A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications
Abstract
Featured Application
Abstract
1. Introduction
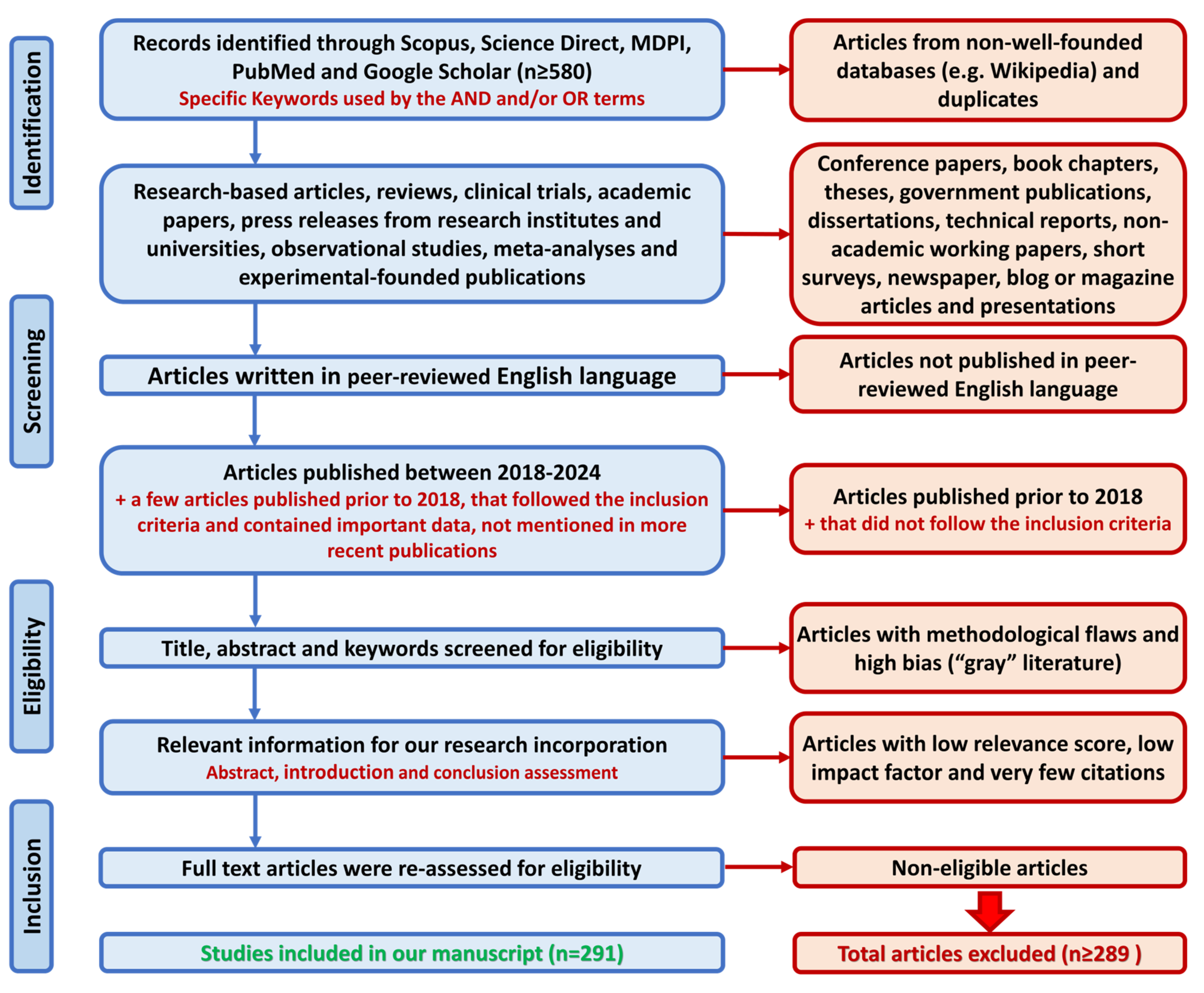
2. Methods
3. Vitamin D’s Structural Profile, Skincare Applications, and Functions
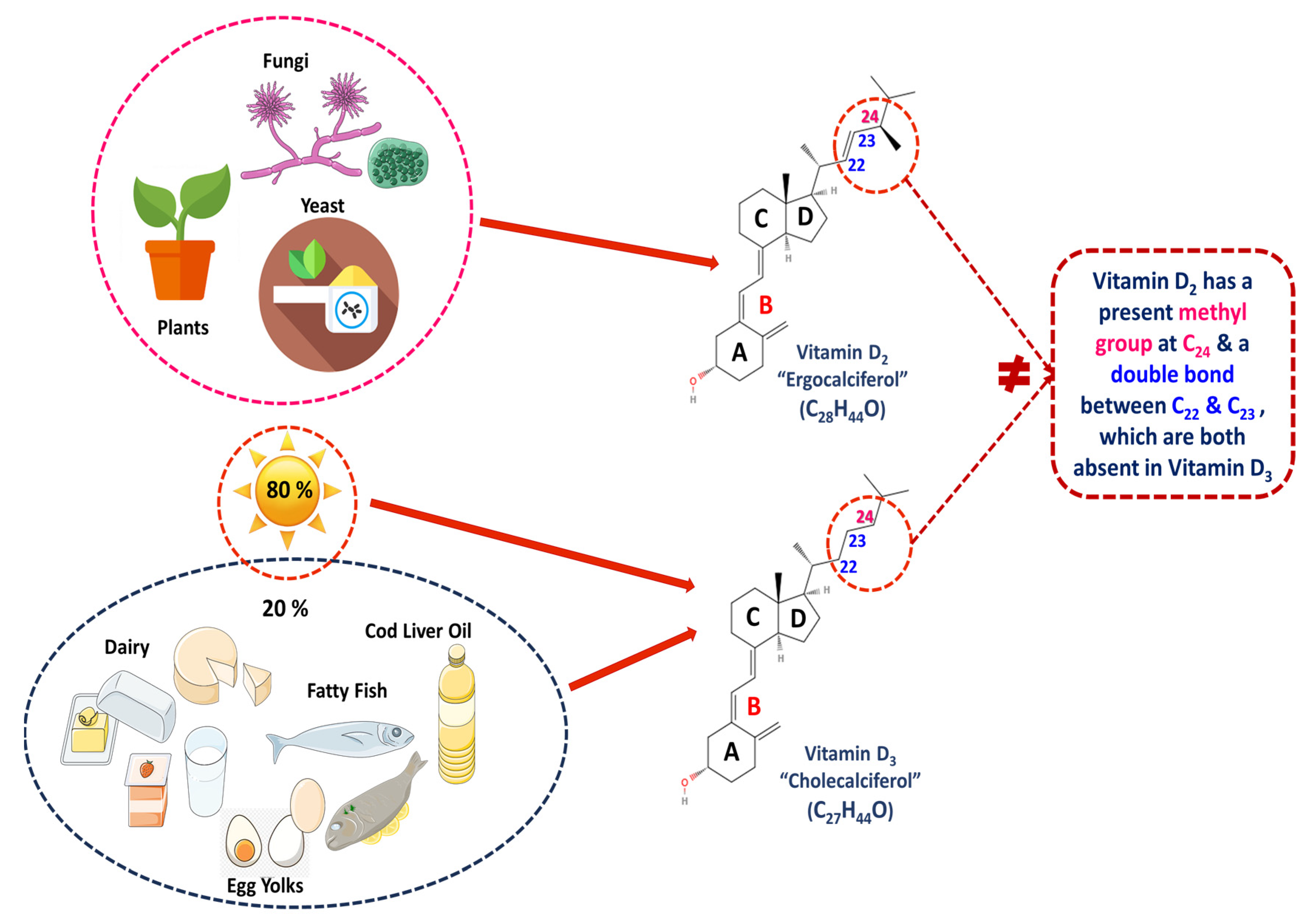
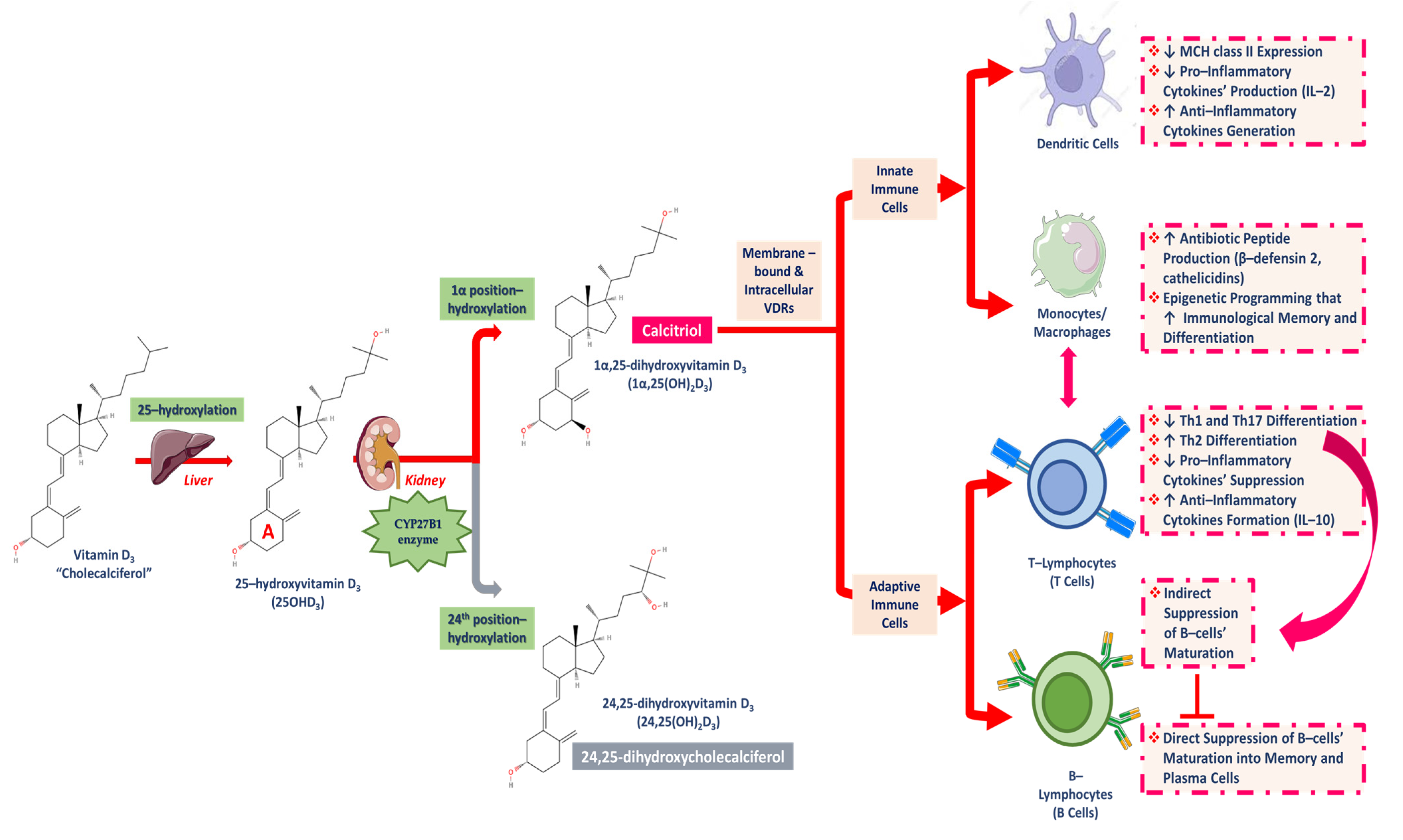
3.1. Chemical Structure and Types of Vitamin D
3.2. Absorption of Vitamin D from Skincare Products
3.3. Topical Vitamin D Analogs
3.4. Indicative Beneficial and Adverse Functions of Vitamin D in the Human Body
4. Skin Biology, Vitamin D Synthesis, and Its Impacting Factors
4.1. General Structure and Function of Skin
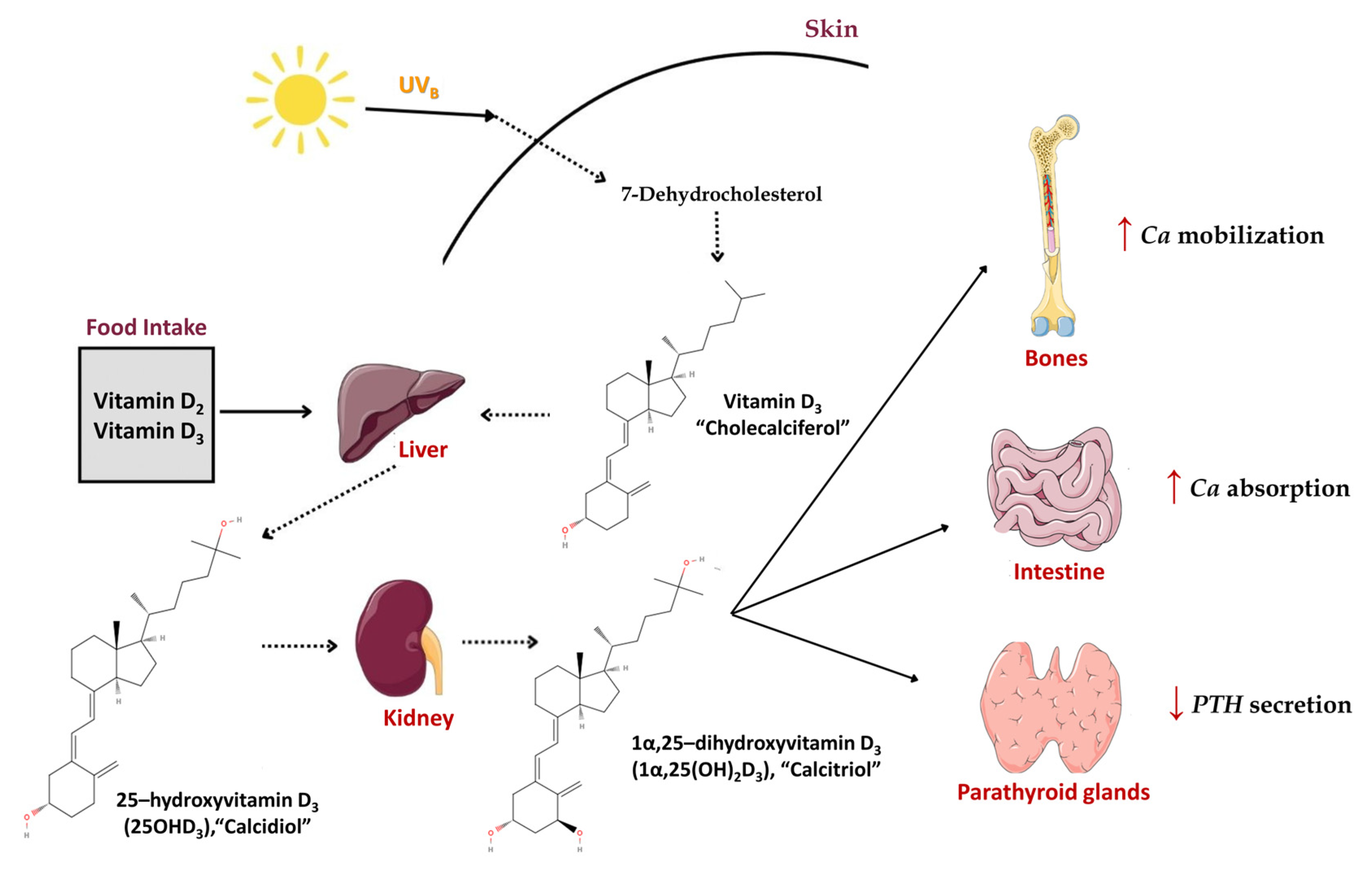
4.2. Synthesis and Metabolism of Vitamin D in Human Skin
4.3. Factors That Affect the Synthesis of Vitamin D
5. Vitamin D Health-Promoting Activities
5.1. Anti-Melanoma and Anti-Cancer Activities
5.2. Anti-UV Activity
5.3. Wound-Healing Activity
5.4. Antioxidant Activity
5.5. Anti-Inflammatory Activity
5.6. Anti-Aging Activity
6. Skin Conditions and Evidence of the Beneficial Role of Vitamin D
6.1. Psoriasis
| Disease | Type of Vitamin D’s Association | References |
|---|---|---|
| Psoriasis |
| [37,89,186,187,188] |
| Atopic dermatitis (AD) |
| [38,39,40,41,195,196,197,198,199] |
| Vitiligo |
| [42,43,89,200] |
| Acne vulgaris |
| [44,89] |
| Acne rosacea |
| [201] |
6.2. Atopic Dermatitis
6.3. Vitiligo
6.4. Acne Vulgaris and Acne Rosacea
7. Vitamin D in Cosmetics and Cosmeceuticals
7.1. Mechanism of Action
7.2. Efficacy of Vitamin D
8. Discussion—Challenges and Controversies
9. Future Directions and Research
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
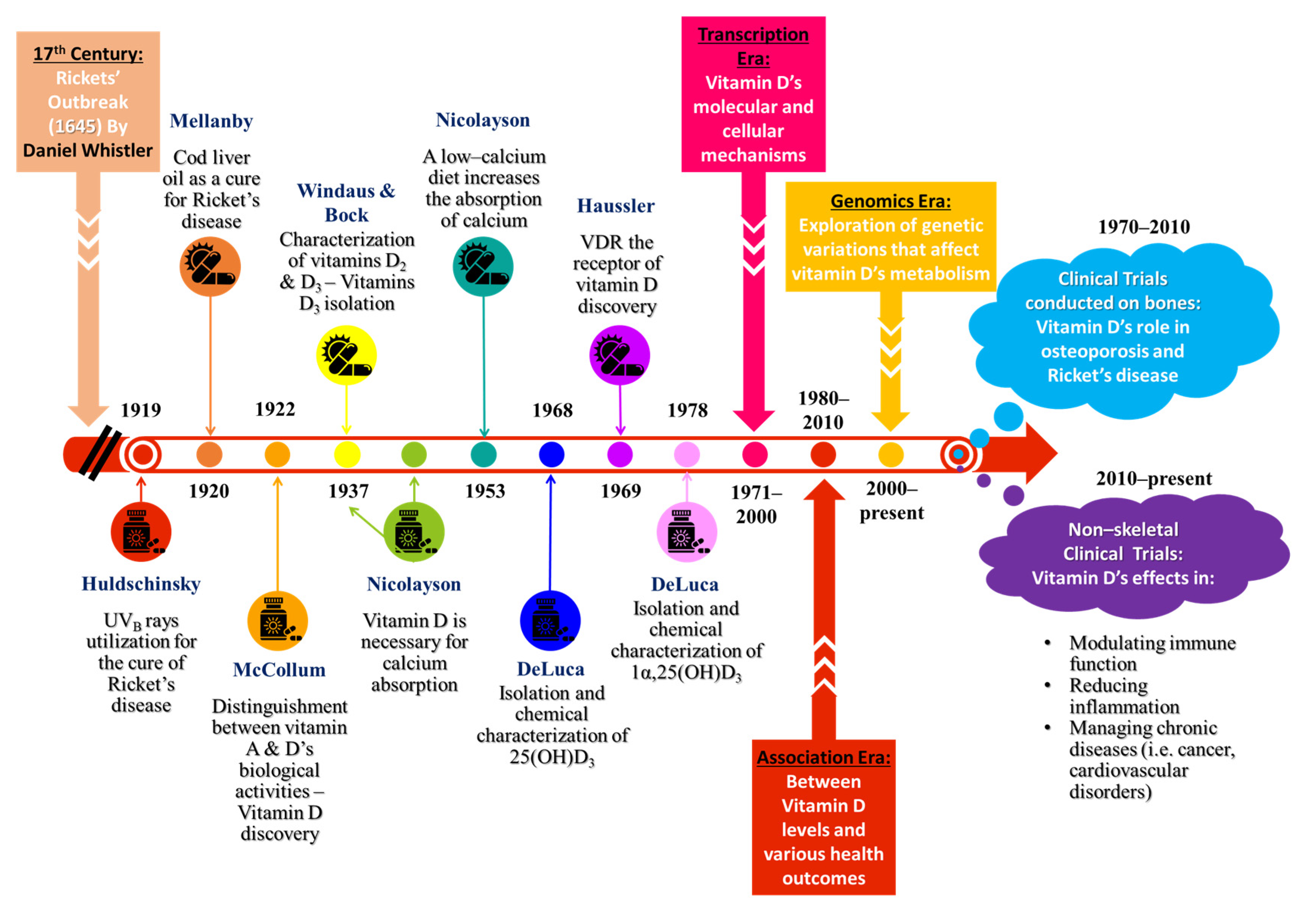
- Holick, M.F. Resurrection of Vitamin D Deficiency and Rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Pettifor, J.M. Nutritional Rickets: Deficiency of Vitamin D, Calcium, or Both? Am. J. Clin. Nutr. 2004, 80, 1725S–1729S. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H. Daniel Whistler: English Physician Who Published the First Book on Rickets in 1645. Br. J. Hosp. Med. 2019, 80, 51. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. 100 Years of Vitamin D: Historical Aspects of Vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, J.L.H.; Bijvoet, O.L.M. Rickets before the Discovery of Vitamin D. BoneKEy Rep. 2014, 3, 478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajakumar, K.; Thomas, S.B. Reemerging Nutritional Rickets: A Historical Perspective. Arch. Pediatr. Adolesc. Med. 2005, 159, 335. [Google Scholar] [CrossRef] [PubMed]
- Huldschinsky, K. Heilung von Rachitis durch künstliche Höhensonne. DMW—Dtsch. Med. Wochenschr. 1919, 45, 712–713. [Google Scholar] [CrossRef]
- Hawgood, B.J. Sir Edward Mellanby (1884–1955) GBE KCB FRCP FRS: Nutrition Scientist and Medical Research Mandarin. J. Med. Biogr. 2010, 18, 150–157. [Google Scholar] [CrossRef]
- Carpenter, K.J.; Zhao, L. Forgotten Mysteries in the Early History of Vitamin D. J. Nutr. 1999, 129, 923–927. [Google Scholar] [CrossRef]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on Experimental Rickets. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Mellanby, E. An Experimental Investigation on Rickets. Lancet 1919, 193, 407–412. [Google Scholar] [CrossRef]
- DeLuca, H.F. History of the Discovery of Vitamin D and Its Active Metabolites. BoneKEy Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.S.; Graciela, E.C. The Saga of Vitamin D Discovery. Anat. Physiol. Biochem. Int. J. 2023, 6, 555685. [Google Scholar] [CrossRef]
- Windans, A.; Bock, F. Über Das Provitamin Aus Dem Sterin Der Schweineschwarte. Biol. Chem. 1936, 245, 168–170. [Google Scholar] [CrossRef]
- Copping, A.M. Origin of Vitamin D in Cod-Liver Oil: Vitamin D Content of Zooplankton. Biochem. J. 1934, 28, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F.; Gray, R.W.; Boyle, I.T.; Suda, T. Isolation and Identification of 24,25-Dihydroxycholecalciferol, a Metabolite of Vitamin D3 Made in the Kidney. Biochemistry 1972, 11, 4251–4255. [Google Scholar] [CrossRef]
- Haussler, M.R.; Norman, A.W. Chromosomal Receptor for a Vitamin D Metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef]
- Norman, A.W.; Myrtle, J.F.; Miogett, R.J.; Nowicki, H.G.; Williams, V.; Popjaák, G. 1,25-Dihydroxycholecalciferol: Identification of the Proposed Active Form of Vitamin D3 in the Intestine. Science 1971, 173, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular Endocrinology of Vitamin D on the Epigenome Level. Mol. Cell. Endocrinol. 2017, 453, 14–21. [Google Scholar] [CrossRef]
- Grant, W.B. Epidemiology of Disease Risks in Relation to Vitamin D Insufficiency. Prog. Biophys. Mol. Biol. 2006, 92, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Epidemiology of Vitamin D in Health and Disease. Nutr. Res. Rev. 2009, 22, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Nardin, S.; Cao, D.; Chiarito, M.; Kedhi, E.; Galasso, G.; Condorelli, G.; De Luca, G. Vitamin D and Cardiovascular Diseases: From Physiology to Pathophysiology and Outcomes. Biomedicines 2024, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Makris, K.; Heijboer, A.C.; Herrmann, M.; Souberbielle, J.-C. Vitamin D: Analytical Advances, Clinical Impact, and Ongoing Debates on Health Perspectives. Clin. Chem. 2024, 70, 1104–1121. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Shapses, S.A. Vitamin D. In Present Knowledge in Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–114. ISBN 978-0-323-66162-1. [Google Scholar]
- Janjetovic, Z.; Slominski, A.T. Promising Functions of Novel Vitamin D Derivatives as Cosmetics: A New Fountain of Youth in Skin Aging and Skin Protection. Cosmetics 2024, 11, 37. [Google Scholar] [CrossRef]
- Bogh, M.K.B.; Schmedes, A.V.; Philipsen, P.A.; Thieden, E.; Wulf, H.C. Vitamin D Production after UVB Exposure Depends on Baseline Vitamin D and Total Cholesterol but Not on Skin Pigmentation. J. Investig. Dermatol. 2010, 130, 546–553. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Tuohimaa, P. Vitamin D and Aging. J. Steroid Biochem. Mol. Biol. 2009, 114, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Cavalier, E. Vitamin D in 2020: An Old Pro-Hormone with Potential Effects beyond Mineral Metabolism. Nutrients 2020, 12, 3378. [Google Scholar] [CrossRef]
- Adamantidi, T.; Maris, G.; Altantsidou, P.; Tsoupras, A. Anti-Inflammatory Benefits of Vitamin D and Its Analogues Against Glomerulosclerosis and Kidney Diseases. Sclerosis 2024, 2, 217–265. [Google Scholar] [CrossRef]
