Oxyanion Recovery from Wastewater with Special Reference to Selenium Uptake by Marine Macroalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Substances and Analytical Methods
2.2. Preparation of Biomass
- (i)
- Arsenic and selenium exhibit a high affinity for iron, and numerous studies in the literature have explored the efficacy of activated carbons and biomaterials pre-treated with iron for the adsorption of these elements [56,57,58,59,60,61]. For the iron pre-treatment in this study, an in-house method was applied, using 10 g L−1 of untreated algae contacted with a 0.05 mol L−1 FeCl3 solution for 24 h under agitation (150–200 rpm) at a pH range of 2.8–3.5. Following treatment, the algae were rinsed with distilled water in multiple cycles until the rinse water reached a neutral pH. The resulting iron-loaded algae, designated as M-Fe (S. muticum) and N-Fe (A. nodosum), were then dried at 60 °C and stored in a desiccator until further use.
- (ii)
- For protonation, the virgin seaweed was treated with an 8 g L−1 concentration of algae in a 1 mol L−1 HNO3 solution for 6 h under agitation at 150–200 rpm. After treatment, the algae were rinsed with distilled water through several cycles until the wash water reached a pH of approximately 4–4.5 [62]. The protonated algae were then dried in an oven at 60 °C and stored in a desiccator. The acid-treated algae were designated as protonated seaweeds: S. muticum (MP), A. nodosum (NP), U. rigida (UP), and C. sericea (CP).
- (iii)
- The chemical modification of the surfaces of Sargassum muticum, Cladophora sericea with ammonium followed the same procedure as described by Filote et al. [63]. The pre-treatment involved stirring the algae (at a dosage of 10 g L−1) with a 0.1 mol L−1 ammonia solution, which was prepared by diluting a commercial 25% NH3 solution (analytical grade), for a period of 24 h. After solid–liquid separation through filtration and washing, the ammonia-treated algae, designated as MA and CA, were dried at 60 °C. This pre-treatment aims to create a positively charged surface, which may enhance the retention of oxyanions.
2.3. Seaweeds Characterization
2.4. Effect of pH
2.5. Biosorption Kinetics and Equilibrium
2.6. Competing Ions Impact
3. Results and Discussion
3.1. Biosorbent Characterization
3.2. Results Attained After the Tested Pre-Treatments
3.3. Cladophora Sericea and Selenium Removal
3.3.1. Effect of pH and Removal Mechanism
3.3.2. Kinetics and Modeling of Biosorption
3.3.3. Equilibrium Study and Comparative Approach
3.3.4. Studies on Interfering Ions Influence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Agents Classifed by the IARC Monographs; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Fernandez-Martinez, A.; Charlet, L. Selenium Environmental Cycling and Bioavailability: A Structural Chemist Point of View. Rev. Environ. Sci. Bio Technol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Sarı, A.; Çıtak, D.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on adsorption of Sb(III) from aqueous solution using low-cost natural diatomite. Chem. Eng. J. 2010, 162, 521–527. [Google Scholar] [CrossRef]
- Jha, P.K.; Tripathi, P. Arsenic and fluoride contamination in groundwater: A review of global scenarios with special reference to India. Groundw. Sustain. Dev. 2021, 13, 100576. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Nath, B.; Stüben, D.; Mallik, S.B.; Chatterjee, D.; Charlet, L. Mobility of arsenic in West Bengal aquifers conducting low and high groundwater arsenic. Part I: Comparative hydrochemical and hydrogeological characteristics. Appl. Geochem. 2008, 23, 977–995. [Google Scholar] [CrossRef]
- Sánchez-Rodas, D.; Luis Gómez-Ariza, J.; Giráldez, I.; Velasco, A.; Morales, E. Arsenic speciation in river and estuarine waters from southwest Spain. Sci. Total Environ. 2005, 345, 207–217. [Google Scholar] [CrossRef]
- Modabberi, S.; Moore, F. Environmental geochemistry of Zarshuran Au-As deposit, NW Iran. Environ. Geol. 2004, 46, 796–807. [Google Scholar] [CrossRef]
- Issanov, A.; Adewusi, B.; Saint-Jacques, N.; Dummer, T.J.B. Arsenic in drinking water and lung cancer: A systematic review of 35 years of evidence. Toxicol. Appl. Pharmacol. 2024, 483, 116808. [Google Scholar] [CrossRef]
- Reimann, C.; Matschullat, J.; Birke, M.; Salminen, R. Antimony in the environment: Lessons from geochemical mapping. Appl. Geochem. 2010, 25, 175–198. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking—Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar] [CrossRef] [PubMed]
- Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals 2017, 7, 46. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Prasad, B.; Gulipalli, S. Adsorption of Selenium Using Bagasse Fly Ash. CLEAN Soil Air Water 2009, 37, 534–543. [Google Scholar] [CrossRef]
- Twidwell, L.; McCloskey, J.; Joyce, H.; Dahlgren, E.; Hadden, A. Removal of Selenium Oxyanions from Mine Waters Utilizing Elemental Iron and Galvanically Coupled Metals. In Innovations in Natural Resoure Processing—Proceedings of the Jan. D. Miller Symposium, SME; Society for Mining, Metallurgy, and Exploration, Incorporated: Englewood, CO, USA, 2005; Volume 2005. [Google Scholar]
- Lemly, A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Mason, P.R.D.; Van Cappellen, P.; Johnson, T.M.; Gill, B.C.; Owens, J.D.; Diaz, J.; Ingall, E.D.; Reichart, G.-J.; Lyons, T.W. Selenium as paleo-oceanographic proxy: A first assessment. Geochim. Cosmochim. Acta 2012, 89, 302–317. [Google Scholar] [CrossRef]
