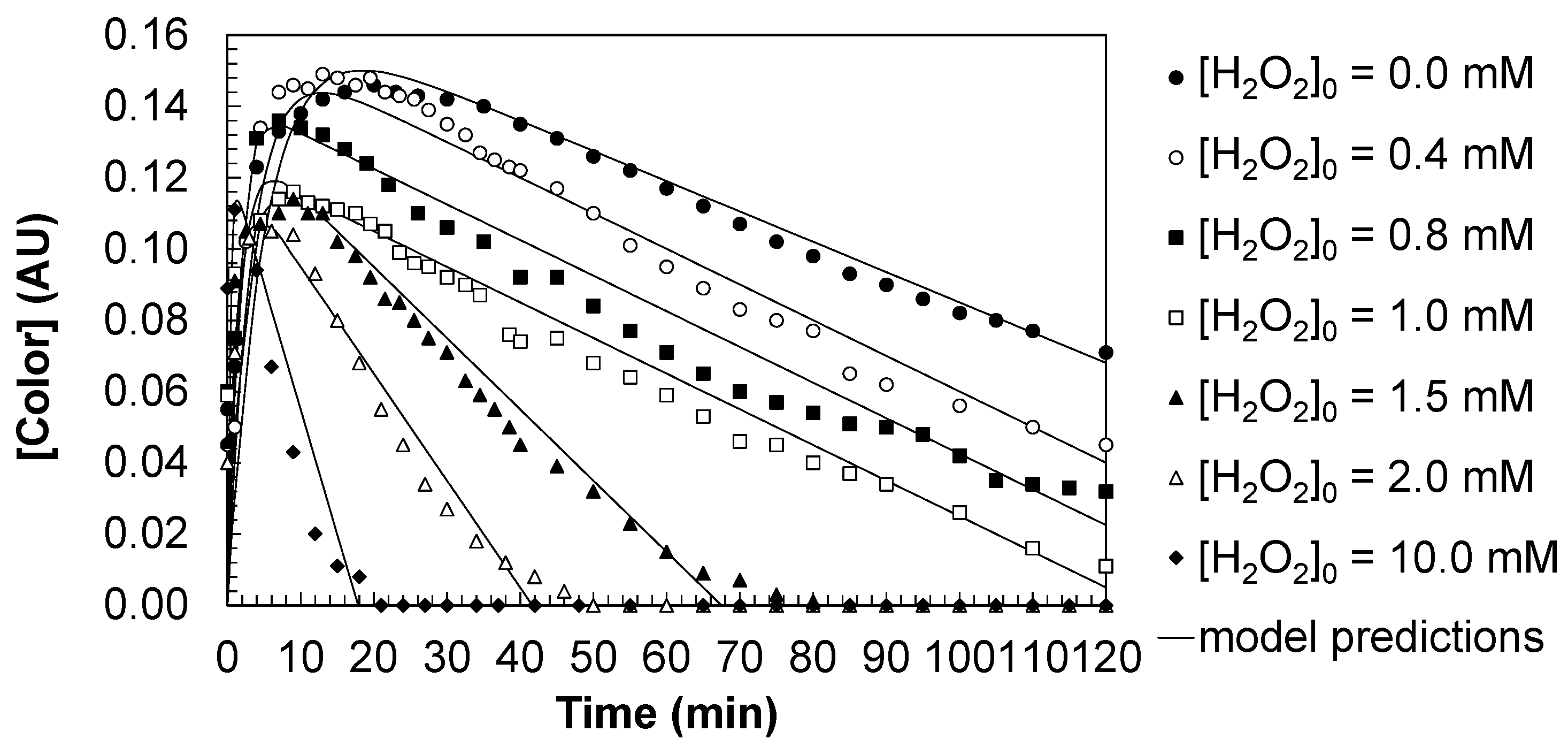
Figure 3 shows the temporal evolution of water color during the oxidation of DCBQ under direct UV photolysis and the UV/H
2O
2 process at different initial oxidant concentrations. It has been observed that DCBQ can be oxidized to the hydroxylated derivative 3-hydroxy-2,6-dichlorobenzoquinone (HO-DCBQ). This compound still retains the quinone structure with chloro and hydroxyl substituents, which gives it chromophore potential, since these functions extend conjugation and can absorb in the visible or near-UV. On the other hand, after hydroxylation, 3,5-dichloro-1,2,4-pyrogallol can be formed. This aromatic structure with several hydroxyl groups can be highly colored by its conjugated system [
17].