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer. Res. 2020, 40, 473–489. [Google Scholar] [CrossRef]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The Impact of Vitamin D on Cancer: A Mini Review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H. Vitamin D Is a Membrane Antioxidant Ability to Inhibit Iron-dependent Lipid Peroxidation in Liposomes Compared to Cholesterol, Ergosterol and Tamoxifen and Relevance to Anticancer Action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Hung, T.; Soung, J. The Role of Vitamin D in Psoriasis: A Review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and Its Role in Psoriasis: An Overview of the Dermatologist and Nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Van Der Schaft, J.; Ariens, L.F.M.; Bruijnzeel-Koomen, C.A.F.M.; De Bruin-Weller, M.S. Serum Vitamin D Status in Adult Patients with Atopic Dermatitis: Recommendations for Daily Practice. J. Am. Acad. Dermatol. 2016, 75, 1257–1259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanmartin, R.; Pardos, C.; Doste, D.; Aguilera, J.; Alijarde, R.; Jesús Agón-Banzo, P.; García-Malinis, A.J.; Puzo, J.; Hernández-Martín, Á.; Gilaberte, Y. The Association between Atopic Dermatitis and Serum 25-hydroxyvitamin D in Children: Influence of Sun Exposure, Diet, and Atopy Features—A Cross-sectional Study. Pediatr. Dermatol. 2020, 37, 294–300. [Google Scholar] [CrossRef]
- Silverberg, J.I. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.L.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.J.; Jouary, T.; et al. Guidelines for the Management of Vitiligo: The European Dermatology Forum Consensus: EDF Vitiligo Guidelines. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Karagüzel, G.; Sakarya, N.P.; Bahadır, S.; Yaman, S.; Ökten, A. Vitamin D Status and the Effects of Oral Vitamin D Treatment in Children with Vitiligo: A Prospective Study. Clin. Nutr. ESPEN 2016, 15, 28–31. [Google Scholar] [CrossRef]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.-H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium Acnes Induces an IL-17 Response in Acne Vulgaris That Is Regulated by Vitamin A and Vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef]
- Alhetheli, G.; Elneam, A.I.A.; Alsenaid, A.; Al-Dhubaibi, M. Vitamin D Levels in Patients with and without Acne and Its Relation to Acne Severity: A Case-Control Study. Clin. Cosmet. Investig. Dermatol. 2020, 13, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Asim, S.A.; Bhatti, S.; Sajid, M.; Mirza, R.; Huma, Z. Association of Vitamin D with Moderate to Severe Acne Vulgaris. J. Coll. Physicians Surg. Pak. 2023, 33, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Algarin, Y.A.; Pulumati, A.; Jaalouk, D.; Tan, J.; Nouri, K. The Role of Vitamins and Nutrients in Rosacea. Arch. Dermatol. Res. 2024, 316, 142. [Google Scholar] [CrossRef] [PubMed]
- Kutner, A.; Brown, G. Vitamins D: Relationship between Structure and Biological Activity. Int. J. Mol. Sci. 2018, 19, 2119. [Google Scholar] [CrossRef]
- Chiellini, G.; DeLuca, H.F. The Importance of Stereochemistry on the Actions of Vitamin D. Curr. Top. Med. Chem. 2011, 11, 840–859. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Jasinghe, V.J.; Perera, C.O. Distribution of Ergosterol in Different Tissues of Mushrooms and Its Effect on the Conversion of Ergosterol to Vitamin D2 by UV Irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Benedik, E. Sources of Vitamin D for Humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the Skin. J. Bone Miner. Metab. 2010, 28, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Al-Smadi, K.; Ali, M.; Alavi, S.E.; Jin, X.; Imran, M.; Leite-Silva, V.R.; Mohammed, Y. Using a Topical Formulation of Vitamin D for the Treatment of Vitiligo: A Systematic Review. Cells 2023, 12, 2387. [Google Scholar] [CrossRef]
- Pilz, S.; Putz-Bankuti, C.; Gaksch, M.; Spindelboeck, W.; Haselberger, M.; Rainer, F.; Posch, A.; Kreuzer, P.; Stojakovic, T.; Stadlbauer, V.; et al. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations in Cirrhotic Patients: A Randomized Controlled Trial. Nutrients 2016, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Ponti, L.; Gabutti, L.; Faré, P.B.; Janett, S.; Bianchetti, M.G.; Schulz, P.J.; Lava, S.A.G.; Agostoni, C.; Milani, G.P. Vitamin D Supply of Multivitamins Commercialized Online by Amazon in Western and Southern Europe: A Labeling Analysis. Nutrients 2023, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef]
- Tóth, B.E.; Takács, I.; Kádár, K.; Mirani, S.; Vecsernyés, M.; Lakatos, P. Safety and Efficacy of Loading Doses of Vitamin D: Recommendations for Effective Repletion. Pharmaceuticals 2024, 17, 1620. [Google Scholar] [CrossRef]
- Iwanowski, T.; Kołkowski, K.; Nowicki, R.J.; Sokołowska-Wojdyło, M. Etiopathogenesis and Emerging Methods for Treatment of Vitiligo. Int. J. Mol. Sci. 2023, 24, 9749. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C.; Zhang, Y.; Kuo, Y.C.; Du, M.; Roh, Y.S.; See, J.; Brix, J.; Gahler, R.J.; Green, T.; et al. A Comparison and Safety Evaluation of Micellar versus Standard Vitamin D3 Oral Supplementation in a Randomized, Double-Blind Human Pilot Study. Nutrients 2024, 16, 1573. [Google Scholar] [CrossRef]
- Galluccio, G.; D’Onghia, M.; Malvaso, D.; Lazzeri, L.; Cinotti, E.; Rubegni, G.; Rubegni, P.; Calabrese, L. Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach. Cosmetics 2024, 11, 11. [Google Scholar] [CrossRef]
- Rusic, D.; Ivic, M.; Slugan, A.; Leskur, D.; Modun, D.; Durdov, T.; Vukovic, D.; Bukic, J.; Bozic, J.; Seselja Perisin, A. Pilot Study on the Effects of a Cosmetic Serum Containing Niacinamide, Postbiotics and Peptides on Facial Skin in Healthy Participants: A Randomized Controlled Trial. Life 2024, 14, 1677. [Google Scholar] [CrossRef]
- Santa, K.; Kumazawa, Y.; Watanabe, K.; Nagaoka, I. The Potential Use of Vitamin D3 and Phytochemicals for Their Anti-Ageing Effects. Int. J. Mol. Sci. 2024, 25, 2125. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Kalafateli, M.; Geramoutsos, G.; Triantos, C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules 2024, 14, 1090. [Google Scholar] [CrossRef]
- Mazur, A.; Koziorowska, K.; Dynarowicz, K.; Aebisher, D.; Bartusik-Aebisher, D. Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review. Nutrients 2022, 14, 3805. [Google Scholar] [CrossRef]
