Abstract
The direct deposition of highly concentrated polyelectrolyte complexes based on poly(ethyleneimine) (PEI) and poly(sodium methacrylate) (PMANa) onto inorganic sand microparticles (F100 and F200) resulted in the formation of versatile core-shell composites with fast removal properties in dynamic conditions toward anionic charged pollutants. Herein, in situ-generated nonstoichiometric PEI/PMANa polyelectrolyte complexes were directly precipitated as a soft organic shell onto solid sand microparticles at a 5% mass ratio (organic/inorganic part = 5%, w/w%). The sorption of an anionic model pollutant (Indigo Carmine (IC)) onto the composite particles in dynamic conditions depended on the inorganic core size, the flow rate, the bed type (fixed or fluidized) and the initial dye concentration. The maximum sorption capacity, after 10 cycles of sorption/desorption of IC onto F100@P5% and F200@P5%, was between 16 and 18 mg IC/mL composite. The newly synthesized core-shell composites could immobilize IC at a high flow rate (8 mL/min), either from concentrated (CIC = 60 mg/L) or very diluted (CIC = 0.2 mg/L) IC aqueous solution, demonstrating that this type of material could be promising in water treatment or efficient in solid-phase extraction (concentration factor of 2000).
1. Introduction
With the rapid pace of technological advancement and industrialization, the global water system has been gradually affected. Anthropogenic activities, including manufacturing, intensive agriculture and the ineffective processing and disposal of waste, have led to the persistent discharge of pollutants into water streams. Numerous studies have documented the presence of a complex mixture of contaminants in the water flows, containing both organic and inorganic pollutants [1,2]. Such compounds were identified in both surface and ground waters, and even in the treated effluents, bringing to light one of the most important problems our society is facing—the pollution of water. The recurrence of pollutants in the water systems poses important concerns in the context of public health but also in the viability of ecosystems, as many of the compounds identified in water are known for their toxicity, chemical stability, persistence and bioaccumulative nature [3,4,5].
The spectrum of pollutants identified in water samples is extremely diverse. Among the identified pollutants that raise concern, one can mention drugs and pharmaceutical compounds (such as doxorubicin, diclofenac and indomethacin) [6,7], personal care products [8], pesticides [9], endocrine disruptors such as bisphenol A, phthalates or persistent organic pollutants. Heavy metal ions (Cu2+, Hg2+, Cd2+, etc.) are also released into the water flows due to industrial activities and mining [10]. Additionally, synthetic dyes such as Malachite Green, Remazol Brilliant blue, Congo red or Indigo Carmine (IC) are of interest due to their wide use in the textile and printing industries [11,12].
To overcome the presence of such compounds in water streams and ensure access to clean water, various treatment methods have been developed. Several conventional methods of treatment have been extensively studied and applied for various classes of pollutants, including coagulation/flocculation, filtration, sedimentation and activated-sludge-based methods. These methods are well established due to their reduced cost, effectiveness in removing bulk contaminants and already-available treatment infrastructure, but their use is limited by their reduced efficiency in the elimination of emerging contaminants [13]. The development of advanced water treatment methods partially overcomes these limitations. Some of the most important advanced water treatment methods include electrochemical methods (electrocoagulation and electrooxidation), advanced oxidation processes and ultra- and nanofiltration methods but also ion exchange and sorption [14,15,16,17,18]. Despite the distinct advantages of each of these methods, their use is often limited by economic, environmental and technological aspects, especially in the context of complex mixtures of pollutants [19].
The sorption of pollutants, including both adsorption (the immobilization of the compound of interest on the surface of the sorbent) and absorption (the immobilization of the compounds on the bulk phase of the sorbent), is one of the most cost-effective and widely studied methods of water treatment [20]. The method relies on the formation of interactions between the targeted compound (named sorbate) and the sorbent, including electrostatic attractions, ion exchange, hydrogen bonding, hydrophobic interactions, π–π stalking interactions or chelation and coordination interactions [21]. A wide range of sorbents has been developed, with some of the most important ones being activated carbon and its derivatives, zeolites, metal oxides, clays and silica, which are highly recognized due to their large surface area and broad applicability [22,23]. Polymer-based materials, including hydrogels, beads, fibers or membranes, are also appreciated due to their versatility in immobilizing various types of pollutants, both organic and inorganic [24,25]. More recently, carbon-based nanomaterials such as graphene oxide and carbon nanotubes and organic/inorganic composite materials have gained attention due to their good sorption capacity and increased selectivity [26,27].
