Determination of the Antioxidant Capacity of Germinated and Yeast-Fermented Sweet and Bitter Lupin Seeds and Sprouts via Cyclic Voltammetry Compared to the Spectrophotometric and Photochemiluminescence Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material, Germination, and Fermentation
2.3. Preparation of Hydrophilic (H) and Lipophilic (L) Extracts
2.4. Determination of the Total Phenolic Compound (TPC) Content
2.5. Cyclic Voltammetric (CV) Experiments
2.6. Photochemiluminescence Assay
2.7. DPPH Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Compound (TPC) Content
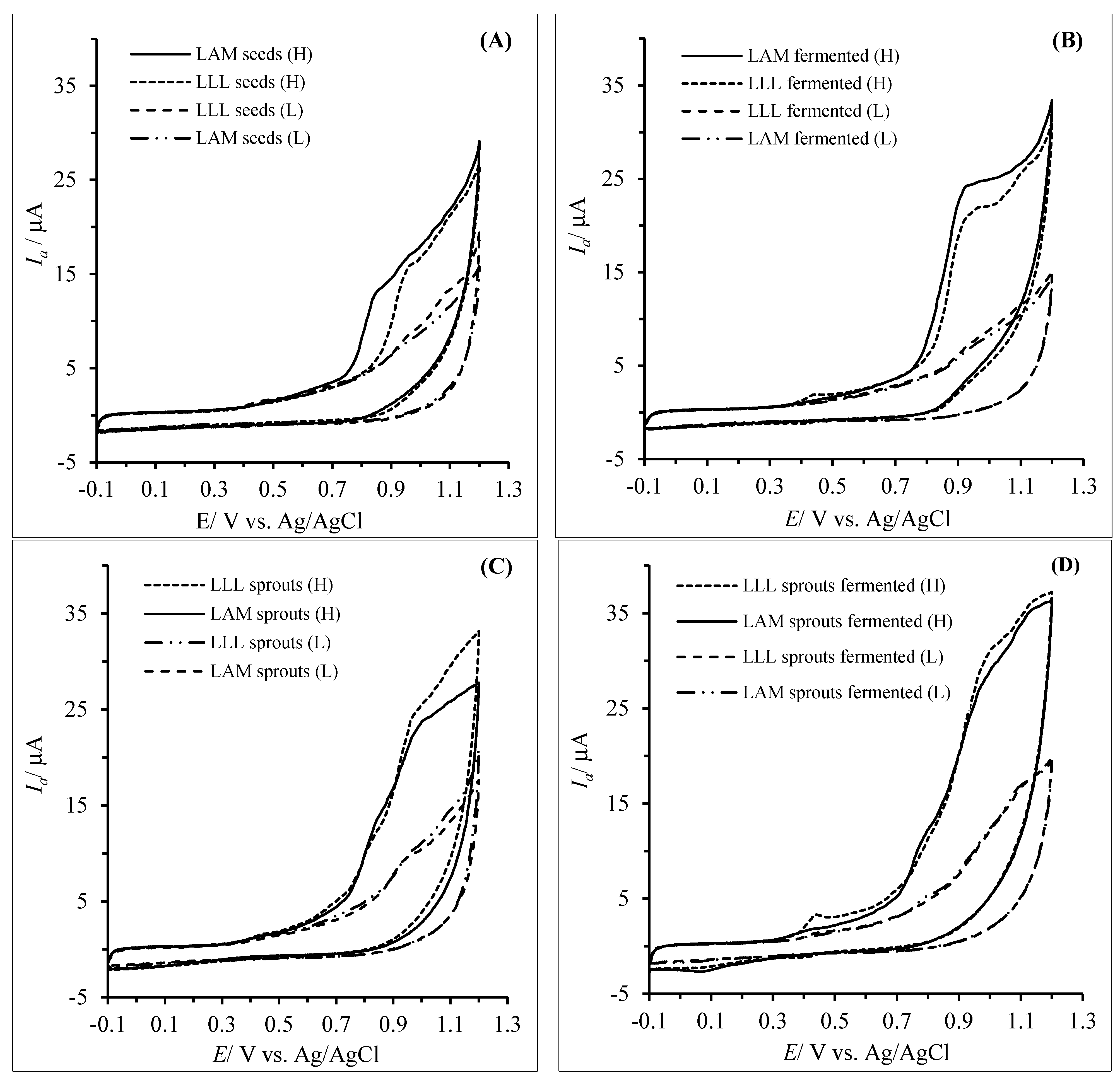
3.2. Antioxidant/Reducing Capacity of the Hydrophilic and Lipophilic Fractions Determined via the Cyclic Voltammetry Method
3.3. Antioxidant Capacity of the Hydrophilic and Lipophilic Fractions Determined by the PCL Method
3.4. Antioxidant Capacity of the Hydrophilic and Lipophilic Fractions Determined Against DPPH⦁ Radicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zander, P.; Amjath-Babu, T.S.; Preissel, S.; Reckling, M.; Bues, A.; Schläfke, N.; Kuhlman, T.; Bachinger, J.; Uthes, S.; Stoddard, F.; et al. Grain legume decline and potential recovery in European agriculture: A review. Agron. Sustain. Dev. 2016, 36, 26. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a source of bioactive antioxidant compounds for food products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Kotlarz, B.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawey, A.A.; Gafar, A.F. Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates. Food Chem. 2001, 74, 455–462. [Google Scholar] [CrossRef]
- Rumiyati, R.; Jayasena, V.; James, A.P. Total Phenolic and Phytosterol Compounds and the Radical Scavenging Activity of Germinated Australian Sweet Lupin Flour. Plant Foods Hum. Nutr. 2013, 68, 352–357. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Piskula, M.K.; Zielinski, H.; Kozlowska, H.; Frias, J.; Vidal-Valverde, C. Germination as a process to improve the antioxidant capacity of Lupinus angustifolius L. var. Zapaton. Eur. Food Res. Technol. 2006, 223, 495–502. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods, and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Głód, B.; Kiersztyn, I.; Piszcz, P. Total antioxidant potential assay with cyclic voltammetry and/or differential pulse voltammetry measurements. J. Electroanal. Chem. 2014, 719, 24–29. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Rad. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Valek, L.; Resetic, J.; Rusic, D.F. Cyclic voltammetry study of plasma antioxidant capacity–comparison with the DPPH and TAS spectrophotometric methods. J. Electrochem. Chem. 2006, 588, 68–73. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC–DAD–MS/MS. Antioxidants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Chilomer, K.; Zaleska, K.; Ciesiołka, D.; Gulewicz, P.; Frankiewicz, A.; Gulewicz, K. Changes in the alkaloid, alpha-galactoside and protein fractions content during germination of different lupin species. Acta Soc. Bot. Pol. 2010, 79, 1. [Google Scholar]
- Shahidi, F.; Naczk, M. Methods of analysis and quantification of phenolic compounds. In Food Phenolic: Sources, Chemistry, Effects and Applications; Shahidi, F., Naczk, M., Eds.; Technomic Publishing Company: Lancaster, PA, USA, 1995; pp. 287–293. [Google Scholar]
- Popov, I.; Lewin, G. Antioxidative homeostasis: Characterisation by means of chemiluminescent technique in methods in enzymology. In Oxidants and Antioxidants; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 300, pp. 96–100. [Google Scholar]
- Zielińska, D.; Frias, J.; Piskuła, M.K.; Kozłowska, H.; Zieliński, H.; Vidal-Valverde, C. Evaluation of the antioxidant capacity of lupin sprouts germinated in the presence of selenium. Eur. Food Res. Technol. 2008, 227, 1711–1720. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compost. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidnera, S. Content of Phenolic Compounds and Antioxidant Properties in Seeds of Sweet and Bitter Cultivars of Lupine (Lupinus angustifolius). Nat. Prod. Commun. 2018, 13, 1341–1344. [Google Scholar]
- Fernandez-Orozco, R.; Frias, J.; Muñoz, R.; Vidal-Valverde, C. Effect of fermentation conditions on the antioxidant compounds and antioxidant capacity of Lupinus angustifolius cv. zapaton. Eur. Food Res. Technol. 2008, 227, 979–988. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus albus L. Var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Cho, Y.S.; Yeum, K.J.; Chen, C.Y.; Beretta, G.; Tang, G.; Krinsky, N.I.; Yoon, S.; Lee-Kim, Y.C.; Blumberg, J.B.; Russell, R.M. Phytonutrients affecting hydrophilic and lipophilic antioxidant activities in fruits, vegetables and legumes. J. Sci. Food Agric. 2007, 87, 1096–1107. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, Antihypertensive, Antidiabetic and Antioxidant Activities of Solid-State Fermented Lupin, Quinoa and Wheat by Bifidobacterium Species: In-Vitro Investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In Vitro Investigation of Bioactivities of Solid-State Fermented Lupin, Quinoa and Wheat Using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.E.; Jayasena, V. Development of a Fermented Product with Higher Phenolic Compounds and Lower Anti-Nutritional Factors from Germinated Lupin (Lupinus angustifolius L.). J. Food Process. Preserv. 2018, 42, e13843. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Piskuła, M.K. An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads. Antioxidants 2022, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.D.; Bains, A.; Sridhar, K.; Sharma, M.; Dhull, S.B.; Goksen, G.; Chawla, P.; Inbaraj, B.S. Recent advances in the analytical methods for quantitative determination of antioxidants in food matrice. Food Chem. 2025, 463, 141348. [Google Scholar] [CrossRef] [PubMed]
| Sample | Total Phenolic Compound Content |
|---|---|
| LLL seeds | 0.76 ± 0.01 a |
| LAM seeds | 6.99 ± 2.31 b |
| LLL sprouts | 3.24 ± 0.01 c |
| LAM sprouts | 6.74 ± 0.04 b |
| fermented LLL | 1.72 ± 0.06 d |
| fermented LAM | 7.05 ± 0.09 b |
| fermented LLL sprouts | 3.44 ± 0.11 b |
| fermented LAM sprouts | 5.66 ± 0.70 e |
| Sample | Hydrophilic Fraction | Lipophilic Fraction | Total Antioxidant Capacity |
|---|---|---|---|
| LLL seeds | 4.56 ± 0.14 a (67.2%) | 2.18 ± 0.20 a (32.3%) | 6.74 ± 0.19 a |
| LAM seeds | 6.24 ± 0.19 b (77.9%) | 1.77 ± 0.12 b (22.1%) | 8.01 ± 0.16 b |
| LLL sprouts | 9.47 ± 0.15 c (76.6%) | 2.90 ± 0.08 c (23.4%) | 12.37 ± 0.17 c |
| LAM sprouts | 8.57 ± 0.07 d (78.0%) | 2.43 ± 0.19 a (22.0%) | 10.99 ± 0.15 d |
| fermented LLL | 7.53 ± 0.27 e (82.8%) | 1.56 ± 0.18 b (17.2%) | 9.09 ± 0.22 e |
| fermented LAM | 9.13 ± 0.15 f (89.0%) | 1.13 ± 0.14 d (11.0%) | 10.26 ± 0.06 f |
| fermented LLL sprouts | 11.62 ± 0.51 g (80.2%) | 2.87 ± 0.24 c (19.8%) | 14.48 ± 0.44 g |
| fermented LAM sprouts | 11.23 ± 0.57 g (78.3%) | 3.11 ± 0.07 c (21.7%) | 14.35 ± 0.49 g |
| Sample | Hydrophilic Fraction | Lipophilic Fraction | Total Antioxidant Capacity |
|---|---|---|---|
| LLL seeds | 0.36 ± 0.02 a (52.9%) | 0.32 ± 0.04 a (47.1%) | 0.68 ± 0.07 a |
| LAM seeds | 0.25 ± 0.02 a (43.1%) | 0.32 ± 0.03 a (56.9%) | 0.58 ± 0.05 a |
| LLL sprouts | 1.37 ± 0.07 b (79.2%) | 0.36 ± 0.01 a (20.8%) | 1.73 ± 0.07 b |
| LAM sprouts | 1.42 ± 0.01 b (78.9%) | 0.38 ± 0.02 a (21.1%) | 1.80 ± 0.03 b |
| fermented LLL | 1.13 ± 0.00 c (84.3%) | 0.20 ± 0.02 b (15.7%) | 1.34 ± 0.02 c |