- McCullough, P.J.; McCullough, W.P.; Lehrer, D.; Travers, J.B.; Repas, S.J. Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients 2021, 13, 1511. [Google Scholar] [CrossRef] [PubMed]
- Kittaneh, M.; Qurt, M.; Malkieh, N.; Naseef, H.; Muqedi, R. Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution. Pharmaceutics 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Vasdeki, D.; Tsamos, G.; Dimakakos, E.; Patriarcheas, V.; Koufakis, T.; Kotsa, K.; Cholewka, A.; Stanek, A. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients 2024, 16, 3651. [Google Scholar] [CrossRef]
- Yan, X.; Lu, E.; Song, Z.; Wu, Y.; Sha, X. Development and In Vivo Evaluation of a Novel Vitamin D3 Oral Spray Delivery System. Pharmaceutics 2023, 16, 25. [Google Scholar] [CrossRef]
- Li, C.-P.; Huang, S.-C.; Hsiao, Y.; Tsai, R.-Y. Evaluating the Role of Vitamin D in Alleviating Chronic Pruritus: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 9983. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.D.C.; Silva, C.J.T.; Gonçalves, M.P.; Borsagli, F.G.L.M. Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery. Processes 2024, 12, 1437. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the Skin: Physiology and Pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Morimoto, S.; Kumahara, Y. A Patient with Psoriasis Cured by 1 Alpha-Hydroxyvitamin D3. Med. J. Osaka Univ. 1985, 35, 51–54. [Google Scholar] [PubMed]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; Von Hurst, P.R. Oral Vitamin D3 Supplementation for Chronic Plaque Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Dermatol. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Yoles, A.; Lombardi, K.; Lou, W. Calcipotriene Ointment and Halobetasol Ointment in the Long-Term Treatment of Psoriasis: Effects on the Duration of Improvement. J. Am. Acad. Dermatol. 1998, 39, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Lebwohl, M.; Stein Gold, L.; Andersen, S.B.; Jensen, K.L.; Pink, A.E.; Segaert, S.; Berg, P.; Calzavara-Pinton, P.G.; De La Cueva Dobao, P.; et al. Itch Relief in Patients with Psoriasis: Effectiveness of Calcipotriol plus Betamethasone Dipropionate Foam. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.; Friedlander, S.F. Corticosteroids: Options in the Era of Steroid-Sparing Therapy. J. Am. Acad. Dermatol. 2005, 53, S50–S58. [Google Scholar] [CrossRef]
- Alia, E.; Kerr, P.E. Vitamin D: Skin, Sunshine, and Beyond. Clin. Dermatol. 2021, 39, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Wasiewicz, T.; Szyszka, P.; Cichorek, M.; Janjetovic, Z.; Tuckey, R.; Slominski, A.; Zmijewski, M. Antitumor Effects of Vitamin D Analogs on Hamster and Mouse Melanoma Cell Lines in Relation to Melanin Pigmentation. Int. J. Mol. Sci. 2015, 16, 6645–6667. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.K.; Castaneda, E.; Antal, M.C.; Jiang, M.; Messaddeq, N.; Meng, X.; Loehr, C.V.; Gariglio, P.; Kato, S.; Wahli, W.; et al. Malignant Transformation of DMBA/TPA-Induced Papillomas and Nevi in the Skin of Mice Selectively Lacking Retinoid-X-Receptor α in Epidermal Keratinocytes. J. Investig. Dermatol. 2007, 127, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Brozyna, A.A.; Tuckey, R.C.; Kim, T.-K.; Nguyen, M.N.; Jozwicki, W.; Pfeffer, S.R.; Pfeffer, L.M.; Slominski, A.T. High Basal NF-κB Activity in Nonpigmented Melanoma Cells Is Associated with an Enhanced Sensitivity to Vitamin D3 Derivatives. Br. J. Cancer 2011, 105, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.-P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized Trial of Calcipotriol Combined with 5-Fluorouracil for Skin Cancer Precursor Immunotherapy. J. Clin. Investig. 2016, 127, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.-K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel Non-Calcemic Secosteroids That Are Produced by Human Epidermal Keratinocytes Protect against Solar Radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–63. [Google Scholar] [CrossRef]
- Goltzman, D. Functions of Vitamin D in Bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Kulling, P.M.; Olson, K.C.; Olson, T.L.; Feith, D.J.; Loughran, T.P. Vitamin D in Hematological Disorders and Malignancies. Eur. J. Haematol. 2017, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the Skin: Focus on a Complex Relationship: A Review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D Receptor and Coactivators SRC2 and 3 Regulate Epidermis-Specific Sphingolipid Production and Permeability Barrier Formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Hawker, N.P.; Pennypacker, S.D.; Chang, S.M.; Bikle, D.D. Regulation of Human Epidermal Keratinocyte Differentiation by the Vitamin D Receptor and Its Coactivators DRIP205, SRC2, and SRC3. J. Investig. Dermatol. 2007, 127, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Pillai, S. Vitamin D, Calcium, and Epidermal Differentiation. Endocr. Rev. 1993, 14, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Rossini, M.; Di Cesare, A.; Idolazzi, L.; Farina, S.; Beltrami, G.; Peris, K.; Girolomoni, G. Vitamin D Status in Patients with Chronic Plaque Psoriasis: Vitamin D Serum Levels in Patients with Psoriasis. Br. J. Dermatol. 2012, 166, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Hewlings, S. Correlation between Vitamin D Levels and Hard-to-Heal Wounds: A Systematic Review. J. Wound Care 2021, 30, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Heimbeck, I.; Wjst, M.; Apfelbacher, C.J. Low Vitamin D Serum Level Is Inversely Associated with Eczema in Children and Adolescents in Germany. Allergy 2013, 68, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Mutgi, K.; Koo, J. Update on the Role of Systemic Vitamin D in Atopic Dermatitis. Pediatr. Dermatol. 2013, 30, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Letavernier, E.; Daudon, M. Vitamin D, Hypercalciuria and Kidney Stones. Nutrients 2018, 10, 366. [Google Scholar] [CrossRef]
- Messa, P.; Castellano, G.; Vettoretti, S.; Alfieri, C.M.; Giannese, D.; Panichi, V.; Cupisti, A. Vitamin D and Calcium Supplementation and Urolithiasis: A Controversial and Multifaceted Relationship. Nutrients 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, J.; Lu, Y.; Zhang, Z.; Qin, B.; Gao, H.; Wang, Y.; Zhu, J.; Wang, Q.; Zhu, Y.; et al. Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 301. [Google Scholar] [CrossRef]