In the absence of hydrogen peroxide, color removal was negligible, reflecting the limited capacity of direct photolysis to disrupt the conjugated chromophores of DCBQ. Upon addition of hydrogen peroxide, a marked enhancement in color elimination was observed. For moderate oxidant levels ([H2O2]0 = 0.4–1.5 mM), color decreased significantly during irradiation, although residual absorbance values persisted after 120 min. At higher oxidant concentrations ([H2O2]0 ≥ 2.0 mM), complete decolorization was achieved, highlighting the dominant role of hydroxyl radicals generated from H2O2 photolysis in breaking down the aromatic structures responsible for water coloration.
Figure 4 summarizes the residual color of water after the treatment. In the absence of H
2O
2, the residual color remained high, indicating the persistence of aromatic moieties. With increasing oxidant concentrations, the residual color decreased progressively, reaching near-zero values for [H
2O
2]
0 ≥ 2.0 mM. At the highest oxidant dose tested (10.0 mM), complete color removal was consistently achieved. These findings demonstrate that, in addition to DCBQ degradation, the UV/H
2O
2 process effectively eliminates the associated water color, thereby improving the quality of the treated effluent.
Practically, these results show that while moderate radical production reduces aromaticity substantially, a critical oxidant threshold (here, ~2.0 mM H2O2 under the specified conditions) is required to fully mineralize or transform chromophore-bearing intermediates to non-aromatic species. This has direct implications for treatment design: achieving color-free effluents from chlorinated quinone contamination requires either sufficiently high oxidant dosing or operating conditions that maximize radical production and minimize radical scavenging or intermediate accumulation.
A first-order consecutive reaction model is proposed (Equation (6)), in which DCBQ is transformed into colored intermediates with a rate constant k
Color,f (1/min). The use of first-order kinetics reflects the direct proportionality between color formation and the concentration of DCBQ or its reactive intermediates. As the generation of chromophoric species is directly governed by the available DCBQ concentration, the rate of color formation progressively decreases as DCBQ is consumed throughout the reaction. To maintain dimensional consistency in the reaction rate expression (Equation (7)), the initial DCBQ concentration contributing to color formation is expressed in absorbance units by introducing the parameter k
Color (AU·L/mg), which establishes the relationship between absorbance at 455 nm and mass concentration.
The mass balance for color changes is determined as follows:
Integrating Equation (7), the kinetic equation for color changes in aqueous DCF solutions oxidized by UV/H
2O
2 treatment is presented as Equation (8).
When sufficient hydrogen peroxide is available to sustain an adequate supply of hydroxyl radicals, the colored intermediates are further oxidized into non-chromophoric end-products, such as low-molecular-weight carboxylic acids and biodegradable inorganic species. This subsequent degradation step is described by a zero-order kinetic model, characterized by the rate constant
kColor,d (AU/min). The adoption of zero-order kinetics indicates that the rate of color removal is independent of the concentration of chromophoric intermediates and is instead limited by the steady availability of reactive radicals. Once chromophore-containing intermediates are formed, their degradation depends primarily on the flux of hydroxyl radicals rather than their own concentration. As long as excess H
2O
2 is present, continuous radical generation through photolysis ensures a constant rate of color degradation. This behavior reflects the non-selective nature of hydroxyl radical attack on aromatic intermediates, which maintains a uniform removal rate under radical-rich conditions. The estimated kinetic parameters, summarized in
Table 2, confirm their dependence on the applied hydrogen peroxide concentration (Equations (9)–(11)). The strong agreement between model predictions and experimental observations validates the suitability of the proposed kinetic model to describe the evolution of color under different oxidative scenarios.