- Stefaniak, J.; Dutta, A.; Verbinnen, B.; Shakya, M.; Rene, E. Selenium removal from mining and process wastewater: A systematic review of available technologies. J. Water Supply Res. Technol. AQUA 2018, 67, 903–918. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Pommier, A.L.; Simon, S.; Buzier, R.; Guibaud, G. Evaluation of a mercapto-functionalized silica binding phase for the selective sampling of Se(IV) by Diffusive Gradients in Thin films. Talanta 2019, 199, 590–595. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Taylor, J.B.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S. Maternal and fetal tissue selenium loads in nulliparous ewes fed supranutritional and excessive selenium during mid- to late pregnancy1,2. J. Anim. Sci. 2009, 87, 1828–1834. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in saltwater fish from New Jersey: Individual and species variability complicate use in human health fish consumption advisories. Environ. Res. 2012, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ni, S.; Pei, C.; Sun, L.; Wu, L.; Xu, A.; Nie, Y.; Liu, Y. Parental treatment with selenium protects Caenorhabditis elegans and their offspring against the reproductive toxicity of mercury. Sci. Total Environ. 2024, 912, 169461. [Google Scholar] [CrossRef]
- Saliba, W.; El Fakih, R.; Shaheen, W. Heart failure secondary to selenium deficiency, reversible after supplementation. Int. J. Cardiol. 2010, 141, e26–e27. [Google Scholar] [CrossRef]
- Li, H.; Jia, L.; Deng, Z.; Sun, X.; Zhang, H.; Li, H. The effects of selenium on the growth and bone development in the weaned rats. Food Biosci. 2023, 55, 103018. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New insights into traditional health risk assessments of mercury exposure: Implications of selenium. Environ. Sci. Technol. 2014, 48, 1206–1212. [Google Scholar] [CrossRef]
- Hammouh, F.; Zein, S.; Amr, R.; Ghazzawi, H.; Muharib, D.; Saad, D.; Subih, H. Assessment of dietary selenium intake of Jordanian adults in Madaba: A cross sectional study. Nutr. Food Sci. 2020, 51, 494–506. [Google Scholar] [CrossRef]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radical Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Herrero Latorre, C.; Barciela García, J.; García Martín, S.; Peña Crecente, R.M. Solid phase extraction for the speciation and preconcentration of inorganic selenium in water samples: A review. Anal. Chim. Acta 2013, 804, 37–49. [Google Scholar] [CrossRef]
- Okonji, S.O.; Achari, G.; Pernitsky, D. Environmental Impacts of Selenium Contamination: A Review on Current-Issues and Remediation Strategies in an Aqueous System. Water 2021, 13, 1473. [Google Scholar] [CrossRef]
- Khakpour, H.; Younesi, H.; Mohammadhosseini, M. Two-stage biosorption of selenium from aqueous solution using dried biomass of the baker’s yeast Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2014, 2, 532–542. [Google Scholar] [CrossRef]
- Somogyi, Z.; Kádár, I.; Kiss, I.; Juríková, T.; Szekeres, L.; Balla, Š.; Nagy, P.; Bakonyi, G. Comparative toxicity of the selenate and selenite to the potworm Enchytraeus albidus (Annelida: Enchytraeidae) under laboratory conditions. Eur. J. Soil Biol. 2012, 50, 159–164. [Google Scholar] [CrossRef]
- Somogyi, Z.; Kiss, I.; Kádár, I.; Bakonyi, G. Toxicity of selenate and selenite to the potworm Enchytraeus albidus (Annelida: Enchytraeidae): A laboratory test. Ecotoxicology 2007, 16, 379–384. [Google Scholar] [CrossRef]
- Albert, M.; Demesmay, C.; Rocca, J.L. Analysis of organic and non-organic arsenious or selenious compounds by capillary electrophoresis. Fresenius’ J. Anal. Chem. 1995, 351, 426–432. [Google Scholar] [CrossRef]
- Amoako, P.O.; Uden, P.C.; Tyson, J.F. Speciation of selenium dietary supplements; formation of S-(methylseleno)cysteine and other selenium compounds. Anal. Chim. Acta 2009, 652, 315–323. [Google Scholar] [CrossRef]
- Ebert, R.; Jakob, F. Selenium deficiency as a putative risk factor for osteoporosis. Int. Congr. Ser. 2007, 1297, 158–164. [Google Scholar] [CrossRef]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi Damuchali, A.; McPhedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Filote, C.; Santos, S.C.R.; Boaventura, R.A.R.; Volf, I.; Botelho, C.M.S. Antimony oxyanions uptake by green marine macroalgae. J. Environ. Chem. Eng. 2016, 4, 3441–3450. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.C.R.; Volf, I.; Boaventura, R.A.R.; Botelho, C.M.S. Biosorption of antimony oxyanions by brown seaweeds: Batch and column studies. J. Environ. Chem. Eng. 2017, 5, 3463–3471. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2010, 158, 200–206. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.-C.; Zhou, C.; Gao, W.; Xia, S.; He, S.; Zhou, W. Development of an algal treatment system for selenium removal: Effects of environmental factors and post-treatment processing of Se-laden algae. J. Hazard. Mater. 2019, 365, 546–554. [Google Scholar] [CrossRef]
- Pereira, L. As Algas Marinhas e Respectivas Utilidades; Universidade de Coimbra: Coimbra, Portugal, 2010. [Google Scholar]
- Petrescu, R. Aurul Verde din Marea Neagra. Green Report. 2010. Available online: http://www.green-report.ro/aurul-verde-din-marea-neagra/ (accessed on 28 February 2025).