- Garunkstiene, R.; Levuliene, R.; Cekuolis, A.; Cerkauskiene, R.; Drazdiene, N.; Liubsys, A. A Prospective Study of Nephrocalcinosis in Very Preterm Infants: Incidence, Risk Factors and Vitamin D Intake in the First Month. Medicina 2024, 60, 1910. [Google Scholar] [CrossRef]
- Gwadera, Ł.; Białas, A.J.; Kumor-Kisielewska, A.; Miłkowska-Dymanowska, J.; Majewski, S.; Piotrowski, W.J. Calcium, Phosphate, and Vitamin D Status in Patients with Sarcoidosis—Associations with Disease Activity and Symptoms. J. Clin. Med. 2023, 12, 4745. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.; Ketteler, M. Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future. Nutrients 2022, 14, 3009. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Massó, E.; Gifre, L.; Alfieri, C.; Soler-Majoral, J.; Fusaro, M.; Calabia, J.; Rodríguez-Pena, R.; Rodríguez-Chitiva, N.; López-Báez, V.; et al. Vitamin D and Chronic Kidney Disease Association with Mineral and Bone Disorder: An Appraisal of Tangled Guidelines. Nutrients 2023, 15, 1576. [Google Scholar] [CrossRef] [PubMed]
- Mamadapur, V.K.; Nagaraju, S.; Prabhu, M.M. Comparative Study of Vitamin D Levels in Newly Diagnosed Tuberculosis and a Normal Population. Medicina 2024, 60, 685. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Tulimilli, S.V.; Bettada, V.G.; Karnik, M.; Uthaiah, C.A.; Anantharaju, P.G.; Nataraj, S.M.; Ramashetty, R.; Sukocheva, O.A.; Tse, E.; et al. Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers 2024, 16, 3211. [Google Scholar] [CrossRef] [PubMed]
- Phiri, C.B.; Davis, C.R.; Grahn, M.; Gannon, B.M.; Kokinos, B.P.; Crenshaw, T.D.; Tanumihardjo, S.A. Vitamin D Maintains Growth and Bone Mineral Density against a Background of Severe Vitamin A Deficiency and Moderate Toxicity in a Swine Model. Nutrients 2024, 16, 2037. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; De Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Tallon, E.; Macedo, J.P.; Faria, A.; Tallon, J.M.; Pinto, M.; Pereira, J. Can Vitamin D Levels Influence Bone Metabolism and Osseointegration of Dental Implants? An Umbrella Review. Healthcare 2024, 12, 1867. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin Biology: Systems That Enhance Drug Delivery—Skin Biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef] [PubMed]
- Woo, W. Skin Structure and Biology. In Imaging Technologies and Transdermal Delivery in Skin Disorders; Xu, C., Wang, X., Pramanik, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–14. ISBN 978-3-527-34460-4. [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Ibrahim, A.A.E.; Bagherani, N.; Smoller, B.R.; Reyes-Baron, C.; Bagherani, N. Functions of the Skin. In Atlas of Dermatology, Dermatopathology and Venereology; Smoller, B., Bagherani, N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–11. ISBN 978-3-319-45134-3. [Google Scholar]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Ishizaki, I.; Kubo, A.; Kawasaki, H.; Nagao, K.; Ohashi, Y.; Amagai, M. The Stratum Corneum Comprises Three Layers with Distinct Metal-Ion Barrier Properties. Sci. Rep. 2013, 3, 1731. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, J.-W.; Kim, Y.-C.; Prausnitz, M.R. The Effect of Heat on Skin Permeability. Int. J. Pharm. 2008, 359, 94–103. [Google Scholar] [CrossRef]
- Relhan, V.; Goel, K.; Kochhar, A.; Garg, V.; Wadhwa, B. Vitamin D and Skin Diseases: A Review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. The Role of the Vitamin D Receptor in the Epidermal Stem Cell Response to Wounding. Receptors 2024, 3, 397–407. [Google Scholar] [CrossRef]
- Veleva, B.I.; Caljouw, M.A.A.; Van Der Steen, J.T.; Mertens, B.J.A.; Chel, V.G.M.; Numans, M.E. The Effect of Ultraviolet B Irradiation Compared with Oral Vitamin D Supplementation on the Well-Being of Nursing Home Residents with Dementia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 1684. [Google Scholar] [CrossRef]
- Lehmann, B.; Querings, K.; Reichrath, J. Vitamin D and Skin: New Aspects for Dermatology. Exp. Dermatol. 2004, 13, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mosca, S.; Morrone, A. Human Skin Pigmentation: From a Biological Feature to a Social Determinant. Healthcare 2023, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Vearing, R.M.; Hart, K.H.; Charlton, K.; Probst, Y.; Blackbourn, D.J.; Ahmadi, K.R.; Lanham-New, S.A.; Darling, A.L. Vitamin D Status of the British African-Caribbean Residents: Analysis of the UK Biobank Cohort. Nutrients 2021, 13, 4104. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.M.; Bocsan, I.C.; Tsiros, G.; Voila, P.; Stanciu, L.A.; Zdrenghea, M. Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children 2021, 8, 111. [Google Scholar] [CrossRef]
- Herdea, A.; Marie, H.; Ionescu, A.; Sandu, D.-M.; Pribeagu, S.-T.; Ulici, A. Vitamin D Deficiency—A Public Health Issue in Children. Children 2024, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism and Function in the Skin. Mol. Cell. Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Koldiri, E.; Beloukas, A.; Rallis, E.; Kefala, V. Deciphering the Effects of Different Types of Sunlight Radiation on Skin Function: A Review. Cosmetics 2024, 11, 80. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Turner, S.C.; Holmes, W.L.; Van Matre, E.T.; Swanson, J.M.; Byerly, S.; Filiberto, D.M.; Fischer, P.E. Reduction in Hypercalcemia Following Readjustment of Target Serum 25-Hydroxy Vitamin D Concentration during Cholecalciferol Therapy in Vitamin D-Deficient Critically Ill Patients. Nutrients 2022, 14, 1650. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, U.; Izquierdo, M.; Tseng, Y.; Prabhu, P.A.J.; Zamorano, M.J.; Robaina, L.; Domínguez, D. Effects of the Interaction between Dietary Vitamin D3 and Vitamin K3 on Growth, Skeletal Anomalies, and Expression of Bone and Calcium Metabolism-Related Genes in Juvenile Gilthead Seabream (Sparus Aurata). Animals 2024, 14, 2808. [Google Scholar] [CrossRef]
- Biasucci, G.; Donini, V.; Cannalire, G. Rickets Types and Treatment with Vitamin D and Analogues. Nutrients 2024, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, M.; Strzelczyk, J.K.; Biernacki, K.; Salatino, S.; Osadnik, T.; Ostrowska, Z. New Variants of the Cytochrome P450 2R1 (CYP2R1) Gene in Individuals with Severe Vitamin D-Activating Enzyme 25(OH)D Deficiency. Biomolecules 2021, 11, 1867. [Google Scholar] [CrossRef]