Among the composites employed in water treatment, core-shell composites have attracted significant interest due to their specific features. These materials typically consist of an inorganic core, which imparts increased mechanical stability, and an organic shell usually obtained by polymer deposition, which ensures a good sorption capacity due to the presence of numerous functional groups. There are various morphologies and chemical compositions of core-shell composites reported as sorbents for pollutants, including magnetic nanoparticles coated with polymers and silica microparticles with a polymeric shell obtained by layer-by-layer deposition of polyelectrolytes or with a coacervate-type polymeric shell. As already evidenced in the literature, core-shell composites are characterized by several key features, including a tailored selectivity, improved chemical and mechanical stability and reusability, which make them highly promising candidates for the development of effective wastewater treatment methods [21]. Their use in such applications is widely documented, with various combinations of composite materials being proposed as sorbents for pollutants, including both organic and inorganic contaminants. Maciel et al. proposed the use of magnetic nanoparticles coated with L-lysine for the removal of acetylsalicylic acid [28]. Similarly, Chkirida et al. reported the use of magnetic alginate-bentonite nanoparticles coated with chitosan for the removal of ciprofloxacin and heavy metal ions from wastewater [29]. Morosanu et al. proposed the use of polymeric shell/silica core microparticles for the sorption of heavy metal ions from simulated polluted water samples [30]. Recently, Bucatariu et al. reported an innovative method for the fabrication of core-shell microparticles intended for water cleaning applications, consisting of the deposition of a coacervate-like polymeric shell on sand microparticles [31]. The obtained materials were successfully employed in the sorption of metal ions from water samples, demonstrating a good capacity of removal for Cd2+, Zn2+, Mn2+, Cu2+, Ni2+ and Co2+.
The current study proposes a comprehensive investigation into the obtaining of sand composites and subsequent application in the removal of a model pollutant—the sulfonic dye IC—from simulated low- and high-concentration wastewater, employing dynamic sorption experiments in columns. The core-shell microparticles were obtained following the deposition from aqueous solution of poly(ethyleneimine) (PEI)/poly(sodium methacrylate) (PMANa) interpolyelectrolyte complexes on the surface of sand microparticles of different sizes, with the polymeric shell being further stabilized through chemical cross-linking. The dynamic sorption of IC was studied in a multi-parameter experimental set-up to evidence the most important parameters (influent concentration, flow rate, pressure drop, particle size) affecting the sorption process. The results obtained highlighted the effective removal of IC from aqueous solution in various experimental conditions as well as a 10-cycle reusability of the materials, demonstrating that the proposed composites can successfully be used in wastewater treatment as well as in solid-phase extraction.
2. Materials and Methods
2.1. Materials
Two polydisperse natural sand samples, F100 and F200, with average particle sizes of 180 μm and 250 μm, respectively, were separated with a Retsch vibratory sieve shaker (Retsch LLC, Haan, Germany). Two complementary polyelectrolytes, branched poly(ethyleneimine) (PEI) (Mw = 25,000 g·mol−1) and PMANa (Mw = 1800 g·mol−1), were purchased from Sigma Aldrich (Darmstadt, Germany) and used without supplementary purification. The model anionic dye, IC (VWR Chemicals, Bucharest, Romania), the glutaraldehyde (GA) (Sigma Aldrich), NaOH and HCl (Merck, Darmstadt, Germany) were used as received.
2.2. PEI/PMANa Interpolyelectrolyte Complex Formation
The aqueous solutions of PEI [0.01 M, ethyleneimine (EI) structural units] and PMANa [0.01 M, sodium methacrylate (MANa) structural units] were prepared through their dissolution in Millipore water at room temperature, for 24 h. Four series, at four pH values, of PEI/PMANa interpolyelectrolyte complexes were prepared using a method involving the stepwise addition of one component (PMANa) in the solution of the second component (PEI). Therefore, several PEI/PMANa complexes were obtained, with different molar ratios between their structural units (10:x, where x = 1, 2, …, 10) at pH 2.5, 6.0, 7.5 and 9.0, respectively.
Dynamic light scattering (DLS) measurements were used to assess the size of the formed complexes. The scattered light intensity and the hydrodynamic diameter of the PEI/PMANa complexes were quantified as a function of the molar ratio between the components, using a Litesizer DLS 500 equipment (Anton Paar, Graz, Austria) (40 mW semiconductor laser diode, λ = 658 nm and at a scattering angle of 173°). The measurements were performed at room temperature.
Dynamic viscosity measurements of the polyelectrolyte solutions and of the PEI/PMANa complexes were carried out with a high-precision MCR 92 Rheometer (Anton Paar, Austria), a modular and versatile instrument designed for advanced rheological analysis, equipped with a cone-plane geometry. The rheological tests for the polyelectrolyte solutions (CPEI = CPMANa = 0.01 M and 1 M) were performed at 25 °C. The dynamic viscosity curves were obtained by measuring the shear stress (mPa) as a function of the shear rate (s−1).