- Akbari, M.; Hallajisani, A.; Keshtkar, A.R.; Shahbeig, H.; Ali Ghorbanian, S. Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)-Co(II) single and binary mixtures on brown algae C. indica. J. Environ. Chem. Eng. 2015, 3, 140–149. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Cukrowska, E.M.; Weiersbye, I.M.; Mihaly-Cozmuta, L.; Peter, A.; Tutu, H. Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.). J. Geochem. Explor. 2014, 144, 492–503. [Google Scholar] [CrossRef]
- Brouers, F.; Al-Musawi, T.J. On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J. Mol. Liq. 2015, 212, 46–51. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Keshtkar, A.R.; Safdari, J.; Abadi, Z. Biosorption of nickel(II) from aqueous solution by brown algae: Equilibrium, dynamic and thermodynamic studies. J. Hazard. Mater. 2010, 175, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xing, J.; Tang, J.; Wang, Z.; Zhang, C.; Wang, Q.; Xiao, X.; Huang, W. Polyethyleneimine-modified iron-doped birnessite as a highly stable adsorbent for efficient arsenic removal. J. Colloid Interface Sci. 2024, 661, 164–174. [Google Scholar] [CrossRef]
- Dechdacho, P.; Howard, S.; Hershey, R.L.; Parashar, R.; Perez, L.J. Effective removal of arsenic from contaminated groundwater using an iron-based metal-organic framework. Environ. Technol. Innov. 2023, 32, 103406. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, W.; Sun, S.; Sun, X.; Zeng, Y.; Hao, R.; Zhang, J.; Li, D. Facile preparation of maghemite based on iron sludge for arsenic removal from water. Sci. Total Environ. 2024, 906, 167575. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, C.; Wang, F.; Zhang, J.; Li, D. Disposal of iron-manganese sludge from waterworks and its potential for Arsenic removal. J. Environ. Chem. Eng. 2022, 10, 108480. [Google Scholar] [CrossRef]
- Gupta, A.R.; Mondal, M.; Bapat, P.S.; Joshi, V.C.; Popat, K.M.; Indurkar, P.D.; Sharma, S. Construction of arsenic selective chelating resin with iron precursor for removal of low-concentration arsenic: Breakthrough modeling and field deployment. J. Hazard. Mater. 2023, 459, 132000. [Google Scholar] [CrossRef]
- Yoon, K.; Kwon, G.; Kim, E.; Rinklebe, J.; Song, H. Production of Fe-biochar from paper-mill sludge and its application to Se(VI) and Se(IV) removal. Chem. Eng. J. 2024, 484, 149470. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Biosorption of antimony by brown algae S. muticum and A. nodosum. Environ. Eng. Manag. J. 2015, 14, 455–463. [Google Scholar] [CrossRef]
- Filote, C.; Ungureanu, G.; Boaventura, R.; Santos, S.; Volf, I.; Botelho, C. Green macroalgae from the Romanian coast of Black Sea: Physico-chemical characterization and future perspectives on their use as metal anions biosorbents. Process Saf. Environ. Prot. 2017, 108, 34–43. [Google Scholar] [CrossRef]
- NREL. Biomass Compositional Analysis Laboratory Providing Detailed and Accurate Characterization; National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy: Golden, CO, USA, 2014.
