A Comparative Study of Advanced Oxidation Processes for the Removal of the Antibiotic Sulfadoxine from Water—Transformation Products and Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. AOPs Setups
2.3. Instrumental Analysis
2.4. Toxicity Assessment
3. Results and Discussion
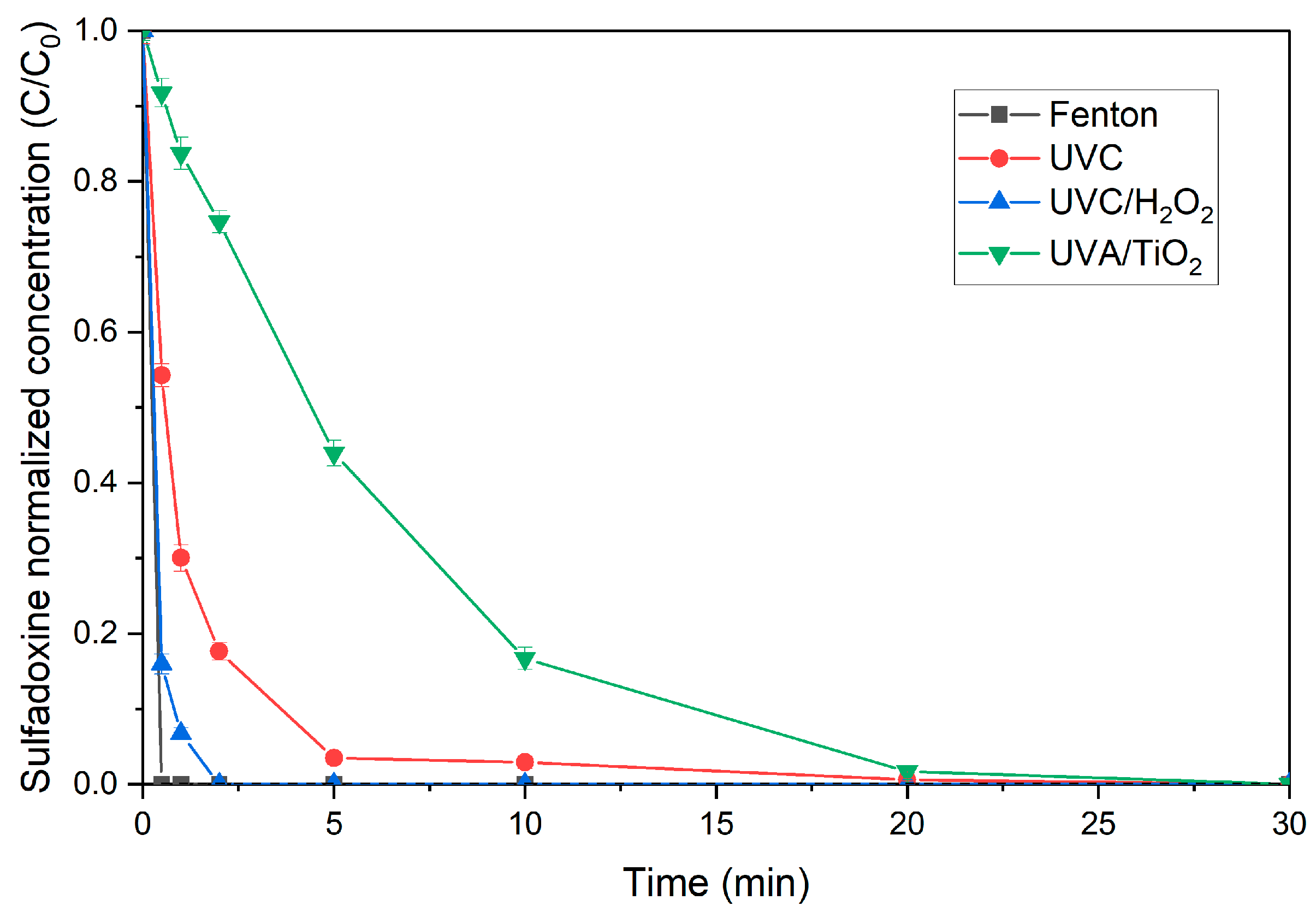
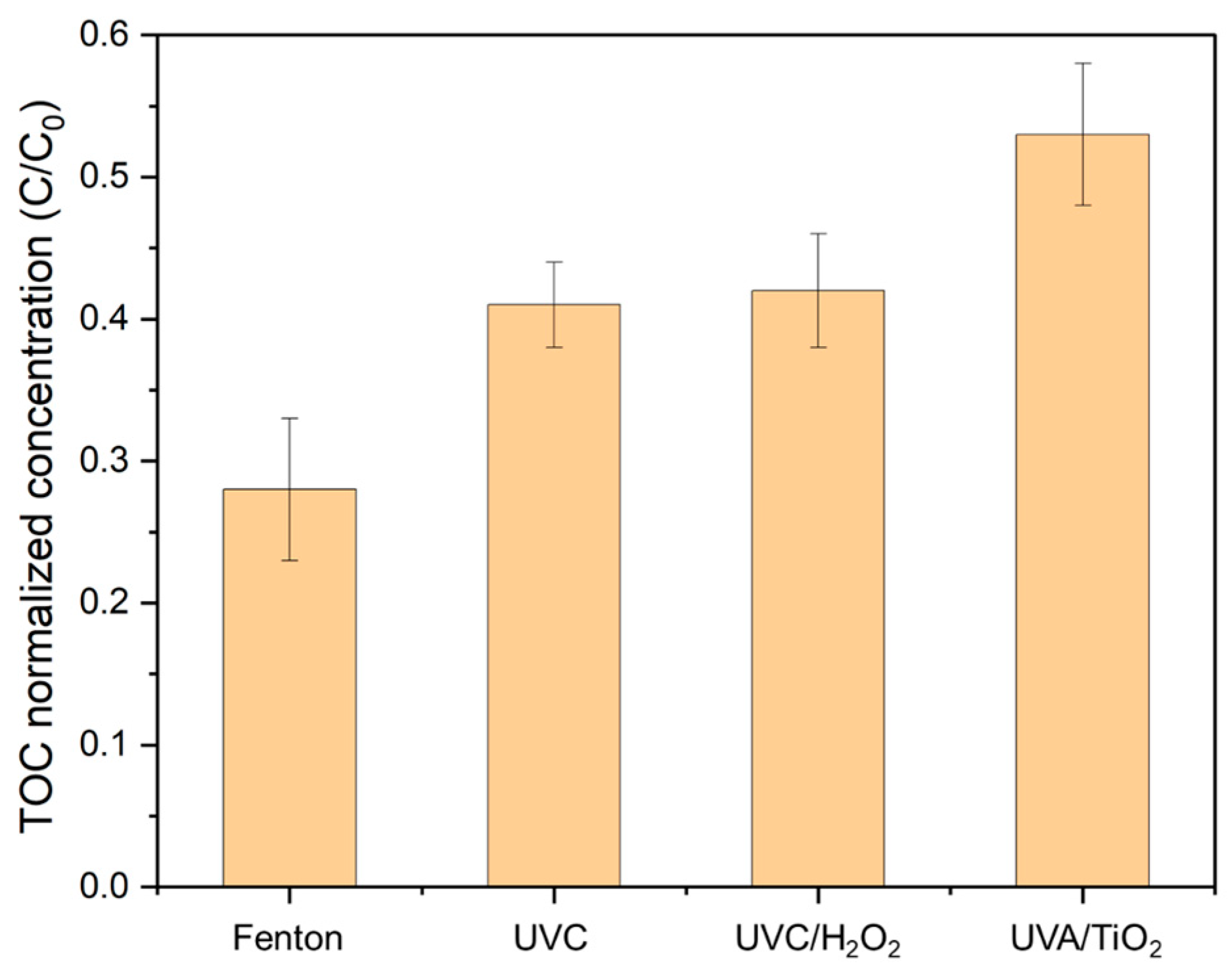
3.1. Screening of Different AOPs
3.1.1. Fenton Reagent
3.1.2. Photolysis Processes
3.1.3. Heterogeneous Photocatalysis Processes
3.2. Parametric Investigation of UV-A/TiO2 Process
3.2.1. The Effect of UV-A Radiant Power per Volume Unit
3.2.2. The Effect of Solution pH
3.2.3. The Effect of Catalyst Loading
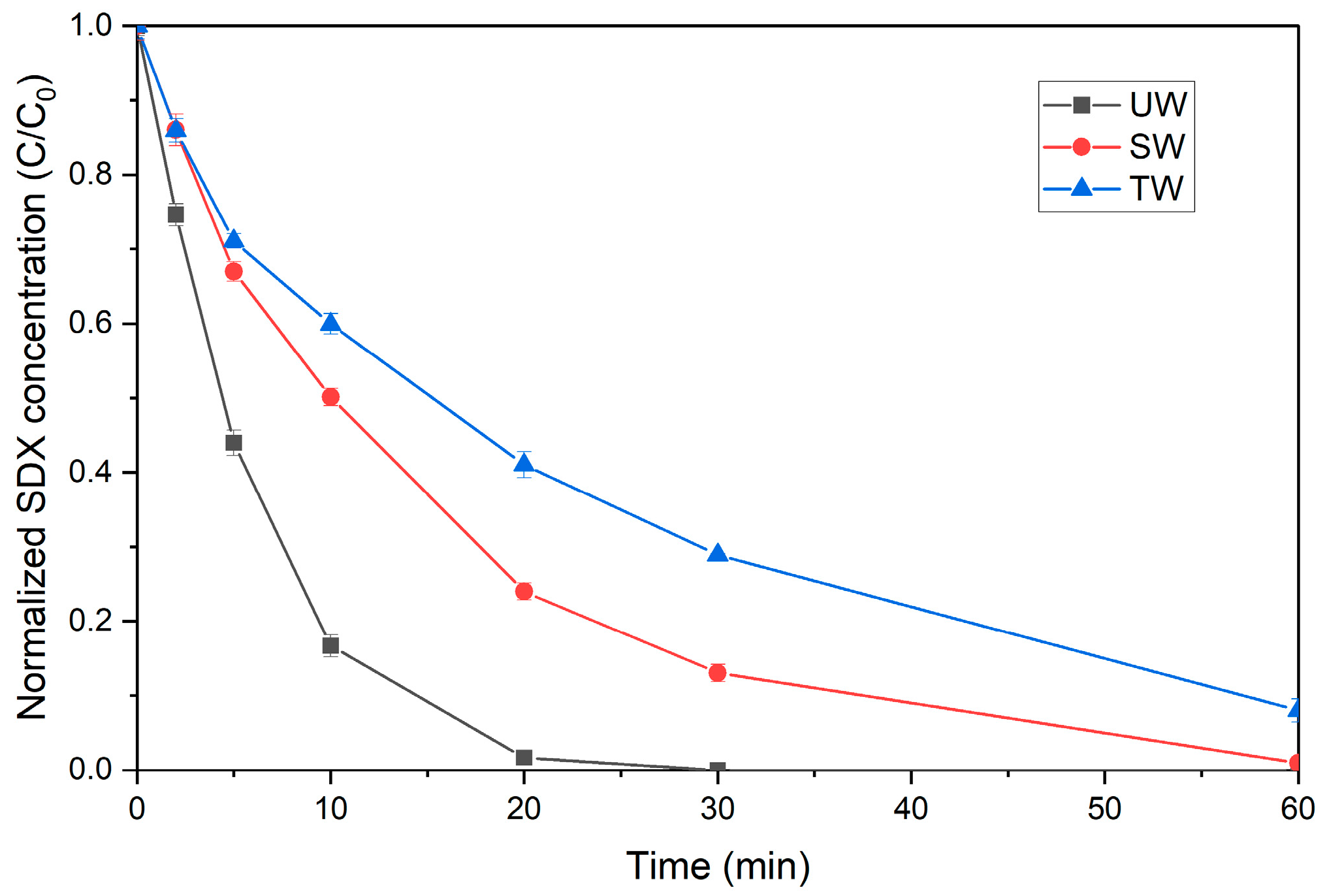
3.3. Investigation of Water Matrix Effect
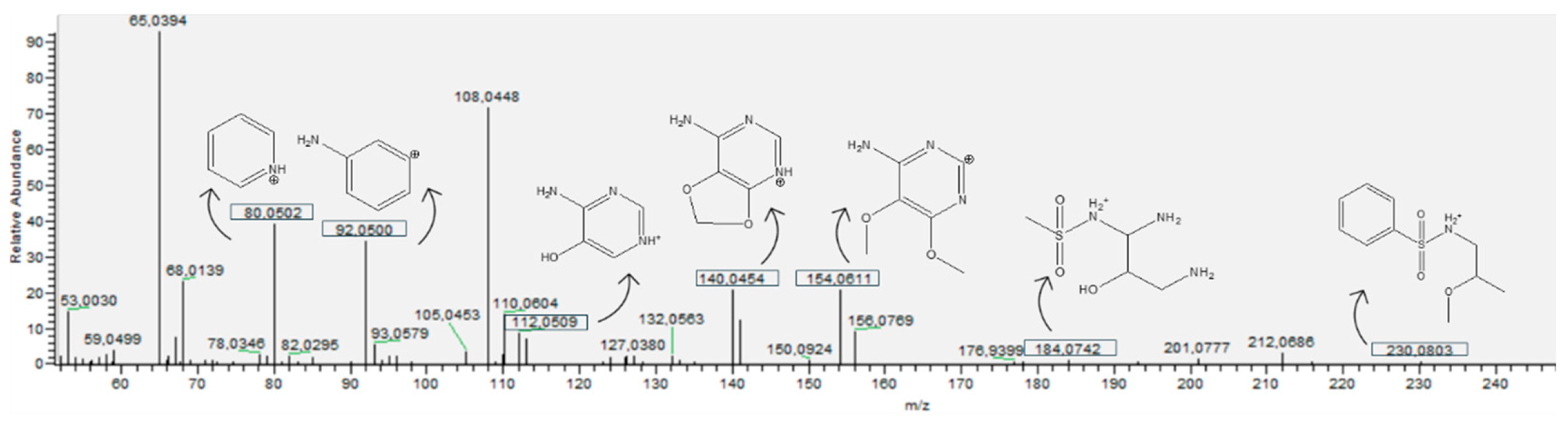
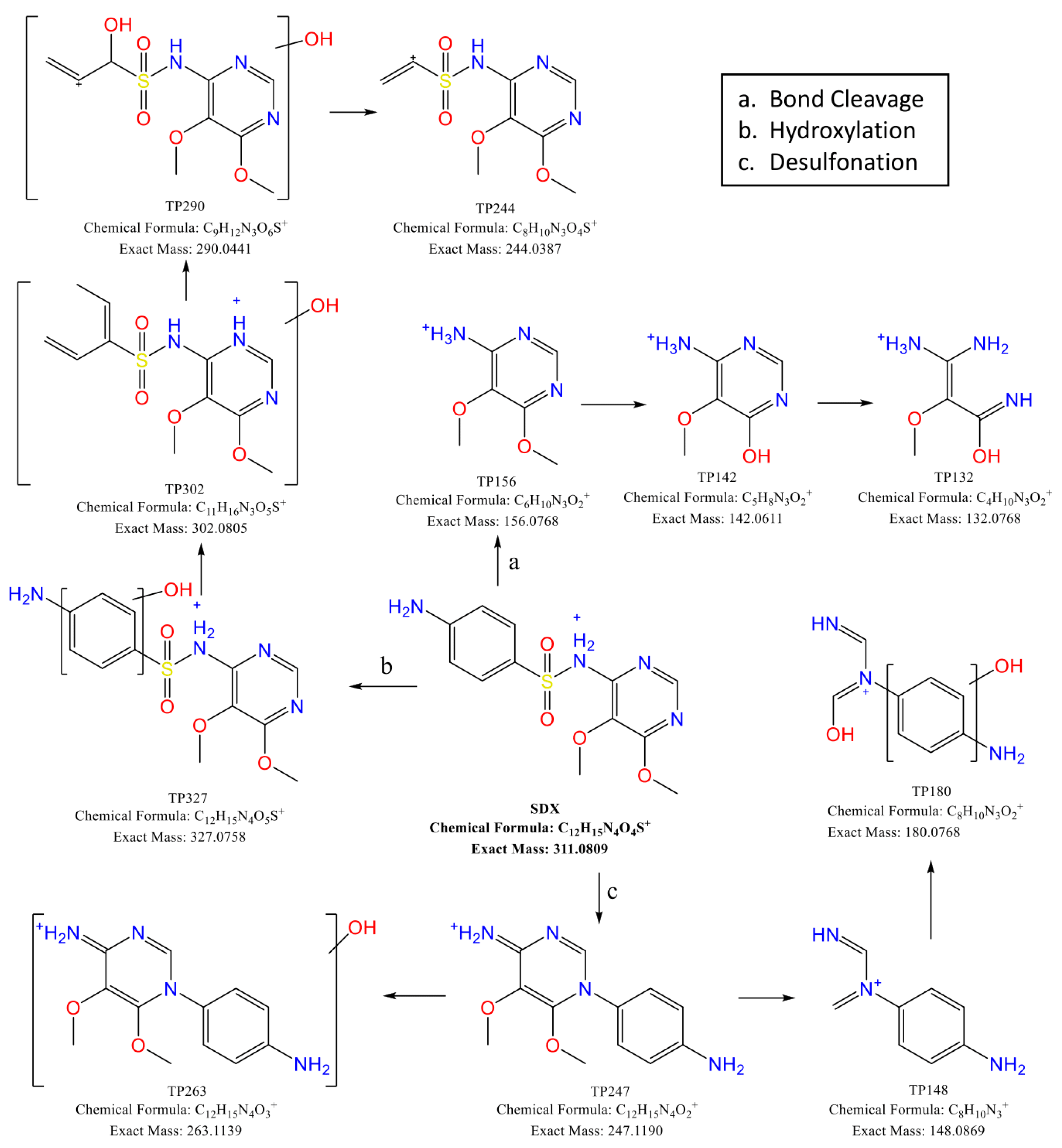
3.4. SDX Transformation Products
3.4.1. Bond Cleavage
3.4.2. Hydroxylation
3.4.3. Desulfonation
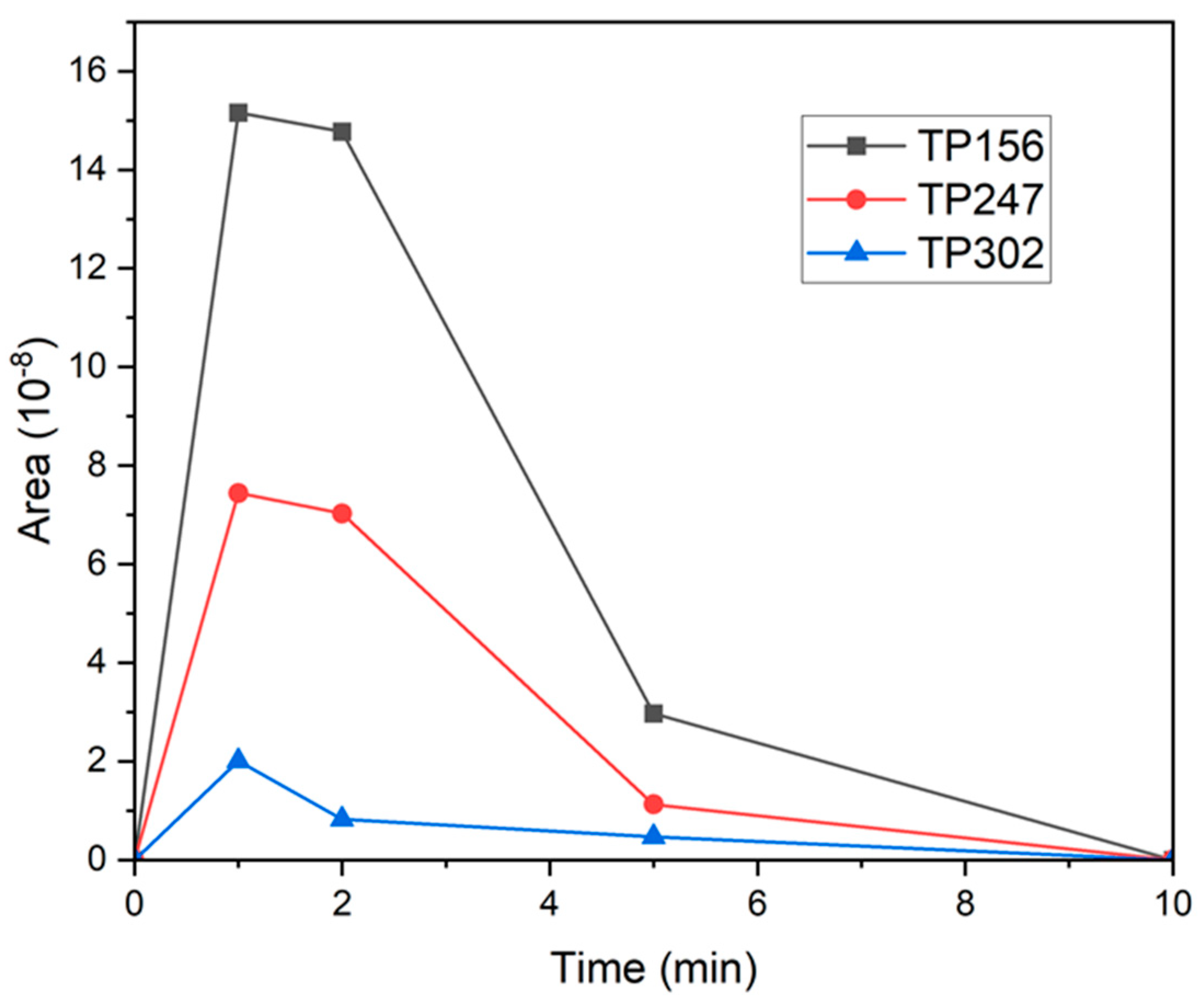
3.5. Evaluation of TP Time Profiles
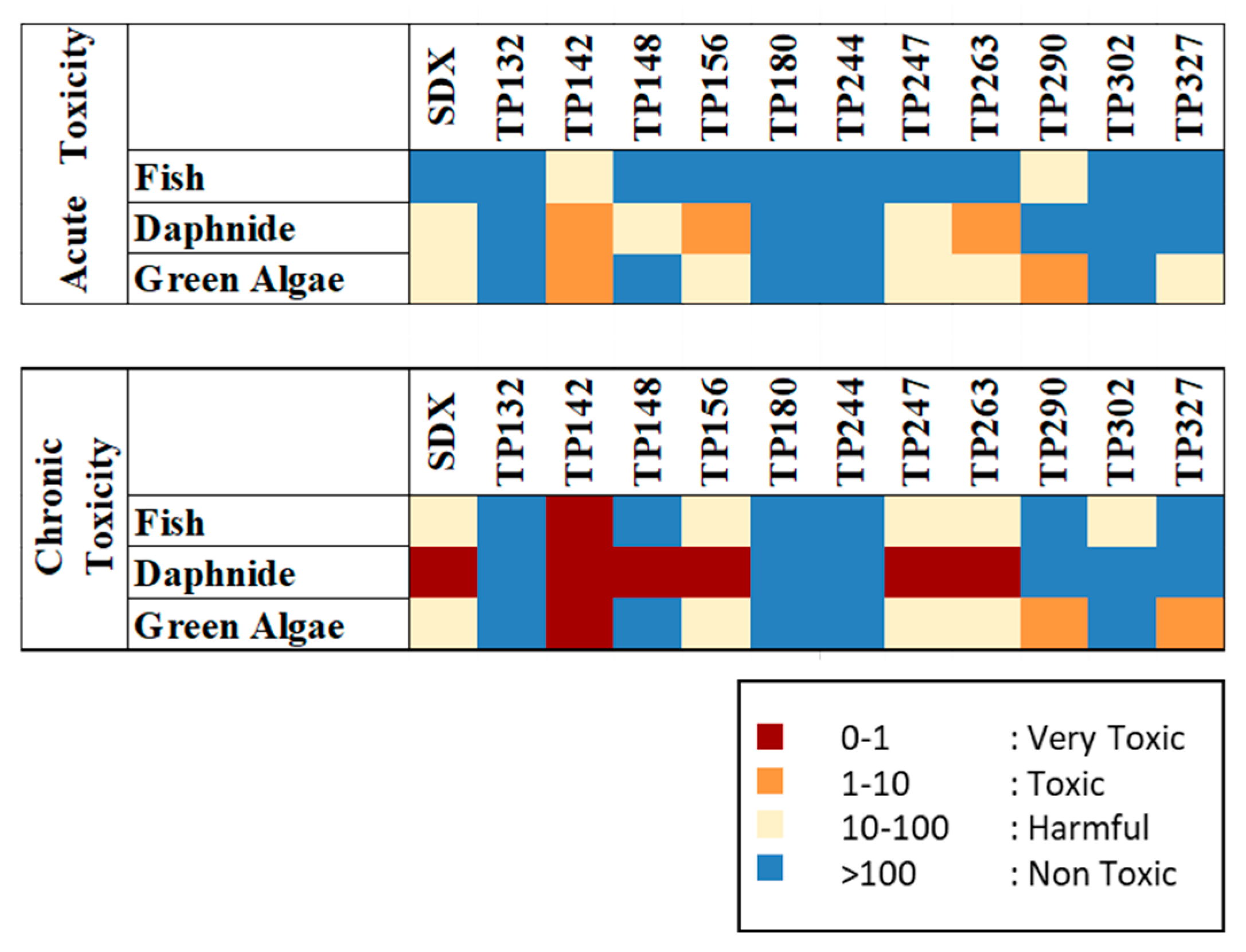
3.6. Assessment of TP Ecotoxicity (In Silico)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moskalik, M.Y. Sulfonamides with Heterocyclic Periphery as Antiviral Agents. Molecules 2023, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Kovalenko, A.; Valtari, A.; Nocentini, A.; Gureev, M.; Urtti, A.; Korsakov, M.; Supuran, C.T.; Krasavin, M. 5-(Sulfamoyl)thien-2-yl 1,3-oxazole inhibitors of carbonic anhydrase II with hydrophilic periphery. J. Enzym. Inhib. Med. Chem. 2022, 37, 1005–1011. [Google Scholar] [CrossRef]