2.3. Fabrication and Characterization of PEI/PMANa Sand Composites
The deposition of PEI/PMANa polyelectrolyte complexes onto two sand sorts, F100 and F200, was performed under stirring, generating a very sticky “ball” formed by all the solid particles bound with the complex, which acted as a “glue”. The deposited amount of polyelectrolytes was calculated taking into account the molar ratio between the polyelectrolyte structural units (SUPEI:SUPMANa = 10:8) and the total amount of the organic shell deposited (5% w/w%) on the final composite. Each type of core-shell composite was cross-linked with GA at a [amine]:[aldehyde] = 1:1 molar ratio, for five hours. The non-cross-linked polyanion and polycation chains were removed in NaOH (0.1 M) to remove PMANa, and HCl (0.1 M) to remove weakly-bound PEI chains. Two types of core-shell sand composites were obtained: F100@P5% and F200@P5%.
The chemical composition of the sand composite material was studied through Fourier-transform infrared (FTIR) spectroscopy using the attenuated total reflectance (ATR) mode. The spectra were obtained with an IRTracer-100 spectrometer (Shimadzu Corporation, Kyoto, Japan) with a GladeATR accessory (PIKE Technologies, Madison, WI, USA). The FTIR-ATR spectra were obtained at 25 °C and over the spectral range of 400–4000 cm−1, with a resolution of 4 cm−1.
The morphology of the sand composite materials was examined by Scanning Electron Microscopy (SEM) (Verios G4 UC, Thermo Scientific, Brno-Černovice, Czech Republic). The samples were lightly coated with a 10 nm thickness layer of platinum in high vacuum on a Leica EM ACE200 sputter coater (Leica, Wetzlar, Germany) immediately before imaging, to prevent electrostatic charging. The imaging employed a secondary electron detector for compositional contrast (CBS, concentric backscattered electrons). The elemental composition of the samples was evaluated semi-quantitatively using an EDAX Octane Elect Super SDD detector (Thermo Scientific, Brno-Černovice, Czech Republic) integrated into the SEM system.
2.4. Column Experiments for Sorption/Desorption of IC onto/from Core-Shell Composites
The IC dynamic sorption properties of the F100@P5% and F200@P5% composite microparticles were investigated in an OMNIFIT chromatographic glass column using a constant fixed-bed height [Volume (mL) = 0.7854 × bed height (cm)]. During all the column experiments, different flow rates (1–20 mL/min) were obtained by a Shenchen peristaltic pump. The IC aqueous influent concentrations were varied during different experiments (concentration IC = 10; 20 and 60 mg/L). The exhausted columns were treated with NaOH 0.1 M for the extraction of the sorbed IC, followed by HCl treatment for composite activation. The dynamic sorption parameters were calculated from the Thomas and Yoon–Nelson models [30].
UV-Vis measurements were performed with a SPEKOL 1300 spectrophotometer (Analytik Jena, Germany) to determine the concentration of IC (maximum absorption at λ = 613 nm) in aqueous solution, before and after loading/release in the column. A calibration curve for IC (yIC = 0.067x) was constructed. The IC amount in each collected fraction was determined using the following equation:
where Ci and Ce are the influent and effluent concentrations (mg/L), Vf is the volume of effluent fraction, and v is the volume of composite material inside the column, which was kept constant during all experiments (3.5 cm × 0.7854 = 2.75 mL).
qe = (Ci − Ce)·(Vf·v)
3. Results
To identify the optimum conditions for polyelectrolyte complexes deposition onto sand particles, such as the pH of the medium and molar ratio of the components, DLS measurements were used to characterize the step-by-step formation of the complexes in aqueous media at a low concentration of polyelectrolytes (CPEI = CPMANa = 0.01 M) (Figure 1).

Figure 1.
(a) Number weighted size distribution (%) of PEI/PMANa complexes dissolved/formed in aqueous solution at pH = 2.5, (b) pH = 6, (c) pH = 7.5 and (d) pH = 9 (CPEI = CPMANa = 0.01 M).
The increase in the size of the complexes, associated with an increase in the number of interactions (electrostatics and hydrophobics), was estimated from the variation of particle number size distribution as a function of pH and molar ratio. The PEI ionization degree, which dictates the polyelectrolyte chain conformation, depends drastically on the pH of the media. At low pH, the PEI chains are fully stretched due to the full ionization of their amino groups, while the PMANa, which is a weak acid, is fully stretched at high values of pH, where its carboxylic groups are ionized. In Figure 1a,d, it is observed that the complexes’ formation is not favored at extreme pH, irrespective of the molar ratio of components. This fact could be attributed to the low number of interactions between the chains, which can be associated with the stretched conformation of one component (stretched chains of PEI in acidic media or stretched PMANa in basic media). At intermediate pH values (low acidic or low basic medium), where both types of polyelectrolyte chains are partly ionized, the successful formation of the complexes can be observed, with the sizes increasing with the decrease in the molar ratio, the optimum molar ratio being PEI:PMANa = 10:8. In these conditions, 6 < pH < 7.5 and 10:8 molar ratio, all the PEI chains interacted with all the PMANa chains in a phase separation process, evidenced by the formation of larger particle populations in the corresponding size distribution profiles (Figure 1b,c). Further, the optimum conditions identified by DLS were kept constant in the dynamic viscosity measurements, only the concentration of the polyelectrolytes being modified (Figure 2).