- Pregl, F.; Roth, H. Quantitative Organische Mikroanalyse; Springer: Berlin/Heidelberg, Germany, 1949. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Schöniger, W. Eine mikroanalytische Schnellbestimmung von Halogen in organischen Substanzen. Microchim. Acta 1955, 43, 123–129. [Google Scholar] [CrossRef]
- Schöniger, W. Die mikroanalytische Schnellbestimmung von Halogenen und Schwefel in organischen Verbindungen. Microchim. Acta 1956, 44, 869–876. [Google Scholar] [CrossRef]
- Bordoloi, S.; Nath, S.K.; Gogoi, S.; Dutta, R.K. Arsenic and iron removal from groundwater by oxidation–coagulation at optimized pH: Laboratory and field studies. J. Hazard. Mater. 2013, 260, 618–626. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Kazapoe, R.W.; Addai, M.O.; Amuah, E.E.Y.; Dankwa, P. Characterization of groundwater in southwest Ghana: Implications for sustainable agriculture and safe water supply in a mining-dominated zone. Environ. Sustain. Indic. 2024, 22, 100341. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Li, G.; Zhang, J.; Xie, F.; Zheng, L. Tracing sulfate sources and transformations of surface water using multiple isotopes in a mining-rural-urban agglomeration area. Ecotoxicol. Environ. Saf. 2024, 269, 115805. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Zhang, R.; Qie, W.; Shao, J.; Zhu, W.; Xu, N. Effects of photoperiod on the growth and physiological responses in Ulva prolifera under constant and diurnal temperature difference conditions. Mar. Environ. Res. 2024, 197, 106477. [Google Scholar] [CrossRef]
- Samarasinghe, M.B.; van der Heide, M.E.; Weisbjerg, M.R.; Sehested, J.; Sloth, J.J.; Bruhn, A.; Vestergaard, M.; Nørgaard, J.V.; Hernández-Castellano, L.E. A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Anim. Feed. Sci. Technol. 2021, 278, 115005. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.-K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef]