3.3. Aromaticity Loss During the DCBQ Oxidation
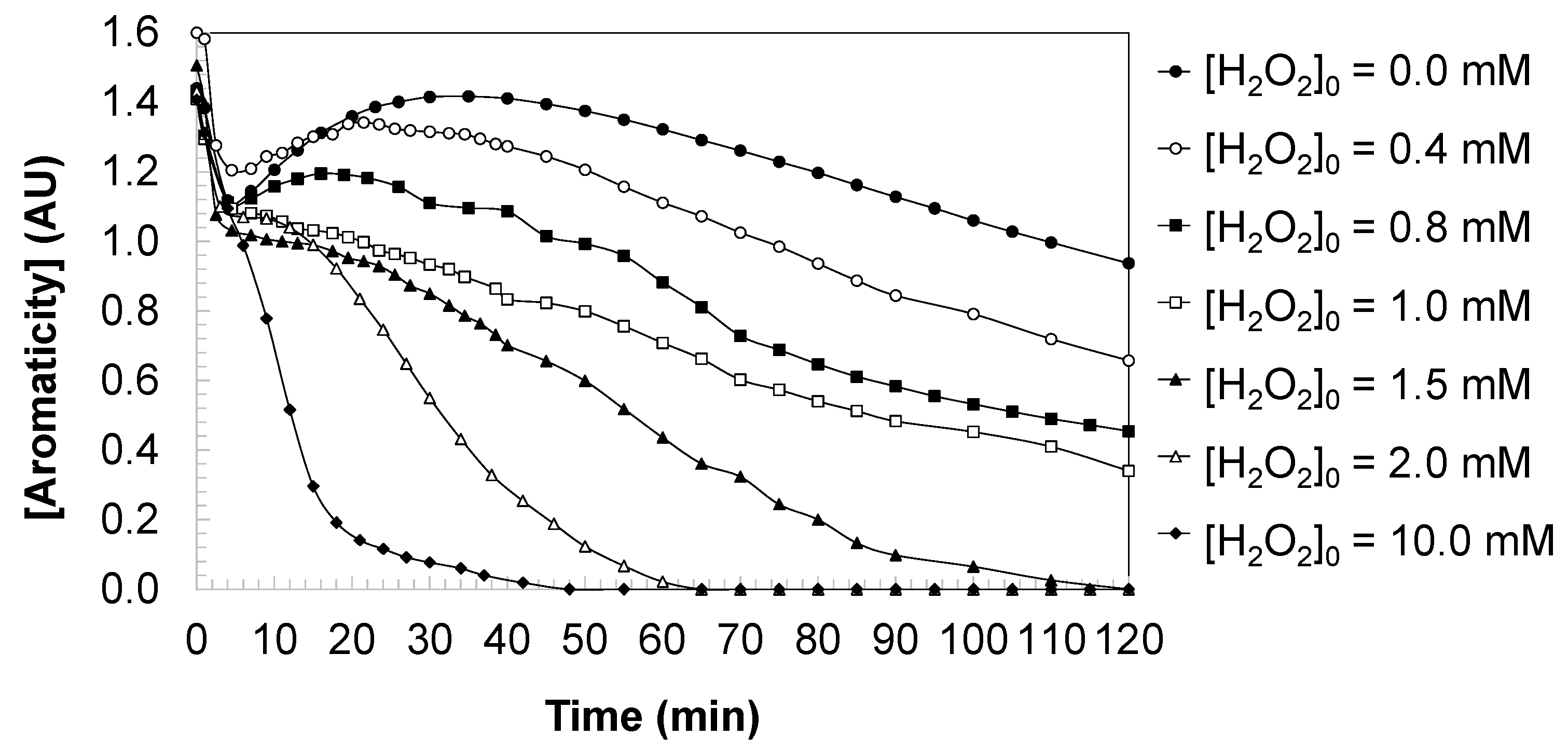
Figure 5 depicts the evolution of sample aromaticity during the oxidation of DCBQ by direct UV photolysis and by the decline of UV/H
2O
2 as irradiation proceeds, and the extent of removal after 120 min is strongly dependent on the initial hydrogen peroxide dose (
Table 2). These results demonstrate a clear, monotonic relationship between oxidant loading and the loss of conjugated chromophores responsible for aromatic UV-Vis absorbance.