Figure 2.
Determination of the dynamic viscosity (mPa·s) of the PEI/PMANa polyelectrolyte complexes obtained from low (a) CPEI = CPMANa = 0.01 M and high (b,c) CPEI = CPMANa = 1 M concentrated polyelectrolyte aqueous solution, at 25 °C and pH = 6, as a function of shear rate (1/s).
The effect of the polyelectrolyte concentration, and the subsequently formed PEI/PMANa complex, on the rheological properties were studied at a constant temperature of 25 °C (Figure 2). The shear viscosity (η) was registered as a function of the shear rate (1 to 100 s−1), in stationary continuous shear conditions. It was observed that at a low concentration of polyelectrolytes (0.01 M, pH = 6), the viscosity decreased at a low shear rate, followed by a plateau region at a high shear rate (Figure 2a). This fact showed reduced interactions between the same polyelectrolyte chains, the non-Newtonian behavior (shear thinning) being attributed to the polyelectrolyte solution at a low shear rate. At a high shear rate, the major component of the solution, which is water, dictated the rheological properties of the solution, a Newtonian behavior being observed. The PEI/PMANa complexes formed at a low concentration of polyelectrolytes presented a slightly lower viscosity compared with the single components, showing a reduced number of chain–solvent interactions due to the new chain–chain interactions in the complex structures. Because the formed complexes have a low concentration in the formed dispersion, the aqueous solvent dictates further the viscosity, with a Newtonian behavior being observed at a high shear rate. At a high concentration of the polyelectrolyte chains in aqueous solution (1 M), an increase in the shear rate threshold between non-Newtonian and Newtonian behavior was observed (Figure 2b). At the same high concentration, the PEI solution is more viscous at a low shear rate, compared with PMANa, due to differences in chain size and conformation of the polyelectrolytes (longer branched PEI versus smaller linear PMANa). Overall, the two polyelectrolyte solutions, low and highly concentrated, presented small differences in the dynamic viscosity (1–200 mP·s), showing that inter-chain polyelectrolyte interactions are more repulsive than attractive interactions for the single-component solutions. Instead, the complexes formed at high concentrations of polyelectrolytes presented a 1,000,000 mPa·s shear viscosity in bulk (characteristic of highly condensed organic matter), demonstrating a high affinity of the complementary polymeric chains, principally based on electrostatic attractive interactions (Figure 2c). Using DLS and rheology measurements, it was possible to roughly identify the optimal conditions (6 < pH < 7.5; molar ratio PEI:PMANa = 10:8 and 1M polyelectrolytes concentration) for direct deposition of a certain amount of PEI/PMANa complexes onto solid surfaces, such as sand particles (Scheme 1).

Scheme 1.
Fabrication of the core-shell sand@P5% composites using a direct deposition of a controlled amount of PEI/PMANa complex (5%, w/w%) onto sand microparticles, followed by chemical cross-linking and a flexibilization process by the extraction of non-cross-linked polyelectrolyte chains in basic and acidic media. Subsequently, the composites were used in IC multiple sorption/desorption cycles.
The main driving forces of the complexes’ deposition onto sand were the attractive electrostatic interactions, hydrogen bonds and hydrophobic interactions. The deposition of the organic shell was demonstrated by FT-IR spectroscopy, comparing the spectra of the sand samples before and after the deposition of the PEI/PMANa complexes (Figure 3).

Figure 3.
FTIR-ATR spectra of sand particles before (F100 and F200) and after PEI/PMANa deposition (F100@P5% and F200@P5%).
The FTIR-ATR spectra clearly confirm that the two sand fractions (F100 and F200) were successfully coated with a polymeric shell, as evidenced by the emergence of multiple new peaks corresponding to functional groups commonly found in PEI and PMANa. The spectra of the sand microparticles with a 180 nm diameter (F100) present the characteristic peaks of amorphous silica observed as follows: 1084 cm−1 (strong Si–O–Si asymmetric stretching (main silica peak)), the band at 792 cm−1 characteristic of the Si–O symmetric stretching and bands typically observed in silica-based materials (Si–O–Si), between 688 and 400 cm−1. In the case of the F100@P5% composite, a new band appeared at 3330 cm−1 (–O–H/–N–H stretching), this signal being a typical indicator of organic polymers. The signal of the carbonyl group from PMANa and GA cross-links appeared in the FTIR-ATR spectrum at 1640 cm−1 (–C=O stretching). New bands appeared also at 1556 cm−1 and 1443 cm−1, characteristic of the –N–H bending and –C–H bending (indicative of amide or aliphatic structures); the signal at 1337 cm−1 can possibly be attributed to the –CH2 wagging or –C–N stretching. Moreover, the band at 1042 cm−1 overlaps with Si–O–Si, but increased intensity or shift is likely due to –C–O or –C–N stretching from the polymer; these shifts and intensity changes in Si–O–Si vibrations suggest interactions between the polymer and the silica surface. Characteristic polyelectrolyte peaks appear in the coated sample (F200@P5%) that are absent in bare F200. The shell deposition introduces new functional groups, –C=O (1734 cm−1), –N–H (1631 and 1532 cm−1) and –C–H (1383 cm−1), effectively indicating the qualitative presence of the organic shell onto the sand surface. Differences in intensity and slight shifts in the Si–O peaks between F100 and F200 may arise from differences in particle sizes, which can affect surface area and its interaction with the polymer. The SEM images showed that the irregular shape of sand particles is not affected by the deposition of the organic shell. Moreover, this shell covered each single solid particle (Figure 4A).