- Toral López, J.; Candia Tenopala, C.; Reyes Mosqueda, A.D.; Fonseca Sánchez, M.Á.; González Huerta, L.M. A Novel Compound Nonsense Variant in CYP27B1 Causes an Atypical Form of Vitamin D-Dependent Rickets Type 1A: A Case Report of Two Siblings in a Mexican Family. Diseases 2024, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Bizerea-Moga, T.O.; Chisavu, F.; Ilies, C.; Olah, O.; Marginean, O.; Gafencu, M.; Doros, G.; Stroescu, R. Phenotype of Idiopathic Infantile Hypercalcemia Associated with the Heterozygous Pathogenic Variant of SLC34A1 and CYP24A1. Children 2023, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Alswailmi, F.K.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular Mechanisms of Vitamin D-Mediated Immunomodulation. Galen Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R. Who, What, Where and When—Influences on Cutaneous Vitamin D Synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef]
- Kimlin, M.G. Geographic Location and Vitamin D Synthesis. Mol. Asp. Med. 2008, 29, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Podgorska, E.; Kim, T.-K.; Janjetovic, Z.; Urbanska, K.; Tuckey, R.C.; Bae, S.; Slominski, A.T. Knocking out the Vitamin D Receptor Enhances Malignancy and Decreases Responsiveness to Vitamin D3 Hydroxyderivatives in Human Melanoma Cells. Cancers 2021, 13, 3111. [Google Scholar] [CrossRef]
- Shariev, A.; Painter, N.; Reeve, V.E.; Haass, N.K.; Rybchyn, M.S.; Ince, F.A.; Mason, R.S.; Dixon, K.M. PTEN: A Novel Target for Vitamin D in Melanoma. J. Steroid Biochem. Mol. Biol. 2022, 218, 106059. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Kwiatkowska, K.; Chodyński, M.; Kutner, A.; Żmijewski, M.A. Antiproliferative Activity of Side-Chain Truncated Vitamin D Analogs (PRI-1203 and PRI-1204) against Human Malignant Melanoma Cell Lines. Eur. J. Pharmacol. 2020, 881, 173170. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.-K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Drigeard Desgarnier, M.-C.; Rochette, P.J. Enhancement of UVB-Induced DNA Damage Repair after a Chronic Low-Dose UVB Pre-Stimulation. DNA Repair 2018, 63, 56–62. [Google Scholar] [CrossRef]
- Raad, H.; Serrano-Sanchez, M.; Harfouche, G.; Mahfouf, W.; Bortolotto, D.; Bergeron, V.; Kasraian, Z.; Dousset, L.; Hosseini, M.; Taieb, A.; et al. NADPH Oxidase-1 Plays a Key Role in Keratinocyte Responses to UV Radiation and UVB-Induced Skin Carcinogenesis. J. Investig. Dermatol. 2017, 137, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Jeayeng, S.; Wongkajornsilp, A.; Slominski, A.T.; Jirawatnotai, S.; Sampattavanich, S.; Panich, U. Nrf2 in Keratinocytes Modulates UVB-Induced DNA Damage and Apoptosis in Melanocytes through MAPK Signaling. Free Radic. Biol. Med. 2017, 108, 918–928. [Google Scholar] [CrossRef]
- Dixon, K.M.; Deo, S.S.; Norman, A.W.; Bishop, J.E.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. In Vivo Relevance for Photoprotection by the Vitamin D Rapid Response Pathway. J. Steroid Biochem. Mol. Biol. 2007, 103, 451–456. [Google Scholar] [CrossRef]
- Mason, R.S.; Sequeira, V.B.; Dixon, K.M.; Gordon-Thomson, C.; Pobre, K.; Dilley, A.; Mizwicki, M.T.; Norman, A.W.; Feldman, D.; Halliday, G.M.; et al. Photoprotection by 1α,25-Dihydroxyvitamin D and Analogs: Further Studies on Mechanisms and Implications for UV-Damage. J. Steroid Biochem. Mol. Biol. 2010, 121, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, L.; Lei, K.; Zeng, J.; Luo, J.; Yin, Y.; Li, Y.; Zhang, L.; Nie, X.; Zuo, D.; et al. Vitamin D3 Analogue Facilitates Epithelial Wound Healing through Promoting Epithelial-Mesenchymal Transition via the Hippo Pathway. J. Dermatol. Sci. 2020, 100, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhao, H.; Fenta, Y.; Kita, H.; Kumar, R.; Juhn, Y.J. Serum 25-Hydroxyvitamin D Is Associated with Enhanced Pneumococcal Antibody Levels in Individuals with Asthma. Allergy Asthma Proc. 2011, 32, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Haensel, D.; Dai, X. Epithelial-to-mesenchymal Transition in Cutaneous Wound Healing: Where We Are and Where We Are Heading. Dev. Dyn. 2018, 247, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, Chemokines and Growth Factors in Wound Healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Role of Vitamin D and Calcium Signaling in Epidermal Wound Healing. J. Endocrinol. Investig. 2022, 46, 205–212. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Hudson, L.; Begg, M.; Wright, B.; Cheek, T.; Jahoda, C.A.B.; Reynolds, N.J. Dominant Effect of Gap Junction Communication in Wound-induced Calcium-wave, NFAT Activation and Wound Closure in Keratinocytes. J. Cell. Physiol. 2021, 236, 8171–8183. [Google Scholar] [CrossRef]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Tu, C.; Bikle, D.D. Combined Deletion of the Vitamin D Receptor and Calcium-Sensing Receptor Delays Wound Re-Epithelialization. Endocrinology 2017, 158, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D, Reactive Oxygen Species and Calcium Signalling in Ageing and Disease. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150434. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Lorente, H.; Herrera-Quintana, L.; Jiménez-Sánchez, L.; Fernández-Perea, B.; Plaza-Diaz, J. Antioxidant Functions of Vitamin D and CYP11A1-Derived Vitamin D, Tachysterol, and Lumisterol Metabolites: Mechanisms, Clinical Implications, and Future Directions. Antioxidants 2024, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Nair-Shalliker, V.; Armstrong, B.K.; Fenech, M. Does Vitamin D Protect against DNA Damage? Mutat. Res. Mol. Mech. Mutagen. 2012, 733, 50–57. [Google Scholar] [CrossRef]
- L Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol Effects on Activities and Metabolism of Platelet Activating Factor and on Inflammatory Cytokines in Hemodialysis Patients. Int. J. Artif. Organs 2013, 36, 87–96. [Google Scholar] [CrossRef]
- Schauber, J.; Gallo, R.L. Antimicrobial Peptides and the Skin Immune Defense System. J. Allergy Clin. Immunol. 2008, 122, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Serpico, D.; Cantelli, M.; Di Sarno, A.; Dalia, C.; Arianna, R.; Lavorgna, M.; Colao, A.; Di Somma, C. Osteoporosis and Dermatoporosis: A Review on the Role of Vitamin D. Front. Endocrinol. 2023, 14, 1231580. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular Matrix Regulation of Fibroblast Function: Redefining Our Perspective on Skin Aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Díaz, E.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. The Role of Vitamin D on Redox Regulation and Cellular Senescence. Free Radic. Biol. Med. 2022, 193, 253–273. [Google Scholar] [CrossRef]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Prtina, A.; Rašeta Simović, N.; Milivojac, T.; Vujnić, M.; Grabež, M.; Djuric, D.; Stojiljković, M.P.; Soldat Stanković, V.; Čolić, M.J.; Škrbić, R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules 2021, 11, 1865. [Google Scholar] [CrossRef]