From a kinetic viewpoint, the observed decrease in aromaticity reflects two complementary processes: (i) direct photochemical modification of the parent quinone chromophore and (ii) radical-mediated oxidative cleavage and transformation of aromatic rings. Under photolysis alone (no added H2O2), aromaticity is only marginally reduced (final 0.737 AU), consistent with the slow, fractional-order degradation of DCBQ previously observed under UV-only conditions (Equation (3)).
When H2O2 is introduced at moderate concentrations (0.4–1.5 mM), the generation of hydroxyl radicals by H2O2 photolysis accelerates aromatic ring oxidation; however, the aromaticity is not completely eliminated at these doses, indicating that partial oxidation products that still retain conjugated structures (hydroxylated or partially dechlorinated aromatics and quinone-like species) accumulate to measurable levels. Kinetically, this regime corresponds to radical-driven reaction dynamics in which the rate of aromaticity loss depends both on the instantaneous concentration of residual DCBQ/partially oxidized intermediates and on the radical flux provided by H2O2 photolysis.
At and above 2.0 mM H2O2 the system attains conditions in which aromaticity is fully abolished (final 0.0 AU). This transition to complete de-aromatization indicates that the oxidative pathway proceeds beyond substitution and partial oxidation to effective ring-opening, fragmentation, and formation of low-molecular-weight, non-conjugated oxidation products (carboxylic acids and inorganic end-products). Kinetically, the disappearance of aromatic signal under excess oxidant is consistent with a regime in which radical availability is sufficiently high and sustained to drive the overall process to completion within the irradiation period; consequently, the apparent reaction order and rate constants inferred from the DCBQ mass-loss kinetics shift toward higher effective rates (and in the highest oxidant case toward pseudo-first-order behavior shown in Equation (5)), which is mirrored by the complete loss of aromaticity.
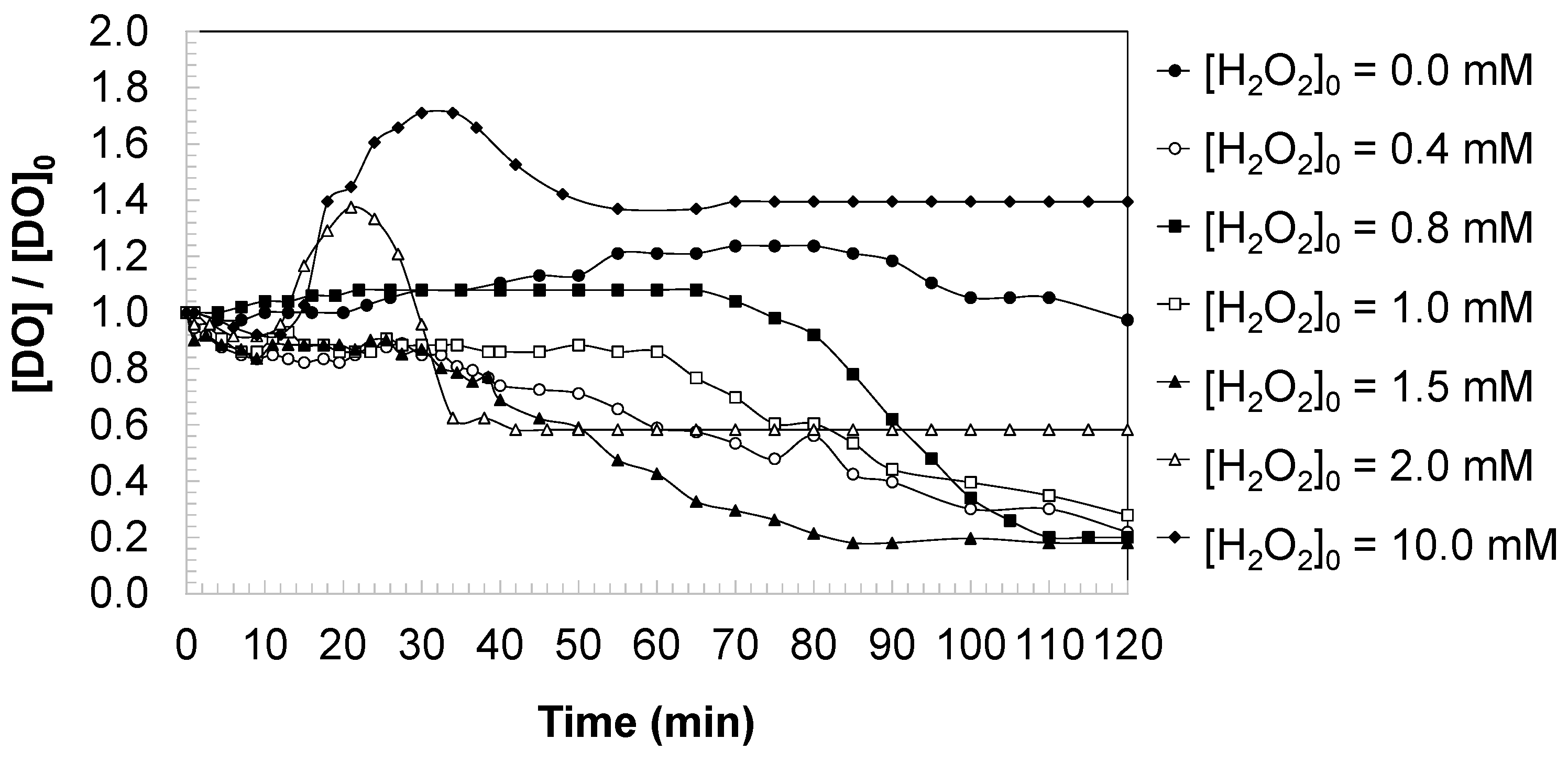
3.4. Dissolved Oxygen Changes During the DCBQ Oxidation
Figure 6 presents the temporal evolution of dissolved oxygen (DO) normalized during the oxidation of DCBQ by UV irradiation and UV/H