- Shen, W.; He, S.; Mu, M.; Cao, B.; Wang, S.; Naqvi, S.R.; Hanelt, D.; Abomohra, A. A comprehensive review on the intricate processes involved in algae pyrolysis mechanism and possible migration of undesirable chemical elements. J. Anal. Appl. Pyrolysis 2024, 177, 106365. [Google Scholar] [CrossRef]
- Kulikova, Y.; Sukhikh, S.; Kalashnikova, O.; Chupakhin, E.; Ivanova, S.; Chubarenko, B.; Gorbunova, J.; Babich, O. Assessment of the Resource Potential of Baltic Sea Macroalgae. Appl. Sci. 2022, 12, 3599. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Sadeh, M.S.; Hallajisani, A.; Hajikhani, M.; Moradi, M.; Alizadeh, O.; Nouralishahi, A. Investigation of catalytic pyrolysis of Azolla filiculoides and Ulva fasciata for bio-oil production. Biochem. Eng. J. 2022, 178, 108278. [Google Scholar] [CrossRef]
- Parsa, M.; Jalilzadeh, H.; Pazoki, M.; Ghasemzadeh, R.; Abdoli, M. Hydrothermal Liquefaction of Gracilaria gracilis and Cladophora glomerata macro-algae for biocrude production. Bioresour. Technol. 2017, 250, 26–34. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Moselhy, K.M.; Amer, A.; Abou El-Naga, E.H.; Mohamedein, L.I.; Al-Prol, A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014, 40, 235–242. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll. 2014, 37, 100–110. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.R.; Vilar, V.J.P. Brown macro-algae as natural cation exchangers for the treatment of zinc containing wastewaters generated in the galvanizing process. J. Clean. Prod. 2016, 119, 38–49. [Google Scholar] [CrossRef]
- Mazur, L.P.; Pozdniakova, T.A.; Mayer, D.A.; de Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Cation exchange prediction model for copper binding onto raw brown marine macro-algae Ascophyllum nodosum: Batch and fixed-bed studies. Chem. Eng. J. 2017, 316, 255–276. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef]