- Ovung, A.; Bhattacharyya, J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef]
- Calderón, F.; Wilson, D.M.; Gamo, F.-J. Chapter Three—Antimalarial Drug Discovery: Recent Progress and Future Directions. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 97–151. [Google Scholar]
- Yun, M.-K.; Wu, Y.; Li, Z.; Zhao, Y.; Waddell, M.B.; Ferreira, A.M.; Lee, R.E.; Bashford, D.; White, S.W. Catalysis and Sulfa Drug Resistance in Dihydropteroate Synthase. Science 2012, 335, 1110–1114. [Google Scholar] [CrossRef]
- Davies, S.C.; Fowler, T.; Watson, J.; Livermore, D.M.; Walker, D. Annual Report of the Chief Medical Officer: Infection and the rise of antimicrobial resistance. Lancet 2013, 381, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.L.; Oliver, K.B. Antibiotic Resistance Threats in the United States: Stepping Back from the Brink. Am. Fam. Physician 2014, 89, 938–941. [Google Scholar]
- Neu, H.C. The Crisis in Antibiotic Resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Barber, M. Staphylococcal Infection due to Penicillin-resistant Strains. Br. Med. J. 1947, 2, 863–865. [Google Scholar] [CrossRef]
- Van, D.-A.; Ngo, T.H.; Huynh, T.H.; Nakada, N.; Ballesteros, F.; Tanaka, H. Distribution of pharmaceutical and personal care products (PPCPs) in aquatic environment in Hanoi and Metro Manila. Environ. Monit. Assess. 2021, 193, 847. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.; Tu, G.; Zhang, S.; Zhang, P. Novel aqueous biphasic system based on ionic liquid for the simultaneous extraction of seven active pharmaceutical ingredients in aquatic environment. Environ. Sci. Pollut. Res. 2021, 28, 17853–17864. [Google Scholar] [CrossRef] [PubMed]