2O
2 under varying oxidant concentrations. In the absence of hydrogen peroxide, the DO level remained nearly constant throughout the reaction time, indicating that direct photolysis of DCBQ did not significantly impact the oxygen balance in the system.
When hydrogen peroxide was introduced, distinct changes in DO concentration were observed, which depended on the oxidant dose. At moderate concentrations ([H2O2]0 = 0.4–1.5 mM), DO/DO0 decreased progressively during irradiation, reflecting the consumption of dissolved oxygen associated with the oxidative degradation of aromatic intermediates. This behavior can be attributed to two processes: (i) the direct involvement of oxygen in radical-driven oxidation reactions and (ii) the formation of partially oxidized intermediates that temporarily sequester oxygen through aromatic hydroxylation or quinone–hydroquinone transformations.
At higher oxidant doses ([H2O2]0 ≥ 2.0 mM), the DO profiles exhibited a different trend. Initially, oxygen levels decreased as in the moderate oxidant regime; however, with continued irradiation, DO values stabilized or even recovered, approaching the initial baseline by the end of the reaction. This recovery suggests that under excess oxidant conditions, the mineralization pathway predominates, leading to the cleavage of aromatic rings and the formation of low-molecular-weight carboxylic acids and inorganic products (e.g., CO2). The stoichiometric release of oxygen from hydrogen peroxide photolysis also contributes to balancing the oxygen demand, resulting in an apparent stabilization of dissolved oxygen levels.
Overall, the observed DO dynamics highlight the interplay between radical-driven oxidation, intermediate accumulation, and final mineralization. Under moderate oxidant supply, oxygen consumption dominates, whereas under excess oxidant, the system tends toward a quasi-steady oxygen balance due to continuous radical generation and the shift toward complete mineralization pathways. The experimental data confirm that hydrogen peroxide concentration not only governs the degradation kinetics of DCBQ and aromaticity but also directly influences the oxygen balance of the treated water, a parameter of environmental relevance for assessing effluent quality.