- Cartes-Velásquez, R.; Vera, A.; Torres-Quevedo, R.; Medrano-Díaz, J.; Pérez, A.; Muñoz, C.; Carrillo-Bestagno, H.; Nova-Lamperti, E. The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer. Nutrients 2024, 16, 4134. [Google Scholar] [CrossRef]
- Mazur, A.; Koziorowska, K.; Dynarowicz, K.; Aebisher, D. Vitamin D Supplementation and Photodynamic Therapy. Biol. Life Sci. Forum 2022, 12, 28. [Google Scholar] [CrossRef]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Redding, T.S.; Thompson, A.D.; Press, A.M.; Upchurch, J.; Williams, C.D.; Choi, Y.M.; Suzuki, A. The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2. Int. J. Environ. Res. Public Health 2021, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of Psoriasis: Recent Advances on IL-23 and Th17 Cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef]
- Cai, Y.; Fleming, C.; Yan, J. New Insights of T Cells in the Pathogenesis of Psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef]
- MacLaughlin, J.A.; Gange, W.; Taylor, D.; Smith, E.; Holick, M.F. Cultured Psoriatic Fibroblasts from Involved and Uninvolved Sites Have a Partial but Not Absolute Resistance to the Proliferation-Inhibition Activity of 1,25-Dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1985, 82, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef] [PubMed]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. [Google Scholar] [CrossRef]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How Does Vitamin D Affect Immune Cells Crosstalk in Autoimmune Diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef]
- Hambly, R.; Kirby, B. The Relevance of Serum Vitamin D in Psoriasis: A Review. Arch. Dermatol. Res. 2017, 309, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Triviño, F.J.; Arias-Santiago, S.; Gilaberte-Calzada, Y. Vitamin D and the Skin: A Review for Dermatologists. Actas Dermo-Sifiliográficas Engl. Ed. 2019, 110, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; Mason, J.; Cork, M.; Hancock, H.; Dooley, G. Topical Treatments for Chronic Plaque Psoriasis: An Abridged Cochrane Systematic Review. J. Am. Acad. Dermatol. 2013, 69, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Fenner, J.; Silverberg, N.B. Oral Supplements in Atopic Dermatitis. Clin. Dermatol. 2018, 36, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Bieber, T. Atopic Dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.-N.; Lee, Y.; Choe, Y.; Ahn, K. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef]
- Li, M.; Hener, P.; Zhang, Z.; Ganti, K.P.; Metzger, D.; Chambon, P. Induction of Thymic Stromal Lymphopoietin Expression in Keratinocytes Is Necessary for Generating an Atopic Dermatitis upon Application of the Active Vitamin D3 Analogue MC903 on Mouse Skin. J. Investig. Dermatol. 2009, 129, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hener, P.; Zhang, Z.; Kato, S.; Metzger, D.; Chambon, P. Topical Vitamin D3 and Low-Calcemic Analogs Induce Thymic Stromal Lymphopoietin in Mouse Keratinocytes and Trigger an Atopic Dermatitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11736–11741. [Google Scholar] [CrossRef]
- Watabe, A.; Yamasaki, K.; Asano, M.; Kanbayashi, Y.; Nasu-Tamabuchi, M.; Terui, H.; Furudate, S.; Kakizaki, A.; Tsuchiyama, K.; Kimura, Y.; et al. Efficacy of Oral Cholecalciferol on Rhododendrol-induced Vitiligo: A Blinded Randomized Clinical Trial. J. Dermatol. 2018, 45, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, Ö.; Balta, İ.; Şen, B.B.; Dikilitaş, M.C.; Özuğuz, P.; Rifaioğlu, E.N. Vitamin D Status in Patients with Rosacea. Cutan. Ocul. Toxicol. 2014, 33, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Chrobak-Chmiel, D.; Golke, A.; Kwiecień, E.; Biegańska, M.J.; Dembele, K.; Dziekiewicz-Mrugasiewicz, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Rzewuska, M. Is Vitamin D3 a Worthy Supplement Protecting against Secondary Infections in Dogs with Atopic Dermatitis? Pathogens 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Garrett, S.; Hong, W.; Zhang, J. Staphylococcus Aureus Infections and Human Intestinal Microbiota. Pathogens 2024, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- AL-smadi, K.; Imran, M.; Leite-Silva, V.R.; Mohammed, Y. Vitiligo: A Review of Aetiology, Pathogenesis, Treatment, and Psychosocial Impact. Cosmetics 2023, 10, 84. [Google Scholar] [CrossRef]
- Elmelid, A.; Vandikas, M.S.; Gillstedt, M.; Alsterholm, M.; Osmancevic, A. The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease. Int. J. Mol. Sci. 2024, 25, 8632. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.J.; Socha, K. Acne Vulgaris and Intake of Selected Dietary Nutrients—A Summary of Information. Healthcare 2021, 9, 668. [Google Scholar] [CrossRef]
- Mao, R.; Zhou, G.; Jing, D.; Liu, H.; Shen, M.; Li, J. Vitamin D Status, Vitamin D Receptor Polymorphisms, and the Risk of Incident Rosacea: Insights from Mendelian Randomization and Cohort Study in the UK Biobank. Nutrients 2023, 15, 3803. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.H. Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies. Int. J. Mol. Sci. 2024, 25, 5302. [Google Scholar] [CrossRef]
- Jasso-Olivares, J.; Diaz-Gonzalez, J.M.; Miteva, M. Horizontal and Vertical Sections of Scalp Biopsy Specimens from Dermatomyositis Patients with Scalp Involvement. J. Am. Acad. Dermatol. 2018, 78, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Mayba, J.N.; Gooderham, M.J. A Guide to Topical Vehicle Formulations. J. Cutan. Med. Surg. 2018, 22, 207–212. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A.G. Vitamin D3 Is More Potent Than Vitamin D2 in Humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 20 December 2024).
- CosIng—Cosmetics—GROWTH—European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/74131 (accessed on 9 January 2025).
- Vitamins in Cosmetics—Special Actives. Available online: https://dermaviduals.de/english/publications/special-actives/vitamins-in-cosmetics.html (accessed on 11 January 2025).