- Barbash, J.E. The geochemistry of pesticides. Treatise Geochem. 2007, 9, 612. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jing, J.; Zhou, M.; Dewil, R. Recent advances in H2O2-based advanced oxidation processes for removal of antibiotics from wastewater. Chin. Chem. Lett. 2023, 34, 107621. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Mohammed, T.J.; Al-Najar, J.A. Advanced Oxidation Processes (AOPs) for treatment of antibiotics in wastewater: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012109. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Zhang, H.; Wang, H.; Sun, P. Enhanced Degradation of Sulfonamide Antibiotics by UV Irradiation Combined with Persulfate. Processes 2021, 9, 226. [Google Scholar] [CrossRef]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E.; Heidari, Z. Degradation of sulfonamide antibiotics using ozone-based advanced oxidation process: Experimental, modeling, transformation mechanism and DFT study. Sci. Total Environ. 2020, 734, 139446. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Nannou, C.; Evgenidou, E.; Lambropoulou, D.A. Does climbazole instigate a threat in the environment as persistent, mobile and toxic compound? Unveiling the occurrence and potential ecological risks of its phototransformation products in the water cycle. J. Hazard. Mater. 2023, 458, 131854. [Google Scholar] [CrossRef] [PubMed]
- Kołecka, K.; Gajewska, M.; Stepnowski, P.; Caban, M. Spatial distribution of pharmaceuticals in conventional wastewater treatment plant with Sludge Treatment Reed Beds technology. Sci. Total Environ. 2019, 647, 149–157. [Google Scholar] [CrossRef]