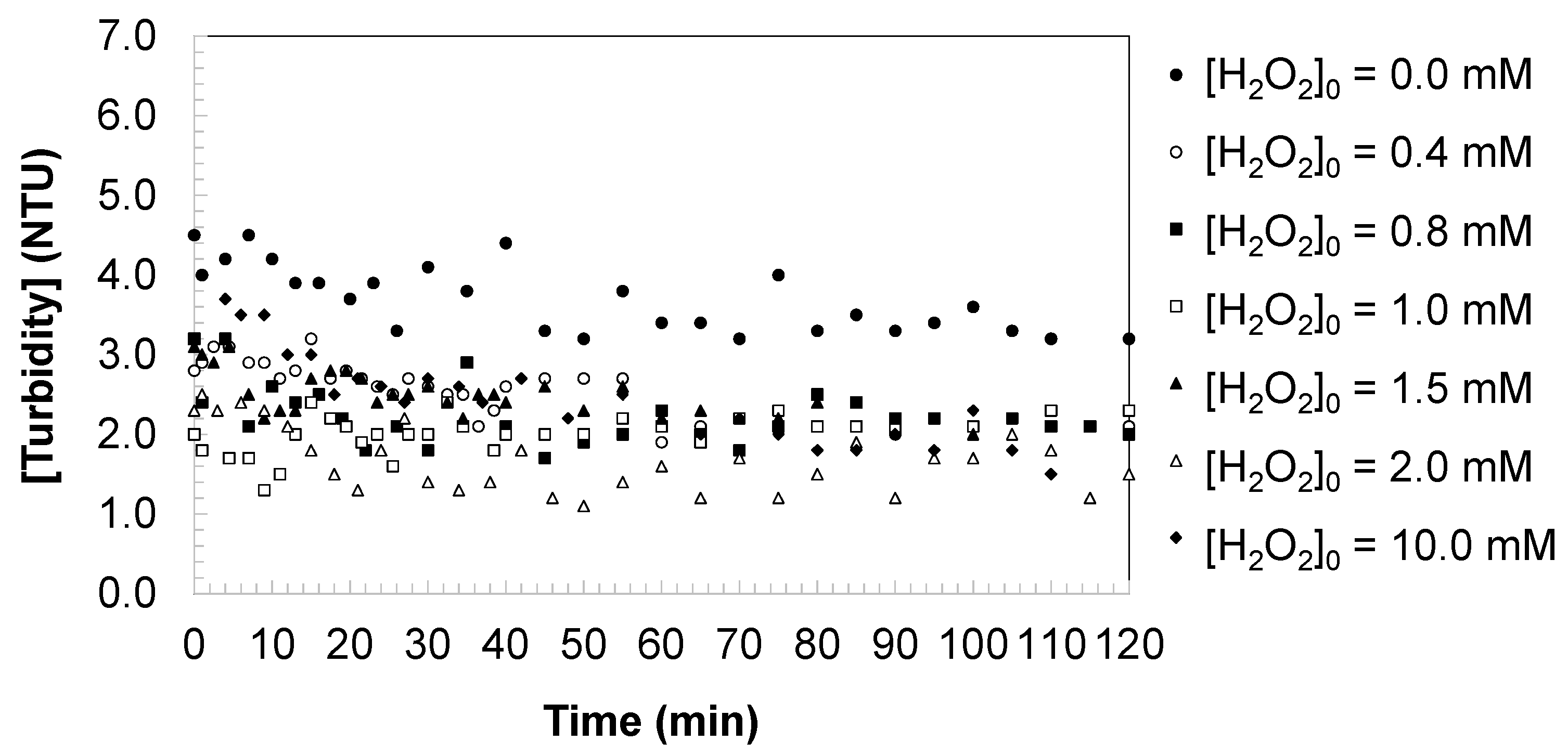
3.5. Turbidity of Water During the DCBQ Oxidation
Figure 7 illustrates the changes in turbidity (NTU) during the oxidation of DCBQ by UV photolysis and the UV/H
2O
2 process under the specified experimental conditions. In the absence of hydrogen peroxide, turbidity remained essentially unchanged throughout irradiation, indicating that direct UV exposure alone does not generate a significant amount of particulate matter or colloidal species.
Upon addition of hydrogen peroxide, the turbidity profiles exhibited a distinct increase during the early stages of the reaction, followed by stabilization or slight decline depending on the oxidant dose. This transient turbidity rise can be attributed to the formation of intermediate oxidation products, including polymeric or colloidal species derived from partial aromatic ring cleavage and condensation reactions. Such intermediates tend to scatter light, thereby increasing turbidity even as the parent compound (DCBQ) undergoes progressive degradation.