- Russell, M. Assessing the Relationship between Vitamin D3 and Stratum Corneum Hydration for the Treatment of Xerotic Skin. Nutrients 2012, 4, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; Bulat, V.; Speeckaert, M.M.; Van Geel, N. The Impact of Antioxidants on Vitiligo and Melasma: A Scoping Review and Meta-Analysis. Antioxidants 2023, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D and Pigmented Skin. Nutrients 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L. Efficacy and Safety of Topical Calcitriol 3 Microg/g Ointment, a New Topical Therapy for Chronic Plaque Psoriasis. J. Drugs Dermatol. JDD 2009, 8, s9–s16. [Google Scholar]
- Segaert, S. Vitamin D Regulation of Cathelicidin in the Skin: Toward a Renaissance of Vitamin D in Dermatology? J. Investig. Dermatol. 2008, 128, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Parsad, D.; Saini, R.; Verma, N. Combination of PUVAsol and Topical Calcipotriol in Vitiligo. Dermatology 1998, 197, 167–170. [Google Scholar] [CrossRef]
- Khullar, G.; Kanwar, A.J.; Singh, S.; Parsad, D. Comparison of Efficacy and Safety Profile of Topical Calcipotriol Ointment in Combination with NB-UVB vs. NB-UVB Alone in the Treatment of Vitiligo: A 24-week Prospective Right–Left Comparative Clinical Trial. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 925–932. [Google Scholar] [CrossRef]
- Gargoom, A.M.; Duweb, G.A.; Elzorghany, A.H.; Benghazil, M.; Bugrein, O.O. Calcipotriol in the Treatment of Childhood Vitiligo. Int. J. Clin. Pharmacol. Res. 2004, 24, 11–14. [Google Scholar] [PubMed]
- Holick, M.F. Skin as the Site of Vitamin D Synthesis and Target Tissue for 1,25-Dihydroxyvitamin D3: Use of Calcitriol (1,25-Dihydroxyvitamin D3) for Treatment of Psoriasis. Arch. Dermatol. 1987, 123, 1677. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Pal, R.; Butani, K.; Singh, S.; Prajapati, B.G. Nanomedicine-Fortified Cosmeceutical Serums for The Mitigation of Psoriasis and Acne. Nanomedicine 2023, 18, 1769–1793. [Google Scholar] [CrossRef]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating Transdermal Delivery of Vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.K. The Rationale behind Topical Vitamin d Analogs in the Treatment of Psoriasis: Where Does Topical Calcitriol Fit In? J. Clin. Aesthetic Dermatol. 2010, 3, 46–53. [Google Scholar]
- Marty, J.; Lafforgue, C.; Grossiord, J.; Soto, P. Rheological Properties of Three Different Vitamin D Ointments and Their Clinical Perception by Patients with Mild to Moderate Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, E.H.; Mumford, R.A. Arylsulfatases A and B in Metachromatic Leukodystrophy and Maroteaux-Lamy Syndrome: Studies with 4-Methylumelliferyl Sulfate. Adv. Exp. Med. Biol. 1976, 68, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Jenssen, M.; Furberg, A.-S.; Jorde, R.; Wilsgaard, T.; Danielsen, K. Effect of Vitamin D Supplementation on Psoriasis Severity in Patients with Lower-Range Serum 25-Hydroxyvitamin D Levels: A Randomized Clinical Trial. JAMA Dermatol. 2023, 159, 518. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, R.; Pourbagheri, H.; Momen-Heravi, M.; Bahmani, F.; Shadi, J.; Soleimani, Z.; Asemi, Z. The Effects of Vitamin D Supplementation on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Diabetes Complicat. 2017, 31, 766–772. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Yan, Y. Vitamin D Status and Efficacy of Vitamin D Supplementation in Acne Patients: A Systematic Review and Meta-analysis. J. Cosmet. Dermatol. 2021, 20, 3802–3807. [Google Scholar] [CrossRef]
- Moukayed, M.; Grant, W.B. The Roles of UVB and Vitamin D in Reducing Risk of Cancer Incidence and Mortality: A Review of the Epidemiology, Clinical Trials, and Mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef]
- Näslund-Koch, C.; Skov, L. New Insights in the Complex Relationship between Psoriasis and Vitamin D…and, Not to Forget, Obesity! Br. J. Dermatol. 2024, 190, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Owczarczyk-Saczonek, A.; Krasowska, D.; Szczerkowska-Dobosz, A. New Insights into Psoriasis. Int. J. Mol. Sci. 2022, 23, 12851. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef]
- Trémezaygues, L.; Reichrath, J. Vitamin D Analogs in the Treatment of Psoriasis: Where Are We Standing and Where Will We Be Going? Dermato-Endocrinology 2011, 3, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, L.; Zhang, Y. Vitamin D and Wound Healing: Assessing Skin Barrier Function and Implications for Chloasma Treatment. Int. Wound J. 2024, 21, e14541. [Google Scholar] [CrossRef] [PubMed]
- Siregar, F.D.; Hidayat, W. The Role of Vitamin D on the Wound Healing Process: A Case Series. Int. Med. Case Rep. J. 2023, 16, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Mohamed, A.; Salah Ahmed, E.M.; Abdel-Aziz, R.T.A.; Eldeeb Abdallah, H.H.; El-Hanafi, H.; Hussein, G.; Abbassi, M.M.; El Borolossy, R. The Impact of Active Vitamin D Administration on the Clinical Outcomes of Acne Vulgaris. J. Dermatol. Treat. 2021, 32, 756–761. [Google Scholar] [CrossRef]
- Paul, S.; Kaushik, R.; Chawla, P.; Upadhyay, S.; Rawat, D.; Akhtar, A. Vitamin-D as a Multifunctional Molecule for Overall Well-Being: An Integrative Review. Clin. Nutr. ESPEN 2024, 62, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef]
- Reichrath, J.; Saternus, R.; Vogt, T. Challenge and Perspective: The Relevance of Ultraviolet (UV) Radiation and the Vitamin D Endocrine System (VDES) for Psoriasis and Other Inflammatory Skin Diseases. Photochem. Photobiol. Sci. 2017, 16, 433–444. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D: Criteria for Safety and Efficacy. Nutr. Rev. 2008, 66, S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Sawarkar, S.; Ashtekar, A. Transdermal Vitamin D Supplementation—A Potential Vitamin D Deficiency Treatment. J. Cosmet. Dermatol. 2020, 19, 28–32. [Google Scholar] [CrossRef]
- Harada, S.; Horisawa, E.; Kano, S.; Sugibayashi, K. Formulation Study of Topically Applied O/W Lotion Containing Vitamin D3 Derivative, Focusing on Skin Permeability of the Drug. Drug Dev. Ind. Pharm. 2011, 37, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Usategui, A.; Criado, G.; Del Rey, M.J.; Faré, R.; Pablos, J.L. Topical Vitamin D Analogue Calcipotriol Reduces Skin Fibrosis in Experimental Scleroderma. Arch. Dermatol. Res. 2014, 306, 757–761. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Sivanathan, S.; Kiepe, G.; Kiepe, T.; Germann, N. Candidate Formulations for a Sustainable Lipstick Supplemented with Vitamin D3: Effects of Wax Type and Concentration on Material Properties. Ind. Eng. Chem. Res. 2021, 60, 2027–2040. [Google Scholar] [CrossRef]
- Khalil, K.; Arnold, N.; Seiger, E. Chronic Eyelid Edema and Xerophthalmia Secondary to Periorbital Hyaluronic Acid Filler Injection. J. Cosmet. Dermatol. 2020, 19, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Trifan, D.F.; Tirla, A.G.; Mos, C.; Danciu, A.; Bodog, F.; Manole, F.; Ghitea, T.C. Involvement of Vitamin D3 in the Aging Process According to Sex. Cosmetics 2023, 10, 114. [Google Scholar] [CrossRef]
- Januszewski, J.; Forma, A.; Zembala, J.; Flieger, M.; Tyczyńska, M.; Dring, J.C.; Dudek, I.; Świątek, K.; Baj, J. Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why. Medicina 2023, 60, 68. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Stoupi, S.; Anastasiou, I.; Nikolaos, V.Z.; Tsakotos, G.; Koutelidakis, A.E. Effect of Vitamin C, D3, Ca Supplements and Olive Paste Enriched with Mountain Tea on Health Biomarkers in Postmenopausal Women with Osteopenia or Osteoporosis: A Prospective Interventional Study. Appl. Sci. 2024, 14, 5610. [Google Scholar] [CrossRef]
- Porcaro, G.; Laganà, A.S.; Neri, I.; Aragona, C. The Association of High-Molecular-Weight Hyaluronic Acid (HMWHA), Alpha Lipoic Acid (ALA), Magnesium, Vitamin B6, and Vitamin D Improves Subchorionic Hematoma Resorption in Women with Threatened Miscarriage: A Pilot Clinical Study. J. Clin. Med. 2024, 13, 706. [Google Scholar] [CrossRef]
- Brennan Laing, B.; Cavadino, A.; Ellett, S.; Ferguson, L. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Le Jan, D.; Siliman Misha, M.; Destrumelle, S.; Terceve, O.; Thorin, C.; Larcher, T.; Ledevin, M.; Desfontis, J.-C.; Betti, E.; Mallem, Y. Omega-3 Fatty Acid and Vitamin D Supplementations Partially Reversed Metabolic Disorders and Restored Gut Microbiota in Obese Wistar Rats. Biology 2024, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; Kim, N. Dietary Supplements in Dermatology: A Review of the Evidence for Zinc, Biotin, Vitamin D, Nicotinamide, and Polypodium. J. Am. Acad. Dermatol. 2021, 84, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The Power of Vitamin D: Is the Future in Precision Nutrition through Personalized Supplementation Plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef]
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory Role of Vitamins A, B3, C, D, and E on Skin Health, Immunity, Microbiome, and Diseases. Pharmacol. Rep. 2023, 75, 1096–1114. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin Safety and Health Prevention: An Overview of Chemicals in Cosmetic Products. J. Prev. Med. Hyg. 2019, 60, E50. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Reply to “The Five Paradoxes of Vitamin D and the Importance of Sunscreen Protection”. Clin. Pediatr. 2013, 52, 994. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Narbutt, J.; Harrison, G.I.; Lawrence, K.P.; Bell, M.; O’Connor, C.; Olsen, P.; Grys, K.; Baczynska, K.A.; Rogowski-Tylman, M.; et al. Optimal Sunscreen Use, during a Sun Holiday with a very High Ultraviolet Index, Allows Vitamin D Synthesis without Sunburn. Br. J. Dermatol. 2019, 181, 1052–1062. [Google Scholar] [CrossRef]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals Vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef]
- Karimi, N. Approaches in Line with Human Physiology to Prevent Skin Aging. Front. Physiol. 2023, 14, 1279371. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Matthan, N.R.; Lamon-Fava, S.; Solano-Aguilar, G.; Turner, J.R.; Walker, M.E.; Chai, Z.; Lakshman, S.; Chen, C.; Dawson, H.; et al. Colon Transcriptome Is Modified by a Dietary Pattern/Atorvastatin Interaction in the Ossabaw Pig. J. Nutr. Biochem. 2021, 90, 108570. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Souza, S. Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods 2022, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Kanazawa, S.; Kajihara, I.; Kobayashi, A.; Watanabe, C.; Ihn, H. Infliximab Improved the Refractory Cutaneous Involvement in a Patient with Dermatomyositis. Dermatol. Ther. 2019, 32, e12859. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Veysey, M.; Beckett, E. The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas. Nutrients 2018, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Ali, A.; Zahedi, F.D.; Ismail, N.A.S. Immunomodulatory Role of Vitamin D on Gut Microbiome in Children. Biomedicines 2023, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldy, N.S.; Al-Musharaf, S.; Aljazairy, E.A.; Hussain, S.D.; Alnaami, A.M.; Al-Daghri, N.; Aljuraiban, G. Serum Vitamin D Level and Gut Microbiota in Women. Healthcare 2023, 11, 351. [Google Scholar] [CrossRef]