- Sinclair, C.J.; Boxall, A.B.A. Assessing the Ecotoxicity of Pesticide Transformation Products. Environ. Sci. Technol. 2003, 37, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-X.; Li, X.-F. Interfacial debonding of an orthotropic half-plane bonded to a rigid foundation. Int. J. Solids Struct. 2019, 161, 1–10. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Nannou, C.; Evgenidou, E.; Lambropoulou, D. Overarching issues on relevant pesticide transformation products in the aquatic environment: A review. Sci. Total Environ. 2022, 815, 152863. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.-S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Akbari, M.Z.; Xu, Y.; Lu, Z.; Peng, L. Review of antibiotics treatment by advance oxidation processes. Environ. Adv. 2021, 5, 100111. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Petsi, P.N.; Sarasidis, V.C.; Plakas, K.V.; Karabelas, A.J. Reduction of nitrates in a photocatalytic membrane reactor in the presence of organic acids. J. Environ. Manag. 2021, 298, 113526. [Google Scholar] [CrossRef]
- George, P. Reaction Between Catalase and Hydrogen Peroxide. Nature 1947, 160, 41–43. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jebalbarezi, B.; Dehghanzadeh, R.; Maryamabadi, A.; Aslani, H. Sulfamethoxazole oxidation in secondary treated effluent using Fe(VI)/PMS and Fe(VI)/H2O2 processes: Experimental parameters, transformation products, reaction pathways and toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 107446. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Xu, J.; Huang, C.-H. Enhanced Direct Photolysis of Organic Micropollutants by Far-UVC Light at 222 nm from KrCl* Excilamps. Environ. Sci. Technol. Lett. 2023, 10, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Sun, P.P.; Araud, E.; Nguyen, T.H. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222 nm. Water Res. 2020, 186, 116386. [Google Scholar] [CrossRef]
- Sharma, V.K.; Demir, H.V. Bright Future of Deep-Ultraviolet Photonics: Emerging UVC Chip-Scale Light-Source Technology Platforms, Benchmarking, Challenges, and Outlook for UV Disinfection. ACS Photonics 2022, 9, 1513–1521. [Google Scholar] [CrossRef]
- Salim, M.M.F.F.; Novack, A.; Soares, P.A.; Medeiros, Â.; Granato, M.A.; Souza, A.A.U.; Vilar, V.J.P.; Souza, S.M.A.G.U. Photochemical UVC/H2O2 oxidation system as an effective method for the decolourisation of bio-treated textile wastewaters: Towards onsite water reuse. RSC Adv. 2016, 6, 90631–90645. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A review on heterogeneous photocatalytic materials: Mechanism, perspectives, and environmental and energy sustainability applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Boncagni, N.T.; Otaegui, J.M.; Warner, E.; Curran, T.; Ren, J.; Fidalgo de Cortalezzi, M.M. Exchange of TiO2 Nanoparticles between Streams and Streambeds. Environ. Sci. Technol. 2009, 43, 7699–7705. [Google Scholar] [CrossRef] [PubMed]
- Şanli, N.; Şanli, S.; Özkan, G.; Denizlic, A. Determination of pKa values of some sulfonamides by LC and LC-PDA methods in acetonitrile-water binary mixtures. J. Braz. Chem. Soc. 2010, 21, 1952–1960. [Google Scholar] [CrossRef]