Figure 4.
(A) SEM images and (B) EDAX [C]/[Si] atomic ratio of bare (F100, F200) and modified (F100@P5%, F200@P5%) sand particles (EDAX results were obtained as averages of four selected areas).
Some composite inter-particle agglomeration could be observed after the drying process at 40 °C due to PMANa stickiness properties, but this phenomenon has a low effect at the macroscopic scale, where composite samples were presented, also, like a powder. To confirm the results from FT-IR spectroscopy, the composite particles were characterized by EDAX (Figure 4B). Considering that the CK photoelectrons from the K atomic layer were the fingerprint of carbon atoms from the organic shell and SiK, the photoelectrons from the Si atoms of the sand, it was possible to estimate the concentration of elements present on the composite surface. From the atomic ratio CK:SiK recorded before and after the deposition of the organic shell, the successful fabrication of the core-shell composites was demonstrated. To test the versatility of the F100@P5% and F200@P5% composite particles toward large molecules, such as the anionic dye IC, sorption/desorption experiments were carried out in dynamic conditions. In the first column test, the influence of the deposited shell on the bed height and on the pressure drop inside the column filled with modified and unmodified sand was investigated as a function of the pure water flow rate (Figure 5).
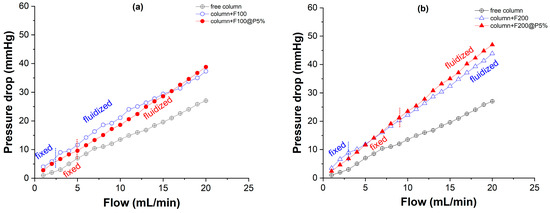

Figure 5.
(a,b) Pressure drop and (c,d) bed height of bare sand (F100 and F200) and sand composites (F100@P5% and F200@P5%) measured inside a column as function of the water flow rate.
Measuring the pressure drop inside a filled column with a digital manometer is very important because this parameter dictates the energy consumption of the pump and offers an estimation of the physical properties (fluidization) of the filler material inside the column. In this study, it was shown that smaller particles (F100, F100@P5%) presented a lower pressure drop (20 mmHg) compared with higher size particles (F200, F200@P5%) at the same flow rate (20 mL/min, Figure 1a,b). Also, small differences (2–4 mmHg) could exist between the unmodified and modified sand particles, demonstrating that the cross-linked organic shell (5 w/w%) has a low effect on the physical macroscopic properties of the composite, such as fluidity. By measuring the bed height as a function of the flow rate, the threshold flow rate of passing from a fixed-bed to a fluidized-bed for the bare sand and composites was determined. In all cases, the composites presented a higher threshold compared with the bare sand, this fact being attributed to the particle aggregation process, which can occur during the deposition of the interpolyelectrolyte complexes (Figure 5c,d). Knowing the relation between pressure drop, bed height and flow rate inside the filled column is very important in the subsequent design of the sorption experiments. Therefore, the composite microparticles F100@P5% and F200@P5% were used in the sorption of a model anionic dye (IC). The IC sorbed amount and pressure drop inside the column were evaluated at two flow rates and constant initial concentration (CIC = 60 mg/L) as a function of effluent volume (Figure 6).
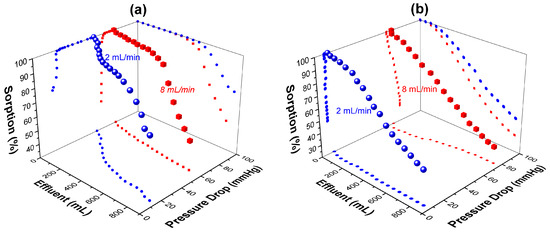

Figure 6.
Evaluation of the IC sorption capacity and pressure drop inside the column at 2 mL/min (spheres) and 8 mL/min (cubes) flow rates for (a) F100@P5% and (b) F200@P5% composite particles.