- Bryson, W.G.; McCormack, A.C.; Plowman, J.E.; Grosvenor, A.J.; Murphy, C.J.; Nagase, S.; Itou, T.; Koike, K. Improved Two-dimensional Electrophoretic Mapping of Japanese Human Hair Proteins; Application to Curved and Straight Japanese Human Hairs; and Protein Identification by MALDI MS and MS/MS Quadrupole Time-of-flight Mass Spectrometry. Int. J. Cosmet. Sci. 2020, 42, 346–358. [Google Scholar] [CrossRef]
- Peñuñuri-Pacheco, N.; Moreno-García, Y.A.; González-Ríos, H.; Astiazarán-García, H.; López-Franco, Y.L.; Tortoledo-Ortiz, O.; Pérez-Báez, A.J.; Dávila-Ramírez, J.L.; Lizardi-Mendoza, J.; Valenzuela-Melendres, M. Optimization of the Encapsulation of Vitamin D3 in Oil in Water Nanoemulsions: Preliminary Application in a Functional Meat Model System. Foods 2024, 13, 2842. [Google Scholar] [CrossRef] [PubMed]
- Akkam, Y.; Rababah, T.; Costa, R.; Almajwal, A.; Feng, H.; Laborde, J.E.A.; Abulmeaty, M.M.; Razak, S. Pea Protein Nanoemulsion Effectively Stabilizes Vitamin D in Food Products: A Potential Supplementation during the COVID-19 Pandemic. Nanomaterials 2021, 11, 887. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Sovrea, A.; Constantin, A.-M.; Samasca, G.; Masalar, A.L.; Ifju, B.; Linga, E.; Neamti, L.; Tranca, R.A.; et al. Nanocarriers for Drug Delivery: An Overview with Emphasis on Vitamin D and K Transportation. Nanomaterials 2022, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chang, C.; Zhang, X.; Zhang, Y.; Radford, M.J.; Gahler, R.J.; Kuo, Y.C.; Wood, S.; Solnier, J. Designing Vitamin D3 Formulations: An In Vitro Investigation Using a Novel Micellar Delivery System. Nutraceuticals 2023, 3, 290–305. [Google Scholar] [CrossRef]
- Zhao, F.; Jia, Z.; Feng, Y.; Li, Z.; Feng, J. Circular RNA Circ_0079593 Enhances Malignant Melanoma Progression by the Regulation of the miR-573/ABHD2 Axis. J. Dermatol. Sci. 2021, 102, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Chang, C.-Y.; Chu, T.-W.; Liang, Y.-J. Using Machine Learning to Identify the Relationships between Demographic, Biochemical, and Lifestyle Parameters and Plasma Vitamin D Concentration in Healthy Premenopausal Chinese Women. Life 2023, 13, 2257. [Google Scholar] [CrossRef] [PubMed]
- Patino-Alonso, C.; Gómez-Sánchez, M.; Gómez-Sánchez, L.; Sánchez Salgado, B.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A. Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters. Mathematics 2022, 10, 616. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Q.; Zhang, W.; Yang, F.; Zhao, K.; Dong, X.; Prakash, S.; Yuan, Y. Advances in the Potential Application of 3D Food Printing to Enhance Elderly Nutritional Dietary Intake. Foods 2023, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Darrigade, A.; Dendooven, E.; Mangodt, E.; Van Dyck, F.; Aerts, O. Airborne Allergic Contact Dermatitis by Proxy Caused by ‘Poppers’. Contact Dermat. 2021, 84, 212–214. [Google Scholar] [CrossRef]
- Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Sienkiewicz-Szłapka, E.; Rozmus, D.; Król-Grzymała, A.; Jarmołowska, B.; Kordulewska, N.; Cieślińska, A. Vitamin D Metabolic Pathway Components in Orthopedic Patientes—Systematic Review. Int. J. Mol. Sci. 2022, 23, 15556. [Google Scholar] [CrossRef] [PubMed]
- Horas, K.; Hoxha, M.; Heinz, T.; Jakuscheit, A.; List, K.; Maier, G.S.; Weißenberger, M.; Rudert, M. Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis. Nutrients 2024, 16, 3060. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.A.; Nakakura, A.M. Nutrition Considerations for Burn Patients: Optimizing Recovery and Healing. Eur. Burn J. 2023, 4, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Yang, B.-S.; Chang, C.-N.; Yu, C.-M.; Chen, K.-H. Additional Vitamin and Mineral Support for Patients with Severe Burns: A Nationwide Experience from a Catastrophic Color-Dust Explosion Event in Taiwan. Nutrients 2018, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Association of Acne Tarda with Endocrinological Disorders. Dermato 2022, 2, 109–120. [Google Scholar] [CrossRef]
- Cammisa, I.; Zona, M.; Guerriero, C.; Cipolla, C.; Rigante, D. Skin Sceneries of Thyroid Disorders and Impact of Thyroid on Different Skin Diseases: A Scoping Review Focused on Pediatric Patients. Children 2024, 11, 1488. [Google Scholar] [CrossRef]
- Varzakas, T.; Antoniadou, M. A Holistic Approach for Ethics and Sustainability in the Food Chain: The Gateway to Oral and Systemic Health. Foods 2024, 13, 1224. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J.; Létinois, U. Adequate Vitamin D Intake Cannot Be Achieved within Carbon Emission Limits Unless Food Is Fortified: A Simulation Study. Nutrients 2021, 13, 592. [Google Scholar] [CrossRef] [PubMed]

| Study Hypothesis | Cosmetic Application | Main Activity | Pathway | Results | Reference |
|---|---|---|---|---|---|
| This study demonstrated the effects of secosteroidal analogs (1α,25(OH)2D3 and 25(OH)D3) and non-calcemic ones (20(OH)D3, calcipotriol, 21(OH)pD, pD, and 20(OH)pL) on proliferation, colony formation in both monolayer and soft agar, and mRNA and protein expression by melanoma cells | In vivo testing | Anti-melanoma |
|
| [82] |
| An investigation of the role of RXRα and its partners in mouse skin tumor formation and malignant progression upon topical mutagen dimethylbenzanthracene (DMBA)/12–O–tetradecanoyl phorbol–13–acetate (TPA) treatment was followed in this study | In vivo testing | Anti-cancer, anti-melanoma |
|
| [83] |
| An examination of the role of the active forms of vitamin D in regulating the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) activity in melanoma cells that are dependent on melanin | In vitro testing | Anti-melanoma |
|
| [84] |
| An investigation of skin cancer therapy in mouse models of skin carcinogenesis with the application of topical calcipotriol was conducted. An additional randomized, double-blind clinical trial investigation for testing the combination of 0.005% calcipotriol ointment together with 5% fluorouracil (5-FU) cream and its comparison with Vaseline and 5-FU used in the treatment of actinic keratoses on the face, scalp, and upper extremities was tested as well | In vivo and in vitro testing | Anti-cancer |
|
| [85] |
| An investigation regarding the ability of novel non-calcemic secosteroids on how they could protect against solar radiation, which can damage human epidermal keratinocytes, melanocytes, and HaCaT keratinocytes, was conducted in this study | In vitro | Anti-melanoma |
|
| [86] |
| Study Hypothesis | Cosmetic Application | Main Activity | Experimental Pathway—Review Target | Results | Ref. |
|---|---|---|---|---|---|
| Vitamin D and calcium are critical for the activation, migration, and function of stem cells during wound healing | In vivo | Wound Healing | Mouse models with full-thickness skin biopsies (control and receptor-deficient) were utilized to assess healing, regarding the fact that vitamin D and calcium aid wound healing by activating VDR and CaSR receptor pathways | Deletion of VDR and CaSR:
| [162] |
| Vitamin D modulates inflammation, supports keratinocyte function, and promotes tissue regeneration to improve wound healing | In vivo | Anti-inflammatory Role in Wound Healing | The key pathway, Vitamin D–VDR signaling, regulates
| Vitamin D aids in successful wound healing, by reducing inflammation, promoting keratinocyte growth and migration, and accelerating re-epithelialization | [181] |
| Vitamin D protects against aging by modulating immunity, reducing inflammation, and enhancing overall health | In vivo and in vitro | Anti-inflammatory and Anti-aging Effects | Vitamin D–VDR signaling regulates immunity and reduces inflammation, while inhibiting NF-kB, and lowers inflammatory cytokine production | Vitamin D contributes to
| [34] |
| Vitamin D’s role as an antioxidant that modulates ROS in keratinocytes, while potentially influencing skin health/disease prevention by reducing oxidative stress | In vivo | Anti-inflammatory and Antioxidant |
| Vitamin D reduces the levels of ROS in keratinocytes, so as to protect cells from oxidative stress and to promote healthier skin, by mainly alleviating inflammation and damage caused by oxidative agents | [170] |
| Vitamin D (especially its D3 metabolite) induces beneficial anti-aging and photoprotective effects on the skin | In vivo | Anti-aging, Photoprotective (Anti-UV), Anti-inflammatory, Antioxidant |
| Vitamin D induces the activation of pathways that reduce ROS, inhibit DNA damage, and promote DNA repair, protecting the skin from premature aging and photoaging, mostly due to its antioxidant and anti-inflammatory profile | [27] |
| Vitamin D can act as a protective agent against aging by regulating cellular processes such as oxidative stress, immune response, and mitochondrial function | In vivo and in vitro | Anti-aging, Photoprotective (Anti-UV), Anti-inflammatory, Antioxidant |
| Vitamin D has several anti-aging-associated properties:
| [34] |
| Cosmetic Type | Purpose of Vitamin D | Effects | Examples | References |
|---|---|---|---|---|
| Moisturizers | Enhances hydration and skin barrier function | Improve moisture retention and sooth dry skin | Vitamin D3 creams, hydrating lotions | [216] |
| Anti-aging products | Supports skin homeostasis, reduces oxidative stress, improves elasticity | Stimulate DNA repair, enhance collagen production, antioxidant and anti-photoaging property, reduce wrinkles | D3 anti-aging serums | [27,28,34,224] |
| Acne treatments | Reduces inflammation and promotes healing | Reduce skin sebum, clear acne scars | Acne serums with vitamin D | [225] |
| Psoriasis treatments | Reduces inflammation | Help keratinocyte regeneration | Ointments | [219,226,227,228] |
| Cleansers | Mild exfoliation and hydration | Cleanse without stripping natural oils | Cleansers with vitamin D derivatives | [121] |
| Scalp treatments | Improves scalp health and prevents dandruff | Reduce flakiness, support hair follicle health | Shampoos, hair oils, and scalp serums | [210] |
| Hand creams | Repairs cracked skin, enhances hydration | Improve moisture retention and sooth dry skin | Hand creams with vitamin D | [229] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, S.N.A.; Anastasiou, E.A.; Adamantidi, T.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications. Appl. Sci. 2025, 15, 796. https://doi.org/10.3390/app15020796
Papadopoulou SNA, Anastasiou EA, Adamantidi T, Ofrydopoulou A, Letsiou S, Tsoupras A. A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications. Applied Sciences. 2025; 15(2):796. https://doi.org/10.3390/app15020796
Chicago/Turabian StylePapadopoulou, Sofia Neonilli A., Elena A. Anastasiou, Theodora Adamantidi, Anna Ofrydopoulou, Sophia Letsiou, and Alexandros Tsoupras. 2025. "A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications" Applied Sciences 15, no. 2: 796. https://doi.org/10.3390/app15020796
APA StylePapadopoulou, S. N. A., Anastasiou, E. A., Adamantidi, T., Ofrydopoulou, A., Letsiou, S., & Tsoupras, A. (2025). A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications. Applied Sciences, 15(2), 796. https://doi.org/10.3390/app15020796