- Ge, P.; Yu, H.; Chen, J.; Qu, J.; Luo, Y. Photolysis mechanism of sulfonamide moiety in five-membered sulfonamides: A DFT study. Chemosphere 2018, 197, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic degradation of sulfa drugs with TiO2, Fe salts and TiO2/FeCl3 in aquatic environment-Kinetics and degradation pathway. Appl. Catal. B Environ. 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Feng, M.; Huang, C.-H.; Sun, P. Abiotic transformation and ecotoxicity change of sulfonamide antibiotics in environmental and water treatment processes: A critical review. Water Res. 2021, 202, 117463. [Google Scholar] [CrossRef]
- Plakas, K.V.; Taxintari, A.; Karabelas, A.J. Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment. Water 2019, 11, 1794. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zahraa, O.; Bouchy, M. Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J. Photochem. Photobiol. A Chem. 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Tang, G.; Chen, Y.; Lin, S.; Li, X. The photo- and microbial degradation kinetics and pathways of sulfadoxine in seawater elucidated by liquid chromatography coupled with time-of-flight mass spectrometry. Chemosphere 2024, 351, 141225. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, N.D.H.; Mahmoud, W.M.M.; Hadad, G.M.; Abdel-Salam, R.A.; Kümmerer, K. Photolysis of sulfamethoxypyridazine in various aqueous media: Aerobic biodegradation and identification of photoproducts by LC-UV–MS/MS. J. Hazard. Mater. 2013, 244–245, 654–661. [Google Scholar] [CrossRef]