- Wu, F.; Sun, F.; Wu, S.; Yan, Y.; Xing, B. Removal of antimony(III) from aqueous solution by freshwater cyanobacteria Microcystis biomass. Chem. Eng. J. 2012, 183, 172–179. [Google Scholar] [CrossRef]
- Neal, R.H.; Sposito, G.; Holtzclaw, K.M.; Traina, S.J. Selenite adsorption on alluvial soils. I. Soil composition and pH effects. Soil Sci. Soc. Am. J. 1987, 51, 1161–1165. [Google Scholar] [CrossRef]
- El-Shafey, E.-S. Removal of Se(IV) from Aqueous Solution Using Sulfuric Acid-Treated Peanut Shell. J. Environ. Manag. 2007, 84, 620–627. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, E.-S. Sorption of Cd(II) and Se(IV) from Aqueous Solution Using Modified Rice Husk. J. Hazard. Mater. 2007, 147, 546–555. [Google Scholar] [CrossRef]
- Jordan, N.; Marmier, N.; Lomenech, C.; Giffaut, E.; Ehrhardt, J.-J. Competition between selenium (IV) and silicic acid on the hematite surface. Chemosphere 2009, 75, 129–134. [Google Scholar] [CrossRef]
- Sharrad, M.O.M.; Liu, H.; Fan, M. Evaluation of FeOOH performance on selenium reduction. Sep. Purif. Technol. 2012, 84, 29–34. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Selenium removal from water and its recovery using iron (Fe3+) oxide/hydroxide-based nanoparticles sol (NanoFe) as an adsorbent. Sep. Purif. Technol. 2013, 103, 167–172. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Zhang, N.; Lin, L.-S.; Gang, D. Adsorptive selenite removal from water using iron-coated GAC adsorbents. Water Res. 2008, 42, 3809–3816. [Google Scholar] [CrossRef]
- Martinez, M.; Giménez, J.; Pablo, J.; Rovira, M.; Duro, L. Sorption of Selenium (IV) and Selenium(VI) onto Magnetite. Appl. Surf. Sci. 2006, 252, 3767–3773. [Google Scholar] [CrossRef]
- Fadaei, A.; Mohammadian-Hafshejani, A. Selenium Removal from Water and Wastewater by Different Technologies: A Systematic Review. Iran. J. Public Health 2023, 52, 64–77. [Google Scholar] [CrossRef]