| fermented LAM | 0.74 ± 0.00 d (82.2%) | 0.17 ± 0.00 b (17.8%) | 0.90 ± 0.01 d |
| fermented LLL sprouts | 4.37 ± 0.06 e (81.1%) | 1.01 ± 0.01 c (18.9%) | 5.39 ± 0.07 e |
| fermented LAM sprouts | 2.24 ± 0.08 f (66.7%) | 1.11 ± 0.03 d (33.3%) | 3.35 ± 0.05 f |
| Sample | Hydrophilic Fraction | Lipophilic Fraction | Total Antioxidant Capacity |
|---|---|---|---|
| LLL seeds | 2.25 ± 0.02 a (80.4%) | 0.54 ± 0.03 a (19.6%) | 2.80 ± 0.03 a |
| LAM seeds | 2.32 ± 0.02 b (84.4%) | 0.44 ± 0.03 a (15.6%) | 2.75 ± 0.03 a |
| LLL sprouts | 2.88 ± 0.01 c (87.0%) | 0.43 ± 0.02 a (13.0%) | 3.31 ± 0.02 b |
| LAM sprouts | 2.59 ± 0.02 d (84.9%) | 0.45 ± 0.02 a (14.8%) | 3.05 ± 0.01 c |
| fermented LLL | 2.25 ± 0.01 a (73.1%) | 0.84 ± 0.03 b (26.9%) | 3.08 ± 0.02 c |
| fermented LAM | 2.29 ± 0.01 e (80.6%) | 0.55 ± 0.02 a (19.4%) | 2.84 ± 0.01 a, f |
| fermented LLL sprouts | 2.54 ± 0.01 f (86.1%) | 0.41 ± 0.03 a (13.9%) | 2.95 ± 0.01 d, f |
| fermented LAM sprouts | 2.11 ± 0.02 g (83.4%) | 0.42 ± 0.02 a (16.6%) | 2.53 ± 0.01 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Gulewicz, P.; Kasprowicz-Potocka, M.; Zieliński, H. Determination of the Antioxidant Capacity of Germinated and Yeast-Fermented Sweet and Bitter Lupin Seeds and Sprouts via Cyclic Voltammetry Compared to the Spectrophotometric and Photochemiluminescence Methods. Appl. Sci. 2025, 15, 729. https://doi.org/10.3390/app15020729
Zielińska D, Gulewicz P, Kasprowicz-Potocka M, Zieliński H. Determination of the Antioxidant Capacity of Germinated and Yeast-Fermented Sweet and Bitter Lupin Seeds and Sprouts via Cyclic Voltammetry Compared to the Spectrophotometric and Photochemiluminescence Methods. Applied Sciences. 2025; 15(2):729. https://doi.org/10.3390/app15020729
Chicago/Turabian StyleZielińska, Danuta, Piotr Gulewicz, Małgorzata Kasprowicz-Potocka, and Henryk Zieliński. 2025. "Determination of the Antioxidant Capacity of Germinated and Yeast-Fermented Sweet and Bitter Lupin Seeds and Sprouts via Cyclic Voltammetry Compared to the Spectrophotometric and Photochemiluminescence Methods" Applied Sciences 15, no. 2: 729. https://doi.org/10.3390/app15020729
APA StyleZielińska, D., Gulewicz, P., Kasprowicz-Potocka, M., & Zieliński, H. (2025). Determination of the Antioxidant Capacity of Germinated and Yeast-Fermented Sweet and Bitter Lupin Seeds and Sprouts via Cyclic Voltammetry Compared to the Spectrophotometric and Photochemiluminescence Methods. Applied Sciences, 15(2), 729. https://doi.org/10.3390/app15020729