- Pang, R.; Li, N.; Hou, Z.; Huang, J.; Yue, C.; Cai, Y.; Song, J. Degradation of sulfonamide antibiotics and a structurally related compound by chlorine dioxide: Efficiency, kinetics, potential products and pathways. Chem. Eng. J. 2023, 451, 138502. [Google Scholar] [CrossRef]
- Shad, A.; Chen, J.; Qu, R.; Dar, A.A.; Bin-Jumah, M.; Allam, A.A.; Wang, Z. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways. Chem. Eng. J. 2020, 398, 125357. [Google Scholar] [CrossRef]
- The European Parliament; Council of the European Union. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA Relevance); The European Parliament: Strasbourg, France; Council of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.-M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; De Vito, S.C. Predicting azo dye toxicity. Crit. Rev. Environ. Sci. Technol. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Fu, W.; Li, B.; Yang, J.; Yi, H.; Chai, L.; Li, X. New insights into the chlorination of sulfonamide: Smiles-type rearrangement, desulfation, and product toxicity. Chem. Eng. J. 2018, 331, 785–793. [Google Scholar] [CrossRef]
- Shad, A.; Li, C.; Zuo, J.; Liu, J.; Dar, A.A.; Wang, Z. Understanding the ozonated degradation of sulfadimethoxine, exploration of reaction site, and classification of degradation products. Chemosphere 2018, 212, 228–236. [Google Scholar] [CrossRef]
| Experiment No. | UV-A Light Dose (W/L) | TOC Removal (%) | EEO (kWh/m3) |
|---|---|---|---|
| R 1 | 2.13 | 54 ± 3 | 30.8 |
| R 2 | 4.26 | 77 ± 2 | 32.2 |
| R 3 | 6.39 | 85 ± 4 | 35.7 |