At a low flow rate (2 mL/min) of influent, both types of composites sorbed the IC in a fixed-bed state inside the column, but the lower-sized composites (F100@P5%) presented the highest sorption capacity compared to the higher-sized composites (F200@P5%). This fact could be attributed to the external surface, which is higher in the case of the smaller composites, therefore more active sorption sites were exposed to the solution flow. At a higher flow rate (8 mL/min), four times higher than the previous, the sorption capacity of both types of composites slightly decreased (by 20%–30%), demonstrating that the sorption efficacy of 100% in non-competitive conditions could be maintained even at high flow rates, irrespective if the composites are in fixed- or fluidized-bed states. During the sorption of the dye on the F100@P5%, the pressure drop inside the column decreased, irrespective if the column bed was fixed or fluidized. This fact could be attributed to the shrinkage of the composite shell under IC influence. The anionic dye, with two sulfonic groups on each molecule, could electrostatically interact with different amino groups from different PEI chains, thus reducing the mobility/flexibility of the entire polymeric network deposited on the sand particles. In the case of high-size composites (F200@P5%), the pressure drop slowly decreased at a low flow rate, confirming the same mechanism as above, but increased at a high flow rate, showing the blocking of capillary flow channels inside the material due to inhomogeneous loading of IC inside the composite organic part. The sorption efficiency of these composites in non-competitive conditions was influenced by the size of the composites, more than the flow rate inside the column, demonstrating that these sand composites can be used under higher mechanical stress (high flow rate). The smaller composites, which showed the best performance, F100@P5%, were further investigated in dynamic conditions. To study the influence of the flow rate, the sorption was investigated at a constant initial concentration (60 mg/L) (Figure 7a). Further, the influence of the IC concentration was studied, at a constant flow rate (15 mL/min) (Figure 7b).

Figure 7.
Evaluation of the sorption capacity of the F100@P5% toward IC as a function of flow rate at (a) constant initial dye concentration (60 mg/L) and (b) as a function of the initial dye concentration at constant flow rate (15 mL/min).
At a high flow rate (8 mL/min and 15 mL/min), where the composite is in a fluidized state, it was observed that the sorption capacity in non-competitive conditions (sorption > 95%) decreased by half compared with the same composite at a low flow rate (1.5 mL/min), this fact being attributed to the lower time of equilibration for the IC sorption onto the composite surface at a high flow rate. Also, in the fluidized-bed state (flow rate of 8 mL/min or 15 mL/min), the composites presented the same shapes for the sorption curves, showing that the active groups on the composite surface are available immediately for the IC molecules’ sorption. The maximum sorption capacity (sorption ~10%) was reached at 1400 mL of effluent and did not depend on the flow rate or the state of material inside the column (fixed or fluidized) (Figure 7a). This fact demonstrates a relatively fast diffusion process inside the organic shell of the composite, all active sorption sites being available for IC interactions, sooner or later. On the other hand, by analyzing the influence of the initial dye concentration on the IC sorbed amount, at a high flow rate (15 mL/min, fluidized-bed), a small variation was observed, with the sorption of 14–16 mg IC/mL composite (Figure 7b). This fact demonstrates that the composite active sorption sites were available for IC molecules and do not depend on the IC concentration in aqueous solution, the diffusion coefficient being very small. The breakthrough volume, which represents the volume where the effluent concentration of IC reached 5% from the initial concentration, decreased with the increase in IC concentration, showing a limit of the sorption process controlled mainly by electrostatic attractive interactions (ionic exchange). The experimental sorption curves of IC onto F100@P5% were fitted to the Thomas and Yoon–Nelson models [32] by the linearization of the experimental data (Figure 8).

Figure 8.
Linear fit of experimental data according to (a) the Thomas and (b) Yoon–Nelson models for F100@P5%, at three IC concentrations (10, 20 and 60 mg/L).
The linear form of the Thomas model is
where Ci = initial dye concentration (influent) (mg·L−1); Ce = effluent concentration (mg·L−1); kTh = Thomas constant [mL·mg−1·min−1)]; qmax = sorption capacity (mg·mL−1); v = sorbent volume inside the column; and F = flow rate (mL/min).
ln(Ci/Ce − 1) = kTh·qmax·v/F − kTh·Ci·t
The Yoon–Nelson model (linear form) is
where Ci = initial dye concentration (mg·L−1); Ce = effluent concentration at time t (mg·L−1); kYN = rate velocity constant (1·min−1); τ = time needed for 50% breakthrough of composite (min). All the parameters obtained according to Thomas and Yoon–Nelson, calculated with (2) and (3), are included in Table 1.
ln(Ce/(Ci − Ce)) = kYN·t − τ·kYN

Table 1.
Parameters of the Thomas and Yoon–Nelson models for IC dynamic sorption onto F100@P5%.