- Rajamohan, N.; Rajasimman, M. Biosorption of Selenium using activated plant based sorbent—Effect of variables, isotherm and kinetic modeling. Biocatal. Agric. Biotechnol. 2015, 4, 795–800. [Google Scholar] [CrossRef]
- Johansson, C.L.; Paul, N.A.; de Nys, R.; Roberts, D.A. Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J. Environ. Manag. 2016, 165, 117–123. [Google Scholar] [CrossRef] [PubMed]

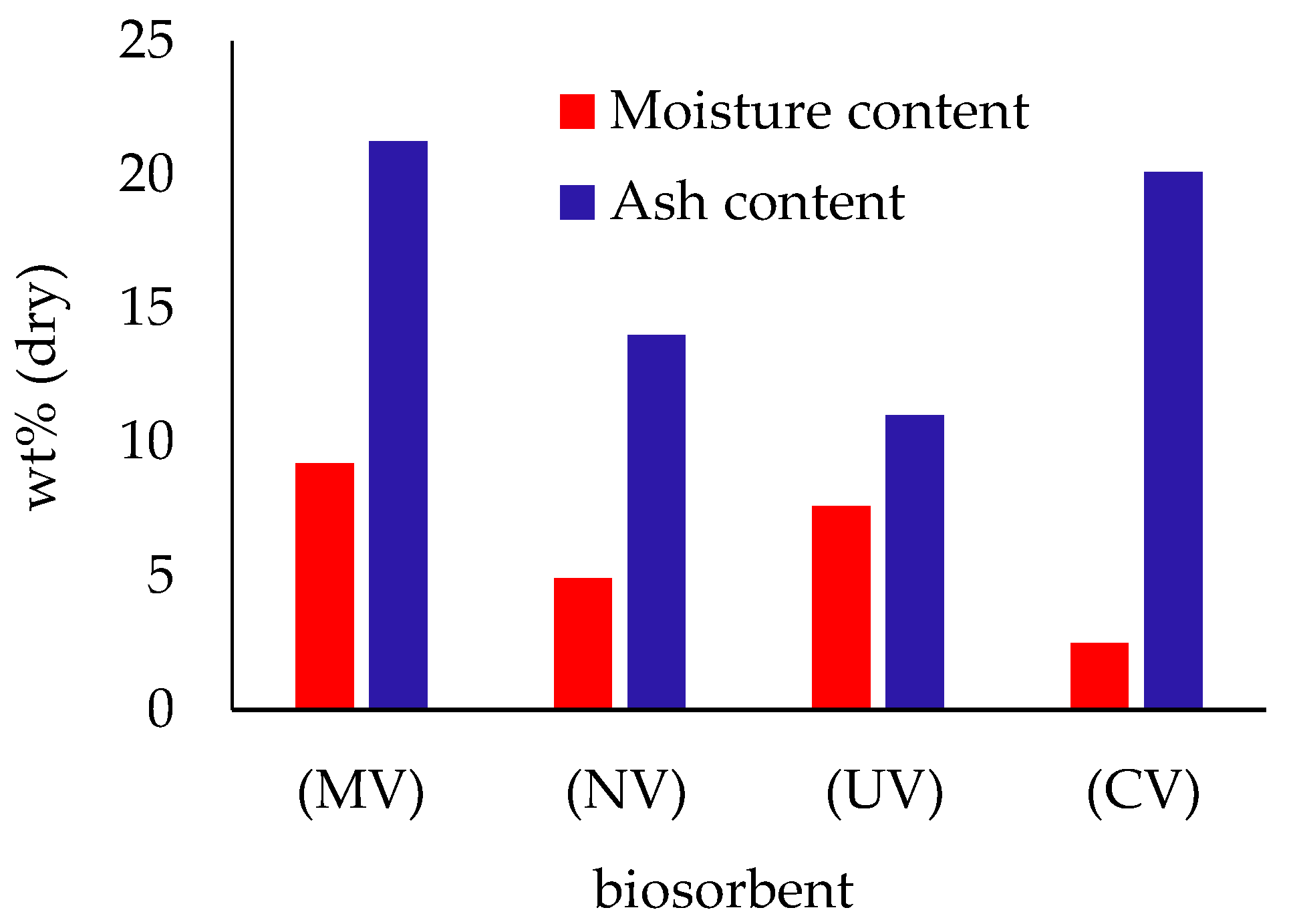
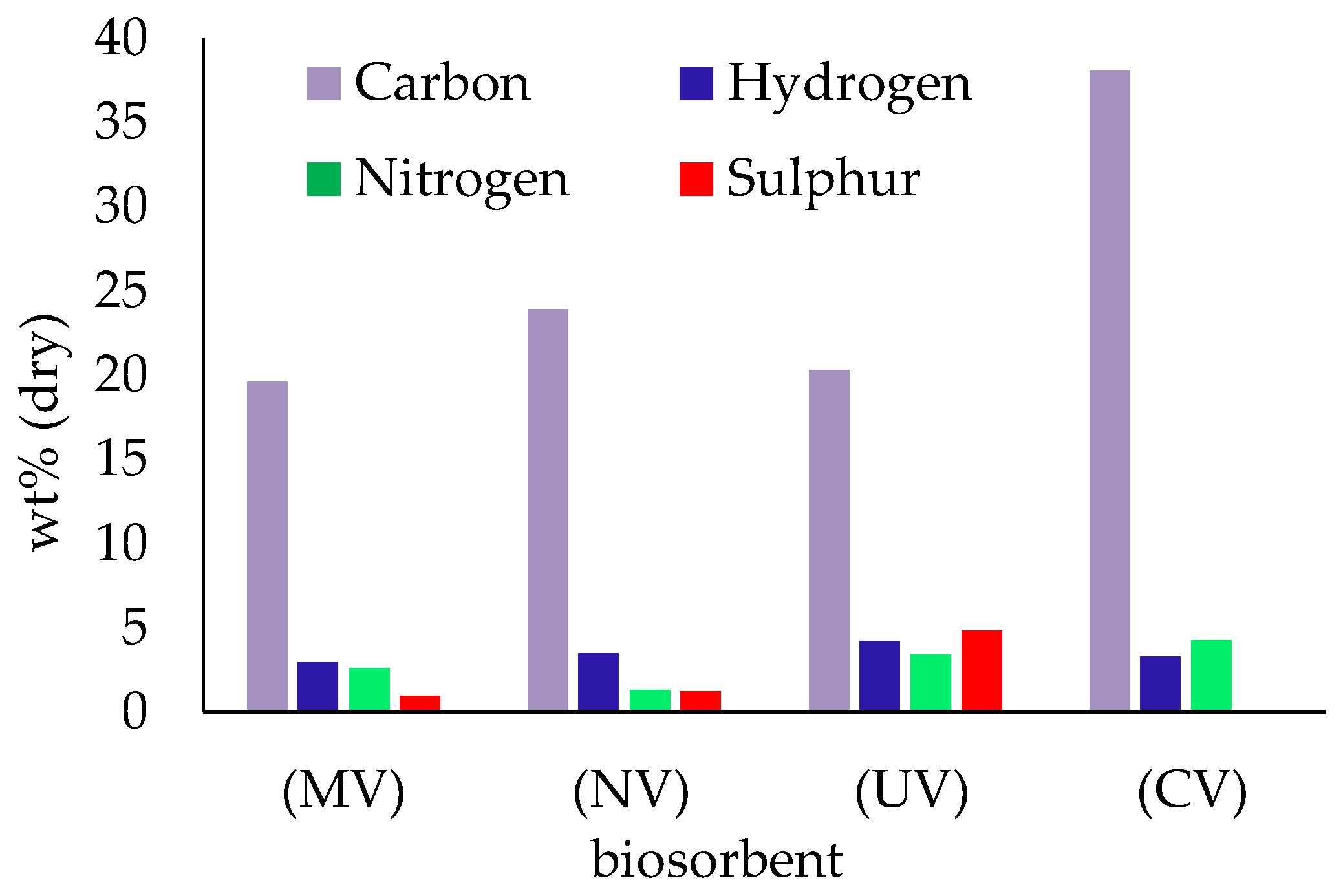

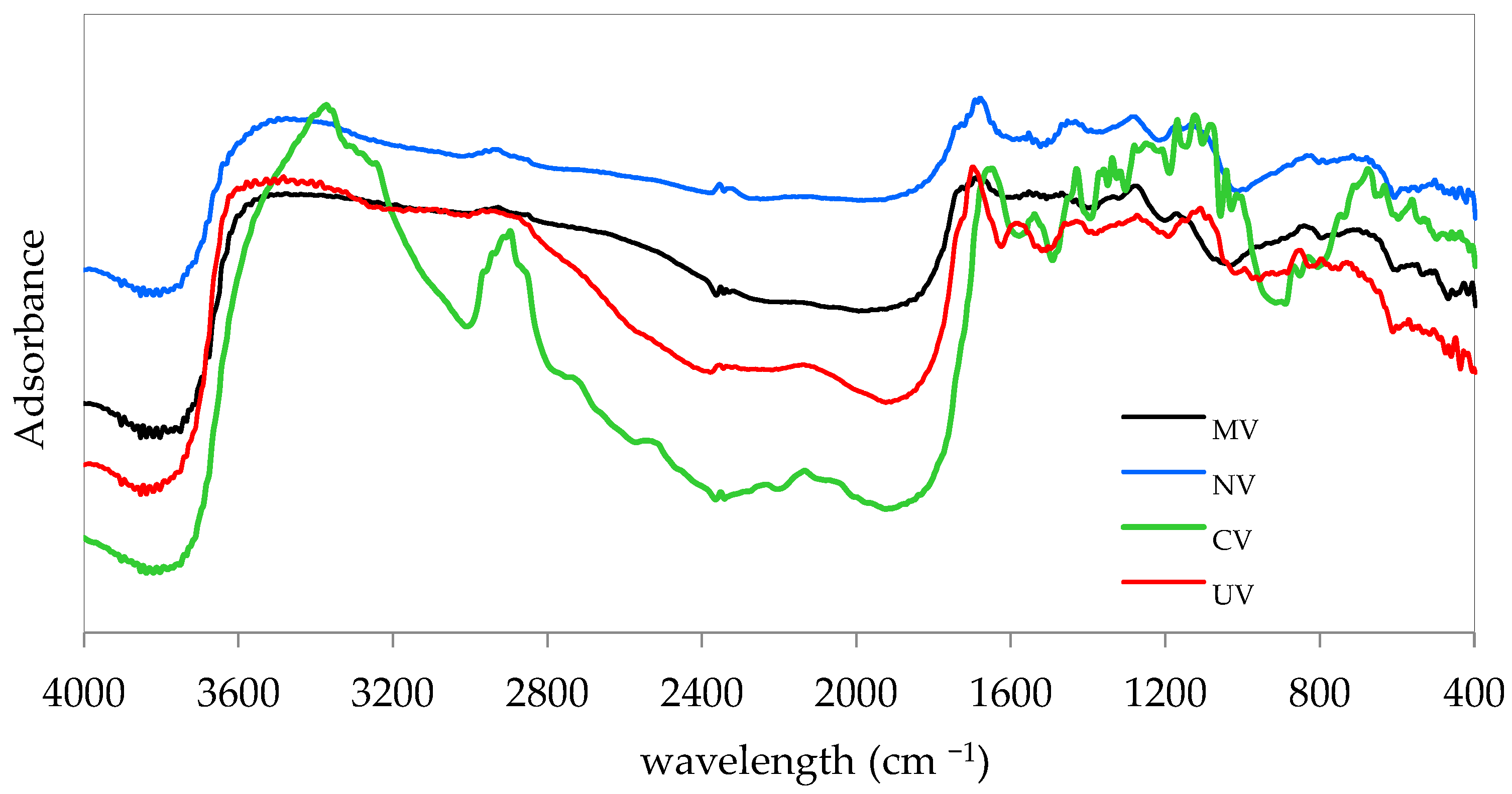


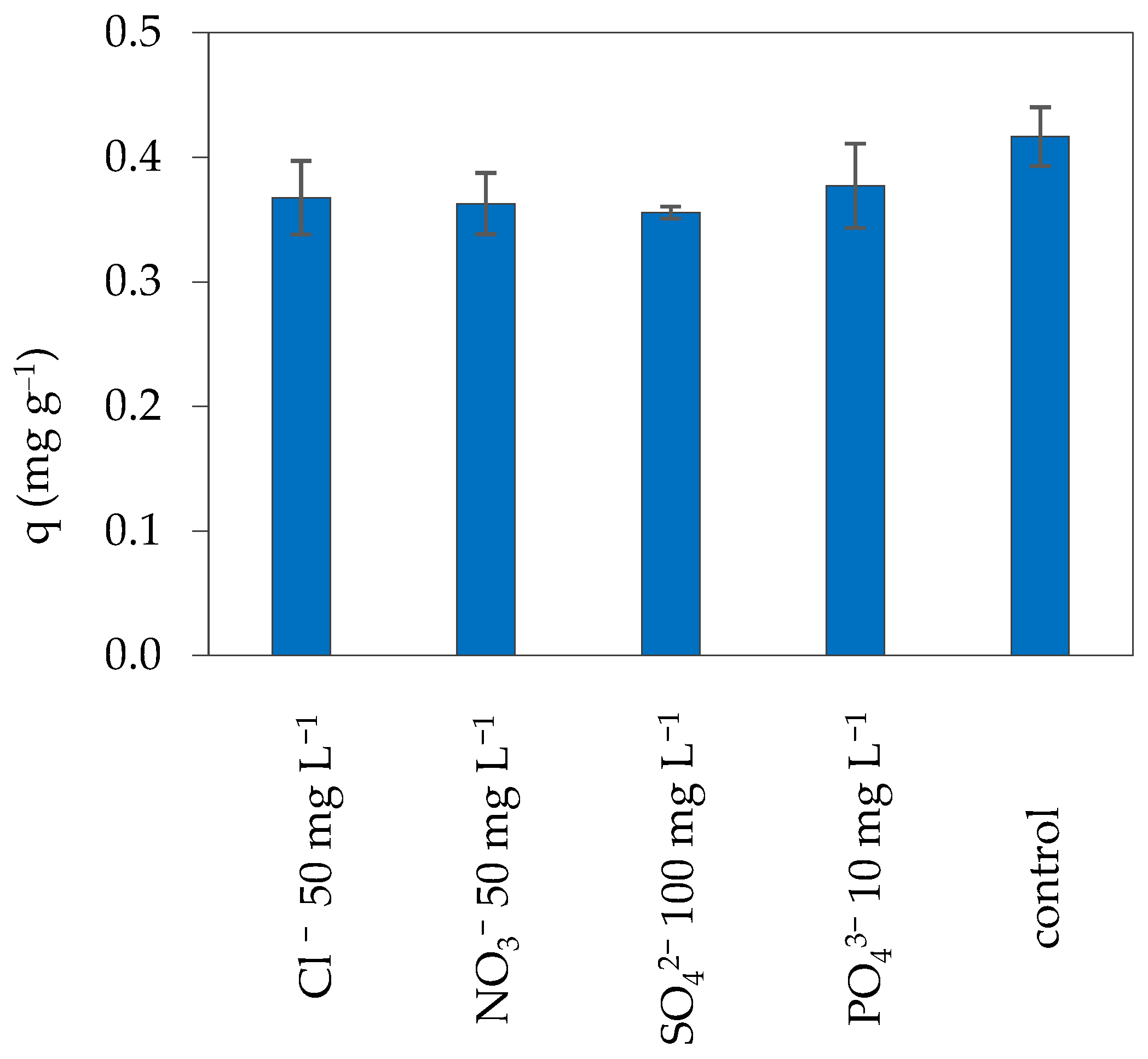
| Adsorbate | Adsorbent | C0 (mg L−1) | CS (g L−1) | pH | q (mg g−1) |
|---|---|---|---|---|---|
| As (III) | NV | 25 | 10 | 5; 7 | ≈0 |
| MV | 25 | 10 | 5; 7 | ≈0 | |
| CV | 25 | 10 | 5; 7 | ≈0 | |
| UV | 25 | 10 | 5; 7 | ≈0 | |
| UP | 25 | 10 | 3 | ≈0 | |
| CP | 25 | 10 | 3 | ≈0 | |
| N-Fe | 10 | 2 | 2; 2.5; 3; 4 | ≈0 | |
| As (V) | NV | 10 | 2 | 6 | ≈0 |
| MV | 10 | 2 | 6 | ≈0 | |
| UV | 25 | 10 | 5; 7 | ≈0 | |
| MP | 10 | 2 | 4 | 0.09 ± 0.01 | |
| NP | 10 | 2 | 4 | ≈0 | |
| UP | 25 | 10 | 3 | ≈0 | |
| CP | 25 | 10 | 3 | ≈0 | |
| MA | 25 | 10 | 7 | ≈0 | |
| CA | 25 | 10 | 7 | ≈0 | |
| Sb (III) | NV | 25 | 10 | 4 | 0.55 ± 0.02 |
| N-Fe | 10 | 2 | 1.5; 2; 3; 4 | 1.4(±0.1); 2.25(±0.04) | |
| MP | 25 | 10 | 2 | 2.21 ± 0.02 | |
| NP | 25 | 10 | 2 | 1.27 ± 0.03 | |
| Sb (V) | MV | 25 | 10 | 5 | 2.005 ± 0.001 |
| UV | 25 | 10 | 5 | 0.66 ± 0.01 | |
| UP | 25 | 10 | 2 | 0.93 ± 0.01 | |
| CP | 25 | 10 | 2 | 0.95 ± 0.02 | |
| Se (VI) | NV | 25 | 10 | 5 | ≈0 |
| MV | 25 | 10 | 5 | ≈0 | |
| CV | 25 | 10 | 5 | 0.12 ± 0.04 | |
| UV | 25 | 10 | 5 | ≈0 | |
| MP | 10 | 2 | 2; 3; 4 | ≈0 | |
| NP | 10 | 20 | 3 | 0.124 ± 0.004 | |
| N-Fe | 10 | 2 | 2; 3; 4 | 0.3–0.6 | |
| M-Fe | 10 | 2 | 2; 3; 4 | 0.5–0.7 |