| R 4 | 8.52 | 87 ± 2 | 49.9 |
| k (min−1) | R2 | |
|---|---|---|
| UW | 0.1972 | 0.9973 |
| TW | 0.0407 | 0.9969 |
| SW | 0.0757 | 0.9964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizirtsakis, P.A.; Anagnostopoulou, K.; Sarasidis, V.C.; Petsi, P.; Moschona, A.; Plakas, K.V.; Lambropoulou, D.A. A Comparative Study of Advanced Oxidation Processes for the Removal of the Antibiotic Sulfadoxine from Water—Transformation Products and Toxicity. Appl. Sci. 2025, 15, 793. https://doi.org/10.3390/app15020793
Bizirtsakis PA, Anagnostopoulou K, Sarasidis VC, Petsi P, Moschona A, Plakas KV, Lambropoulou DA. A Comparative Study of Advanced Oxidation Processes for the Removal of the Antibiotic Sulfadoxine from Water—Transformation Products and Toxicity. Applied Sciences. 2025; 15(2):793. https://doi.org/10.3390/app15020793
Chicago/Turabian StyleBizirtsakis, Panagiotis A., Kyriaki Anagnostopoulou, Vasilis C. Sarasidis, Panagiota Petsi, Alexandra Moschona, Konstantinos V. Plakas, and Dimitra A. Lambropoulou. 2025. "A Comparative Study of Advanced Oxidation Processes for the Removal of the Antibiotic Sulfadoxine from Water—Transformation Products and Toxicity" Applied Sciences 15, no. 2: 793. https://doi.org/10.3390/app15020793
APA StyleBizirtsakis, P. A., Anagnostopoulou, K., Sarasidis, V. C., Petsi, P., Moschona, A., Plakas, K. V., & Lambropoulou, D. A. (2025). A Comparative Study of Advanced Oxidation Processes for the Removal of the Antibiotic Sulfadoxine from Water—Transformation Products and Toxicity. Applied Sciences, 15(2), 793. https://doi.org/10.3390/app15020793