At moderate oxidant concentrations ([H2O2]0 = 0.4–1.5 mM), the turbidity increase was more pronounced and persisted throughout the reaction time, suggesting that incomplete oxidation led to the accumulation of poorly soluble intermediates. In contrast, at higher oxidant concentrations ([H2O2]0 ≥ 2.0 mM), turbidity values were significantly lower and in some cases declined toward baseline levels by the end of irradiation. This trend reflects the predominance of mineralization pathways under excess oxidant conditions, which transform aromatic intermediates into low-molecular-weight, soluble products (e.g., carboxylic acids and inorganic ions) that do not contribute to light scattering.
From a kinetic standpoint, the evolution of turbidity provides indirect evidence of the reaction pathway. At sub-stoichiometric oxidant levels, radical-driven oxidation yields chromophoric and colloidal by-products that temporarily increase turbidity, whereas under excess oxidant, continuous hydroxyl radical production promotes the rapid breakdown of these intermediates, suppressing turbidity formation. The observed turbidity patterns therefore support the mechanistic interpretation that hydrogen peroxide dosage controls not only the degradation rate of DCBQ and its aromaticity but also the extent to which intermediate products accumulate or are further oxidized toward complete mineralization.
From an environmental perspective, residual turbidity in treated water is a critical parameter, as it directly impacts not only the aesthetic quality but also the safety of potable water. Increased turbidity may shield microorganisms from disinfection, reduce treatment efficiency in downstream processes, and indicate the presence of partially oxidized aromatic by-products that could exert additional ecological or toxicological effects. The results obtained here demonstrate that operating under excess oxidant conditions minimizes turbidity by driving the reaction toward complete mineralization, thereby producing a clearer effluent. This finding underscores the importance of optimizing oxidant dosage in UV/H2O2 systems to ensure both effective contaminant degradation and compliance with drinking water quality standards.
From a practical perspective, the transient turbidity increase observed at moderate oxidant concentrations may represent a challenge for downstream treatment steps in full-scale facilities. Increased turbidity could hinder disinfection efficiency by shielding microorganisms from exposure to disinfectants and may also place additional demands on filtration units. In contrast, operation under excess oxidant conditions was shown to suppress turbidity formation, producing a clearer effluent that is more compatible with conventional disinfection and filtration processes. These observations highlight the importance of selecting operational conditions that not only maximize contaminant removal but also ensure compatibility with subsequent stages of drinking water treatment.
4. Conclusions
This work systematically investigated the degradation of 2,6-dichlorobenzoquinone (DCBQ) under direct UV photolysis and UV/H2O2 treatment, focusing on kinetics and water quality outcomes. The results confirmed that UV irradiation alone achieved only partial DCBQ removal, whereas the addition of hydrogen peroxide significantly enhanced efficiency, leading to complete elimination at [H2O2]0 ≥ 2.0 mM. The proposed kinetic model reproduced the observed profiles under different oxidant regimes, supporting the predominance of radical-mediated pathways and capturing not only parent compound decay but also changes in color and aromaticity.
In addition to pollutant removal, the study provides important insights into broader water quality parameters. Dissolved oxygen dynamics indicated a balance between oxygen-consuming reactions and mineralization, while turbidity measurements revealed that insufficient oxidant doses can lead to the transient accumulation of colloidal intermediates. These findings highlight the dual importance of achieving efficient micropollutant degradation and maintaining desirable water quality characteristics. Overall, the work demonstrates the robustness of the UV/H2O2 process for treating halogenated benzoquinones and underscores the practical need to optimize oxidant dosage. Future research should extend this evaluation to environmentally realistic concentrations and neutral pH conditions, and eventually to pilot-scale systems, in order to confirm applicability in drinking water treatment practice.
While UV/H2O2 is highly effective for DCBQ removal, attention should be given to the possible transient formation of hydroxylated intermediates at suboptimal oxidant doses, as these species may retain significant toxicological relevance. Our findings underscore the need to balance treatment efficiency with by-product control. Compared with previous investigations on other halobenzoquinones, this study highlights the novel contribution of correlating degradation kinetics with water quality indicators, thereby strengthening the basis for applying UV/H2O2 to the control of emerging halogenated benzoquinones in drinking water treatment.