| pH Range | Eh Range (mV) | Predominant Selenium Species |
|---|---|---|
| <3 | ≈500< | H2SeO3/+4 (Se (IV)) |
| 3–7 | ≈500–700 | HSeO3−/+4 (Se (IV)) |
| 7–14 | ≈300–500 | SeO32−/+4 (Se (IV)) |
| 4–10 | ≈700< | SeO42−/+6 (Se (VI)) |
| <2 | ≈1000< | HSeO4−/+6 (Se (VI)) |
| 3–10 | ≈200–400 | Se (elemental) |
| 2–4 | <−200 | H2Se/−2 (Se(-II)) |
| 4< | <−200 | HSe−/−2 (Se(-II)) |
| Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|
| C0 (mg L−1) | Cs (g L−1) | k1 (min−1) | qe (mg g−1) | SE (mg g−1) | k2 (g mg−1 min−1) | qe (mg g−1) | SE (mg g−1) |
| 25 | 5 | 0.03 ± 0.01 | 0.50 ± 0.06 | 0.03 | 0.07 ± 0.03 | 0.57 ± 0.05 | 0.02 |
| 25 | 10 | 0.07 ± 0.02 | 0.34 ± 0.03 | 0.02 | 0.2 ± 0.1 | 0.38 ± 0.04 | 0.02 |
| 25 | 20 | 0.016 ± 0.006 | 0.21 ± 0.03 | 0.01 | 0.07 ± 0.05 | 0.25 ± 0.05 | 0.01 |
| 10 | 10 | 0.03 ± 0.01 | 0.12 ± 0.01 | 0.009 | 0.3 ± 0.1 | 0.13 ± 0.01 | 0.005 |
| 1 | 10 | 0.13 ± 0.08 | 0.037 ± 0.003 | 0.001 | 8 ± 8 | 0.039 ± 0.003 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, G.; Pavel, V.L.; Volf, I. Oxyanion Recovery from Wastewater with Special Reference to Selenium Uptake by Marine Macroalgae. Appl. Sci. 2025, 15, 10946. https://doi.org/10.3390/app152010946
Ungureanu G, Pavel VL, Volf I. Oxyanion Recovery from Wastewater with Special Reference to Selenium Uptake by Marine Macroalgae. Applied Sciences. 2025; 15(20):10946. https://doi.org/10.3390/app152010946
Chicago/Turabian StyleUngureanu, Gabriela, Vasile Lucian Pavel, and Irina Volf. 2025. "Oxyanion Recovery from Wastewater with Special Reference to Selenium Uptake by Marine Macroalgae" Applied Sciences 15, no. 20: 10946. https://doi.org/10.3390/app152010946
APA StyleUngureanu, G., Pavel, V. L., & Volf, I. (2025). Oxyanion Recovery from Wastewater with Special Reference to Selenium Uptake by Marine Macroalgae. Applied Sciences, 15(20), 10946. https://doi.org/10.3390/app152010946