As presented in Table 1, the R-square values, higher than 0.92, suggest a good fit to the experimental data, while the other parameters showed very good sorption properties of the modified sand in dynamic conditions under strong mechanical stress.
This demonstrated fact opens a new possibility of using these core-shell composite materials in solid-phase extraction, where traces of a certain molecule could be concentrated in a solid support, followed by a fast extraction in a small amount of solvent. Therefore, 6 L of a very diluted IC solution (0.2 mg/L) were passed through the column filled with sand composites (Figure 9).

Figure 9.
Solid-phase extraction of IC at a high flow rate from a diluted aqueous solution (0.2 mg/L) onto composite particles, followed by NaOH elution.
More than 95% of the amount of IC immobilized on the composite surface was eluted in approximately 3 mL of NaOH 0.1 M, the calculated concentration factor of 2000 times being more than satisfying. This single example underlines the above conclusion, highlighting the versatility of these composites toward anionic organic molecules, even at very low concentration in aqueous solutions, as well as their potential use in solid-phase extraction processes.
The fully loaded column, filled with sand composites and sorbed IC, was treated with NaOH 0.1 M (~30 mL) to remove the IC molecules and to regenerate the sorbent inside the column. The reusability capacity of this type of composite was tested in 10 consecutive sorption/desorption cycles (Figure 10).

Figure 10.
IC sorbed amount during multiple cycles onto F100@P5% sand composite (example: column cycle carried out on a 2.6 mL fluidized bed, under 8 mL/min flow).
The results obtained showed that the composites maintained their sorption capacity with very good yields, with less than 10% of the sorption capacity being lost after 10 cycles of use. Thus, the results presented above recommend this type of composite in water treatment and solid-phase extraction processes.
4. Conclusions
This study investigated the optimum conditions for the deposition of an interpolyelectrolyte complex based on PEI/PMANa on sand microparticles. Parameters such as pH, the component molar ratio and the polyelectrolyte solution concentration were investigated using DLS and rheology measurements. FT-IR and SEM/EDAX characterization studies showed that all the sand solid particles were covered with a good yield with a shell formed from a cross-linked organic network with a high number of functional groups. The newly synthesized sand@P5% composites were used in multiple sorption/desorption cycles of a model anionic dye (IC) in dynamic conditions. IC dynamic sorption tests showed that the amount of dye sorbed depended on the flow rate, initial dye concentration and composite size. The IC dynamic desorption process took place with very good yields (>98%) and low consumption of NaOH 0.1 M effluent solutions. This type of composite, based on sand and polyelectrolytes, presented average sorption properties toward anionic organic molecules (tens of mg/mL), which is rather low compared with other types of materials (hydrogels, nanoparticles, beads) where hundreds of mg/g support have been reported. Instead, our type of composite could be used in extreme conditions of high flow (10–20 mL/min) and low height fluidized bed, where sorption properties were the same as in the batch conditions for other diffusion-controlled sorbents (high waiting times for reaching sorption equilibrium). The obtained results demonstrated that sand composites based on cross-linked polyelectrolyte complexes could be very promising materials, inexpensive, environmentally friendly and cost-efficient, potentially being used during multiple sorption cycles for the immobilization of anionic organic molecules from aqueous media.
Author Contributions
Conceptualization, F.B.; methodology, F.B.; software, F.B.; validation, F.B.; formal analysis, F.B., L.-M.P., T.-A.C. and M.-M.Z.; investigation, F.B., L.-M.P., T.-A.C. and M.-M.Z.; resources, F.B.; data curation, F.B., L.-M.P., T.-A.C. and M.-M.Z.; writing—original draft preparation, F.B.; writing—review and editing, F.B. and M.M.; visualization, F.B.; supervision, F.B. and M.M.; project administration, F.B.; funding acquisition, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P1-PCE-2023-1545, within PNCDI IV.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IC | Indigo Carmine |
| PEI | Poly(ethyleneimine) |
| PMANa | Poly(sodium methacrylate) |
| DLS | Dynamic light scattering |
| EI | Ethyleneimine |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| SU | Structural unit |
| MANa | Sodium methacrylate |
| GA | Glutharaldehyde |
| ATR | Attenuated total reflectance |
References
- Álvarez-Ruiz, R.; Picó, Y. Analysis of emerging and related pollutants in aquatic biota. Trends Environ. Anal. Chem. 2020, 25, e00082. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Ruhí, A.; Acuña, V.; Barceló, D.; Huerta, B.; Mor, J.R.; Rodríguez-Mozaz, S.; Sabater, S. Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci. Total Environ. 2016, 540, 250–259. [Google Scholar] [CrossRef]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on ecotoxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Dovlatabadi, A.; Estiri, E.H.; Najafi, M.L.; Ghorbani, A.; Rezaei, H.; Behmanesh, M.; Momeni, E.; Gholizadeh, A.; Cristaldi, A.; Mancini, G.; et al. Bioaccumulation and health risk assessment of exposure to potentially toxic elements by consuming agricultural products irrigated with wastewater effluents. Environ. Res. 2022, 205, 112479. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dash, S.; Mahlknecht, J.; Kolok, A.; Dogra, S.; Kuroda, K.; Tobino, T.; Mora, A.; Kazmi, A.A.; Singh, R.; et al. Understanding the pathways, pollution and potential solutions pertaining to pesticides: Circular engineering for persistent chemicals. Curr. Opin. Environ. Sci. Health 2025, 46, 100638. [Google Scholar] [CrossRef]
- Sheraz, N.; Shah, A.; Haleem, A.; Iftikhar, F.J. Comprehensive assessment of carbon-, biomaterial- and inorganic-based adsorbents for the removal of the most hazardous heavy metal ions from wastewater. RSC Adv. 2024, 14, 11284–11310. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Bharti, M.; Das, P.P.; Purkait, M.K. A review on the treatment of water and wastewater by electrocoagulation process: Advances and emerging applications. J. Environ. Chem. Eng. 2023, 11, 111558. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhou, W.; Shen, L.; Li, B.; Sun, H.; Zeng, Q.; Tang, C.Y.; Lin, H.; Chung, T.S. New directions on membranes for removal and degradation of emerging pollutants in aqueous systems. Water Res. 2024, 251, 121111. [Google Scholar] [CrossRef]
- Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research progress of graphene-based nanomaterials for the environmental remediation. Chin. Chem. Lett. 2020, 31, 1462–1473. [Google Scholar] [CrossRef]
- Pan, X.; Kong, F.; Xing, M. Spatial separation of photo-generated carriers in g-C3N4/MnO2/Pt with enhanced H2 evolution and organic pollutant control. Res. Chem. Interm. 2022, 48, 2837–2855. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Hou, J.; Wang, Y.; Lin, D. A review on the industrial waste based adsorbents for the removal of pollutants from water: Modification methods and adsorption study. Resour. Environ. Sustain. 2025, 19, 100183. [Google Scholar] [CrossRef]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An Overview on Composite Sorbents Based on Polyelectrolytes Used in Advanced Wastewater Treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process Eng. 2022, 46, 102641. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Alkhaldi, H.; Alharthi, S.; Alharthi, S.; AlGhamdi, H.A.; AlZahrani, Y.M.; Mahmoud, S.A.; Amin, L.G.; Al-Shaalan, N.H.; Boraie, W.E.; Attia, M.S.; et al. Sustainable polymeric adsorbents for adsorption-based water remediation and pathogen deactivation: A review. RSC Adv. 2024, 14, 33143–33190. [Google Scholar] [CrossRef]
- He, Y.; Shi, Y.; Li, L.; Zhang, Z.; Cao, N.; Zhang, L.; Zhang, Y.; Zhang, K. Recent progress on the porous cyclodextrin polymers in water treatment. Coord. Chem. Rev. 2025, 541, 216826. [Google Scholar] [CrossRef]
- Donga, C.; Ratshiedana, R.; Kuvarega, A.T.; Masunga, N.; Vallabhapurapu, V.S.; Mbule, P. Photocatalytic degradation of organic pollutants in wastewater using magnetic functionalized reduced graphene oxide nanocomposites. A review. Talanta 2025, 295, 128318. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Z.; Wang, S.; Chen, J.; Hu, B.; Shen, C.; Wang, X. Carbon-based nanocomposites for the elimination of inorganic and organic pollutants through sorption and catalysis strategies. Sep. Purif. Technol. 2023, 308, 122862. [Google Scholar] [CrossRef]
- Maciel, A.P.; Gomide, G.; Da Silva, F.G.A.A.M.; Guerra, A.M.; Estevez, G.; Maciel, A.P.; Gomide, G.; Da Silva, A.A.A.M.; Guerra, J.; Depeyrot, A.; et al. L-Lysine-Coated Magnetic Core–Shell Nanoparticles for the Removal of Acetylsalicylic Acid from Aqueous Solutions. Nanomaterials 2023, 13, 514. [Google Scholar] [CrossRef]
- Chkirida, S.; El Mernissi, N.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. In-situ magnetic alginate coated chitosan core@shell beads with excellent performance in simulated and real wastewater treatment: Behavior, mechanisms, and new perspectives. Int. J. Biol. Macromol. 2024, 260, 129389. [Google Scholar] [CrossRef]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping polyelectrolyte composites for heavy metals adsorption from wastewater: Experimental assessment and equilibrium studies. J. Environ. Manage. 2022, 321, 115999. [Google Scholar] [CrossRef]
- Bucatariu, F.; Zaharia, M.M.; Petrila, L.M.; Simon, F.; Mihai, M. Sand/polyethyleneimine composite microparticles: Eco-friendly, high selective and efficient heavy metal ion catchers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129540. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).